Abstract
Both homoharringtonine (HHT) and curcumin exhibit anti-proliferative effects on lymphoma cells, but the effects of combined HHT and curcumin treatment remain unclear. Here, we investigated the effects of HHT/curcumin combination on the proliferation, apoptosis, and invasion in lymphoma cells. CCK-8, flow cytometry, and transwell assays were used to assess proliferation, apoptosis, and invasion of U937 and Raji cells. p-Smad3, E-cadherin, and N-cadherin expression were also measured in Raji cells using Western blot assays. Combination of HHT and curcumin synergistically inhibited U937 and Raji cell proliferation and invasion. In addition, the combination treatment markedly increased apoptosis of Raji cells as evidenced by increased Bax, cleaved caspase 3, and cleaved caspase 9 expression. Meanwhile, the combination treatment promoted anti-tumor mechanisms in Raji cells as indicated by decreases in p-Smad3 and N-cadherin and increases in E-cadherin. In vivo experiments showed that the combination treatment suppressed tumor growth in a mouse Raji xenograft model. Our findings indicate that combination of HHT and curcumin inhibited lymphoma cell growth by downregulating the TGF-β/Smad3 pathway. These results suggest that HHT combined with curcumin might be a promising therapeutic approach for the treatment of lymphoma.
Keywords: lymphomas, homoharringtonine, curcumin, apoptosis, combination treatment
INTRODUCTION
Lymphomas are a heterogeneous group of lymphoid malignancies [1]. Mature lymphoid neoplasms are classified by the WHO as either non-Hodgkin’s lymphoma (NHL) or Hodgkin’s lymphoma (HL) [1, 2]. Burkitt’s lymphoma is a highly invasive NHL that primarily affects children and adolescents [3, 4]. Burkitt’s lymphoma is also an aggressive B cell acute lymphoblastic leukemia [5]. At present, chemotherapy, immunotherapy, and radiotherapy are used to treat lymphomas [6–8]. However, the prognosis of lymphoma patients remains poor due to chemoradiotherapy resistance [9, 10]. Therefore, the development of novel treatment strategies has become a major focus of research.
Homoharringtonine (HHT) is a compound that was initially extracted from the Cephalotaxus hainanensis Li plant used in traditional Chinese medicine [11]. HHT was approved by the US Food and Drug Administration (FDA) for treatment of patients with chronic myeloid leukemia in 2012 [12]. Since the 1970s, HHT has been used in the treatment of hematological malignancies in China [13, 14]. Nguyen et al. found that HHT combined with bortezomib could kill diffuse large B-cell lymphoma (DLBCL) cells [15]. In addition, Klanova et al. found that HHT significantly inhibited cell growth in murine xenograft models of DLBCL [16].
Curcumin is a polyphenol derived from the rhizomes of the Chinese medicinal herb Curcuma Longa L [17, 18]. Curcumin has strong anti-oxidative and anti-inflammatory activities [19], and studies have shown that curcumin exhibits strong anti-tumor effects on colorectal, prostate, breast, and gastric cancers [20–23]. Guorgui et al. found that curcumin exhibited an anti-proliferative effect in HL [24]. In addition, curcumin could be taken up by cells in a 1/20 ratio and then induced the apoptosis of cancer cells [25–28]. Although HHT and curcumin have shown anti-tumor effects in lymphoma cells, little is known about the effects of a combination of these two compounds.
Epithelial-mesenchymal transition (EMT) is an essential process for acquisition of aggressiveness and metastatic capacity in tumor, which is characterized by loss of epithelial markers (such as E-cadherin) and gain of mesenchymal markers (such as N-cadherin). [29]. In addition, transforming growth factor-beta1 (TGF-β1)/Smads signaling has been found to exert an important role in EMT [30]. Evidences have found that activation of TGF-β/Smad3 signaling pathway could promote the metastasis and EMT in tumor cells [31, 32]. In this study, we found that HHT combined with curcumin could exert tumoricidal effects in lymphoma cells in vitro and in vivo via inhibition of TGF-β/Smad3 signaling pathway. These results suggested that HHT combined with curcumin might be a promising therapeutic approach for the treatment of lymphoma.
RESULTS
Combination of curcumin and HHT synergistically inhibited lymphoma cell proliferation
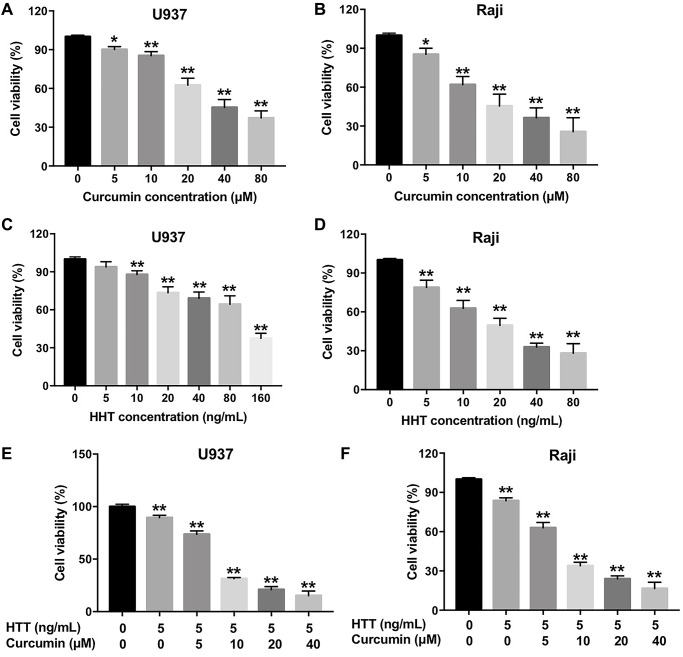
A CCK-8 assay was used to determine the effects of curcumin and HHT on the viability of U937 and Raji cells. As shown in Figure 1A–1D, curcumin or HHT treatment inhibited U937 and Raji cell viability in a dose-dependent manner. In addition, combined HHT and curcumin treatment significantly inhibited U937 and Raji cell viability (Figure 1E and 1F). The IC50 values of curcumin when administered alone were 39.52 μM and 19.79 μM in U937 and Raji cells, respectively; when HHT (5 ng/mL) was combined with curcumin, the latter’s IC50 value was decreased to 7.14 μM and 6.71 μM, respectively (Table 1). Moreover, the CI values for the combination of HHT and curcumin in U937 cells were less than 0.4, which indicated a strong synergistic effect (Table 1). The CI values for combined HHT and curcumin in Raji cells were less than 0.6, also indicating a synergistic effect (Table 1). These data indicate that combination of curcumin and HHT synergistically inhibited lymphoma cell proliferation.
Figure 1.
Combination of curcumin with HHT synergistically inhibited lymphoma cell proliferation. (A) U937 or (B) Raji cells were treated with 0, 5, 10, 20, 40, or 80 μM curcumin for 72 h. CCK-8 assay was used to measure cell viability. (C) U937 or (D) Raji cells were treated with 0, 5, 10, 20, 40, 80, or 160 ng/mL HHT for 72 h. CCK-8 assay was used to measure cell viability. (E) U937 or (F) Raji cells were treated with 5 ng/mL HHT plus curcumin (0, 5, 10, 20, or 40 μM) for 72 h. CCK-8 assay was used to measure cell viability. *P < 0.05, **P < 0.01 compared to control group.
Table 1. Evaluation of combination of HHT with curumin in U937 and raji cells (72 h treatment).
| Drug combination | U937 cells | Raji cells | ||
| IC 50 value | CI values | IC 50 value | CI values | |
| Curumin (range 0 from 40 μM) | IC50 = 39.52 μM | – | IC50 = 19.79 μM | – |
| Curumin + 5 ng/mL HHT | IC50 = 7.14 μM | 0.23 | IC50 = 6.71 μM | 0.59 |
Combination of curcumin and HHT induced apoptosis and suppressed migratory and invasive ability in lymphoma cells
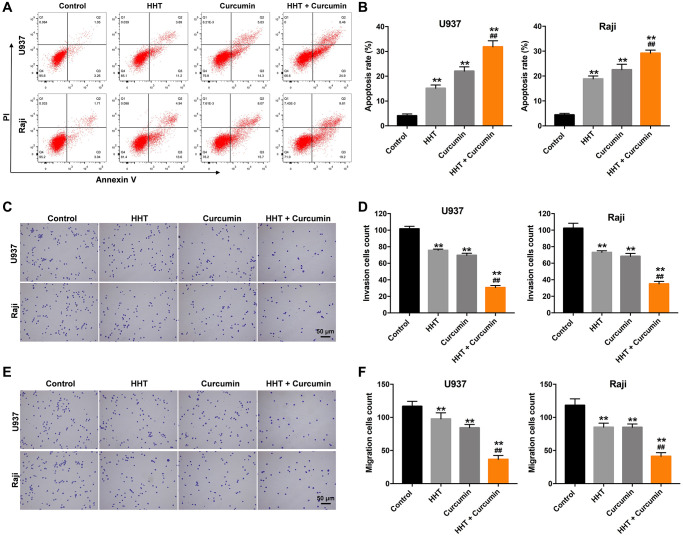
A flow cytometry assay was used to investigate the effects of curcumin and HHT on apoptosis in lymphoma cells. As shown in Figure 2A and 2B, treatment with either HHT (15.1%) or curcumin (22.0%) significantly increased the apoptosis of U937 cells compared with control (4.1%) group, and HHT (18.9%) or curcumin (22.5%) notably increased the apoptosis of Raji cells compared with control (4.4%) group. As expected, the apoptotic cells increased up to 31.8% for U937 cell treated with the combination (5 ng/mL HHT plus 10 μM curcumin), compared to 15.1% with HHT (5 ng/mL) alone, and the apoptotic cells increased up to 29.2% for Raji cell treated with the combination (5 ng/mL HHT plus 10 μM curcumin), compared to 18.9% with HHT (5 ng/mL) alone (Figure 2A and 2B). In addition, HHT treatment showed 25.0% and 28.6% decreases in invasion cells count in U937 and Raji cells, respectively, and curcumin caused 31.3% and 33.2% decreases, while combined HHT and curcumin treatment produced 69.8% and 65.8% decreases in invasion cells count after 24 h of treatment (Figure 2C and 2D). Meanwhile, HHT treatment showed 16.3% and 28.2% decreases in migration cells count in U937 and Raji cells, respectively, and curcumin caused 27.7% and 28.2% decreases, while the combined treatment produced 68.6% and 65.1% decreases in migration cells count (Figure 2E and 2F). These results indicate that the combination of curcumin and HHT induced apoptosis and suppressed migratory and invasive ability in lymphoma cells.
Figure 2.
Combination of curcumin with HHT induced apoptosis and suppressed invasive ability of lymphoma cells. (A, B) U937 and Raji cells were treated with 5 ng/mL HHT or/and 10 μM curcumin for 72 h. Apoptotic cells were quantified by flow cytometry. (C, D) U937 and Raji cells were treated with 5 ng/mL HHT or/and 10 μM curcumin for 24 h. Cell invasion was assessed in a transwell invasion assay. (E, F) Cell migration was assessed in a transwell migration assay. **P < 0.01 compared to control group; ##P < 0.01 compared to HHT group.
Combination of curcumin with HHT induced apoptosis in lymphoma cells via the mitochondrial-mediated apoptosis pathway
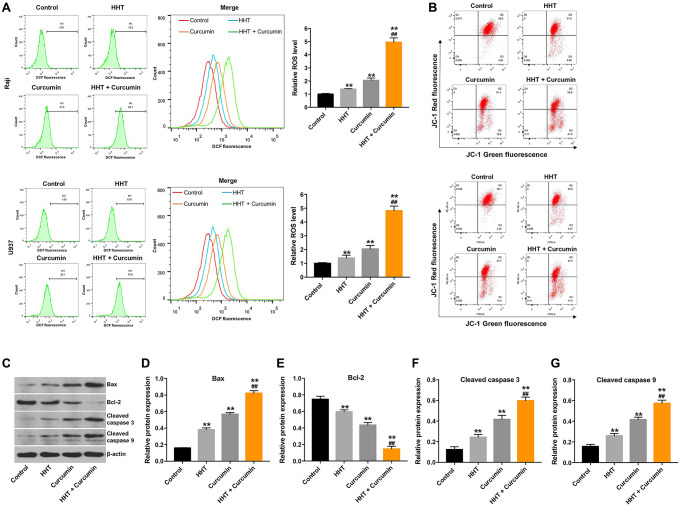
To assess whether lymphoma cell death induced by HHT/curcumin combination was accompanied by enhanced ROS generation, Raji cells were stained with DCFH-DA. As shown in Figure 3A, combination of HHT and curcumin remarkedly increased ROS generation in Raji cells (4.95 fold) and U937 cells (4.82 fold) compared to the HHT (1.38 fold and 1.39 fold, respectively) alone treatment group (Figure 3A). In addition, JC-1 staining indicated that the combination treatment obviously decreased MMP in Raji and U937 cells (Figure 3B). Meanwhile, combination of HHT and curcumin markedly increased Bax, cleaved caspase 3, and cleaved caspase 9 expression and decreased Bcl-2 expression in Raji cells (Figure 3C–3G). These data demonstrate that combination of curcumin and HHT induced apoptosis of lymphoma cells via the mitochondrial-mediated apoptosis pathway.
Figure 3.
Combination of curcumin with HHT induced apoptosis of lymphoma cells via the mitochondrial-mediated apoptosis pathway. Raji and U937 cells were treated with 5 ng/mL HHT or/and 10 μM curcumin for 72 h. (A) Intracellular ROS generation was measured by DCF fluorescence. (B) MMP loss was determined via JC-1 staining. (C) Bax, Bcl-2, cleaved caspase 3, and cleaved caspase 9 expression were measured in Raji cells using Western blotting. (D, E, F, G) Relative cellular Bax, Bcl-2, cleaved caspase 3, and cleaved caspase 9 expression normalized to β-actin. **P < 0.01 compared to control group; ##P < 0.01 compared to HHT group.
Combination of curcumin and HHT inhibited the EMT in lymphoma cells by inhibiting the TGF-β/Smad3 signaling pathway
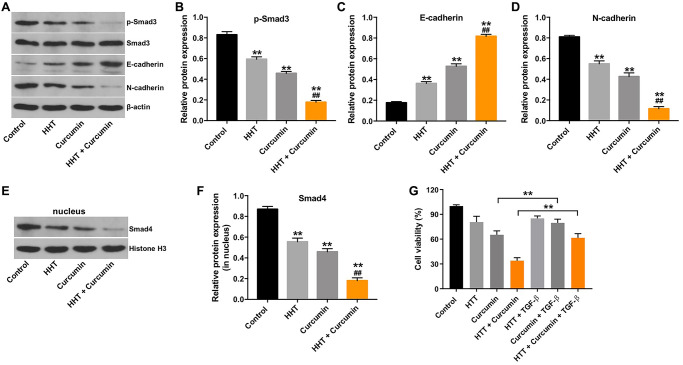
TGF-β/Smad signaling participates in various biological processes, including cancer cell growth, migration, and invasion [33]. We therefore assessed p-Smad3, E-cadherin, and N-cadherin expression in Raji cells using a Western blotting assay. As indicated in Figure 4A–4D, combination of HHT and curcumin significantly decreased p-Smad3 and N-cadherin levels and markedly increased E-cadherin levels in Raji cells. Meanwhile, the combination treatment decreased nuclear accumulation of Smad4 in Raji cells (Figure 4E and 4F). To investigate whether TGF-β signaling is involved in combination treatment-mediated lymphoma cell growth, rescue experiments were performed. As shown in Figure 4G, the inhibitory effect of combination treatment on the viability of Raji cells was reversed by the administration of TGF-β. These data suggest that combination of curcumin and HHT inhibited lymphoma cell growth by inhibiting the TGF-β/Smad3 signaling pathway.
Figure 4.
Combination of curcumin with HHT inhibited the EMT in lymphoma cells by inhibiting the TGF-β1/Smad3 signaling pathway. Raji cells were treated with 5 ng/mL HHT or/and 10 μM curcumin for 72 h. (A) p-Smad3, Smad3, E-cadherin, and N-cadherin expression were measured in Raji cells using Western blotting. (B, C, D) Relative cellular p-Smad3, E-cadherin, and N-cadherin normalized to Smad3, β-actin, and β-actin, respectively. (E, F) Nuclear Smad4 expression in Raji cells was measured by Western blotting. Relative Smad4 expression was determined by normalizing to Histone H3. **P < 0.01 compared to control group; ##P < 0.01 compared to HHT group. (G) Raji cells were treated with HHT and curcumin for 72 h or treated with HHT, curcumin and TGF-β. CCK-8 assay was used to measure cell viability. **P < 0.01.
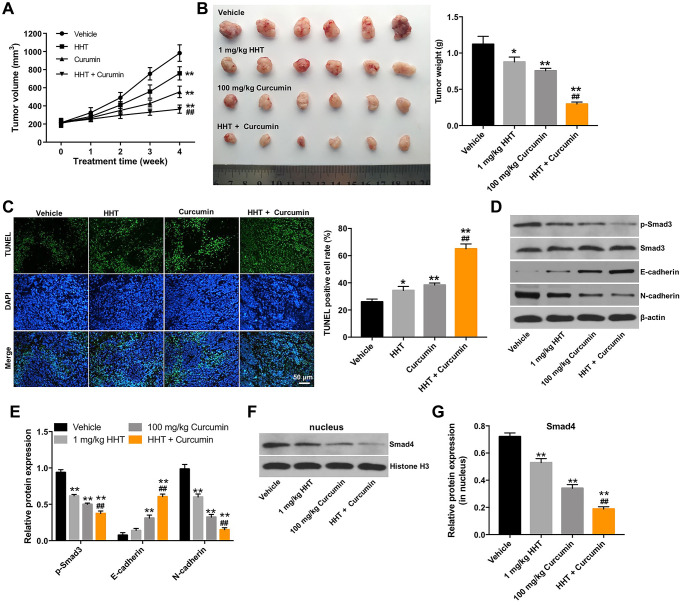
Combination of curcumin and HHT inhibited tumorigenesis in Raji xenograft in vivo
Next, we further assessed the effects of HHT combined with curcumin in a mouse Raji xenograft in vivo model. As shown in Figure 5A and 5B, the combination treatment markedly decreased the tumor volume and tumor weight of transplanted Raji tumors in vivo. In addition, a TUNEL assay revealed that combined treatment notably increased cell apoptosis in tumor tissues compared to the HHT alone treatment group (Figure 5C). Meanwhile, Western blots indicated that the combination treatment decreased the expressions of p-Smad3 and N-cadherin, increased the expression of E-cadherin, and reduced nuclear accumulation of Smad4 in tumor tissues (Figure 5D–5G). These results indicate that combination of curcumin with HHT inhibited tumorigenesis in Raji xenografts in vivo.
Figure 5.
Combination of curcumin with HHT inhibits tumorigenesis in Raji xenografts in vivo. (A) Tumor volume was calculated. (B) Raji xenograft tumors were excised and photographed. Tumor weights were calculated for each group of mice. (C) A TUNEL assay was used to assess cell apoptosis in tumor tissues. (D) p-Smad3, Smad3, E-cadherin and N-cadherin expressions were measured in tumor tissues using Western blotting. (E) Relative p-Smad3, E-cadherin and N-cadherin expressions in tumor tissues normalized to Smad3, β-actin and β-actin. (F, G) Smad4 expression in tumor tissues was measured by Western blotting. Relative Smad4 was determined by normalizing to Histone H3. *P < 0.05, **P < 0.01 compared to control group; ##P < 0.01 compared to HHT group.
DISCUSSION
In this study, we found that combination of curcumin with HHT synergistically inhibited lymphoma cell proliferation. Moreover, this combination treatment suppressed migration and invasion and induced apoptosis of lymphoma cells by inhibiting the TGF-β1/Smad3 pathway.
HHT is widely used as an antineoplastic drug in the treatment of hematological malignancies [34]. In addition, curcumin inhibits lymphoma cell growth [35]. Previous studies have shown that combining anti-cancer agents can reduce the toxicity and improve the effectiveness of anti-cancer therapies [35, 36]. In particular, addition of curcumin can enhance the efficacy of conventional anticancer therapies [37, 38]. For example, Guo et al. found that curcumin increased the efficacy of doxorubicin treatment in lymphoma cells by inducing apoptosis [35]. In addition, Li et al. found that combination of HHT with triptolide synergistically inhibited KG-1a cell proliferation [39]. In this study, we found that combination of curcumin with HHT synergistically inhibited lymphoma cell proliferation. In addition, the combined treatment with HHT and curcumin induced apoptosis and inhibited invasive ability of lymphoma cells to a significantly greater extent than treatment with either curcumin or HHT alone. These data indicate that combination of curcumin and HHT could suppress lymphoma cell growth to a greater extent than either compound alone.
TGF-β1 is a pleiotrophic cytokine that plays roles in cell differentiation, growth, and apoptosis [40]. Chen et al. found that exogenous TGF-β1 suppressed cellular growth in B-cell lymphoma [41]. However, Jung et al. found that TGF-β1 promoted lymphoma cell survival by regulating Fas-mediated apoptosis signaling [40]. In addition, Chang et al. found that suppression of TGF-β1 induced apoptosis in T cell lymphoma [42]. TGF-β might therefore function as both a pro- and anti-apoptotic regulatory factor in lymphoma cells. Zhang et al. found that curcumin inhibited metastasis in papillary thyroid carcinoma cells by downregulating the TGF-β/Smad3 pathway [43]. In addition, Yin et al. demonstrated that combination of curcumin with oxaliplatin significantly suppressed colorectal cancer cell growth by suppressing TGF-β/Smad3 signaling [44]. Consistent with these previous findings, we found that combination treatment decreased Smad3 phosphorylation and reduce nuclear accumulation of Smad4 in lymphoma cells in vitro and in vivo. These data suggest that combination of curcumin and HHT may suppress lymphoma cell growth and EMT by downregulating the TGF-β/Smad3 pathway. Zhang et al. found that curcumin could suppress the proliferation of endometrial carcinoma cells by downregulating ERK/c-Jun signaling [45]. In addition, curcumin could inhibit the proliferation and EMT in colon cancer cells via inactivating the Wnt signaling pathway [46]. Thus, further study is needed to identify whether curcumin enhance the anti-tumor effect of HHT on lymphoma cells via mediating ERK/c-Jun signaling or Wnt signaling.
In conclusion, combination treatment of curcumin with HHT exerted anti-lymphoma effects in Raji cells by inactivating the TGF-β/Smad3 pathway. Combined curcumin and HHT treatment might therefore be a promising therapeutic approach for the treatment of lymphomas. However, in the future, further investigation into the mechanistic aspects of the synergism between HHT and curcumin may provide novel therapeutic approaches for the treatment of lymphomas.
MATERIALS AND METHODS
Cell culture
The human leukemic monocyte lymphoma cell line U937 and Burkitt's lymphoma cell line Raji were purchased from American Type Culture Collection (ATCC, Rockville, MD, USA). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS, Thermo Fisher Scientific), 100 μg/mL of penicillin, and 100 units/mL of streptomycin. Cells were incubated in a humidified incubator containing 5% CO2 at 37°C. Curcumin was purchased from MedChem Expression Co. Ltd (Shanghai, China).
Cell Counting Kit-8 assay
The Cell Counting Kit-8 (CCK8) (Dojindo Laboratories, Japan) was used to assess cell viability after 3 days of curcumin or HHT treatment. U937 and Raji cells (5 × 103 cells per well) were plated onto 96-well plates at 37°C, and 10 μL of CCK-8 reagent was added to each well followed by incubation for 2 h. Subsequently, a Multiskan™ FC Microplate Photometer (Thermo Fisher Scientific) was used to determine the optical density (OD) of each well at 450 nm.
Combination studies
The Chou–Talalay method was used to calculate combination index (CI) values for the drug combination [47]. U937 and Raji cells were exposed to solutions containing curcumin (0, 5, 10, 20, or 40 μM) combined with HHT (5 ng/mL). The CI value for the combination of curcumin and HHT in lymphoma was defined as CI = DA/ICx, A + DB/ICx, B [48].
Flow cytometry analysis
Analysis of apoptosis rates in U937 and Raji cells was carried out using the fluorescein isothiocyanate (FITC) Annexin V Apoptosis Detection Kit (BD Biosciences, Franklin Lake, NJ, USA). Cells (5 × 105/ml) were resuspended in binding buffer and then stained with Annexin V-FITC and propidium iodide (PI) for 20 min in the dark. Numbers of apoptotic cells were then measured using a BD FACSCalibur flow cytometer (BD Biosciences) and analyzed using the CellQuest Pro software (BD Biosciences).
Transwell assays
Cell migration or invasion was examined using Matrigel-uncoated or Matrigel-coated transwell inserts (8 μm pore size, Corning, USA). U937 and Raji cells (4 × 104 cells/well) were suspended in 150 μL serum-starved culture medium and then added to the upper chambers, and the lower chambers were filled with 700 μL of DMEM medium containing 10% FBS. Twenty-four hours later, cells that had moved through the transwell membrane and invaded the lower chamber were fixed with 70% methanol and stained with 1% crystal violet. The migrated or invaded cells were imaged using a fluorescence microscope and counted in five randomly selected fields.
ROS detection
The DCFDA/H2DCFDA-Cellular ROS Assay kit (Abcam, Cambridge, MA, USA) was used to assess intracellular ROS generation according to the manufacturer’s instructions. Raji cells were stained with 10 μM DCFH-DA for 30 min in the dark at 37°C. Subsequently, fluorescence signals were analyzed using a FACSCalibur flow cytometer (BD Biosciences).
Mitochondrial membrane potential assay
Raji cells were incubated with JC-1 staining reagent (2 mL, Beijing Leagene Biotech. Co., Ltd., Beijing, China) for 20 min in the dark at 37°C. Cells were then washed three times with PBS, and JC-1 fluorescence was assessed using a FACSCalibur flow cytometer (BD Biosciences).
Western blot assay
The BCA protein assay kit (Bio-Rad, Hercules, CA, USA) was used to determine protein concentrations. Equal amounts of total protein were separated by 10% SDS-PAGE and transferred onto a polyvinylidene difluoride (PVDF) membrane (Thermo Fisher Scientific). The membrane was then blocked in 5% skim milk at room temperature for 1 h and incubated with primary antibodies against Bax (cat. no. ab182733; 1:1000), Bcl-2 (cat. no. ab32124; 1:1000), cleaved caspase 3 (cat. no. ab32042; 1:1000), cleaved caspase 9 (cat. no. ab2324; 1:1000), p-Smad3 (cat. no. ab52903; 1:1000), Smad3 (cat. no. ab40854; 1:1000), E-cadherin (cat. no. ab227639; 1:1000), N-cadherin (cat. no. ab76011; 1:1000), Smad4 (cat. no. ab230815; 1:1000), Histone H3 (cat. no. ab1791; 1:1000), and anti-β-actin (cat. no. ab8227; 1:1000) at 4°C overnight. Next, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (cat. no. ab97051; 1:5000) at room temperature for 1 h. Immunoreactive bands were then visualized using an electrochemiluminescence instrument (Thermo Fisher Scientific). All antibodies were obtained from Abcam (Cambridge, MA, USA). β-actin and histone H3 were used as internal controls.
Animal study
BALB/c nude mice (6–8 weeks old) were obtained from the Shanghai Slac Animal Center (Shanghai, China). Raji cells (5 × 106 cells resuspended in 50 μL PBS) were injected subcutaneously into the left flanks of nude mice. When tumor volumes reached 180 mm3, animals were randomized into four groups: vehicle, 1 mg/kg HHT, 100 mg/kg curcumin, or HHT + curcumin. The vehicle group received normal saline only. HTT was administered in daily intraperitoneal (ip) injections at 1 mg/kg. Curcumin was administered via oral gavage at 100 mg/kg/day. Tumor volumes were calculated based on caliper measurements every week. After four weeks, animals were sacrificed under anesthesia according to the recommended procedures of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All animal experiments were approved by the First Affiliated Hospital of Zhejiang Chinese Medical University.
TUNEL staining
Tumor tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5-μm sections. Cell apoptosis was assessed using a terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) assay with an In Situ Cell Apoptosis Detection Kit V (Boster Biological Technology Co. Ltd, Wuhan, China) according to the manufacturer’s protocol.
Statistical analysis
All statistical analyses were performed using GraphPad Prism software (version 7.0, La Jolla, CA, USA). One-way analysis of variance (ANOVA) and Tukey’s tests were carried out for multiple group comparisons. All experiments were repeated in triplicate. Data are presented as the mean ± standard deviation (S.D.). *P < 0.05 was considered statistically significant.
Ethical statement
All animal experiments were performed with approval from the First Affiliated Hospital of Zhejiang Chinese Medical University.
Footnotes
AUTHOR CONTRIBUTIONS: Yu Zhang conceived and supervised the study. Jingjing Xiang, Ni Zhu, Hangping Ge, and Xianfu Sheng designed the study. Shu Deng, Junfa Chen, Lihong Yu, and Yan Zhou performed the experiments and analyzed the data. Jianping Shen made substantial contributions to conception and design of the study and revised the manuscript critically for important intellectual content. All authors reviewed the results and approved the final version of the manuscript.
CONFLICTS OF INTEREST: The authors declare no conflicts of interest related to this study.
FUNDING: Zhejiang Provincial Natural Science Foundation (No. Y19H270018, LY15H29004). Special project for the modernization of traditional Chinese medicine in Zhejiang Province (No. 2020ZX007). National TCM clinical research base construction project (No. 2015H0105). National Natural Science Foundation of China (No. 81800138).
This corresponding author has a verified history of publications using a personal email address for correspondence
REFERENCES
- 1.Jiang M, Bennani NN, Feldman AL. Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms. Expert Rev Hematol. 2017; 10:239–49. 10.1080/17474086.2017.1281122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JY, Yu J, Chan WC. Gene expression profiling in non-Hodgkin lymphomas. Cancer Treat Res. 2015; 165:97–123. 10.1007/978-3-319-13150-4_4 [DOI] [PubMed] [Google Scholar]
- 3.Wang ZC, Liu Y, Wang H, Han QK, Lu C. Research on the relationship between artesunate and Raji cell autophagy and apoptosis of Burkitt's lymphoma and its mechanism. Eur Rev Med Pharmacol Sci. 2017; 21:2238–43. [PubMed] [Google Scholar]
- 4.Zheng C, Xiao Y, Li Y, He D. Knockdown of long non-coding RNA PVT1 inhibits the proliferation of Raji cells through cell cycle regulation. Oncol Lett. 2019; 18:1225–34. 10.3892/ol.2019.10450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mrdenovic S, Zhang Y, Wang R, Yin L, Chu GC, Yin L, Lewis M, Heffer M, Zhau HE, Chung LWK. Targeting Burkitt lymphoma with a tumor cell-specific heptamethine carbocyanine-cisplatin conjugate. Cancer. 2019; 125:2222–32. 10.1002/cncr.32033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu Y, Hochberg J, Yahr A, Ayello J, van de Ven C, Barth M, Czuczman M, Cairo MS. Targeting CD20+ Aggressive B-cell Non-Hodgkin Lymphoma by Anti-CD20 CAR mRNA-Modified Expanded Natural Killer Cells In Vitro and in NSG Mice. Cancer Immunol Res. 2015; 3:333–44. 10.1158/2326-6066.CIR-14-0114 [DOI] [PubMed] [Google Scholar]
- 7.Lapalombella R, Zhao X, Triantafillou G, Yu B, Jin Y, Lozanski G, Cheney C, Heerema N, Jarjoura D, Lehman A, Lee LJ, Marcucci G, Lee RJ, et al. A novel Raji-Burkitt's lymphoma model for preclinical and mechanistic evaluation of CD52-targeted immunotherapeutic agents. Clin Cancer Res. 2008; 14:569–78. 10.1158/1078-0432.CCR-07-1006 [DOI] [PubMed] [Google Scholar]
- 8.Qiao Q, Jiang Y, Li G. Inhibition of the PI3K/AKT-NF-κB pathway with curcumin enhanced radiation-induced apoptosis in human Burkitt's lymphoma. J Pharmacol Sci. 2013; 121:247–56. 10.1254/jphs.12149fp [DOI] [PubMed] [Google Scholar]
- 9.Miles RR, Arnold S, Cairo MS. Risk factors and treatment of childhood and adolescent Burkitt lymphoma/leukaemia. Br J Haematol. 2012; 156:730–43. 10.1111/j.1365-2141.2011.09024.x [DOI] [PubMed] [Google Scholar]
- 10.Rizzieri DA, Johnson JL, Byrd JC, Lozanski G, Blum KA, Powell BL, Shea TC, Nattam S, Hoke E, Cheson BD, Larson RA, and Alliance for Clinical Trials In Oncology (ACTION). Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: cancer and Leukemia Group B study 10 002. Br J Haematol. 2014; 165:102–11. 10.1111/bjh.12736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen J, Mu Q, Li X, Yin X, Yu M, Jin J, Li C, Zhou Y, Zhou J, Suo S, Lu D, Jin J. Homoharringtonine targets Smad3 and TGF-β pathway to inhibit the proliferation of acute myeloid leukemia cells. Oncotarget. 2017; 8:40318–26. 10.18632/oncotarget.16956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvandi F, Kwitkowski VE, Ko CW, Rothmann MD, Ricci S, Saber H, Ghosh D, Brown J, Pfeiler E, Chikhale E, Grillo J, Bullock J, Kane R, et al. U.S. Food and Drug Administration approval summary: omacetaxine mepesuccinate as treatment for chronic myeloid leukemia. Oncologist. 2014; 19:94–99. 10.1634/theoncologist.2013-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harringtonine in acute leukemias. Clinical analysis of 31 cases. Chin Med J (Engl). 1977; 3:319–24. [PubMed] [Google Scholar]
- 14.Yakhni M, Briat A, El Guerrab A, Furtado L, Kwiatkowski F, Miot-Noirault E, Cachin F, Penault-Llorca F, Radosevic-Robin N. Homoharringtonine, an approved anti-leukemia drug, suppresses triple negative breast cancer growth through a rapid reduction of anti-apoptotic protein abundance. Am J Cancer Res. 2019; 9:1043–60. [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen T, Parker R, Zhang Y, Hawkins E, Kmieciak M, Craun W, Grant S. Homoharringtonine interacts synergistically with bortezomib in NHL cells through MCL-1 and NOXA-dependent mechanisms. BMC Cancer. 2018; 18:1129. 10.1186/s12885-018-5018-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klanova M, Andera L, Brazina J, Svadlenka J, Benesova S, Soukup J, Prukova D, Vejmelkova D, Jaksa R, Helman K, Vockova P, Lateckova L, Molinsky J, et al. Targeting of BCL2 Family Proteins with ABT-199 and Homoharringtonine Reveals BCL2- and MCL1-Dependent Subgroups of Diffuse Large B-Cell Lymphoma. Clin Cancer Res. 2016; 22:1138–49. 10.1158/1078-0432.CCR-15-1191 [DOI] [PubMed] [Google Scholar]
- 17.Lopresti AL. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv Nutr. 2018; 9:41–50. 10.1093/advances/nmx011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giordano A, Tommonaro G. Curcumin and Cancer. Nutrients. 2019; 11:2376. 10.3390/nu11102376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, inflammation, and chronic diseases: how are they linked? Molecules. 2015; 20:9183–213. 10.3390/molecules20059183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanmugam MK, Rane G, Kanchi MM, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, Tan BK, Kumar AP, Sethi G. The multifaceted role of curcumin in cancer prevention and treatment. Molecules. 2015; 20:2728–69. 10.3390/molecules20022728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calibasi-Kocal G, Pakdemirli A, Bayrak S, Ozupek NM, Sever T, Basbinar Y, Ellidokuz H, Yigitbasi T. Curcumin effects on cell proliferation, angiogenesis and metastasis in colorectal cancer. J BUON. 2019; 24:1482–87. [PubMed] [Google Scholar]
- 22.Mbese Z, Khwaza V, Aderibigbe BA. Curcumin and Its Derivatives as Potential Therapeutic Agents in Prostate, Colon and Breast Cancers. Molecules. 2019; 24:4386. 10.3390/molecules24234386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng R, Deng Q, Liu Y, Zhao P. Curcumin Inhibits Gastric Carcinoma Cell Growth and Induces Apoptosis by Suppressing the Wnt/β-Catenin Signaling Pathway. Med Sci Monit. 2017; 23:163–71. 10.12659/msm.902711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guorgui J, Wang R, Mattheolabakis G, Mackenzie GG. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin's lymphoma in mice. Arch Biochem Biophys. 2018; 648:12–19. 10.1016/j.abb.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 25.Moustapha A, Pérétout PA, Rainey NE, Sureau F, Geze M, Petit JM, Dewailly E, Slomianny C, Petit PX. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015; 1:15017. 10.1038/cddiscovery.2015.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rainey NE, Moustapha A, Petit PX. Curcumin, a Multifaceted Hormetic Agent, Mediates an Intricate Crosstalk between Mitochondrial Turnover, Autophagy, and Apoptosis. Oxid Med Cell Longev. 2020; 2020:3656419. 10.1155/2020/3656419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sala de Oyanguren FJ, Rainey NE, Moustapha A, Saric A, Sureau F, O'Connor JE, Petit PX. Highlighting Curcumin-Induced Crosstalk between Autophagy and Apoptosis as Supported by Its Specific Subcellular Localization. Cells. 2020; 9:361. 10.3390/cells9020361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainey N, Motte L, Aggarwal BB, Petit PX. Curcumin hormesis mediates a cross-talk between autophagy and cell death. Cell Death Dis. 2015; 6:e2003. 10.1038/cddis.2015.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grant CM, Kyprianou N. Epithelial mesenchymal transition (EMT) in prostate growth and tumor progression. Transl Androl Urol. 2013; 2:202–11. 10.3978/j.issn.2223-4683.2013.09.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai G, Sun B, Gong T, Pan Z, Meng Q, Ju W. Ginsenoside Rb2 inhibits epithelial-mesenchymal transition of colorectal cancer cells by suppressing TGF-β/Smad signaling. Phytomedicine. 2019; 56:126–35. 10.1016/j.phymed.2018.10.025 [DOI] [PubMed] [Google Scholar]
- 31.Xue J, Lin X, Chiu WT, Chen YH, Yu G, Liu M, Feng XH, Sawaya R, Medema RH, Hung MC, Huang S. Sustained activation of SMAD3/SMAD4 by FOXM1 promotes TGF-β-dependent cancer metastasis. J Clin Invest. 2014; 124:564–79. 10.1172/JCI71104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Risolino M, Mandia N, Iavarone F, Dardaei L, Longobardi E, Fernandez S, Talotta F, Bianchi F, Pisati F, Spaggiari L, Harter PN, Mittelbronn M, Schulte D, et al. Transcription factor PREP1 induces EMT and metastasis by controlling the TGF-β-SMAD3 pathway in non-small cell lung adenocarcinoma. Proc Natl Acad Sci U S A. 2014; 111:E3775–84. 10.1073/pnas.1407074111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon HS, Jen J. TGF-beta signaling and the role of inhibitory Smads in non-small cell lung cancer. J Thorac Oncol. 2010; 5:417–19. 10.1097/JTO.0b013e3181ce3afd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kantarjian HM, Talpaz M, Santini V, Murgo A, Cheson B, O'Brien SM. Homoharringtonine: history, current research, and future direction. Cancer. 2001; 92:1591–605. [DOI] [PubMed] [Google Scholar]
- 35.Guo W, Song Y, Song W, Liu Y, Liu Z, Zhang D, Tang Z, Bai O. Co-delivery of Doxorubicin and Curcumin with Polypeptide Nanocarrier for Synergistic Lymphoma Therapy. Sci Rep. 2020; 10:7832. 10.1038/s41598-020-64828-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ravindranathan P, Pasham D, Balaji U, Cardenas J, Gu J, Toden S, Goel A. A combination of curcumin and oligomeric proanthocyanidins offer superior anti-tumorigenic properties in colorectal cancer. Sci Rep. 2018; 8:13869. 10.1038/s41598-018-32267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansouri K, Rasoulpoor S, Daneshkhah A, Abolfathi S, Salari N, Mohammadi M, Rasoulpoor S, Shabani S. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer. 2020; 20:791. 10.1186/s12885-020-07256-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Batra H, Pawar S, Bahl D. Curcumin in combination with anti-cancer drugs: A nanomedicine review. Pharmacol Res. 2019; 139:91–105. 10.1016/j.phrs.2018.11.005 [DOI] [PubMed] [Google Scholar]
- 39.Li X, Liu JY, Yuan XH, Lin ZX, Wu Y. [Effects of Triptolide Combined with Homoharringtonine on Proliferation and Apoptosis of KG-1α Cells]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2018; 26:347–53. [DOI] [PubMed] [Google Scholar]
- 40.Jung YJ, Kim JY, Park JH. TGF-beta1 inhibits Fas-mediated apoptosis by regulating surface Fas and cFLIPL expression in human leukaemia/lymphoma cells. Int J Mol Med. 2004; 13:99–104. [PubMed] [Google Scholar]
- 41.Chen G, Ghosh P, O'Farrell T, Munk R, Rezanka LJ, Sasaki CY, Longo DL. Transforming growth factor β1 (TGF-β1) suppresses growth of B-cell lymphoma cells by p14(ARF)-dependent regulation of mutant p53. J Biol Chem. 2012; 287:23184–95. 10.1074/jbc.M112.351411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang TP, Poltoratsky V, Vancurova I. Bortezomib inhibits expression of TGF-β1, IL-10, and CXCR4, resulting in decreased survival and migration of cutaneous T cell lymphoma cells. J Immunol. 2015; 194:2942–53. 10.4049/jimmunol.1402610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang L, Cheng X, Gao Y, Zhang C, Bao J, Guan H, Yu H, Lu R, Xu Q, Sun Y. Curcumin inhibits metastasis in human papillary thyroid carcinoma BCPAP cells via down-regulation of the TGF-β/Smad2/3 signaling pathway. Exp Cell Res. 2016; 341:157–65. 10.1016/j.yexcr.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 44.Yin J, Wang L, Wang Y, Shen H, Wang X, Wu L. Curcumin reverses oxaliplatin resistance in human colorectal cancer via regulation of TGF-β/Smad2/3 signaling pathway. Onco Targets Ther. 2019; 12:3893–903. 10.2147/OTT.S199601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Yi P, Tu C, Zhan J, Jiang L, Zhang F. Curcumin Inhibits ERK/c-Jun Expressions and Phosphorylation against Endometrial Carcinoma. Biomed Res Int. 2019; 2019:8912961. 10.1155/2019/8912961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Z, Chen H, Xu C, Song L, Huang L, Lai Y, Wang Y, Chen H, Gu D, Ren L, Yao Q. Curcumin inhibits tumor epithelial-mesenchymal transition by downregulating the Wnt signaling pathway and upregulating NKD2 expression in colon cancer cells. Oncol Rep. 2016; 35:2615–23. 10.3892/or.2016.4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984; 22:27–55. 10.1016/0065-2571(84)90007-4 [DOI] [PubMed] [Google Scholar]
- 48.Chen S, Zhang Z, Zhang J. Emodin enhances antitumor effect of paclitaxel on human non-small-cell lung cancer cells in vitro and in vivo. Drug Des Devel Ther. 2019; 13:1145–53. 10.2147/DDDT.S196319 [DOI] [PMC free article] [PubMed] [Google Scholar]