Abstract
Extracellular vesicles (EVs) are small lipid bound structures released from cells containing bioactive cargoes. Both the type of cargo and amount loaded varies compared to that of the parent cell. The characterisation of EVs in cancers of the male urogenital tract has identified several cargoes with promising diagnostic and disease monitoring potential. EVs released by cancers of the male urogenital tract promote cell-to-cell communication, migration, cancer progression and manipulate the immune system promoting metastasis by evading the immune response.
Their use as diagnostic biomarkers represents a new area of screening and disease detection, potentially reducing the need for invasive biopsies. Many validated EV cargoes have been found to have superior sensitivity and specificity than current diagnostic tools currently in use. The use of EVs to improve disease monitoring and develop novel therapeutics will enable clinicians to individualise patient management in the exciting era of personalised medicine.
Keywords: Extracellular vesicles, Male reproductive tract, Prostasomes, Epididymosomes, Cancer, Biomarker, Liquid biopsy
1. Introduction
Extracellular vesicles (EVs) are small, cell-derived, lipid bilayer structures secreted by virtually all cell types [1]. Following their release, EVs constitute a heterogeneous population, incapable of replication. Initially believed to be a means of cellular waste removal, they are now understood to have numerous and significant functions [2,3]. The cargoes of EVs include mRNA, non-coding RNAs such as microRNA, long non-coding RNA, DNA, lipids, proteins and metabolites, the loading of which is influenced by the health, state and lineage of the parent cell [[4], [5], [6], [7], [8], [9]]. Variable expression of these cargoes within EVs has led many to speculate their loading within EVs is a highly regulated process, however, although given this variation, the reverse may also be true [[10], [11], [12], [13], [14]]. Fundamental changes to cell and tissue DNA is reflected in EV cargoes, as demonstrated by Lee et al., showing DNA alterations in the tumour profile of bladder cancer was reflected in the DNA profile of urinary EVs [9]. Analysis of copy number variant between the tumour tissue and EVs demonstrated 12 somatic mutations were identified with an allele frequency of 65.6% between tissue and EV DNA [9].
Although the precise mechanisms which govern loading of individual proteins, metabolites and lipids into EVs has yet to be fully understood, RNA loading into EVs is thought to be based on recognition of a specific sequence within the nucleotide strand. On the identification of the ‘loading’ sequence motif, RNA incorporation within the EV is co-ordinated by the ubiquitously expressed RNA binding protein sumoylated heterogeneous nuclear ribonucleoprotein A2B1 (hnRNPA2B1) [15]. Variable expression of hnRNPA2B1 alters RNA loading in EVs which further demonstrates the dynamic nature of this process [15]. More recent work on RNA loading into EVs by Temoche-Diaz et al., has identified two distinct pathways in which microRNAs are loaded into EVs by a metastatic breast cancer cell line. Both a non-selective and selective pathway were identified, the latter regulated by the activity of the RNA binding protein Lupus La governing miR-122 loading, believed to be primarily due to the sub-cellular origin of the EV [16].
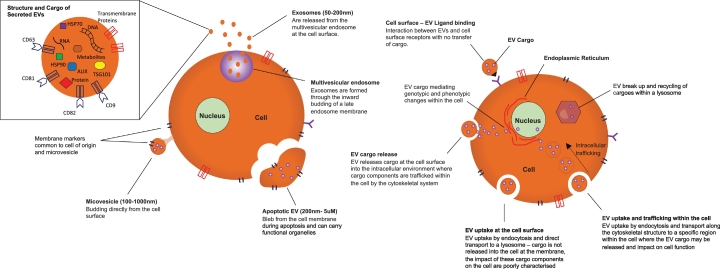
Following their release into the extracellular environment, EVs interact with recipient cells via numerous receptors on the cell surface including, but not limited to, tetraspanins, clathrin and integrins [[17], [18], [19], [20]]. Interactions may also be receptor-independent. Cellular targeting mechanisms fall broadly into four categories: 1) interaction with cell surface ligands; 2) fusion with cell membranes and release of cargo into the cell; 3) endocytic uptake and transport to lysosomes and 4) endocytic uptake and transport to a specific area of the cell [21,22]. It is through interaction with cell surface receptors or release of cargoes into cells that EVs mediate their effect on recipient cells (Fig. 1) [23]. Precisely how EVs are targeted to recipient cells, however, is yet to be elucidated in detail.
Fig. 1.
Sites of EV production, interaction with recipient cells, EV intracellular fate and EV structure and cargo.
Attempts to characterise EVs have been met with challenges owing to their diverse size, function and biogenesis. Ranging in size from 50 nm – 5um they are known to be produced by one of three pathways [24]. The first are formed within multivesicular endosomes through inward budding of the early endosome, following fusion of the endosome with the plasma membrane, these vesicles are released into the extracellular space and termed exosomes [25]. Formation of exosomes within a MVB leads to a lipid bilayer enriched with lipid components including glycophospholipid, cholesterol, ceramide and sphingomyelin [26]. The biogenesis of exosomes is highly regulated by the endosomal sorting complex required for transport (ESCORT) machinery in addition to other factors; this in turn leads to a rich complement of tetraspanins identified on exosomes, due to their endosomal origin [27]. Frequently used markers to identify exosomes include cluster of differentiation (CD) 9, 63 and 81, tumour suppressor gene 101, ALG-2-interacting protein X (ALIX) and heat shock proteins 60, 70 and 90 [27,28].
The second species of EV is from direct budding of the plasma membrane to form microparticles resulting in a lipid bilayer enriched with phosphatidylserine. They are best characterised by their surface markers including annexin A5, integrins and selectins [[29], [30], [31]]. The third species of EV is formed through the blebbing of the cell membrane as it undergoes apoptosis to produce apoptotic bodies which often contain cellular organelles and nuclear fragments [[32], [33], [34]]. Although considered a distinct cohort of EVs and the largest sub-type, they are only released during apoptosis unlike exosomes or microparticles which are released by healthy cells [35,36]. Despite their release occurring during programmed cell death, apoptotic EVs have numerous roles in disease progression in particular the tumour microenvironment, including enhanced tumour progression and disease resistance [37,38].
Although derived through distinct biogenesis pathways, there is substantial overlap between exosomes, microparticles and apoptotic bodies in their characterisation based on size, surface markers and cargoes. Once released into the extracellular environment, identification of specific subsets of EVs is challenging given this overlap. To address this, the International Society of Extracellular vesicles has developed consensus statements on how to classify EVs, which advocates describing EVs based on their size, biogenesis, tissue of origin and the presence of surface markers, outlined in Fig. 1 [1]. A simpler nomenclature for EVs is now advocated and they can be referred to as either small EVs (<200 nM) or large EVs (>200uM) and referencing their known surface markers such as CD81+ve/ TSG101+ve 1.
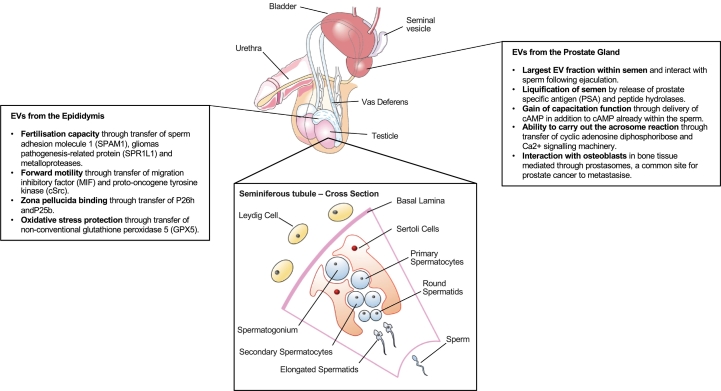
The preferential loading of cargoes into EVs frequently results in EV cargo being significantly different to that of their cell of origin [[39], [40], [41], [42]]. The study of EVs, in particular the characterisation of these cargoes, has grown dramatically over the years in numerous pathologies, especially in understanding their role in cancer [43]. Of particular interest is their use as novel biomarkers, tracking disease progression and predicting response to treatment. There is limited knowledge as to their release from the male reproductive tract [44]. However, the role of EVs in the maturation of sperm and fertility potential should not be underestimated, as they confer essential modifications in cargo and function [45]. These include acquisition of forward motility, capability to fertilise an oocyte and protection from oxidative stress, conferred by EVs for the epididymis or epididymosomes. Transfer of cAMP and Ca2+ signalling machinery to sperm which facilitate motility and the ability to carry out the acrosome reaction is mediated by prostate EVs or prostasomes [46]. The importance of the interaction between EVs of the male reproductive tract and sperm is best seen when comparing the function of sperm in vasectomised men, compared to healthy controls, who have impaired capacitation, motility and fertilisation potential [[47], [48], [49], [50], [51]].
Interaction between EVs from the male reproductive tract not only alters the function of sperm but also the female reproductive tract. EVs trigger increased endometrial prolactin stimulation, enhanced decidualisation and endometrial receptivity to a developing embryo and ameliorate the local immune response to sperm, reducing phagocytotic activity of neutrophils and monocytes [52,53]. A brief overview of the role of EVs in the male reproductive tract, in both health and disease is outlined in Fig. 2.
Fig. 2.
Adult male reproductive tract, seminiferous tubule and summary of the release of EVs.
EVs play an important role in the development of urological cancers [54]. Their sustained production and bespoke cargo loading implies their production is highly regulated and contributes to their ability to manipulate individual cells and tissue [55]. Their widespread distribution throughout the body via the circulatory and lymphatic systems gives them the potential to ‘prime’ various sites for future metastatic spread [20,56]. This is best characterised by their contribution to the development and maintenance of the pre-metastatic niche and regulation of the tumour microenvironment [57,58] through to manipulation of the immune system, attenuating its response to metastasising cancer cells [59,60]. Cancer-derived EVs mediate their effects through interaction with recipient cells, modulating their function to transform the tissue into a supportive pro-metastatic environment [61]. Some examples of this include the transformation of macrophages into tumour-supporting macrophages, promotion of angiogenesis through activation of endothelial cells and development of cancer-associated fibroblasts [[61], [62], [63], [64], [65], [66], [67], [68]]. In addition to modification of the pre-metastatic environment, transformation of recipient cells by EV uptake leads to increased metastatic organotropism to these tissues, as demonstrated in a mouse model [19].
EVs confer chemotherapy resistance to recipient cells [[69], [70], [71]], through delivery of proteins such as ATP-binding cassette sub-family B member 1 (ABC1) [72] transfer of apoptosis inhibitors [73], increasing tumour invasiveness and metastasis [74,75]. Higher numbers of EVs released in response to chemotherapy highlight the surge in EV mediated intracellular communication in response to cellular stress [74]; potentially conferring pro-survival characteristics to recipient cells [76].
Many EV functions are mediated through their cargoes, the characterisation of which is growing rapidly with advances in their isolation and detection [77,78]. A growing number of studies have reported EV cargoes in relation to diagnostic accuracy, treatment prognosis, treatment response as well as numerous biological processes. At first, this may appear to herald a new era of precision medicine in relation to urological malignancies, however, the methodologies to isolate these EVs as well as likely co-precipitated molecules must be taken into account. A common EV isolation technique such as ultracentrifugation pellets EVs based on their biophysical properties, however, proteins of similar sizes, often reported as novel biomarkers, may also be precipitated. Variation in EV isolation methodologies is outlined in Table 1, Table 2 and discussed further below.
Table 1.
Extracellular vesicle cargo, known functions and clinical validation.
| Disease | No. of Patients | No. of Controls | Source of EV | Isolation Techniques | EV Characterisation | Storage | Clinical Application / Role of Cargo | Cargo Generic | Cargo Specific | Cargo Identification | Sensitivity | Specificity | ROC- Area Under Curve | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Renal Cancer | 82 | 80 | Serum | Centrifugation, Immuno-affinity beads & commercial EV precipitation kit | Flow cytometry & immunostaining | Serum frozen —80C pre-analysis | Diagnosis | miRNA | miR-210 miR- 1233 |
Candidate screening | miR-210 (70%) miR-1233 (81%) |
miR-210 (62.2%) miR-1233 (76%) |
miR-210 - 0.69 miR-1233 - 0.82 |
Zhang W, Ni M, Su Y, Wang H, Zhu S, Zhao A and Li G. MicroRNAs in Serum Exosomes as Potential Biomarkers in Clear-cell Renal Cell Carcinoma. Eur Urol Focus. 2018:4;412–419. |
| Renal Cancer | 40 | 30 | Serum | Centrifugation & commercial EV precipitation kit | TEM, Western Blot | Serum frozen —80C pre-analysis | Diagnosis | miRNA | miR-210 | High throughput screening |
82.50% | 80% | 0.8779 | Wang X, Wang T, Chen C, Wu Z, Bai P, Li S, Chen B, Liu R, Zhang K, Li W, et al. Serum exosome miR-210 as a potential biomarker for clear cell renal cell carcinoma. Journal of Cellular Biochemistry. 2019:120;1492–1502. |
| Renal Cancer | 28 | 18 | Urine, Cell Lines | Commercial spin column for urinary EVs, commercial isoelectric precipitation | TEM | Urine frozen —80C pre-analysis | Diagnosis | miRNA | miR-126-3p miR-449a miR-34b-5p |
Candidate analysis following high throughput screening | – | – | miR-126-3p - miR-449a: 0.84 miR-126-3p – miR-34b-5p: AUC: 0.79 |
Butz H, Nofech-Mozes R, Ding Q, Khella HWZ, Szabó PM, Jewett M, Finelli A, Lee J, Ordon M, Stewart R, et al. Exosomal MicroRNAs Are Diagnostic Biomarkers and Can Mediate Cell-Cell Communication in Renal Cell Carcinoma. Eur Urol Focus. 2016:2;210–218. |
| Renal Cancer | 109 | 0 | Plasma | Commercial EV precipitation kit | None | Plasma frozen —80C pre-analysis | Prognosis | miRNA | miR-let-7i-5p | Candidate analysis following high throughput screening | – | – | 0.64 | Du M, Giridhar KV, Tian Y, Tschannen MR, Zhu J, Huang CC, Kilari D, Kohli M and Wang L. Plasma exosome miRNAs-based prognosis in metastatic kidney cancer. Oncotarget. 2017:8;63,703–63,714. |
| Renal Cancer | 108 | 0 | Serum, Cell Lines | Centrifugation, Immuno-affinity beads & commercial EV precipitation kit | TEM, Western Blot | None | Progression, Prognosis | miRNA | miR-224 | Candidate screening | – | – | Progression: 0.833 Prognosis: 0.857 |
Fujii N, Hirata H, Ueno K, Mori J, Oka S, Shimizu K, Kawai Y, Inoue R, Yamamoto Y, Matsumoto H, et al. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget. 2017:8;109,877–109,888. |
| Bladder Cancer | 28 | 12 | Urine | Centrifugation | TEM, Western Blot, Flow cytometry | Urine supernatant stored at —80C before EV isolation | Diagnosis, discrimination between high and low grade disease | Protein | APOA1, CD5L, FGA, FGB, FGG, HPR, HP | Candidate analysis following high throughput screening | – | – | Range: 0.762–0.830 | Chen CL, Lai YF, Tang P, Chien KY, Yu JS, Tsai CH, Chen HW, Wu CC, Chung T, Hsu CW, et al. Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J Proteome Res. 2012:11;5611–29. |
| Bladder Cancer | 80 | 80 | Urine | Centrifugation | TEM, Western Blot, NTA, Flow cytometry | Urine supernatant stored at —80C before EV isolation | Diagnosis, Prognosis | lncRNA | MALT1, PCAT-1, SPRY4-IT1 | Candidate screening | MALT1: 78.7% PCAT-1: 71.2% SPRY4-IT1: 87.5% Combined: 70.2% |
MALT1: 67.5% PCAT-1: 80.0% SPRY4-IT1: 65.0% Combined: 85.6% |
MALT1: 0.785 PCAT-1: 0.810 SPRY4-IT1: 0.799 Combined: 0.854 |
Zhan Y, Du L, Wang L, Jiang X, Zhang S, Li J, Yan K, Duan W, Zhao Y, Wang L, et al. Expression signatures of exosome long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol Cancer. 2018:17;142. |
| Bladder Cancer | 129 | 62 | Urine | Centrifugation | TEM, Western Blot | Urine supernatant stored at —80C before EV isolation | Diagnosis, Prognosis | Protein | Alpha 1-antitrypsin, H2BK1 | Candidate analysis following high throughput screening | Alpha 1-antitrypsin: 50.4% H2BK1: 62.0% Combined: 62.7% |
Alpha 1-antitrypsin: 96.9% H2BK1: 92.3% Combined: 87.59% |
Alpha 1-antitrypsin: 0.736 H2BK1: 0.772 Combined: 0.87 |
Lin SY, Chang CH, Wu HC, Lin CC, Chang KP, Yang CR, Huang CP, Hsu WH, Chang CT and Chen CJ. Proteome Profiling of Urinary Exosomes Identifies Alpha 1-Antitrypsin and H2B1K as Diagnostic and Prognostic Biomarkers for Urothelial Carcinoma. Sci Rep. 2016:6;34,446. |
| Bladder Cancer | 260 | 260 | Serum | Commercial EV precipitation kit | TEM, NTA, Western Blot, | Urine supernatant stored at —80C before EV isolation | Diagnosis | lncRNA | PACT-1, UBC1, SNHG16 | Candidate screening | Combined: 85% | Combined: 78% | PACT-1: 0.753 UBC1: 0.751 SNHG16: 0.681 Combined: 0.857 |
Zhang S, Du L, Wang L, Jiang X, Zhan Y, Li J, Yan K, Duan W, Zhao Y, Wang L, et al. Evaluation of serum exosome LncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J Cell Mol Med. 2019:23;1396–1405. |
| Bladder Cancer | 59 | 49 | Urine | Commercial EV precipitation kit | DLS, SEM, Western blot | Urine stored at 4C prior to EV isolation | Diagnosis | Protein | MAGE B4 | Candidate screening | 71.00% | 66.00% | 0.67 | Yazarlou F, Mowla SJ, Oskooei VK, Motevaseli E, Tooli LF, Afsharpad M, Nekoohesh L, Sanikhani NS, Ghafouri-Fard S and Modarressi MH. Urine exosome gene expression of cancer-testis antigens for prediction of bladder carcinoma. Cancer Manag Res. 2018:10;5373–5381. |
| Bladder Cancer | 206 | 36 | Urine | Commercial EV isolation kit | None | Urine stored at —80C prior to EV isolation | Diagnosis | mRNA | SLC2A1, GPRC5A and KRT17 | Candidate analysis following high throughput screening | SLC2A1: 64% GPRC5A: 54% KRT17: 58% |
SLC2A1: 75% GPRC5A: 72% KRT17: 58% |
SLC2A1: 0.70 GPRC5A: 0.64 KRT17: 0.64 |
Murakami T, Yamamoto CM, Akino T, Tanaka H, Fukuzawa N, Suzuki H, Osawa T, Tsuji T, Seki T and Harada H. Bladder cancer detection by urinary extracellular vesicle mRNA analysis. Oncotarget. 2018:9. |
| Bladder Cancer | 85 | 45 | Urine | Centrifugation | Western Blot | Urine supernatant stored at —20C before EV isolation, EVs frozen at -80 after isolation Plasma frozen —80C pre-analysis, urine supernatant frozen —80C pre-analysis |
Diagnosis | miRNA | miR-26a, miR-93, miR-191, and miR-940 | High throughput screening |
Combined: 70% | Combined: 84% | 0.858 | Long JD, Sullivan TB, Humphrey J, Logvinenko T, Summerhayes KA, Kozinn S, Harty N, Summerhayes IC, Libertino JA, Holway AH, et al. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am J Transl Res. 2015:7;2500–9. |
| Bladder Cancer | 6 | 3 | Urine, Cell Line | Centrifugation | TEM, NTA, Western Blot | EVs stored at —80C prior to analysis | Diagnosis | miRNA | miR-21-5p | High throughput screening |
75% | 95.80% | 0.9 | Matsuzaki K, Fujita K, Jingushi K, Kawashima A, Ujike T, Nagahara A, Ueda Y, Tanigawa G, Yoshioka I, Ueda K, et al. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget. 2017:8;24,668–24,678. |
| Bladder Cancer | 16 | 8 | Urine | Centrifugation, Microfluidic filtration | DLS, ELISA, TEM | None | Diagnosis | Protein | CD63 and EV signal intensity | Candidate screening | 81.30% | 90% | 0.96 | Liang L-G, Kong M-Q, Zhou S, Sheng Y—F, Wang P, Yu T, Inci F, Kuo WP, Li L-J, Demirci U, et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Scientific Reports. 2017:7;46,224. |
| Prostate Cancer | 16 | 15 | Urine | Centrifugation, Size exclusion filtration | TEM, DSL, Western Blot, Protein Quantification | EVs stored at —80C prior to analysis | Diagnosis | Protein | 17 proteins including: ADIRF, TM256, PCYOX1, LAMTOR1 | High throughput screening |
– | – | TM256 and LAMTOR1: 0.94 | Øverbye A, Skotland T, Koehler CJ, Thiede B, Seierstad T, Berge V, et al. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6(30):30357–76. |
| Prostate Cancer | 152 | 189 | Urine | Centrifugation, Size exclusion filtration, commercial EV precipitation kit | TEM, NTA, Western Blot | Urine supernatant stored at —80C before EV isolation | Diagnosis, Discrimination between low and high grade tumours | Protein | TGM4, ADSV, CD63, GLPK5, PSA, PPAP, SPHM | Candidate screening | – | – | Diagnosis TGM4: 0.58 ADSV: 0.58 Combined: 0.65 Discrimination between high and low grade disease CD63: 0.65 GLPK5: 0.64 PSA: 0.66 PPAP: 0.64 SPHM: 0.61 Combined: 0.70 |
Sequeiros T, Rigau M, Chiva C, Montes M, Garcia-Grau I, Garcia M, Diaz S, Celma A, Bijnsdorp I, Campos A, et al. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget. 2017:8;4960–4976. |
| Prostate Cancer | 89 | 106 | Urine | Commercial EV concentrator | TEM, NTA, Western Blot | Urine stored 2-8C for up to 2 weeks prior to EV isolation, EVs stored at —80C prior to analysis | Discrimination between benign and high grade disease | RNA | PCA3, ERG | Candidate screening | – | – | Discrimination between high and low grade disease using: PSA, age, race, family history: 0.6723 RNA, PSA, age, race, family history: 0.803 |
Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O'Neill V, Cochran JS and Brown GA. A molecular signature of PCA3 and ERG exosome RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer and Prostatic Diseases. 2015:18;370–375. |
| Prostate Cancer | 60 | 24 | Urine, Serum | Centrifugation, Size exclusion concentration, Commercial EV precipitation kit | TEM, NTA, Western Blot, Protein quantification | Urine and serum supernatant stored at —80C before EV isolation | Diagnosis | miRNA | miR-1290, miR-145 | Candidate screening | – | – | miR-1290: 0.613 miR-145: 0.623 miR-145 and PSA: 0.863 |
Xu Y, Qin S, An T, Tang Y, Huang Y and Zheng L. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate. 2017:77;1167–1175. |
| Prostate Cancer | 9 | 4 | Urine | Centrifugation, Size exclusion concentration, Commercial EV precipitation kit | None | EVs stored at —80C prior to analysis | Diagnosis | isomiRNA | miR-21, miR-204, miR-375 | Candidate analysis following high throughput screening | 72.90% | 88% | Combined isomiRs: 0.821 Combines PSA and isomiRs: 0.866 |
Koppers-Lalic D, Hackenberg M, de Menezes R, Misovic B, Wachalska M, Geldof A, Zini N, de Reijke T, Wurdinger T, Vis A, et al. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget. 2016:7;22,566–78. |
| Prostate Cancer | 78 | 28 | Urine, Plasma | Filtration and size exclusion concentration | None | Plasma and urine supernatant stored at —80C prior to EV isolation | Diagnosis, identification of metastatic disease | miRNA | miR-141, miR-375, miR-107, miR574-3p | Candidate analysis following high throughput screening | miR-107: 67% | miR-107: 43% | miR-107: 0.62 miR-574-30: 0.66 |
Bryant RJ, Pawlowski T, Catto JW, Marsden G, Vessella RL, Rhees B, Kuslich C, Visakorpi T and Hamdy FC. Changes in circulating microRNA levels associated with prostate cancer. Br J Cancer. 2012:106;768–74. |
| Prostate Cancer | 44 | 8 | Cell Lines, Serum | Centrifugation & commercial EV precipitation kit | NTA | None | Tumour suppression | miRNA | miR-1246 | High throughput screening |
75% | 100% | 0.926 | Bhagirath D, Yang TL, Bucay N, Sekhon K, Majid S, Shahryari V, Dahiya R, Tanaka Y and Saini S. microRNA-1246 Is an Exosomal Biomarker for Aggressive Prostate Cancer. Cancer Res. 2018:78;1833–1844. |
| Prostate Cancer | 30 | 49 | Urine | Centrifugation | None | Urine supernatant stored at —80C before EV isolation | Diagnosis | lncRNA | lncRNA-p21 | Candidate screening | 67% | 63% | 0.663 | Işın M, Uysaler E, Özgür E, Köseoğlu H, Şanlı Ö, Yücel Ö B, Gezer U and Dalay N. Exosomal lncRNA-p21 levels may help to distinguish prostate cancer from benign disease. Front Genet. 2015:6;168. |
| Prostate Cancer | 60 | 10 | Urine | Centrifugation | TEM | EVs stored at —80C prior to analysis | Diagnosis | miRNA | miR-21, miR-141, miR-375, miR-214, let-7c | Candidate screening | – | – | miR-21: 0.713 miR-141: 0.652 miR-214: 0.542 miR-375: 0.799 let-7c: 0.679 |
Foj L, Ferrer F, Serra M, Arévalo A, Gavagnach M, Giménez N and Filella X. Exosomal and Non-Exosomal Urinary miRNAs in Prostate Cancer Detection and Prognosis. Prostate. 2017:77;573–583. |
| Prostate Cancer | 50 | 22 | Plasma | Size exclusion chromatography, size exclusion concentration | TEM, NTA, Western Blot | Plasma supernatant stored at —80C before EV isolation | Diagnosis | miRNA | miR-200c-3p, miR-21-5p, Let-7a-5p | Candidate screening | – | – | miR-200c-3p: 0.68 miR-21-5p: 0.67 Let-7a-5p: 0.68 |
Endzeliņš E, Berger A, Melne V, Bajo-Santos C, Soboļevska K, Ābols A, Rodriguez M, Šantare D, Rudņickiha A, Lietuvietis V, et al. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017:17;730. |
| Prostate Cancer | 30 | 34 | Cell Lines, Plasma | Centrifugation, Immuno-affinity beads, Commercial EV precipitation kit | TEM, DSL, Western Blot | Plasma supernatant stored at —80C before EV isolation | Diagnosis | lncRNA | SChLAP1, SAP30L-AS1 | Candidate screening | SChLAP1: 87.9% SAP30L-AS1: 61.1% |
SChLAP1: 76.7% SAP30L-AS1: 82.1% |
SChLAP1: 0.8697 SAP30L-AS1: 0.6587 Combined: 0.9224 |
Wang YH, Ji J, Wang BC, Chen H, Yang ZH, Wang K, Luo CL, Zhang WW, Wang FB and Zhang XL. Tumour-Derived Exosomal Long Noncoding RNAs as Promising Diagnostic Biomarkers for Prostate Cancer. Cell Physiol Biochem. 2018:46;532–545. |
| Prostate Cancer | 123 | 0 | Plasma | Centrifugation, commercial EV precipitation kit | NTA | Plasma supernatant stored at —80C before EV isolation | Prognosis | miRNA | miR-1290, miR-1246, miR-375 | High throughput screening |
– | – | miR-1290 and miR-375: 0.68 | Huang X, Yuan T, Liang M, Du M, Xia S, Dittmar R, Wang D, See W, Costello BA, Quevedo F, et al. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur Urol. 2015:67;33–41. |
| Prostate Cancer | 30 | 0 | Urine | Centrifugation, size exclusion concentration | TEM | Urine supernatant stored at —80C before EV isolation | Diagnosis | miRNA | PCA3 | Candidate screening | – | – | 0.534 | Dijkstra S, Birker IL, Smit FP, Leyten GHJM, de Reijke TM, van Oort IM, Mulders PFA, Jannink SA and Schalken JA. Prostate Cancer Biomarker Profiles in Urinary Sediments and Exosomes. The Journal of Urology. 2014:191;1132–1138. |
| Prostate Cancer | 519 | – | Urine | Size exclusion filtration, Commercial EV precipitation kit | None | Urine stored at 2-8C and —80C prior to EV isolation | Diagnosis, Discrimination between low and high grade disease | RNA | ERG, PCA3, SPDEF | Candidate screening | – | – | Combined: 0.74 | McKiernan J, Donovan MJ, O'Neill V, Bentink S, Noerholm M, Belzer S, Skog J, Kattan MW, Partin A, Andriole G, et al. A Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer at Initial Biopsy. JAMA Oncol. 2016:2;882–9. |
| Prostate Cancer | 503 | – | Urine | Size exclusion filtration, Commercial EV precipitation kit | None | Urine stored at 4C prior to EV isolation and —80C after filtration | Diagnosis, Discrimination between low and high grade disease | RNA | ERG, PCA3, SPDEF | Candidate screening | – | – | Combined: 0.70 | McKiernan J, Donovan MJ, Margolis E, Partin A, Carter B, Brown G, Torkler P, Noerholm M, Skog J, Shore N, et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2-10 ng/mL at Initial Biopsy. Eur Urol. 2018:74;731–738. |
| Prostate Cancer | 20 | 9 | Urine | Centrifugation, Size exclusion filtration | None | EVs stored at —80C post analysis, pre application | Diagnosis | miRNA | miR-196a-5p, miR-34a-5p, miR-143-3p, miR-501-3p and miR-92a-1-5p | High throughput screening |
miR-196a-5p: 100% | miR-196a-5p: 89% | miR-196a-5p: 0.92 miR-143-3p: 0.72 |
Rodríguez M, Bajo-Santos C, Hessvik NP, Lorenz S, Fromm B, Berge V, Sandvig K, Linē A and Llorente A. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Molecular Cancer. 2017:16;156. |
| Prostate Cancer | 51 | 40 | Serum | Centrifugation, Size exclusion filtration, Commercial EV precipitation kit | TEM, Flow cytometry, Western Blot | None | Discriminating between local and metastatic disease | miRNA | miR-141 | Candidate screening | 80% | 87.10% | 0.8694 | Li Z, Ma YY, Wang J, Zeng XF, Li R, Kang W and Hao XK. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco Targets Ther. 2016:9;139–48. |
| Prostate Cancer | 50 | 21 | Serum | Centrifugation, Size exclusion filtration | TEM, Western Blot | Serum supernatant stored at —80C before EV isolation, EVs stored at —80C prior to analysis | Diagnosis, discrimination between prostate cancer and BPH | Protein | EphrinA2 | Candidate screening | 80.59% | 88% | 0.9062 | Li S, Zhao Y, Chen W, Yin L, Zhu J, Zhang H, Cai C, Li P, Huang L and Ma P. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J Cancer. 2018:9;2659–2665. |
| Prostate Cancer | 15 | 13 | Urine | Centrifugation, Size exclusion filtration | Protein and Lipid measurements | EVs stored at —80C following analysis of purity and yield | Diagnosis | Lipids | Lactosylceramide: Phosphatidylserine ratio | Candidate screening | – | – | 0.989 | Skotland T, Ekroos K, Kauhanen D, Simolin H, Seierstad T, Berge V, Sandvig K and Llorente A. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur J Cancer. 2017:70;122–132. |
| Prostate Cancer | 35 | 35 | Urine, Cell Lines | Centrifugation, Lectin induced precipitation | ATM, DSL, Western Blot | EVs stored at —80C prior to analysis | Diagnosis | miRNA | miR-574-3p, miR-141-5p, miR-21-5p | Candidate screening | miR-574-3p: 71% miR-141-5p: 66% miR-21-5p: 46% |
– | miR-574-3p: 0.85 miR-141-5p: 0.86 miR-21-5p: 0.65 |
Samsonov R, Shtam T, Burdakov V, Glotov A, Tsyrlina E, Berstein L, Nosov A, Evtushenko V, Filatov M and Malek A. Lectin-induced agglutination method of urinary exosomes isolation followed by mi-RNA analysis: Application for prostate cancer diagnostic. Prostate. 2016:76;68–79. |
| Prostate Cancer | 14 | 20 | Urine | Centrifugation, Size exclusion filtration | TEM | Urine supernatant stored at —80C before EV isolation | Diagnosis | miRNA | miR-19b, miR-16 | Candidate screening | miR-19b: 100% miR-16: 95% |
miR-19b: 93% miR-16: 79% |
– | Bryzgunova OE, Zaripov MM, Skvortsova TE, Lekchnov EA, Grigor'eva AE, Zaporozhchenko IA, Morozkin ES, Ryabchikova EI, Yurchenko YB, Voitsitskiy VE, et al. Comparative Study of Extracellular Vesicles from the Urine of Healthy Individuals and Prostate Cancer Patients. PLoS One. 2016:11;e0157566. |
| Prostate Cancer | 19 | 16 | Urine | Centrifugation | None | Urine stored 2-8C before EV isolation | Diagnosis | mRNA | GATA2 | Candidate screening | – | – | 0.74 | Woo J, Santasusagna S, Banks J, Pastor-Lopez S, Yadav K, Carceles-Cordon M, Dominguez-Andres A, Den RB, Languino LR, Pippa R, et al. Urine Extracellular Vesicle GATA2 mRNA Discriminates Biopsy Result in Men with Suspicion of Prostate Cancer. J Urol. 2020:204;691–700. |
| Prostate Cancer | 26 | 16 | Urine | Centrifugation, Size exclusion filtration | Western Blot, ELISA | EVs stored at —80C following analysis | Diagnosis | Protein | TMEM256, flotillin 2, Rab3B, PARK7, LAMTOR1 | Candidate screening | Flotillin 2: 88% Flotillin 2 and PARK7: 68% |
Flotillin 2: 94% Flotillin 2 and PARK7: 93% |
Flotillin 2: 0.91 | Wang L, Skotland T, Berge V, Sandvig K and Llorente A. Exosomal proteins as prostate cancer biomarkers in urine: From mass spectrometry discovery to immunoassay-based validation. European Journal of Pharmaceutical Sciences. 2017:98;80–85. |
| Prostate Cancer | 24 | 23 | Urine | Centrifugation | TEM, Western Blot | Urine supernatant stored at —80C before EV isolation | Diagnosis, Prognosis | Protein | 11 proteins including: FABP5, Granulin, AMBP, CHMP4A, CHMP4C | High throughput screening |
FABP5: 60% | FABP5: 100% | FABP5: 0.856 | Fujita K, Kume H, Matsuzaki K, Kawashima A, Ujike T, Nagahara A, Uemura M, Miyagawa Y, Tomonaga T and Nonomura N. Proteomic analysis of urinary extracellular vesicles from high Gleason score prostate cancer. Scientific Reports. 2017:7;42,961. |
| Prostate Cancer | 24 | 15 | Cell Lines, urine | Centrifugation, Size exclusion filtration | TEM, Western Blot | Urine stored at 4C until processed | Diagnosis | RNA | AGR2 SV-G, AGR2 wt, AGR2 SV-G | Candidate screening | – | – | AGR2 SV-H: 0.96 AGR2 SV-G: 0.94 AGR2 wt: 0.91 |
Neeb A, Hefele S, Bormann S, Parson W, Adams F, Wolf P, Miernik A, Schoenthaler M, Kroenig M, Wilhelm K, et al. Splice variant transcripts of the anterior gradient 2 gene as a marker of prostate cancer. Oncotarget. 2014:5. |
| Prostate Cancer | 47 | 39 | Urine | Size exclusion filtration, Centrifugation | None | Urine stored at 4C prior to EV isolation | Diagnosis | RNA | TMPRSS2:ERG, BIRC5, ERG, PCA3, TMPRSS2 | Candidate screening | – | – | BIRC5: 0.674 ERG: 0.785 PCA3: 0.681 TMPRSS2: 0.637 TMPRSS2:ERG: 0.744 |
Motamedinia P, Scott AN, Bate KL, Sadeghi N, Salazar G, Shapiro E, Ahn J, Lipsky M, Lin J, Hruby GW, et al. Urine Exosomes for Non-Invasive Assessment of Gene Expression and Mutations of Prostate Cancer. PLOS ONE. 2016:11;e0154507. |
Abbreviations
ATM - Atomic force microscopy
DSL - Dynamic light scattering
CyroEM - Cryo electron microscopy.
TEM – Transmission electronic microscopy.
NTA – Nanoparticle tracking analysis.
Table 2.
Extracellular vesicle cargo and known functions.
| Disease | No. of Patients | No. of Controls | Source of EV | Isolation Techniques | EV Characterisation | Storage | Clinical Application / Role of Cargo | Cargo Generic | Cargo Specific | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Renal Cancer | 71 | 0 | Plasma | Centrifugation, Size exclusion filtration | TEM, NTA, Western Blot | Plasma frozen —80C pre-analysis | Drug Resistance | lncRNA | lncARSR - lncRNA Activated in RCC with Sunitinib Resistance | Qu L, Ding J, Chen C, Wu ZJ, Liu B, Gao Y, Chen W, Liu F, Sun W, Li XF, et al. Exosome-Transmitted lncARSR Promotes Sunitinib Resistance in Renal Cancer by Acting as a Competing Endogenous RNA. Cancer Cell. 2016:29;653–668. |
| Renal Cancer | – | – | Cell Line | Centrifugation, concentration | Western Blot | Isolated EVs stored at -80C | Proliferation | Protein | HepaCAM | Jiang X, Zhang Y, Tan B, Luo C and Wu X. Renal tumour-derived exosomes inhibit hepaCAM expression of renal carcinoma cells in ap-AKT-dependent manner. Neoplasma. 2014:61;416. |
| Renal Cancer | – | – | Cell Line | Centrifugation, Size exclusion filtration, Density graded centrifugation, Immuno-affinity beads | Western Blot | None | Angiogenesis | Protein | Carbonic anhydrase IX | Horie K, Kawakami K, Fujita Y, Sugaya M, Kameyama K, Mizutani K, Deguchi T and Ito M. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochemical and biophysical research communications. 2017:492;356–361. |
| Renal Cancer | – | – | Cell Line | Centrifugation, Size exclusion concentration, Density graded centrifugation | TEM, Western Blot | None | Cell migration | Protein | CXCR4 & MM9 | Chen G, Zhang Y and Wu X. 786–0 Renal cancer cell line-derived exosomes promote 786–0 cell migration and invasion in vitro. Oncology letters. 2014:7;1576–1580. |
| Renal Cancer | – | – | Cell Line | Centrifugation | TEM, size and zeta potential assessment | None | Angiogenesis and development of the pre-metastatic niche | mRNA, miRNA | miR-29a, miR-650, miR-15, miR-19b, miR-29c, miR-151 | Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B and Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer research. 2011:71;5346–5356. |
| Renal Cancer | – | – | Cell Line | Centrifugation | Flow cytometry, NTA | Isolated EVs stored at -80C | Immune system modulation | Protein | HLA-G | Grange C, Tapparo M, Tritta S, Deregibus MC, Battaglia A, Gontero P, Frea B and Camussi G. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC cancer. 2015:15;1–11. |
| Renal Cancer | 36 | 36 | Plasma | Centrifugation | TEM, Western Blot | None | Immune system modulation | Protein | TGF-β1 | Xia Y, Zhang Q, Zhen Q, Zhao Y, Liu N, Li T, Hao Y, Zhang Y, Luo C and Wu X. Negative regulation of tumour-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosome pathway. Oncotarget. 2017:8;37,783. |
| Renal Cancer | 29 | 23 | Urine | Centrifugation, Density graded centrifugation | TEM, Western Blot | Urine frozen —80C pre-analysis and EVs frozen at -80 after isolation | Diagnosis | Protein | MMP9, CP, PODXL, DKK4, CAIX, AQP1, EMMPRIN, CD10, dipeptidase 1, syntenin-1 | Raimondo F, Morosi L, Corbetta S, Chinello C, Brambilla P, Della Mina P, Villa A, Albo G, Battaglia C, Bosari S, et al. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol Biosyst. 2013:9;1220–33. |
| Renal Cancer | 8 | 8 | Urine | Centrifugation, Size exclusion concentration | Western Blot | EVs frozen at -80 after isolation | Diagnosis | Lipids | Various Lipids | Del Boccio P, Raimondo F, Pieragostino D, Morosi L, Cozzi G, Sacchetta P, Magni F, Pitto M and Urbani A. A hyphenated microLC-Q-TOF-MS platform for exosome lipidomics investigations: application to RCC urinary exosomes. Electrophoresis. 2012:33;689–96. |
| Renal Cancer | – | – | Cell Line | Centrifugation, Size exclusion concentration, Density graded centrifugation | TEM | EVs frozen at -80 after isolation | Angiogenesis | mRNA | mRNA regulating VEGF expression | Zhang L, Wu X, Luo C, Chen X, Yang L, Tao J and Shi J. The 786–0 renal cancer cell-derived exosomes promote angiogenesis by downregulating the expression of hepatocyte cell adhesion molecule. Mol Med Rep. 2013:8;272–276. |
| Renal Cancer | 33 | 22 | Urine | Centrifugation, Size exclusion concentration | None | None | Transcription, Metabolism | esRNA | GSTA1, CEBPA and PCBD1 | De Palma G, Sallustio F, Curci C, Galleggiante V, Rutigliano M, Serino G, Ditonno P, Battaglia M and Schena FP. The Three-Gene Signature in Urinary Extracellular Vesicles from Patients with Clear Cell Renal Cell Carcinoma. J Cancer. 2016:7;1960–1967. |
| Bladder Cancer | – | – | Cell Line | Centrifugation, Size exclusion filtration, Density graded centrifugation | TEM | None | Cell Migration, Angiogenesis | Protein | EDIL-3 | Beckham CJ, Olsen J, Yin PN, Wu CH, Ting HJ, Hagen FK, Scosyrev E, Messing EM and Lee YF. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J Urol. 2014:192;583–92. |
| Bladder Cancer | – | – | Cell Line, Urine | Centrifugation, Density graded centrifugation | TEM, Flow cytometry, Western Blot | EVs frozen at -80 after isolation | Various functions including: Diagnosis & Cell Adhesion | Protein | 353 proteins | Welton JL, Khanna S, Giles PJ, Brennan P, Brewis IA, Staffurth J, Mason MD and Clayton A. Proteomics analysis of bladder cancer exosomes. Mol Cell Proteomics. 2010:9;1324–38. |
| Bladder Cancer | – | – | Cell Line | Centrifugation | NTA, Western Blot | None | Tumour suppression | miRNA | miR23b, miR921, mmiR224 | Ostenfeld MS, Jeppesen DK, Laurberg JR, Boysen AT, Bramsen JB, Primdal-Bengtson B, Hendrix A, Lamy P, Dagnaes-Hansen F, Rasmussen MH, et al. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014:74;5758–71. |
| Bladder Cancer | 27 | 0 | Urine, Plasma, Tumour | Centrifugation, Commercial EV isolation kit | None | Plasma frozen —80C pre-analysis, urine supernatant frozen —80C pre-analysis | Diagnosis | miRNA | miR 21, miR4454, miR720, miR205-5p, miR200c-3p, miR200-3p, miR21-5p, miR29b-3q, miR548ai, miR548aa, miR223, miR338-3p, miR378e, miR548n, miR1290, miR16, miR451a, let-7a-5p, let-7b-5p | Armstrong DA, Green BB, Seigne JD, Schned AR and Marsit CJ. MicroRNA molecular profiling from matched tumour and bio-fluids in bladder cancer. Molecular Cancer. 2015:14;194. |
| Bladder Cancer | – | – | Cell Line, Urine | Centrifugation, Commercial EV isolation kit | NTA, Western Blot, TEM | None | Prognosis | lncRNA | HOTAIR | Berrondo C, Flax J, Kucherov V, Siebert A, Osinski T, Rosenberg A, Fucile C, Richheimer S and Beckham CJ. Expression of the Long Non-Coding RNA HOTAIR Correlates with Disease Progression in Bladder Cancer and Is Contained in Bladder Cancer Patient Urinary Exosomes. PLoS One. 2016:11;e0147236. |
| Bladder Cancer | 21 | – | Urine, Cell Lines | Centrifugation, Commercial EV isolation kit | TEM, Western Blot | Urine supernatant stored at —80C before EV isolation | Diagnosis, Prognosis | miRNA | miR-200-3p | Baumgart S, Hölters S, Ohlmann CH, Bohle R, Stöckle M, Ostenfeld MS, Dyrskjøt L, Junker K and Heinzelmann J. Exosomes of invasive urothelial carcinoma cells are characterised by a specific miRNA expression signature. Oncotarget. 2017:8;58,278–58,291. |
| Bladder Cancer | 34 | 9 | Urine, Cell Lines | Centrifugation, Size exclusion filtration | NTA, TEM, Western Blot | None | Diagnosis, Prognosis | miRNA | miR-375 miR-let7c miR-194 miR-146a miR-30c-2 |
Andreu Z, Otta Oshiro R, Redruello A, López-Martín S, Gutiérrez-Vázquez C, Morato E, Marina AI, Olivier Gómez C and Yáñez-Mó M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur J Pharm Sci. 2017:98;70–79. |
| Bladder Cancer | 26 | 0 | Urine | None | None | None | Diagnosis, Proliferation, Migration, Invasive Phenotype | miRNA | miR-141-3p miR-146b-5p miR-200a-3p miR-200b-3p |
Baumgart S, Meschkat P, Edelmann P, Hartmann A, Bohle R, Pryalukhin A, Heinzelmann J, Stöckle M and Junker K. Invasion-associated miRNAs S as possible diagnostic biomarkers of muscle invasive bladder cancer in tumour tissues and urinary exosomes. Journal of Urology. 2018:199;e1038-e1038. |
| Bladder Cancer | 89 | 50 | Urine, Serum | Centrifugation | TEM, Western Blot | Urine and serum snap frozen in liquid nitrogen prior to EV isolation. | Clinical Staging, Survival | circRNA | circPRMT5 | Chen X, Chen R-X, Wei W—S, Li Y—H, Feng Z-H, Tan L, Chen J-W, Yuan G-J, Chen S-L, Guo S-J, et al. PRMT5 Circular RNA Promotes Metastasis of Urothelial Carcinoma of the Bladder through Sponging miR-30c to Induce Epithelial–Mesenchymal Transition. Clinical Cancer Research. 2018:24;6319–6330. |
| Bladder Cancer | – | – | Cell Line | Centrifugation, Size exclusion filtration, Commercial EV isolation kit | TEM, NTA, Western Blot | None | Proliferation, Migration, Invasion | lncRNA | lncRNA-UCA1 | Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, Pang H, An H, Wang X, Hou H, et al. Hypoxic exosomes facilitate bladder tumour growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017:16;143. |
| Bladder Cancer | – | – | Cell Line | Centrifugation | Western Blot | None | Development of the pre-metastatic niche | Protein | ErbB2, CRK | Yoshida K, Tsuda M, Matsumoto R, Semba S, Wang L, Sugino H, Tanino M, Kondo T, Tanabe K and Tanaka S. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019:110;2119–2132. |
| Bladder Cancer | 8 | 11 | Urine | Centrifugation, Size exclusion filtration | CryoEM, NTA, | Urine supernatant stored at —80C before EV isolation | Diagnosis | mRNA | LASS2, GALNT1, ARHGEF39, FOXO3 | Perez A, Loizaga A, Arceo R, Lacasa I, Rabade A, Zorroza K, Mosen-Ansorena D, Gonzalez E, Aransay AM, Falcon-Perez JM, et al. A Pilot Study on the Potential of RNA-Associated to Urinary Vesicles as a Suitable Non-Invasive Source for Diagnostic Purposes in Bladder Cancer. Cancers (Basel). 2014:6;179–92. |
| Bladder Cancer | 46 | – | Urine | Centrifugation, Commercial EV isolation kit | None | Urine supernatant stored at 4C prior to EV isolation | Diagnosis | miRNA | miR-141-3p, miR-141-3p | Poli G, Egidi MG, Cochetti G, Brancorsini S and Mearini E. Relationship between cellular and exosomal miRNAs targeting NOD-like receptors in bladder cancer: preliminary results. Minerva Urol Nefrol. 2020:72;207–213. |
| Bladder Cancer | 6 | 5 | Urine, Plasma | Centrifugation | Western Blot | Urine supernatant stored at —80C before EV isolation, Urine micro pellet frozen at —80C prior to analysis | Diagnosis | Protein | Resistin, GTPase NRas, EPS8L2, Mucin 4, EPS8L1, Retinoic acid-induced protein 3, Alpha subunit of GsGTP binding protein, EH-domain-containing protein 4, Galectin-3-binding protein | Smalley DM, Sheman NE, Nelson K and Theodorescu D. Isolation and Identification of Potential Urinary Microparticle Biomarkers of Bladder Cancer. Journal of Proteome Research. 2008:7;2088–2096. |
| Bladder Cancer | – | – | Urine, Cell Line | Centrifugation | NTA, Western Blot | Urine supernatant stored at —80C before EV isolation | Invasive disease | Protein | Periostin | Silvers CR, Liu Y-R, Wu C—H, Miyamoto H, Messing EM and Lee Y—F. Identification of extracellular vesicle-borne periostin as a feature of muscle-invasive bladder cancer. Oncotarget. 2016:7. |
| Bladder Cancer | 6 | 6 | Urine | Centrifugation | TEM, NTA, Western Blot | Cell culture & urine supernatant stored at —80C prior to EV isolation | Cell membrane, extracellular matrix, inflammation & angiogenesis signalling pathways | Protein, mRNA | HEXB, S100A4, SND1, TALD01, EHd4 | Silvers CR, Miyamoto H, Messing EM, Netto GJ and Lee Y—F. Characterisation of urinary extracellular vesicle proteins in muscle-invasive bladder cancer. Oncotarget. 2017:8. |
| Bladder Cancer | 34 | 9 | Urine | Size exclusion filtration, Centrifugation | TEM, NTA, Western Blot | None | Diagnosis, Prognosis | miRNA | miR-375, miR-146a, apoB | Andreu Z, Otta Oshiro R, Redruello A, López-Martín S, Gutiérrez-Vázquez C, Morato E, Marina AI, Olivier Gómez C and Yáñez-Mó M. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. European Journal of Pharmaceutical Sciences. 2017:98;70–79. |
| Prostate Cancer | 12 | – | Tissue from metastatic disease | Tissue homogenisation, centrifugation | None | Tissue frozen prior to EV isolation | Diagnosis, Disease Progression, Angiogenesis | Protein | 25 proteins including: annexin A1, A3, A5, DDAH 1 | Ronquist KG, Ronquist G, Larsson A and Carlsson L. Proteomic analysis of prostate cancer metastasis-derived prostasomes. Anticancer Research. 2010:30;285–90. |
| Prostate Cancer | 8 | 5 | Cell Lines, Urine | Centrifugation | Western Blot | Urine supernatant stored at —80C before EV isolation | Diagnosis, Cell Adhesion, Cell Motility | Protein | ITGA3, ITGB1 | Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJ and Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles. 2013:2. |
| Prostate Cancer | 90 | 50 | Urine | Centrifugation & commercial EV precipitation kit | SEM, Western Blot | None | Diagnosis | miRNA | miR-2909 | Wani S, Kaul D, Mavuduru RS, Kakkar N and Bhatia A. Urinary-exosome miR-2909: A novel pathognomonic trait of prostate cancer severity. J Biotechnol. 2017:259;135–139. |
| Prostate Cancer | – | – | Cell Lines | Centrifugation, Density graded centrifugation | TEM, Western Blot | None | Diagnosis | Protein | ALIX, FASN, XPO1, ENO1 | Duijvesz D, Burnum-Johnson KE, Gritsenko MA, Hoogland AM, Vredenbregt-van den Berg MS, Willemsen R, Luider T, Paša-Tolić L and Jenster G. Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS One. 2013:8;e82589. |
| Prostate Cancer | – | – | Cell Lines | Centrifugation | TEM, NTA, Western Blot | None | Cell Differentiation | Protein | Ets1, PTHrP | Itoh T, Ito Y, Ohtsuki Y, Ando M, Tsukamasa Y, Yamada N, Naoe T and Akao Y. Microvesicles released from hormone-refractory prostate cancer cells facilitate mouse pre-osteoblast differentiation. J Mol Histol. 2012:43;509–15. |
| Prostate Cancer | 11 | 0 | Urine | Centrifugation, Density graded centrifugation | Immunoelectron microscopy | None | Diagnosis, Disease Progression | miRNA | PCA3, ERG | Nilsson J, Skog J, Nordstrand A, Baranov V, Mincheva-Nilsson L, Breakefield XO and Widmark A. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br J Cancer. 2009:100;1603–7. |
| Prostate Cancer | 10 | 0 | Cell Lines, Serum | Centrifugation | Western Blot | Serum supernatant stored at —80C before EV isolation, EVs stored at —20C before characterisation | Prediction of Chemotherapy Response | Protein | P-glycoprotein | Kato T, Mizutani K, Kameyama K, Kawakami K, Fujita Y, Nakane K, Kanimoto Y, Ehara H, Ito H, Seishima M, et al. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urologic Oncology: Seminars and Original Investigations. 2015:33;385.e15–385.e20. |
| Prostate Cancer | – | – | Cell Lines, Plasma | Centrifugation, Density graded centrifugation, commercial EV precipitation kit | TEM, Flow cytometry, Western Blot | EVs stored at —80C prior to analysis | Diagnosis, Tumour Progression, Anti-apoptosis, Cell Proliferation, Chemotherapy Resistance, Cell Migration, Angiogenesis | Protein | 103 proteins, differentially expressed | Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria P-J, Cavallini L, Ciardiello C, Sobreiro MR, Morello M, et al. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumour-derived extracellular vesicles. Oncotarget. 2015:6. |
| Prostate Cancer | 70 | 51 | Urine | Centrifugation, Size exclusion filtration | TEM, NTA, Western Blot | Urine supernatant stored at —80C before EV analysis | Diagnosis and Tumour Suppressive Effects | mRNA | CMTM3, CDH3 | Royo F, Zuñiga-Garcia P, Torrano V, Loizaga A, Sanchez-Mosquera P, Ugalde-Olano A, González E, Cortazar AR, Palomo L, Fernández-Ruiz S, et al. Transcriptomic profiling of urine extracellular vesicles reveals alterations of CDH3 in prostate cancer. Oncotarget. 2016:7;6835–46. |
| Prostate Cancer | 47 | 16 | Plasma, Serum | Centrifugation, Commercial EV precipitation kit | Protein levels, Western Blot | Plasma cryopreserved prior to EV isolation, EVS stored at —80C prior to analysis | Diagnosis, Prognosis | Protein | Survivin | Khan S, Jutzy JMS, Valenzuela MMA, Turay D, Aspe JR, Ashok A, Mirshahidi S, Mercola D, Lilly MB and Wall NR. Plasma-Derived Exosomal Survivin, a Plausible Biomarker for Early Detection of Prostate Cancer. PLOS ONE. 2012:7;e46737. |
| Prostate Cancer | 36 | 0 | Plasma | Centrifugation & Commercial EV precipitation kit | None | Plasma supernatant stored at —80C before EV isolation | Resistance to Hormone Treatment | mRNA | Androgen receptor splice variant | Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. Eur Urol. 2017:71;680–687. |
| Prostate Cancer | 15 | 30 | Plasma, Cell Lines | Centrifugation, Size exclusion filtration | NTA, Flow Cytometry | Plasma stored at —80C before EV isolation | Screening and Diagnosis | Protein | PSA | Logozzi M, Angelini DF, Iessi E, Mizzoni D, Di Raimo R, Federici C, Lugini L, Borsellino G, Gentilucci A, Pierella F, et al. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017:403;318–329. |
| Prostate Cancer | 31 | 8 | Serum, Cell Lines | Centrifugation, Size exclusion filtration, Immuno-affinity beads, size exclusion chromatography, Density graded centrifugation | Western Blot | EVs stored at —80C prior to analysis, Serum supernatant stored at —80C prior to EV isolation | Diagnosis | Protein | Gamma-glutamyltransferase 1 | Kawakami K, Fujita Y, Matsuda Y, Arai T, Horie K, Kameyama K, Kato T, Masunaga K, Kasuya Y, Tanaka M, et al. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer. 2017:17;316. |
| Prostate Cancer | 31 | 14 | Urine | Centrifugation, Size exclusion filtration | NTA, CryoEM | Urine supernatant stored at —80C before EV analysis | Disease monitoring | Metabolites | 76 individual metabolites with differential abundance between prostate cancer a BPH | Clos-Garcia M, Loizaga-Iriarte A, Zuñiga-Garcia P, Sánchez-Mosquera P, Rosa Cortazar A, González E, Torrano V, Alonso C, Pérez-Cormenzana M, Ugalde-Olano A, et al. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J Extracell Vesicles. 2018:7;1,470,442. |
| Prostate Cancer | – | – | Cell Lines | Commercial EV precipitation kit | TEM, Western Blot | None | Cell Proliferation, Invasion | cicrRNA | circ_SLC19A1 | Zheng Y, Li J-x, Chen C-j, Lin Z-y, Liu J-x and Lin F-j. Extracellular vesicle-derived circ_SLC19A1 promotes prostate cancer cell growth and invasion through the miR-497/septin 2 pathway. Cell Biology International. 2020:44;1037–1045. |
| Prostate Cancer | 3 | 3 | Urine | Centrifugation, Size exclusion filtration | TEM, Western Blot, NTA, | Urine stored at 4C prior to centrifugation and supernatant frozen in LN, isolated EVs stored at -80C | Diagnosis | Metabolites | 11 metabolites specific to urinary EVs | Puhka M, Takatalo M, Nordberg ME, Valkonen S, Nandania J, Aatonen M, Yliperttula M, Laitinen S, Velagapudi V, Mirtti T, et al. Metabolomic Profiling of Extracellular Vesicles and Alternative Normalization Methods Reveal Enriched Metabolites and Strategies to Study Prostate Cancer-Related Changes. Theranostics. 2017:7;3824–3841. |
| Prostate Cancer | 22 | 23 | Urine, Tumour conditioned medium | Density graded centrifugation, Size based concentration, Size exclusion chromatography, commercial precipitation kit | NTA, TEM, Western Blot | Urine supernatant stored at —80C, EV pellets stored at —80C prior to analysis | Diagnosis, protein synthesis, nucleic acid synthesis, autophagy, immune system activation | Protein | 705 differentially EV enriched proteins including HRAS, AKT1, CUL3, NKX3–1, PTEN | Dhondt B, Geeurickx E, Tulkens J, Van Deun J, Vergauwen G, Lippens L, Miinalainen I, Rappu P, Heino J, Ost P, et al. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. Journal of Extracellular Vesicles. 2020:9;1,736,935. |
| Prostate Cancer | 16 | 15 | Cell Lines, Urine | Centrifugation | Western Blot, TEM | None | Transcription, Diagnosis | Protein | Catenin | Lu Q, Zhang J, Allison R, Gay H, Yang W-X, Bhowmick NA, Frelix G, Shappell S and Chen Y—H. Identification of extracellular δ-catenin accumulation for prostate cancer detection. The Prostate. 2009:69;411–418. |
| Prostate Cancer | 10 | 10 | Cell Lines, Urine | Size exclusion filtration, Density graded centrifugation | Western Blot | None | Diagnosis, Disease Monitoring | Protein | PSA, PSMA, 5 T4 | Mitchell PJ, Welton J, Staffurth J, Court J, Mason MD, Tabi Z and Clayton A. Can urinary exosomes act as treatment response markers in prostate cancer? Journal of Translational Medicine. 2009:7;4. |
| Prostate Cancer | – | – | Urine, plasma | Size exclusion filtration, size exclusion chromatography, centrifugation, Cryo-EM | NTA, Western Blot, ELISA | Urine supernatant and platelet free plasma stored at —80C before EV isolation | Prognosis | Protein | Differentia expression of 643 proteins including: protein S, kininogen-1, insulin-like binding proteins, Afamin, cardiotrophin-1 | Welton JL, Brennan P, Gurney M, Webber JP, Spary LK, Carton DG, Falcón-Pérez JM, Walton SP, Mason MD, Tabi Z, et al. Proteomics analysis of vesicles isolated from plasma and urine of prostate cancer patients using a multiplex, aptamer-based protein array. Journal of Extracellular Vesicles. 2016:5;31,209. |
| Prostate Cancer | 29 | – | Urine | Centrifugation, Size exclusion filtration | None | Urine stored at 4C prior to EV isolation, EVs stroed at —70C following isolation | Diagnosis | mRNA | PCA3, ERG | Hendriks RJ, Dijkstra S, Jannink SA, Steffens MG, van Oort IM, Mulders PFA and Schalken JA. Comparative analysis of prostate cancer specific biomarkers PCA3 and ERG in whole urine, urinary sediments and exosomes. Clinical Chemistry and Laboratory Medicine (CCLM). 2016:54;483–492. |
| Prostate Cancer | 46 | 17 | Urine | Size exclusion filtration, Centrifugation | TEM | Urine supernatant stored at —80C before EV isolation | Diagnosis | RNA | PCA3, ERG | Pellegrini KL, Patil D, Douglas KJS, Lee G, Wehrmeyer K, Torlak M, Clark J, Cooper CS, Moreno CS and Sanda MG. Detection of prostate cancer-specific transcripts in extracellular vesicles isolated from post-DRE urine. The Prostate. 2017:77;990–999. |
| Prostate Cancer | 4 | 4 | Urine | Centrifugation, field flow fractionation | TEM, Western Blot | Urine stored at —80C prior to EV isolation | Diagnosis | Lipids | 22:6/22:6-phosphatidylglycerol 16:0, 16:0 - diacylglycerol 16:1, 18:1-diacylglycerol Triacylglycerol species |
Yang JS, Lee JC, Byeon SK, Rha KH and Moon MH. Size Dependent Lipidomic Analysis of Urinary Exosomes from Patients with Prostate Cancer by Flow Field-Flow Fractionation and Nanoflow Liquid Chromatography-Tandem Mass Spectrometry. Analytical Chemistry. 2017:89;2488–2496. |
Abbreviations
ATM - Atomic force microscopy
DSL - Dynamic light scattering
CyroEM - Cryo electron microscopy.
TEM – Transmission electronic microscopy.
NTA – Nanoparticle tracking analysis.
In cancer, the identification of EV cargoes has found applicability in diagnostics, disease monitoring and response to treatment [[79], [80], [81], [82]]. Although EVs are unlikely to mediate all these processes in their entirety, the identification of cancer specific RNAs which are poorly related to their cell of origin outlines their potential role in cancer development [[83], [84], [85]]. Altered EV loading into cancer-derived EVs may be driven in part by enhanced expression of hnRNPA2B1, upregulated in several cancers including breast [86] and pancreatic [87]. The unique cargoes within cancer derived EVs and their distribution in plasma and urine makes them appealing targets for identification of novel biomarkers [88], similar to what has been achieved with circulating tumour cells and cell free DNA [89,90]. EVs themselves express numerous surface markers including proteins which may themselves be misattributed as biomarkers of disease, highlighting the importance of validation in larger cohorts and in comparison to non-cancer controls [91].In addition to characterisation of cargoes, increased EV production from cancer cells has led to speculation that this in itself may be of clinical utility to detect disease [92,93] and off-target effects of chemotherapy [94].
The role of EVs in many diseases has yet to be elucidated and the characterisation of their cargoes to identify novel diagnostic biomarkers has yet to be undertaken. In addition to this, the tissue of origin of many EVs is not always clear, whether released only from the diseased tissue or also from other tissues influenced by downstream signalling or systemic changes due to the disease. Evidence of the majority of EVs originating from malignant tissue, however, is demonstrated by a reduction in circulating EVs following removal of the diseased tissue [95]. Furthermore, lower pre- and post-operative EV levels correlate with greater survival [95]. Other potential sources of elevated EVs are from the systemic response to the disease or the subsequent medical treatment on diseased and non-diseased tissues [41]. The role of EVs in urogenital malignancies is poorly understood, especially in comparison to other diseases such as breast cancer. We provide an overview of the current level of understanding below.
2. Evidence acquisition
We undertook a literature search of PubMed articles written in English using 56 MeSH search terms, relating to extracellular vesicles, reproductive tissues and urological malignancies, as outlined in Supplementary Fig. 1. Search terms were combined using the Boolean operators AND/OR from inception until 3rd of February 2021.
Titles and abstracts of 9532 articles were screened against our inclusion criteria of studies reporting on EVs from male reproductive tract and in urogenital malignancies. We screened the bibliographies of review articles identified in our search to identify relevant studies not captured in out electronic search. We identified 91 original research articles reporting on EV cargoes in renal, prostate and bladder cancer. We report these studies narratively with a focus on lipids, proteins and RNAs, summarising EV cargoes and their identified cellular functions and clinical validation and use as potential biomarkers for disease.
3. Evidence synthesis
3.1. Renal cancer
EVs released from the kidney both in normal physiology, and in renal cancer (RC), have been identified in both plasma and urine [96]. Primary renal cancer is a heterogeneous spectrum of malignancies and accounts for around 90% of all kidney malignancies. Renal cancer preferentially metastasises to the lungs, bone, brain and lymph nodes, suggesting a potential role of EVs in signalling to distant tissues to prepare and maintain the metastatic niche [[97], [98], [99]].
3.2. RNA in renal cancer EVs
Although EV isolation from urine represents a truly non-invasive biopsy, one limitation of examining urinary EVs is the challenge in identifying their tissue of origin and many studies report on the isolation of EVs from plasma and serum. Despite this, urinary and serum RC EVs carry microRNAs capable of diagnosing RC with high levels of sensitivity and specificity (Table 1, Table 2) [100]. Further interrogation of their cargoes has identified numerous other micro-RNAs, lncRNAs, lipids and proteins with promising potential as biomarkers. MicroRNA cargoes with promising clinical diagnostic applications include miR-1233–1, the pro-angiogenic miR-210 and miR-224 with known roles in cell proliferation and migration [89,[101], [102], [103], [104], [105], [106], [107]]. Comparing micro-RNA EVs cargo from RC patients to healthy controls demonstrated the ability to distinguish RC patients with a sensitivity and specificity of 70% and 62.2% respectively for miR-210 and 81% and 76% respectively for miR-1233–1 [101]. Following tumour resection in a subset of 10 patients, 7-days post operatively, significantly lower exosomal levels of miR-210 and miR-1233 where found, p < 0.01 [101]. Although many micro-RNAs identified are upregulated in other diseases, miR-1233–1 has yet to be identified in pathologies other than RC. Similar findings were reported by Wang et al. outlining diagnostic potential for miR-210, with a sensitivity and specificity of 82.5% and 80% respectively, again reporting a reduction in serum exosomal miR-210 at 1, 4 and 12 weeks post-operatively [102].
To increase diagnostic efficacy, the use of micro-RNA combinations have been studied. Of particular interest, is miR-126-3p in combination with either miR-449a or miR-34b-5p, with an Area Under the Curve (AUC) of 0.84 and 0.79 respectively, in discriminating between RC patients and healthy controls [103]. Micro-RNAs have found utility in diagnostics and also survival prediction; in particular miR-let-7i-5p, combined with clinical and biochemical features, discriminates between patients with a survival of 14 vs 39 months, AUC 0.64 [89].
Similar findings and diagnostic accuracy have been reported following examination of the RNA cargoes of plasma EVs, notably micro-RNA miR-224, whose known cellular functions include cellular proliferation and migration [101,104]. Higher levels of EV miR-224 have been associated with poorer clinical outcomes in RC patients, and may have future prognostic biomarker applicability [104]. Other RNAs of interest in RC include lncARSR, a lncRNA associated with a poor response to the tyrosine kinase inhibitor sunitinib, which is used in treatment of RC. Intracellular exchange of lncARSR via EVs leads to upregulation of AXL and c-MET receptors, both of which positively corelate with sunitinib resistance [105].
In addition to miRNA cargos, EV-incorporated mRNA has been studied for its diagnostic biomarker potential. Three genes in particular were identified with lower urinary EV expression compared to healthy controls. The known functions of these genes GSTA1, CEBPA and PCBD1 include transcription and metabolism and warrant further investigation including validation in large patient cohorts [108]. Following radical nephrectomy, levels of GSTA1, CEBPA and PCBD1 within urinary EVs increased and were found to be similar to health controls, however, pointing to their release from malignant tissues [108]. Work by Zhang et al. identified tumour cell derived EVs co-culturered with human umbilical vein endothelial cells (HUVEC) showed increased vascular endothelial growth factor mRNA and protein expression, this in turn increased tubular formation on within HUVEC, which in vivo may promote angiogenesis [107]. Although this work was carried out in cell lines and validation of this finding in a patient population has yet to be undertaken.
3.3. Protein in renal cancer EVs
Despite substantial work to characterise RNA cargoes of EVs, proteins have also been studied revealing numerous biological roles of the identified cargoes. Raimondo et al. reported numerous protein cargoes within urinary EVs including matrix metallopeptidase-9 (MMP-9), podocalyxin-like protein 1 (PODXL), carbonic anhydrase IX (CAIX) and syntenin-1 [109]. Although their differential expression makes them potential biomarkers for diagnosis, validation in large patient cohorts is still required.
Interrogation of EVs released from renal cancer cell lines has also led to the identification of numerous cargoes. The RC cell line, OS-RC-2 mediate reduced expression of the tumour suppressor hepatic and glial cell adhesion molecule, which in turn causes a reciprocal increase in cell proliferation and disease progression [110]. In addition to RNA, RC EVs also transport proteins such as CAIX, a cellular hypoxia marker and common feature of the tumour microenvironment, which induces hypoxia inducible factor 1 (HIF1) [111]. CAIX is overexpressed in several cancers and in vitro studies have demonstrated it promotes endothelial tube formation, a prerequisite for angiogenesis [111]. Our understanding of the role of EVs in RC metastasis is generally limited. However, matrix MMP-9 and chemokine receptor CXCR-4 were both upregulated in the RC cell line 786-O following co-culture with EVs derived from the same malignant cell line [112]. This finding is suggestive of ‘closed loop’ communication between cells, driving malignant change in adjacent cells, through EVs. In addition to local changes, EVs promote development of the pre-metastatic niche. RC derived EVs from human CD105 positive cancer stem cells have pro-angiogenic RNA cargo when studied in vitro. Administration of these EVs lead to enhanced development of lung metastasis in a mouse model, when renal cancer cells were injected at a later time point [63].
As well as the direct action of EVs on local cells within the renal parenchyma or sites of metastatic spread, RC derived EVs also interact with the immune system, the modulation of which is an important feature of cancer progression. Impaired maturation of monocyte derived dendritic cells and T cell activation is mediated through transfer of HLA-G to these cells [113]. Dendritic cell infiltration into developing tumours results in a significant immune response leading to reduced progression of disease. Conversely, inhibition of dendritic call maturation, ameliorates this immune response and allows the cancer to progress unhindered [114]. EVs derived from the renal cell adenocarcinoma cell line, ACHN, co-cultured with the immortalised T cell line, Jurkat, induced apoptosis in a dose dependent manner via the caspase pathway. EVs derived from ACHN cells carry Fas Ligand, a type-II transmembrane protein of the tumour necrosis factor (TNF) receptor family known to induce caspase activation and apoptosis. EVs treated with soluble Fas reduced EV mediated apoptosis in Jurkat T cells. Natural Killer (NK) cells form part of the innate immune system and have strong anti-tumorigenic properties through their ability to discriminate between self and cancerous ligands on the cell surface. This can be disrupted, however, by the EV-mediated immunosuppressive cytokine TGF- β. Following interaction between RC derived EVs and NK cells, NK cell function is disrupted, leading to reduced cytotoxic activity towards cancer cells [115].
3.4. Lipids in renal cancer EVs
Although less frequently reported on than RNAs and proteins, EV-incorporated lipid cargoes can be utilised in clinical applications. Del Boccio et al. identified differences in the lipid components of urinary EVs between RC patients and healthy controls [106]. Although this work was undertaken in a clinical cohort, the small numbers of patients and controls (n = 8 in each group) means this work must be validated in a larger sample size prior to its use as a lipid-based diagnostic biomarker.
Together, this evidence demonstrates the significant role EVs play in RC, highlighting numerous opportunities to exploit them as novel diagnostic biomarkers and potentially to risk-stratify patients with RC in response to treatment and prognosis. Validation of these markers in large clinical cohorts has the potential to facilitate superior monitoring and guide treatment for individual patients, with greater accuracy than is currently achievable.
3.5. Bladder cancer
Bladder cancer is the most common malignancy of the urinary tract and diagnosed by cystoscopy and tissue biopsy, which are invasive and carries associated procedural risk to the patient [116]. Although urinary cytology has been explored as an alternative diagnostic tool, the variation in sensitivity has limited its clinical use. As such, there is an urgent unmet need for screening test to facilitate early disease detection and non-invasive diagnostic biomarkers to reduce the need for invasive testing.
One potential avenue for this is through interrogation of the EVs released from bladder cancer identified to be elevated in both plasma and urine, compared to healthy controls [117] (Table 1, Table 2).
EVs derived from bladder cancer cell lines confer tumorigenic changes in cells though a range of pathways inducing genome instability and increased invasiveness. Interrogation of their cargoes has led to the identification of several potential biomarkers [118]. Despite previous reports indicating that EVs return to their pre-malignant levels both in terms of composition and cargo [95], a persistent cancer phenotype was recently identified by Hiltbrunner et al. Over-expression of exosomal proteins regulating glycolysis and glycolytic shift were identified, in patients post cystectomy, both of which play important roles in cancer metabolism [119]; altered EV mediated glucose metabolism also being a known feature of the pre-metastatic niche [120]. This was in a small cohort of 13 patients however, and must be confirmed in larger cohorts and for different pathologies.
3.6. RNA in bladder cancer EVs
The lncRNA transcriptase antisense RNA, HOTAIR, has been identified within urinary EVs in patients with bladder cancer. HOTAIR facilitates tumour progression and is associated with poor prognosis and high recurrence rates in patients with invasive high-grade disease [121]. Epigenetic modification and silencing of miR-205 by HOTAIR leads to increased cell proliferation, migration and invasion of bladder cancer cell lines and represents a potential prognostic marker for disease activity and recurrence. Screening other candidate lncRNAs within EVs has identified metastasis-associated lung adenocarcinoma transcript 1, prostate cancer associated transcript-1 (PCAT-1) and sprouty receptor tyrosine kinase signalling antagonist 4-intronic transcript 1 as diagnostic and prognostic biomarkers [122]. Combining these markers has led to the development of a diagnostic panel with a sensitivity and specificity of 70.2% and 85.6% respectively in discriminating bladder cancer from controls [122].
As with RC, examination of urinary exosomes has revealed several candidate markers for prognosis in bladder cancer. Of particular interest are the EV cargoes H2BK1 and alpha 1-antitrypsin as both prognostic and diagnostic biomarkers, with a combined sensitivity and specificity of 62.7% and 87.59% respectively in discriminating patients with bladder cancer from controls [90]. The diagnostic potential of lncRNAs incorporated in serum EVs was investigated by screening 11 lncRNAs against 200 cases of bladder cancer. Following validation of these lncRNAs, three were found to have a high diagnostic accuracy for bladder cancer: PCAT-1, upregulated in bladder cancer 1 (UBC1) and small nucleolar RNA host gene 16 (SNHG16). A screening panel of all three lncRNAs showed they were highly diagnostic of bladder cancer with an AUC of 0.857 [123]. However, widespread validation of these lncRNAs in clinical studies has yet to be undertaken despite a sensitivity and specificity of 80% and 75%, respectively (AUC: 08.26), discriminating between patients with bladder cancer and healthy controls, when validated in an independent training set [123]. Another combined RNA panel including miR-26a, miR-93, miR-191 and miR-940, had a sensitivity of 70%, specificity of 84% and diagnostic value of AUC: 0.858 for identifying bladder cancer in a cohort of 85 patients and 45 controls [124].
Further EV cargoes explored for their diagnostic potential include three mRNAs identified through RNAseq analysis and further validated in a cohort of 206 patients and 36 controls. The diagnostic value of these RNAs was SLC2A1 AUC 0.70, GPRC5A AUC 0.64 and KRT17 AUC 0.64, when these mRNAs were combined with conventional urinary cytology, their diagnostic accuracy rose to AUC 0.93 [125]. This highlights the importance of utilising established diagnostic methods in conjunction with newly established markers to improve test accuracy.
Interestingly EVs released from bladder cancer tissue express high levels of the tumour suppressor micro-RNAs miR-23b and miR-921 [126]. This finding suggests that malignant cells have exploited EVs to dispose of intracellular tumour-supression molecules, thus allowing the malignancy to evade cellular checkpoints which would typically slow or stop the progression of disease. Characterisation of micro-RNAs in EVs from serum, tissue, urine and white blood cells in bladder cancer patients revealed 19 upregulated micro-RNAs of which miR-2 and miR-4454 were upregulated in EVs from all sources [127].
In addition to RNA cargo, EVs derived from bladder malignancies and isolated from urine, carry endothelial locus 1 (EDIL-3), a glycoprotein secreted by endothelial cells mediating endothelial cell attachment and migration [128]. EDIL-3 has been identified in other malignancies including hepatocellular carcinoma and pancreatic cancer, conferring poor prognosis in both. Studies of EDIL-3 in high-grade bladder cancer EVs promoted cell migration and angiogenesis in bladder cancer cells. This was confirmed with short hairpin RNA interference to knockdown EDIL-3, which inhibited migration and angiogenesis [128].
A feasibility study by Perez et al. identified LAG1 homolog ceramide synthase-2 and Polypeptide N-acetylgalactosaminyltransferase 1 were upregulated in urinary EVs in a small cohort of bladder cancer patients, while chromosome 9 open reading frame 100 (ARHGEF39) and FOXO3 were found only in EVs derived from benign tissue [129]. A further urinary EV mRNA, miR-21-5p was validated for its diagnostic potential and found to be highly sensitive with an AUC of 0.89, however this was only in a small cohort of 6 patients with 3 controls [130].
EV cargoes not only have diagnostic potential but also a possible role in disease monitoring and progression. Andreu et al. examined miRNAs within urinary EVs identifying miR-30c-2 was predictive for disease relapse whereas the EV incorporated miRNas let-7c, miR-375 and miR-194 where both found to be downregulated in bladder cancer patients while miR-146a was significantly upregulated [131]. Both miR-375 and miR-146a were validated as potential biomarkers for high grade and low grade disease respectively in small cohort of patients.
3.7. Proteins in bladder cancer EVs
Initial examination of EV cargo released by bladder cancer cell lines revealed over 350 protein cargoes, with 72 previously unidentified in other human EV proteomic studies [132]. Further validation of these cargoes revealed a subset of 7 proteins: apolipoprotein A1, CD5 antigen-like, fibrinogen alpha chain, fibrinogen beta chain, fibrinogen gamma chain, phosphocarrier protein and haptoglobin, which could discriminate between low- and high-grade disease [133]. Although the function of many of these proteins in bladder cancer has yet to be defined, EVs released from invasive bladder cancer increase migration of urothelial cells co-cultured with these EVs, a crucial transitionary step from localised to invasive disease [134]. Further pro-oncogenic roles identified within EVs include promotion of the pre-metastatic niche, identified through the effects of the protein C10 regulator of kinase (CRK) and mRNA ErbB2 [135]. Knock down of CRK impaired disease progression, reducing local and metastatic tumour progression in a mouse cell line [135].
In bladder cancer cells lines, unique EV cargoes have been identified, including MAGE B4 which was overexpressed in urinary exosomes of transitional cell carcinoma of the bladder [136]. One notable role of MAGE B4 is inhibition of apoptosis and tumour cell survival [137], however urinary exosome levels of MAGE B4 in bladder cancer patents was lower than that identified in small cohort (n = 8) of BHP patient controls, outlining other roles for MAGE B4 in different tissues and also the risk of false positives when screening for bladder cancer. The small numbers used in these validation studies mean their results should be interpreted with caution, and larger cohorts are needed to further validate these markers.
Despite the identification of numerous EV cargoes derived from both bladder cancer tissue and cell lines, there is limited scope for their routine use in clinical practice at present. Further validation of these potential markers as well as the feasibility of their use with established diagnostic tools must be undertaken to better define their applicability in a clinical setting.
3.8. Prostate cancer
Prostate cancer is a common malignancy diagnosed in aging men and a significant cause of mortality. Although survival is improving through early detection of latent disease and improving treatment outcomes, for many patients, their disease is first recognised once it has metastasised. The subjective and poorly specific nature of digital rectal examination and serum prostate specific antigen (PSA) means there is an urgent unmet need for sensitive and specific biomarkers.
Both benign and malignant prostate cells release EVs which have been detected in plasma and urine with significant diagnostic potential [138,139]. The term prostasome has been used to describe EVs released by the prostate and these likely represent a mixed cohort of EVs derived from numerous pathways [43,[140], [141], [142], [143], [144]].
Initial studies characterising the cargo of these EVs have identified over 200 potential candidates for biomarkers with various roles in cell adhesion, differentiation, migration and transcription [[145], [146], [147], [148], [149], [150], [151]]. Exploration of their diagnostic ability has shown them to discriminate between benign and malignant disease, but also high and low grade disease using a panel of EV incorporated proteins including transglutaminase-4, adseverin, cluster differentiation 63, putative glycerol kinase 5, prostate-specific antigen, prostatic acid phosphatase and N-sulphoglucosamine sulphohydrolase [147].
The function and impact of prostasomes as potent mediators in the development of cancer should not be underestimated. EV incorporated circ_SLC18A1, increased in prostate cancer derived EVs, downregulated miR-497 with a reciprocal rise in expression of miR-497's target gene septin 2 [152]. This over expression leads to important EV regulated phenotypic changes associated with prostate cancer progression, notably cell growth. In addition to impacts on local tissue, prostate cancer derived EVs play an important role in disease progression in tissues remote from the prostate. The mechanisms by which prostate cancer metastasises to bone is yet to be fully elucidated but the development of the pre-metastatic nice is, in part, developed through the action of prostasomes. Several examples of how these EVs impact metastasis and their mechanisms have been identified. Dai et al., demonstrated prostasome transfer of pyruvate kinase M2 (PKM2) to bone marrow stromal cells in culture. Transfer of PKM2 to recipient cells effectively ‘primes' the tissue for subsequent metastatic spread through up-regulation of CXCL12 [153]. In addition to direct protein transfer to tissues, EV incorporated microRNAs also play a crucial role, including has-miR-940 which promoted osteogenic differentiation of human mesenchymal stem cell to osteoblasts in culture [154]. siRNA knockdown of potential candidate genes through which has-miR-940 mediates its action revealed osteogenesis was regulated through ARHGAP1 and FAM134A [154]. Another microRNA identified in the regulation of osteoblastic activity is miR-141-3p,previously identified in as a biomarker for prostate cancer (Table 1 and Table 2). Ye et al., demonstrated EV mediated delivery of miR-141-3p showed preferential targeting to bone tissues and suppressed the gene DLC1, which in turn activates the p38MAPK pathway leading to promotion of osteoblast activity through increased expression of osteoprotegerin [155].
EV cargo loading is dynamic and reflective of their cell of origin, this feature continues as these cells mutate both in terms of acquisition of malignant potential but also other features such as development of chemotherapy resistance. Corcoran et al., demonstrated docetaxel-resistant cell lines conveyed this resistance through transfer of MDR-1/P-gp to docetaxel-sensitive cells. In addition to enhanced cell proliferation and invasion from cells incubated with docetaxel-resistant EVs, correlations were identified between cellular response and patients response to docetaxel treatment, when cells were cultured with patients EVs [156].
3.9. RNA in prostate cancer EVs
The diagnostic potential of urinary EV-incorporated RNA has also been explored. Of particular note are the RNA panels: miR-572, miR-1290, miR-141, miR-145 157 and miR-21, miR-204, miR-375 158 combined with serum PSA, which demonstrate good discrimination between benign and malignant disease with an AUC of 0.863 and 0.866 respectively. The use of miR's in addition to PSA offered superior diagnostic potential than PSA alone. Using a combination of urinary EV mRNA levels of Erythroblast transformation-specific related gene (ERG), prostate cancer antigen 3 (PCA3) and PSA with patient characteristics including age, race and family history improved the diagnostic accuracy from AUC 0.6723 to 0.803 159 highlighting the applicability of ERG, PCA3 and PSA as diagnostic biomarkers [160]. In addition to the biomarker potential of microRNAs, miR-1908 interaction with spermidine synthase (SRM) was found to regulate EV release in prostate cancer cells, confirmed by reduced secretion following SRM knockdown [161].
Numerous other micro-RNAs and lncRNAS with altered expression in prostate cancer have been identified in both urine [[157], [158], [159], [160],162] and plasma [[163], [164], [165], [166]] (Table 1, Table 2). Some of these micro-RNAs have been investigated for their correlation with the Gleason score as alternative diagnostic biomarkers, although none have been as robustly validated as ERG. Gleason scores are used as a prognostic marker of prostate adenocarcinoma with scores <6 considered to have good prognosis, while scores >8 imply aggressive disease. Other RNA molecules with good correlation to the Gleason score include: (a) elevated miR-145 in the EVs in both serum and urine predictive of a score > 8 157 and (b) lower levels of urinary let-7c, an RNA precursor which correlates well with clinical staging [162]. Serum EV cargoes also show good discrimination between patients with high and low grade disease despite it representing only a small fraction of circulating RNA. In particular, levels of let-7a-5p were able to discriminate between patients with Gleason scores of <6 and those with scores of >8 with an AUC of 0.68 [164].
Interestingly, not all micro-RNAs identified within cancer-derived EVs are upregulated. The downregulation of tumour suppressor micro-RNAs including miR-1246 165 and miR-214, which have numerous roles, has been identified compared to disease free, or benign prostatic hyperplasia controls [162]. Despite their potential, the routine use of EVs to detect urogenital malignancies is not yet commonplace, and further work must be undertaken to determine their clinical applicability and utility. To date the combination of the lncRNAs SChLAP1 and SAP30L-AS1 have the highest diagnostic accuracy in prostate cancer with a AUC of 0.9224, when compared to PSA alone which has a sensitivity and specificity ranging from 78 to 100% and 6–66% respectively [166,167]. Serum exosomal levels of miR-141 were elevated in patients with metastatic disease compared to localised disease and [168]. This is likely due to the presence of more diseased tissue, but also makes miR-141 a potential target for further evaluation in monitoring response to treatment or disease progression.
Urinary EV-incorporated ERG, PCA3, SPDEF was validated in two studies for their diagnostic potential to discriminate between low and high grade disease with an AUC of 0.74 [169] and 0.70 [170]. Additional work on the diagnostic potential of urinary EV cargoes was undertaken by Bryzgunova et al. identifying miRNA-19b and miR-16 to have a sensitivity and specificity of 100%/93% and 95/79% respectively in distinguishing prostate cancer from healthy controls [171].
Examination of the potential roles of EV cargoes has identified their ability to alter the function of the immune system. Angelis and Spiliotis et al. identified circular RNA, specifically circ_SLC19A1, within EVs interacts with miR-497, regulating the expression of septin 2, the dysregulated expression of which is identified in numerous cancers [172]. EV mediated circ_SLC19A1 indirectly alters the regulation of septin 2, promoting growth and invasion of prostate cancer cells [152]. Modulation of the immune system was reported by Salimu et al. reporting EV mediated delivery of prostaglandin E2 to antitumour CD8+ T cells suppressed their dendritic function [173]. Further EV - T cell interaction leads to downregulation of the transmembrane receptor NKG2D on NK cells and CD8+ T cells [174,175]. While EV-mediated transfer of adenosine reduces T cell response to IL-2 [176].
3.10. Proteins in prostate cancer EVs
The protein cargoes of EVs have been reported by numerous authors with validation for diagnosis, discrimination between low- and high-grade disease and disease progression.
In EVs isolated from serum, prostasomal EphrinA2, a member of the protein-tyrosine kinase family has been validated in a cohort of 50 patients and 21 controls showing a high sensitivity (80.59%) and specificity (88%) in discriminating between prostate cancer and benign prostate hyperplasia with an AUC of 0.9062 [177]. Other serum and plasma EV markers identified within prostasomes include PSA, survivin and P-glycoprotein with potential roles in diagnosis screening and predicting response to chemotherapy [[178], [179], [180]]. Gamma-glutamyltransferase 1 levels, initially identified within EVs released in cell culture has subsequently been identified in serum EVs derived from prostate cancer patients and may have potential use as a diagnostic marker [181]. In addition to diagnostic markers, EV cargoes have the potential to discriminate between malignant and benign prostate disease. Khan et al. demonstrated the apoptosis inhibitor, survivin, was significantly raised in EVs derived from prostate cancer patents compared to a cohort of patients with benign prostatic hyperplasia (BPH) and healthy controls [179]. In addition to survivin, EV-incorporated PSA can also be used to screen for prostate cancer when compared to healthy and BPH controls [178].
None of these markers have been validated in large patient cohorts, limiting their clinical applicability at present. A proteomic analysis undertaken by Minciacchi et al. revealed 103 differentially expressed proteins with numerous known functions including cell proliferation, transmembrane transport, vesicle mediated transport, angiogenesis and chemotherapy resistance [182]. As with other cargoes the numerous functions identified within EVs signals their potential use not only clinically but also give important clues as to potential mechanisms at a cellular level through which they mediate their effects. Although the clinical utility of these markers is important and has great potential to advance diagnostics, screening and disease monitoring, identification of mechanistic pathways may lead to new treatment to disrupt these pathways. Mass spectrometry-based proteomic assessment of urinary EVs by Dhondt et al. demonstrated 1134 enriched proteins within EVs including known drivers of prostate cancer HRAS, AKT1, CUL3, NKX3–1 and PTEN [183]. Comparisons between prostate cancer patients and healthy controls revealed 705 of these proteins were differentially enriched within EVs. Following resection of prostate disease, analysis of urinary EVs demonstrated an increase in the number containing bladder and kidney markers, suggesting prostate cancer leads to increased EV release into urine. This work was carried out in a small cohort, however, and requires further validation prior to clinical application.
As well as their identification within clinical samples, cultured cell-line EVs have been found to carry protein cargoes such as ALIX, FASN, XPO1 and ENO1 [148]. Further work by Itoh et al. also revealed Ets1 and PTHrP were incorporated within EVs [149]. These were found to promote cell differentiation within osteoblasts in a mouse model and give important insights as to the possible function of prostasomes within other tissues and may be of significant clinical relevance, given prostate cancer preferentially metastasises to bone.
3.11. Lipids in prostate cancer EVs
Lipid cargoes of prostasomes have received little attention in comparison to protein and RNA, however Skotland et al. corelated the ratio between Lactosylceramideand Phosphatidylserine in a small cohort of 15 prostate cancer patients and 13 healthy controls, with an AUC of 0.989 [184]. The use of mass spectrometry in healthcare settings is currently limited to the identifcaiton of specific markers, rarther than large scale screening for numerous compounds which will limit the use of EV lipid profiling, despite its high diagnostic accuracy. The small cohort size included in this study however means that despite promising results, they require validation in larger cohorts prior to their use in clinical settings. However, this is the first study to report the use of EV incorporated lipids as a possible biomarker for prostate cancer diagnosis.
3.12. Metabolites in prostate cancer EVs
Changes in EV incorporated metabolites have been identified in a cohort of 31 prostate cancer patients and 14 controls. Of these metabolites, 76 showed differential expression levels within EVs. Of particular interest are two androgen precursors 3beta-hydroxyandors-5-en-17-one-3-sulphate and estrone sulphate, implicated in the progression of prostate cancer [185]. A small study of urine samples from three patients pre- and post-prostatectomy also identified differential expression of EV incorporated metabolites compared to healthy controls [186]. However, as with many EV studies, these findings are reported in a small number of patients and require validation in larger cohorts.
3.13. Testicular cancer
Although EVs are reported in renal, bladder and prostate cancer, EV identification and functional appreciation have yet to be achieved in testicular cancer. Testicular cancer is the commonest cancer in young men globally. The majority of cases arise due to the persistence of foetal germ cells which should differentiate into spermatogonia during fetal and early postnatal life, but instead transform into pre-neoplastic Germ Cell Neoplasia In-Situ (GCNIS). Interestingly, the presence of GCNIS cells in early postnatal life, and the peak age of onset for testicular germ cell tumours being 25–29 years, means that the pre-malignant phase of this cancer can last over 20 years. Prognosis for testicular cancer is good once diagnosed, but an effective diagnostic test prior to malignant transformation during the pre-malignant phase has yet to be developed. The potential capability of EVs in providing material for detecting testicular cancer is currently unknown. Although a unique micro-RNA signature has been identified in testicular cancer, its presence within EVs has not been investigated [187].
3.14. Methodological challenges in EV isolation and sample preparation
The heterogeneous nature of EVs means their isolation from a range of biological media including serum, plasma and urine as well as cell culture supernatant leads to challenges in achieving replicable standards. As no ‘gold stand’ methodology exists, efforts to build consensus driven reporting on EVs have been led by the International Society for Extracellular Vesicles (ISEV), developing two minimum consensus positions [1,188]. The majority of this heterogeneity is based on the separation techniques used and as such is of upmost importance, as this effectively generates the material to be analysed and will influence results and conclusions drawn [189]. Frequently used techniques include ultracentrifugation, size exclusion chromatography, filtration, immunoaffinity capture, microfluidic separation and polymer precipitation [[190], [191], [192], [193], [194]]. As well as the EV separation technique used, numerous other factors can influence EV purity include pre-analytical variables such as the medium in which EVs are to be isolated from, sample acquisition and storage. Many of these issues were apparent in the studies included in this review and are outlined in Table 1, Table 2.
3.15. Pre-analytical variables
Despite development of a consensus position on EV isolation and characterisation, substantial variation exists when reporting biomarker discovery and validation. In addition to the heterogeneity from EVs themselves, inherent pre-analytical variation can impact on the results obtained [195].
How samples are obtained and stored can affect their molecular and proteomic profiles. This is of great significance given reporting of novel biomarkers is in part based on the measurement of these factors [196]. Many studies included in their review used EVs in blood to conduct their analysis. However, the way blood is processed varies greatly. Additives within the phlebotomy bottles into which blood is collected varies greatly and often based on the intended use of the clinical sample. These additives can include ethylenediaminetetraacetic acid which is suitable for a range of DNA and protein-based studies whereas heparin binds to numerous proteins and may impact protein-based assays.
[195,197]. In addition to interaction between additives and presumptive EV cargoes, the number of EVs identified within a blood sample can be altered after only a few hours, with Lacroix et al. reporting a 80% increase in EVs after 4 h [198]. Variation in the number of additional EVs generated through interaction with additives to transport blood may be reduced by storing and transporting samples of serum as opposed to plasma. This would require further sample preparation which may delay downstream analysis to isolate EVs and inadvertently lead to unwanted sample variation. In many studies reported in this review, often a single sample of blood was obtained as opposed to repeated samples, accounting for variation in sample acquisition or unknown physiological processes which may impact EVs number [199].
In addition to blood, urine is a frequently collected clinical sample in which to separate EVs for further analysis. The bladder, unlike many organs, changes in volume and contents throughout the day, the contents of which originate from the kidney as it filters waste from blood. As such it represents a potentially rich and diverse source of EVs, either through the direct release of EVs from the bladder and kidneys or through co-contamination with semen comprising testicular, prostate and seminal fluid EVs. Many authors reported using freshly voided urine often centrifuging the sample to remove large pellets of co-contaminants, reduced the heterogeneity of the sample and the presumed effect of urine pooled within the bladder for longer periods. A notable contaminant of urine is the presence of uromodulin, a major protein component of urine with the potential to interact with EVs [200]. Uromodulin has been demonstrated to interact and bind to EVs, forming a uromodulin-EV complex, this is both larger and heavier than single EVs, and thus size based EV isolation methods may not accurately capture these particles [201]. How uromodulin interacts with EVs when stored post void, is currently unknown.
Acquisition of clinical samples is further compounded by a lack of consensus driven protocols in how to obtain these samples and process them. This variation, often compounded by a small cohort size leads to challenges in the reliability of the results obtained with much work reported in this review requiring validation in larger patient cohorts.
3.16. EV separation from liquid biopsy and cell culture media
In addition to pre-analytical variables impacting EV yield and quality, the aforementioned methodologies to separate EVs from these samples often produces results with variable EV heterogeneity, structure and co-precipitates. Many of these factors in isolation, let alone in relation to other poorly controlled for variables, have the potential to confound any subsequent analysis [202]. This in part can be due to the nature of the sample preparation but also the technique used such as the shear stresses placed on EVs during pellet formation in ultracentrifugation, as well as the co-precipitation of non-vesicular debris [203]. Many of these techniques depend on the purity of the sample with size exclusion chromatography or density graded centrifugation reliant on the size profile of EVs, leading to the risk of co-precipitation of other particles with a similar size [204]. Despite the intention of differential ultracentrifugation being to pellet larger debris e.g. cells, with a higher buoyant density, while EVs remain suspended in the media to be pelleted in subsequent centrifugation steps, this methodology is somewhat limited in its ability generate a homogenous sample of EVs [205]. Particles of a similar size may be co-precipitated and contaminate the EV sample, assessing the size of the particles obtained through nanoparticle tracking analysis for example, may lead to false confidence in the purity of the sample [192]. Sample purity can be improved somewhat by using differential ultracentrifugation, followed by additional purification steps to allow large volumes of sample to be processed and remove co-precipitated particles; however, this is both time consuming and associated with large equipment costs and inevitably leads to reduced numbers of EVs obtained by the final preparation [206,207].
Gradient centrifugation again reduces sample heterogeneity and uses a dense medium through which the sample passes with particles of similar density held within the sample. Filtration of samples through membranes with a pre-specified pore size allows a low cost alternative to centrifugation and is more suited to processing of smaller samples and has been reported by some to produce EVs with greater functionality than centrifugation [208]. However, membrane deterioration allowing the passage of larger particles as well as mechanical stress to EVs passing through the membrane, can lead to contaminants within the sample. Although this technique does exclude larger particles, it fails to produce a concentrated sample of EVs as with centrifugation, thus additional steps may be required in order to prepare the EVs for analysis [209]. Isolation techniques which place EVs under less stress during their preparation include immunoaffinity capture and precipitation which make use of the known surface markers of EVs and their lipid bilayer membrane [210]. Immunoaffinity requires the use of high cost antibodies but can facilitate the capture of certain populations of EVs and with high purity. Precipitation also generates a high yield of EVs; however, this can be contaminated with protein aggregates and other lipid based molecules. More recently, microfluidic devices have been developed to isolate EVs from a range of suspensions including cell culture media, plasma and urine [211]. These devices are diverse and make use of the EV size, EV density and surface antibodies to separate them from their suspension as they flow through the device. Preferable for small sample sizes, microfluidic isolation of EVs is costly yet offers both a high yield of EVs and low co-capture of contaminates.
3.17. EV storage and analysis
Following sample acquisition or EV isolation many studies included in this review stored samples at different temperatures and for different lengths of time. Methods of storage for serum, plasma and urine prior to EVs isolation varies greatly between studies and may impact the quantity and integrity of the EVs isolated. While this may be due to practicalities of obtaining clinical samples, variation in their storage at different temperatures can affect both quantity and integrity of EVs. Following the isolation of EVs, there is variation as to when they were characterised and interrogated for their cargoes. The impact of freeze-thawing is disputed with some authors reporting minimal impact on EV yield [212], others identifying a reduction in EV number, but minimal impact on EV uptake [213], and others reporting the addition of the cryoprotectant DMSO as improving EV yield and function [214].
Work to establish the optimal storage conditions for EVs has suggested −80C may be suitable with Enderle et al. reporting the isolation of EVs from plasma with minimally degraded RNA levels after 12 years of storage at −80C [215]. Interestingly, EVs stored at 4C were found to have a greater reduction in concentration than those stored at 60C, −20C and −80C [213]. Many of the studies included in this review stored EVs at -80C; however, several studies reported storage at 4C and this may impact on the reliability of the results obtained.
Following the presumptive isolation of EVs, substantial variation in validation of EV yield and purity has been identified. The most commonly used technique is transmission electron microscopy which enables the size and morphological appearance of the EVs to be identified. This was frequently accompanied by western blotting for common EV surface markers. Interestingly, some studies did not undertake any specific analysis of their sample to assess the yield of EVs or ensure their presumptive EVs were not contaminated. This raises issues with subsequent analysis and conclusions drawn as cargoes reportedly within EVs may have been identified by samples with a low number of EVs. Comparisons of identified cargoes to the EV depleted fraction should also be undertaken to ensure similar results or novel findings aren't reported in fractions which do not contain, or are not meant to contain, EVs. Again, lack of consistent reporting across studies and variation in the methodologies used means the identification of many biomarkers require further validation in larger cohorts and reliable identification using standardised techniques.
Variable reliability and cost associated with current EV isolation and storage techniques outlined above have led to the development of platforms which directly analyse EVs from biological samples without prior need for EV isolation steps. Novel platforms to profile EVs from patient blood samples have been developed using antibody capture EVs for identification of colorectal cancer allowing analysis within 2 h and requiring only 5 μL of serum [216]. More recently, microfluid EV capture devices have been developed again using small volume (100 μL – 5 mL) blood samples from glioblastoma patients utilising antibody based capture of EVs to isolate tumour specific EV incorporated RNA within 3 h [217]. Although the aforementioned studies do not focus on urological malignancies, similar devices have been developed for the characterisation of EVs in prostate cancer. Sunkara et al., developed a device utilising both sequential and tangential flow filtration of 30 μL blood samples to characterise mRNA of EVs between prostate cancer and healthy controls. Comparison of this technique to ultracentrifugation demonstrated superior EV isolation mRNA yield, with sample analysis only taking 40 min [218]. These platforms, should they be by clinically reliable and financially viable, may lead to development of novel multi-disease screening or point of care testing platforms for application in numerous health care settings.
4. Conclusions
The field of EVs has grown in the understanding of their widespread distribution, identification in numerous body fluids and their role in health and disease. The advent of technologies, to characterise EVs in vivo, but also isolate and study them in vitro, has led to significant advances in our understanding of their potential. Appreciation and application of the roles EVs play in cancer development, progression and response to chemotherapy are exciting and fast-moving areas.
Advances in the characterisation of their cargo has revolutionised our understanding of EVs over the past 10 years. Proteomic, metabolomic and RNAseq studies have enabled us to appreciate individual molecules leading to further research on the properties of individual proteins and micro-RNAs. Identification of unique cargo within EVs offers new opportunities to develop novel diagnostic tools in order to recognise diseases which currently lack sensitive and specific tests. Variation in methodologies used and the way in which biomarkers are identified means some novel reported cargoes should be treated with caution and validated in larger patient cohorts, prior to their consideration for use in healthcare settings. Of the 91 primary studies identified in our review, only 5 made references to ISEV standards when reporting data on EVs [182,[219], [220], [221], [222]]. This highlights further work is required to standardise reporting across studies and fully implement minimum reporting standards.
Increasing use of EVs both in science and healthcare, rapidly expanding cataloguing of their cargo and falling costs of underlying technologies, will likely remove barriers to the routine use of EVs in healthcare settings to facilitate early disease detection and monitoring in the future. The correlation of EV cargo to current diagnostic techniques has demonstrated the exciting potential of EVs in ushering in a new era of precision medicine in relation to cancer diagnostics. This is of particular importance for urological malignancies which remain a significant health care burden. In addition to their diagnostic potential, EVs are a growing area of therapeutic interest. The widespread distribution and the development of new techniques to manipulate them through artificial incorporation of molecules such as siRNA or modulating their interaction with cells represents a new potential field of clinical therapeutics.
Author contributions
MPR – conceived the idea for the article, undertook the literature search, wrote the manuscript, developed the figures and approved the manuscript for submission.
CDG & RTM – conceived the idea for the manuscript, undertook editorial changes and approved it for submission.
Funding
MPR and RTM are funded by a MRC Centre for Reproductive Health Grant No: MR/N022556/1.
RTM is funded by a UK Research and Innovation (UKRI) Future Leaders Fellowship MR/S017151/1.
Declaration of Competing Interest
Michael P Rimmer, Christopher D Gregory and Rod T Mitchell have no decelerations of interest to declare.
Contributor Information
Michael P. Rimmer, Email: Michael.Rimmer@ed.ac.uk.
Rod T. Mitchell, Email: Rod.Mitchell@ed.ac.uk.
Appendix A. Supplementray data
Supplementray material.
References
- 1.Théry C. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracel. Vesicl. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 3.Kalluri R., LeBleu V.S. The biology, sfunction, and biomedical applications of exosomes. Science. 2020;367 doi: 10.1126/science.aau6977. eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pallet N. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics. 2013;13:1108–1120. doi: 10.1002/pmic.201200531. [DOI] [PubMed] [Google Scholar]
- 5.Han L., Lam E.W.F., Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol. Cancer. 2019;18:59. doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Neill C.P., Gilligan K.E., Dwyer R.M. Role of extracellular vesicles (EVs) in cell stress response and resistance to cancer therapy. Cancers. 2019;11 doi: 10.3390/cancers11020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. Cardiac extracellular vesicles in normal and infarcted heart. Int. J. Mol. Sci. 2016;17 doi: 10.3390/ijms17010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Z. Emerging role of exosome-derived long non-coding RNAs in tumor microenvironment. Mol. Cancer. 2018;17:1–9. doi: 10.1186/s12943-018-0831-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee D.H. Urinary Exosomal and cell-free DNA detects somatic mutation and copy number alteration in urothelial carcinoma of bladder. Sci. Rep. 2018;8:14707. doi: 10.1038/s41598-018-32900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittelbrunn M. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valadi H. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 12.Skog J. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guduric-Fuchs J. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genomics. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Squadrito M.L. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep. 2014;8:1432–1446. doi: 10.1016/j.celrep.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Villarroya-Beltri C. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Temoche-Diaz M.M. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. eLife. 2019;8 doi: 10.7554/eLife.47544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horibe S., Tanahashi T., Kawauchi S., Murakami Y., Rikitake Y. Mechanism of recipient cell-dependent differences in exosome uptake. BMC Cancer. 2018;18:47. doi: 10.1186/s12885-017-3958-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nazarenko I. Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation. Cancer Res. 2010;70:1668–1678. doi: 10.1158/0008-5472.Can-09-2470. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino A. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S.E. Extracellular vesicles and metastasis. Cold Spring Harb. Perspect. Med. 2020;10 doi: 10.1101/cshperspect.a037275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rappa G. Nuclear transport of cancer extracellular vesicle-derived biomaterials through nuclear envelope invagination-associated late endosomes. Oncotarget. 2017;8:14443–14461. doi: 10.18632/oncotarget.14804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abels E.R., Breakefield X.O. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell. Mol. Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulcahy L.A., Pink R.C., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracel. Vesicl. 2014;3 doi: 10.3402/jev.v3403.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Niel G., D’Angelo G., Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018;19:213–228. doi: 10.1038/nrm.2017.125. [DOI] [PubMed] [Google Scholar]
- 25.Akers J.C., Gonda D., Kim R., Carter B.S., Chen C.C. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J. Neuro-Oncol. 2013;113:1–11. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subra C., Laulagnier K., Perret B., Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Hessvik N.P., Llorente A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci.: CMLS. 2018;75:193–208. doi: 10.1007/s00018-017-2595-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshioka Y. Comparative marker analysis of extracellular vesicles in different human cancer types. J. Extracel. Vesicl. 2013;2 doi: 10.3402/jev.v2i0.20424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duijvesz D., Luider T., Bangma C.H., Jenster G. Exosomes as biomarker treasure chests for prostate cancer. Eur. Urol. 2011;59:823–831. doi: 10.1016/j.eururo.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 30.Van der Pol E., Böing A., Gool E., Nieuwland R. Recent developments in the nomenclature, presence, isolation, detection and clinical impact of extracellular vesicles. J. Thromb. Haemost. 2016;14:48–56. doi: 10.1111/jth.13190. [DOI] [PubMed] [Google Scholar]
- 31.Muralidharan-Chari V., Clancy J.W., Sedgwick A., D’Souza-Schorey C. Microvesicles: mediators of extracellular communication during cancer progression. J. Cell Sci. 2010;123:1603–1611. doi: 10.1242/jcs.064386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dieudé M. The 20S proteasome core, active within apoptotic exosome-like vesicles, induces autoantibody production and accelerates rejection. Sci. Transl. Med. 2015;7 doi: 10.1126/scitranslmed.aac9816. 318ra200. [DOI] [PubMed] [Google Scholar]
- 33.Fujita Y., Yoshioka Y., Ochiya T. Extracellular vesicle transfer of cancer pathogenic components. Cancer Sci. 2016;107:385–390. doi: 10.1111/cas.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holmgren L. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood. 1999;93:3956–3963. [PubMed] [Google Scholar]
- 35.Hauser P., Wang S., Didenko V.V. Springer; 2017. Signal Transduction Immunohistochemistry; pp. 193–200. [Google Scholar]
- 36.Gregory C.D., Dransfield I. Apoptotic tumor cell-derived extracellular vesicles as important regulators of the onco-regenerative niche. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford C.A. Oncogenic properties of apoptotic tumor cells in aggressive B cell lymphoma. Curr. Biol. 2015;25:577–588. doi: 10.1016/j.cub.2014.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Q. Caspase 3–mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nat. Med. 2011;17:860–866. doi: 10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldie B.J. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42:9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raposo G., Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J. Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasconcelos M.H., Caires H.R., Ābols A., Xavier C.P.R., Linē A. Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updat. 2019;47 doi: 10.1016/j.drup.2019.100647. 100647. [DOI] [PubMed] [Google Scholar]
- 42.Turchinovich A., Drapkina O., Tonevitsky A. Transcriptome of extracellular vesicles: State-of-the-art. Front. Immunol. 2019;10 doi: 10.3389/fimmu.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vickram A.S. Human prostasomes an extracellular vesicle - biomarkers for male infertility and prostrate cancer: the journey from identification to current knowledge. Int. J. Biol. Macromol. 2020;146:946–958. doi: 10.1016/j.ijbiomac.2019.09.218. [DOI] [PubMed] [Google Scholar]
- 44.Foster B.P. Extracellular vesicles in blood, milk and body fluids of the female and male urogenital tract and with special regard to reproduction. Crit. Rev. Clin. Lab. Sci. 2016;53:379–395. doi: 10.1080/10408363.2016.1190682. [DOI] [PubMed] [Google Scholar]
- 45.Simon C. Extracellular vesicles in human reproduction in health and disease. Endocr. Rev. 2018;39:292–332. doi: 10.1210/er.2017-00229. [DOI] [PubMed] [Google Scholar]
- 46.Park K.-H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011;4:31. doi: 10.1126/scisignal.2001595. [DOI] [PubMed] [Google Scholar]
- 47.Martin-DeLeon P., Epididymal A. SPAM1 and its impact on sperm function. Mol. Cell. Endocrinol. 2006;250:114–121. doi: 10.1016/j.mce.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Griffiths G.S., Galileo D.S., Reese K., Martin-Deleon P.A. Investigating the role of murine epididymosomes and uterosomes in GPI-linked protein transfer to sperm using SPAM1 as a model. Mol. Reprod. Dev. 2008;75:1627–1636. doi: 10.1002/mrd.20907. [DOI] [PubMed] [Google Scholar]
- 49.Park K.H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011;4 doi: 10.1126/scisignal.2001595. ra31. [DOI] [PubMed] [Google Scholar]
- 50.Alasmari W. Ca2+ signals generated by CatSper and Ca2+ stores regulate different behaviors in human sperm. J. Biol. Chem. 2013;288:6248–6258. doi: 10.1074/jbc.M112.439356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan R. Epididymosomes: role of extracellular microvesicles in sperm maturation. Front. Biosci. 2016;8:106–114. doi: 10.2741/s450. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez-Caro H. In vitro decidualisation of human endometrial stromal cells is enhanced by seminal fluid extracellular vesicles. J. Extracel. Vesicl. 2019;8:1565262. doi: 10.1080/20013078.2019.1565262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lazarevic M., Skibinski G., Kelly R.W., James K. Immunomodulatory effects of extracellular secretory vesicles isolated from bovine semen. Vet. Immunol. Immunopathol. 1995;44:237–250. doi: 10.1016/0165-2427(94)05320-R. [DOI] [PubMed] [Google Scholar]
- 54.Yáñez-Mó M. Biological properties of extracellular vesicles and their physiological functions. J. Extracel. Vesicl. 2015;4:27066. doi: 10.3402/jev.v4.27066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han L., Lam E.W., Sun Y. Extracellular vesicles in the tumor microenvironment: old stories, but new tales. Mol. Cancer. 2019;18:59. doi: 10.1186/s12943-019-0980-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoshino A. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ab Razak N.S., Ab Mutalib N.S., Mohtar M.A., Abu N. Impact of chemotherapy on extracellular vesicles: understanding the chemo-EVs. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egeblad M., Nakasone E.S., Werb Z. Tumors as organs: complex tissues that interface with the entire organism. Dev. Cell. 2010;18:884–901. doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Whiteside T.L. Springer; 2017. Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy; pp. 81–89. [Google Scholar]
- 60.Raimondo S., Pucci M., Alessandro R., Fontana S. Extracellular vesicles and tumor-immune escape: biological functions and clinical perspectives. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21072286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kosaka N., Yoshioka Y., Fujita Y., Ochiya T. Versatile roles of extracellular vesicles in cancer. J. Clin. Invest. 2016;126:1163–1172. doi: 10.1172/jci81130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skog J. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grange C. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 64.Shoucair I., Weber Mello F., Jabalee J., Maleki S., Garnis C. The role of cancer-associated fibroblasts and extracellular vesicles in tumorigenesis. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21186837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webber J.P. Differentiation of tumour-promoting stromal myofibroblasts by cancer exosomes. Oncogene. 2015;34:290–302. doi: 10.1038/onc.2013.560. [DOI] [PubMed] [Google Scholar]
- 66.Fabbri M. MicroRNAs bind to toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang D. Exosomes derived from Piwil2-induced cancer stem cells transform fibroblasts into cancer-associated fibroblasts. Oncol. Rep. 2020;43:1125–1132. doi: 10.3892/or.2020.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y. Exosomal transfer of miR-124 inhibits normal fibroblasts to cancer-associated fibroblasts transition by targeting sphingosine kinase 1 in ovarian cancer. J. Cell. Biochem. 2019;120:13187–13201. doi: 10.1002/jcb.28593. [DOI] [PubMed] [Google Scholar]
- 69.Richards K.E. Cancer-associated fibroblast exosomes regulate survival and proliferation of pancreatic cancer cells. Oncogene. 2017;36:1770–1778. doi: 10.1038/onc.2016.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lobb R.J. Exosomes derived from mesenchymal non-small cell lung cancer cells promote chemoresistance. Int. J. Cancer. 2017;141:614–620. doi: 10.1002/ijc.30752. [DOI] [PubMed] [Google Scholar]
- 71.Qu L. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 72.Wang X. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Mol. Cancer. 2019;18:182. doi: 10.1186/s12943-019-1114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kreger B.T., Johansen E.R., Cerione R.A., Antonyak M.A. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers. 2016;8 doi: 10.3390/cancers8120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keklikoglou I. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019;21:190–202. doi: 10.1038/s41556-018-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Milman N., Ginini L., Gil Z. Exosomes and their role in tumorigenesis and anticancer drug resistance. Drug Resist. Updat. 2019;45:1–12. doi: 10.1016/j.drup.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 76.Samuel P. Cisplatin induces the release of extracellular vesicles from ovarian cancer cells that can induce invasiveness and drug resistance in bystander cells. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2018;373 doi: 10.1098/rstb.2017.0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ding D., Han D., Li J., Tan W. Improving early detection of cancers by profiling extracellular vesicles. Expert. Rev. Proteom. 2019;16:545–547. doi: 10.1080/14789450.2019.1624531. [DOI] [PubMed] [Google Scholar]
- 78.Ludwig N. Isolation and analysis of tumor-derived exosomes. Curr. Protoc. Immunol. 2019;127 doi: 10.1002/cpim.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kok V.C., Yu C.C. Cancer-derived exosomes: their role in cancer biology and biomarker development. Int. J. Nanomedicine. 2020;15:8019–8036. doi: 10.2147/ijn.S272378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ruhen O., Meehan K. Tumor-derived extracellular vesicles as a novel source of protein biomarkers for cancer diagnosis and monitoring. Proteomics. 2019;19 doi: 10.1002/pmic.201800155. [DOI] [PubMed] [Google Scholar]
- 81.Soekmadji C., Rockstroh A., Ramm G.A., Nelson C.C., Russell P.J. Extracellular vesicles in the adaptive process of prostate cancer during inhibition of androgen receptor signaling by enzalutamide. Proteomics. 2017;17:1600427. doi: 10.1002/pmic.201600427. [DOI] [PubMed] [Google Scholar]
- 82.Yu Q. Nano-vesicles are a potential tool to monitor therapeutic efficacy of carbon ion radiotherapy in prostate cancer. J. Biomed. Nanotechnol. 2018;14:168–178. doi: 10.1166/jbn.2018.2503. [DOI] [PubMed] [Google Scholar]
- 83.Pigati L. Selective release of microRNA species from normal and malignant mammary epithelial cells. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nolte-’t Hoen E.N. Deep sequencing of RNA from immune cell-derived vesicles uncovers the selective incorporation of small non-coding RNA biotypes with potential regulatory functions. Nucleic Acids Res. 2012;40:9272–9285. doi: 10.1093/nar/gks658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mittelbrunn M. Unidirectional transfer of microRNA--loaded exosomes from T cells to antigen--presenting cells. Nat. Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hu Y. Splicing factor hnRNPA2B1 contributes to tumorigenic potential of breast cancer cells through STAT3 and ERK1/2 signaling pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017;39 doi: 10.1177/1010428317694318. [DOI] [PubMed] [Google Scholar]
- 87.Qiao L. Identification of Upregulated HNRNPs associated with poor prognosis in pancreatic cancer. Biomed. Res. Int. 2019;2019:5134050. doi: 10.1155/2019/5134050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Urabe F. Extracellular vesicles as biomarkers and therapeutic targets for cancer. Am. J. Phys. Cell Phys. 2020;318:C29–c39. doi: 10.1152/ajpcell.00280.2019. [DOI] [PubMed] [Google Scholar]
- 89.Du M. Plasma exosomal miRNAs-based prognosis in metastatic kidney cancer. Oncotarget. 2017;8:63703–63714. doi: 10.18632/oncotarget.19476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin S.Y. Proteome profiling of urinary exosomes identifies alpha 1-antitrypsin and H2B1K as diagnostic and prognostic biomarkers for urothelial carcinoma. Sci. Rep. 2016;6:34446. doi: 10.1038/srep34446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sandfeld-Paulsen B. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016;10:1595–1602. doi: 10.1016/j.molonc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cappello F. Exosome levels in human body fluids: a tumor marker by themselves? Eur. J. Pharm. Sci. 2017;96:93–98. doi: 10.1016/j.ejps.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 93.Wu Z. Extracellular vesicles in urologic malignancies—implementations for future cancer care. Cell Prolif. 2019;52 doi: 10.1111/cpr.12659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bar-Sela G., Cohen I., Avisar A., Loven D., Aharon A. Circulating blood extracellular vesicles as a tool to assess endothelial injury and chemotherapy toxicity in adjuvant cancer patients. PLoS One. 2020;15 doi: 10.1371/journal.pone.0240994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rodríguez Zorrilla S. A pilot clinical study on the prognostic relevance of plasmatic exosomes levels in oral squamous cell carcinoma patients. Cancers. 2019;11:429. doi: 10.3390/cancers11030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Junker K., Heinzelmann J., Beckham C., Ochiya T., Jenster G. Extracellular vesicles and their role in urologic malignancies. Eur. Urol. 2016;70:323–331. doi: 10.1016/j.eururo.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 97.Shuch B. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur. Urol. 2015;67:85–97. doi: 10.1016/j.eururo.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 98.Capitanio U., Montorsi F. Renal cancer. Lancet (London, England) 2016;387:894–906. doi: 10.1016/s0140-6736(15)00046-x. [DOI] [PubMed] [Google Scholar]
- 99.Qin Z., Xu Q., Hu H., Yu L., Zeng S. Extracellular vesicles in renal cell carcinoma: multifaceted roles and potential applications identified by experimental and computational methods. Front. Oncol. 2020;10:724. doi: 10.3389/fonc.2020.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Butz H. Exosomal MicroRNAs are diagnostic biomarkers and can mediate cell–cell communication in renal cell carcinoma. Eur. Urol. Focus. 2016;2:210–218. doi: 10.1016/j.euf.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Zhang W. MicroRNAs in serum exosomes as potential biomarkers in clear-cell renal cell carcinoma. Eur. Urol. Focus. 2018;4:412–419. doi: 10.1016/j.euf.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 102.Wang X.G. Serum exosomal miR-210 as a potential biomarker for clear cell renal cell carcinoma. J. Cell. Biochem. 2019;120:1492–1502. doi: 10.1002/jcb.27347. [DOI] [PubMed] [Google Scholar]
- 103.Butz H. Exosomal MicroRNAs are diagnostic biomarkers and can mediate cell-cell communication in renal cell carcinoma. Eur. Urol. Focus. 2016;2:210–218. doi: 10.1016/j.euf.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 104.Fujii N. Extracellular miR-224 as a prognostic marker for clear cell renal cell carcinoma. Oncotarget. 2017;8:109877–109888. doi: 10.18632/oncotarget.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qu L. Exosome-transmitted lncARSR promotes sunitinib resistance in renal cancer by acting as a competing endogenous RNA. Cancer Cell. 2016;29:653–668. doi: 10.1016/j.ccell.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Del Boccio P. A hyphenated microLC-Q-TOF-MS platform for exosomal lipidomics investigations: application to RCC urinary exosomes. Electrophoresis. 2012;33:689–696. doi: 10.1002/elps.201100375. [DOI] [PubMed] [Google Scholar]
- 107.Zhang L. The 786-0 renal cancer cell-derived exosomes promote angiogenesis by downregulating the expression of hepatocyte cell adhesion molecule. Mol. Med. Rep. 2013;8:272–276. doi: 10.3892/mmr.2013.1458. [DOI] [PubMed] [Google Scholar]
- 108.De Palma G. The three-gene signature in urinary extracellular vesicles from patients with clear cell renal cell carcinoma. J. Cancer. 2016;7:1960–1967. doi: 10.7150/jca.16123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raimondo F. Differential protein profiling of renal cell carcinoma urinary exosomes. Mol. BioSyst. 2013;9:1220–1233. doi: 10.1039/c3mb25582d. [DOI] [PubMed] [Google Scholar]
- 110.Jiang X., Zhang Y., Tan B., Luo C., Wu X. Renal tumor-derived exosomes inhibit hepaCAM expression of renal carcinoma cells in ap-AKT-dependent manner. Neoplasma. 2014;61:416. doi: 10.4149/neo_2014_051. [DOI] [PubMed] [Google Scholar]
- 111.Horie K. Exosomes expressing carbonic anhydrase 9 promote angiogenesis. Biochem. Biophys. Res. Commun. 2017;492:356–361. doi: 10.1016/j.bbrc.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 112.Chen G., Zhang Y., Wu X. 786-0 renal cancer cell line-derived exosomes promote 786-0 cell migration and invasion in vitro. Oncol. Lett. 2014;7:1576–1580. doi: 10.3892/ol.2014.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Grange C. Role of HLA-G and extracellular vesicles in renal cancer stem cell-induced inhibition of dendritic cell differentiation. BMC Cancer. 2015;15:1–11. doi: 10.1186/s12885-015-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tran Janco J.M., Lamichhane P., Karyampudi L., Knutson K.L. Tumor-infiltrating dendritic cells in cancer pathogenesis. J. Immunol. 2015;194:2985–2991. doi: 10.4049/jimmunol.1403134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Xia Y. Negative regulation of tumor-infiltrating NK cell in clear cell renal cell carcinoma patients through the exosomal pathway. Oncotarget. 2017;8:37783. doi: 10.18632/oncotarget.16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kamat A.M. Bladder cancer. Lancet. 2016;388:2796–2810. doi: 10.1016/S0140-6736(16)30512-8. [DOI] [PubMed] [Google Scholar]
- 117.Elsharkawi F., Elsabah M., Shabayek M., Khaled H. Urine and serum exosomes as novel biomarkers in detection of bladder cancer. Asian Pac. J. Cancer Prevent.: APJCP. 2019;20:2219–2224. doi: 10.31557/apjcp.2019.20.7.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu C.H., Silvers C.R., Messing E.M., Lee Y.F. Bladder cancer extracellular vesicles drive tumorigenesis by inducing the unfolded protein response in endoplasmic reticulum of nonmalignant cells. J. Biol. Chem. 2019;294:3207–3218. doi: 10.1074/jbc.RA118.006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hiltbrunner S. Urinary exosomes from bladder cancer patients show a residual cancer phenotype despite complete pathological downstaging. Sci. Rep. 2020;10:5960. doi: 10.1038/s41598-020-62753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fong M.Y. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 2015;17:183–194. doi: 10.1038/ncb3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berrondo C. Expression of the long non-coding RNA HOTAIR correlates with disease progression in bladder cancer and is contained in bladder cancer patient urinary exosomes. PLoS One. 2016;11 doi: 10.1371/journal.pone.0147236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhan Y. Expression signatures of exosomal long non-coding RNAs in urine serve as novel non-invasive biomarkers for diagnosis and recurrence prediction of bladder cancer. Mol. Cancer. 2018;17:142. doi: 10.1186/s12943-018-0893-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang S. Evaluation of serum exosomal LncRNA-based biomarker panel for diagnosis and recurrence prediction of bladder cancer. J. Cell. Mol. Med. 2019;23:1396–1405. doi: 10.1111/jcmm.14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Long J.D. A non-invasive miRNA based assay to detect bladder cancer in cell-free urine. Am. J. Transl. Res. 2015;7:2500–2509. [PMC free article] [PubMed] [Google Scholar]
- 125.Murakami T. Bladder cancer detection by urinary extracellular vesicle mRNA analysis. Oncotarget. 2018;9 doi: 10.18632/oncotarget.25998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ostenfeld M.S. Cellular disposal of miR23b by RAB27-dependent exosome release is linked to acquisition of metastatic properties. Cancer Res. 2014;74:5758–5771. doi: 10.1158/0008-5472.Can-13-3512. [DOI] [PubMed] [Google Scholar]
- 127.Armstrong D.A., Green B.B., Seigne J.D., Schned A.R., Marsit C.J. MicroRNA molecular profiling from matched tumor and bio-fluids in bladder cancer. Mol. Cancer. 2015;14:194. doi: 10.1186/s12943-015-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beckham C.J. Bladder cancer exosomes contain EDIL-3/Del1 and facilitate cancer progression. J. Urol. 2014;192:583–592. doi: 10.1016/j.juro.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 129.Perez A. A pilot study on the potential of RNA-associated to urinary vesicles as a suitable non-invasive source for diagnostic purposes in bladder cancer. Cancers. 2014;6:179–192. doi: 10.3390/cancers6010179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matsuzaki K. MiR-21-5p in urinary extracellular vesicles is a novel biomarker of urothelial carcinoma. Oncotarget. 2017;8:24668–24678. doi: 10.18632/oncotarget.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Andreu Z. Extracellular vesicles as a source for non-invasive biomarkers in bladder cancer progression. Eur. J. Pharm. Sci. 2017;98:70–79. doi: 10.1016/j.ejps.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 132.Welton J.L. Proteomics analysis of bladder cancer exosomes. Mol. Cell. Proteom. MCP. 2010;9:1324–1338. doi: 10.1074/mcp.M000063-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen C.L. Comparative and targeted proteomic analyses of urinary microparticles from bladder cancer and hernia patients. J. Proteome Res. 2012;11:5611–5629. doi: 10.1021/pr3008732. [DOI] [PubMed] [Google Scholar]
- 134.Franzen C.A. Urothelial cells undergo epithelial-to-mesenchymal transition after exposure to muscle invasive bladder cancer exosomes. Oncogenesis. 2015;4:e163. doi: 10.1038/oncsis.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoshida K. Exosomes containing ErbB2/CRK induce vascular growth in premetastatic niches and promote metastasis of bladder cancer. Cancer Sci. 2019;110:2119–2132. doi: 10.1111/cas.14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yazarlou F. Urine exosome gene expression of cancer-testis antigens for prediction of bladder carcinoma. Cancer Manag. Res. 2018;10:5373–5381. doi: 10.2147/cmar.S180389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yang B. Epigenetic control of MAGE gene expression by the KIT tyrosine kinase. J. Invest. Dermatol. 2007;127:2123–2128. doi: 10.1038/sj.jid.5700836. [DOI] [PubMed] [Google Scholar]
- 138.Ronquist K.G., Ronquist G., Larsson A., Carlsson L. Proteomic analysis of prostate cancer metastasis-derived prostasomes. Anticancer Res. 2010;30:285–290. [PubMed] [Google Scholar]
- 139.Sahlén G.E. Ultrastructure of the secretion of prostasomes from benign and malignant epithelial cells in the prostate. Prostate. 2002;53:192–199. doi: 10.1002/pros.10126. [DOI] [PubMed] [Google Scholar]
- 140.Stridsberg M., Fabiani R., Lukinius A., Ronquist G. Prostasomes are neuroendocrine-like vesicles in human semen. Prostate. 1996;29:287–295. doi: 10.1002/(SICI)1097-0045(199611)29:5<287::AID-PROS3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 141.Carlsson L. Characteristics of human prostasomes isolated from three different sources. Prostate. 2003;54:322–330. doi: 10.1002/pros.10189. [DOI] [PubMed] [Google Scholar]
- 142.Aalberts M., Stout T.A., Stoorvogel W. Prostasomes: extracellular vesicles from the prostate. Reproduction. 2014;147 doi: 10.1530/REP-13-0358. R1–14. [DOI] [PubMed] [Google Scholar]
- 143.Zhang X., Vos H.R., Tao W., Stoorvogel W. Proteomic profiling of two distinct populations of extracellular vesicles isolated from human seminal plasma. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21217957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rikkert L.G. Detection of extracellular vesicles in plasma and urine of prostate cancer patients by flow cytometry and surface plasmon resonance imaging. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bijnsdorp I.V. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J. Extracel. Vesicl. 2013;2 doi: 10.3402/jev.v2i0.22097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Øverbye A. Identification of prostate cancer biomarkers in urinary exosomes. Oncotarget. 2015;6:30357–30376. doi: 10.18632/oncotarget.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sequeiros T. Targeted proteomics in urinary extracellular vesicles identifies biomarkers for diagnosis and prognosis of prostate cancer. Oncotarget. 2017;8:4960–4976. doi: 10.18632/oncotarget.13634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Duijvesz D. Proteomic profiling of exosomes leads to the identification of novel biomarkers for prostate cancer. PLoS One. 2013;8 doi: 10.1371/journal.pone.0082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Itoh T. Microvesicles released from hormone-refractory prostate cancer cells facilitate mouse pre-osteoblast differentiation. J. Mol. Histol. 2012;43:509–515. doi: 10.1007/s10735-012-9415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wang J. Exosomal microRNAs as liquid biopsy biomarkers in prostate cancer. Crit. Rev. Oncol. Hematol. 2020;145:102860. doi: 10.1016/j.critrevonc.2019.102860. doi:j.critrevonc.2019.102860. [DOI] [PubMed] [Google Scholar]
- 151.Rodríguez M. Identification of non-invasive miRNAs biomarkers for prostate cancer by deep sequencing analysis of urinary exosomes. Mol. Cancer. 2017;16:156. doi: 10.1186/s12943-017-0726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zheng Y. Extracellular vesicle-derived circ_SLC19A1 promotes prostate cancer cell growth and invasion through the miR-497/septin 2 pathway. Cell Biol. Int. 2020;44:1037–1045. doi: 10.1002/cbin.11303. [DOI] [PubMed] [Google Scholar]
- 153.Dai J. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019;216:2883–2899. doi: 10.1084/jem.20190158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hashimoto K. Cancer-secreted hsa-miR-940 induces an osteoblastic phenotype in the bone metastatic microenvironment via targeting ARHGAP1 and FAM134A. Proc. Natl. Acad. Sci. U. S. A. 2018;115:2204–2209. doi: 10.1073/pnas.1717363115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ye Y. Exosomal miR-141-3p regulates osteoblast activity to promote the osteoblastic metastasis of prostate cancer. Oncotarget. 2017;8:94834–94849. doi: 10.18632/oncotarget.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Corcoran C. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS One. 2012;7 doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Y. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate. 2017;77:1167–1175. doi: 10.1002/pros.23376. [DOI] [PubMed] [Google Scholar]
- 158.Koppers-Lalic D. Non-invasive prostate cancer detection by measuring miRNA variants (isomiRs) in urine extracellular vesicles. Oncotarget. 2016;7:22566–22578. doi: 10.18632/oncotarget.8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Donovan M.J. A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 2015;18:370–375. doi: 10.1038/pcan.2015.40. [DOI] [PubMed] [Google Scholar]
- 160.Nilsson J. Prostate cancer-derived urine exosomes: a novel approach to biomarkers for prostate cancer. Br. J. Cancer. 2009;100:1603–1607. doi: 10.1038/sj.bjc.6605058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Urabe F. The miR-1908/SRM regulatory axis contributes to extracellular vesicle secretion in prostate cancer. Cancer Sci. 2020;111:3258–3267. doi: 10.1111/cas.14535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Foj L. Exosomal and non-exosomal urinary miRNAs in prostate cancer detection and prognosis. Prostate. 2017;77:573–583. doi: 10.1002/pros.23295. [DOI] [PubMed] [Google Scholar]
- 163.Huang X. Exosomal miR-1290 and miR-375 as prognostic markers in castration-resistant prostate cancer. Eur. Urol. 2015;67:33–41. doi: 10.1016/j.eururo.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Endzeliņš E. Detection of circulating miRNAs: comparative analysis of extracellular vesicle-incorporated miRNAs and cell-free miRNAs in whole plasma of prostate cancer patients. BMC Cancer. 2017;17:730. doi: 10.1186/s12885-017-3737-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhagirath D. microRNA-1246 is an exosomal biomarker for aggressive prostate cancer. Cancer Res. 2018;78:1833–1844. doi: 10.1158/0008-5472.Can-17-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang Y.H. Tumor-derived exosomal long noncoding RNAs as promising diagnostic biomarkers for prostate cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018;46:532–545. doi: 10.1159/000488620. [DOI] [PubMed] [Google Scholar]
- 167.Harvey P. A systematic review of the diagnostic accuracy of prostate specific antigen. BMC Urol. 2009;9:14. doi: 10.1186/1471-2490-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Z. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco. Targets Ther. 2016;9:139–148. doi: 10.2147/ott.S95565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McKiernan J. A novel urine exosome gene expression assay to predict high-grade prostate cancer at initial biopsy. JAMA Oncol. 2016;2:882–889. doi: 10.1001/jamaoncol.2016.0097. [DOI] [PubMed] [Google Scholar]
- 170.McKiernan J. A prospective adaptive utility trial to validate performance of a novel urine exosome gene expression assay to predict high-grade prostate cancer in patients with prostate-specific antigen 2-10ng/ml at initial biopsy. Eur. Urol. 2018;74:731–738. doi: 10.1016/j.eururo.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 171.Bryzgunova O.E. Comparative study of extracellular vesicles from the urine of healthy individuals and prostate cancer patients. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Angelis D., Spiliotis E.T. Septin mutations in human cancers. Front. Cell. Dev. Biol. 2016;4:122. doi: 10.3389/fcell.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Salimu J. Dominant immunosuppression of dendritic cell function by prostate-cancer-derived exosomes. J. Extracel. Vesicl. 2017;6:1368823. doi: 10.1080/20013078.2017.1368823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Clayton A., Mitchell J.P., Linnane S., Mason M.D., Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J. Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 175.Lundholm M. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9 doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Clayton A., Mitchell J.P., Mason M.D., Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 177.Li S. Exosomal ephrinA2 derived from serum as a potential biomarker for prostate cancer. J. Cancer. 2018;9:2659–2665. doi: 10.7150/jca.25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Logozzi M. Increased PSA expression on prostate cancer exosomes in in vitro condition and in cancer patients. Cancer Lett. 2017;403:318–329. doi: 10.1016/j.canlet.2017.06.036. [DOI] [PubMed] [Google Scholar]
- 179.Khan S. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kato T. Serum exosomal P-glycoprotein is a potential marker to diagnose docetaxel resistance and select a taxoid for patients with prostate cancer. Urol. Oncol. Semin. Original Invest. 2015;33 doi: 10.1016/j.urolonc.2015.04.019. 385.e315–385.e320. [DOI] [PubMed] [Google Scholar]
- 181.Kawakami K. Gamma-glutamyltransferase activity in exosomes as a potential marker for prostate cancer. BMC Cancer. 2017;17:316. doi: 10.1186/s12885-017-3301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Minciacchi V.R. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6 doi: 10.18632/oncotarget.3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dhondt B. Unravelling the proteomic landscape of extracellular vesicles in prostate cancer by density-based fractionation of urine. J. Extracel. Vesicl. 2020;9:1736935. doi: 10.1080/20013078.2020.1736935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Skotland T. Molecular lipid species in urinary exosomes as potential prostate cancer biomarkers. Eur. J. Canc. (Oxford, England : 1990) 2017;70:122–132. doi: 10.1016/j.ejca.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 185.Clos-Garcia M. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J. Extracel. Vesicl. 2018;7:1470442. doi: 10.1080/20013078.2018.1470442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Puhka M. Metabolomic profiling of extracellular vesicles and alternative normalization methods reveal enriched metabolites and strategies to study prostate cancer-related changes. Theranostics. 2017;7:3824–3841. doi: 10.7150/thno.19890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ling H., Krassnig L., Bullock M.D., Pichler M. MicroRNAs in testicular cancer diagnosis and prognosis. Urol. Clin. North Am. 2016;43:127–134. doi: 10.1016/j.ucl.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 188.Lötvall J. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J. Extracel. Vesicl. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Dong L. Comprehensive evaluation of methods for small extracellular vesicles separation from human plasma, urine and cell culture medium. J. Extracel. Vesicl. 2020;10 doi: 10.1002/jev2.12044. e12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gardiner C. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J. Extracel. Vesicl. 2016;5:32945. doi: 10.3402/jev.v5.32945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Théry C., Amigorena S., Raposo G., Clayton A. Isolation AND CHARACTERIZATION OF EXOSOMES FROM CELL CULTURE SUPERNATANTS AND BIOLOGICAL Fluids. Curr. Protocols Cell Biol. 2006;30 doi: 10.1002/0471143030.cb0322s30. 3.22.21–23.22.29. [DOI] [PubMed] [Google Scholar]
- 192.Tauro B.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods (San Diego, Calif.) 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 193.Böing A.N. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracel. Vesicl. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Karttunen J. Precipitation-based extracellular vesicle isolation from rat plasma co-precipitate vesicle-free microRNAs. J. Extracel. Vesicl. 2019;8:1555410. doi: 10.1080/20013078.2018.1555410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shabihkhani M. The procurement, storage, and quality assurance of frozen blood and tissue biospecimens in pathology, biorepository, and biobank settings. Clin. Biochem. 2014;47:258–266. doi: 10.1016/j.clinbiochem.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gillio-Meina C., Cepinskas G., Cecchini E.L., Fraser D.D. Translational research in pediatrics II: blood collection, processing, shipping, and storage. Pediatrics. 2013;131:754–766. doi: 10.1542/peds.2012-1181. [DOI] [PubMed] [Google Scholar]
- 197.Elliott P., Peakman T.C. The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int. J. Epidemiol. 2008;37:234–244. doi: 10.1093/ije/dym276. [DOI] [PubMed] [Google Scholar]
- 198.Lacroix R. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J. Thromb. Haemost. 2012;10:437–446. doi: 10.1111/j.1538-7836.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 199.Witwer K.W. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracel. Vesicl. 2013;2 doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Devuyst O., Olinger E., Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nat. Rev. Nephrol. 2017;13:525–544. doi: 10.1038/nrneph.2017.101. [DOI] [PubMed] [Google Scholar]
- 201.Hogan M.C. Characterization of PKD protein-positive exosome-like vesicles. J. Am. Soc. Nephrol. 2009;20:278–288. doi: 10.1681/asn.2008060564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Yang D. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–3707. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Brennan K. A comparison of methods for the isolation and separation of extracellular vesicles from protein and lipid particles in human serum. Sci. Rep. 2020;10:1039. doi: 10.1038/s41598-020-57497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Lobb R.J. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracel. Vesicl. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Konoshenko M.Y., Lekchnov E.A., Vlassov A.V., Laktionov P.P. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed. Res. Int. 2018;2018:8545347. doi: 10.1155/2018/8545347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Livshits M.A. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci. Rep. 2015;5:17319. doi: 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kırbaş O.K. Optimized isolation of extracellular vesicles from various organic sources using aqueous two-phase system. Sci. Rep. 2019;9:19159. doi: 10.1038/s41598-019-55477-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Mol E.A., Goumans M.-J., Doevendans P.A., Sluijter J.P.G., Vader P. Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine. 2017;13:2061–2065. doi: 10.1016/j.nano.2017.03.011. doi:j.nano.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 209.Nordin J.Z. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 210.Brett S.I. Immunoaffinity based methods are superior to kits for purification of prostate derived extracellular vesicles from plasma samples. Prostate. 2017;77:1335–1343. doi: 10.1002/pros.23393. [DOI] [PubMed] [Google Scholar]
- 211.Talebjedi B., Tasnim N., Hoorfar M., Mastromonaco G.F., De Almeida Monteiro Melo Ferraz M. Exploiting microfluidics for extracellular vesicle isolation and characterization: potential use for standardized embryo quality assessment. Front. Veterin. Sci. 2021;7 doi: 10.3389/fvets.2020.620809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bæk R., Søndergaard E.K., Varming K., Jørgensen M.M. The impact of various preanalytical treatments on the phenotype of small extracellular vesicles in blood analyzed by protein microarray. J. Immunol. Methods. 2016;438:11–20. doi: 10.1016/j.jim.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 213.Cheng Y., Zeng Q., Han Q., Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein & Cell. 2019;10:295–299. doi: 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Tegegn T.Z. Characterization of procoagulant extracellular vesicles and platelet membrane disintegration in DMSO-cryopreserved platelets. J. Extracel. Vesicl. 2016;5:30422. doi: 10.3402/jev.v5.30422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Enderle D. Characterization of RNA from exosomes and other extracellular vesicles isolated by a novel spin column-based method. PLoS One. 2015;10 doi: 10.1371/journal.pone.0136133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yoshioka Y. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014;5:3591. doi: 10.1038/ncomms4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Reátegui E. Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat. Commun. 2018;9:175. doi: 10.1038/s41467-017-02261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sunkara V. Fully automated, label-free isolation of extracellular vesicles from whole blood for cancer diagnosis and monitoring. Theranostics. 2019;9:1851–1863. doi: 10.7150/thno.32438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Silvers C.R., Miyamoto H., Messing E.M., Netto G.J., Lee Y.-F. Characterization of urinary extracellular vesicle proteins in muscle-invasive bladder cancer. Oncotarget. 2017;8 doi: 10.18632/oncotarget.20043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Welton J.L. Proteomics analysis of vesicles isolated from plasma and urine of prostate cancer patients using a multiplex, aptamer-based protein array. J. Extracel. Vesicl. 2016;5:31209. doi: 10.3402/jev.v5.31209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Yang J.S., Lee J.C., Byeon S.K., Rha K.H., Moon M.H. Size dependent Lipidomic analysis of urinary Exosomes from patients with prostate cancer by flow field-flow fractionation and nanoflow liquid chromatography-tandem mass spectrometry. Anal. Chem. 2017;89:2488–2496. doi: 10.1021/acs.analchem.6b04634. [DOI] [PubMed] [Google Scholar]
- 222.Poli G., Egidi M.G., Cochetti G., Brancorsini S., Mearini E. Relationship between cellular and exosomal miRNAs targeting NOD-like receptors in bladder cancer: preliminary results. Minerva urologica e nefrologica = Itali. J. Urol. Nephrol. 2020;72:207–213. doi: 10.23736/s0393-2249.19.03297-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementray material.