Abstract
Background
Atopic dermatitis is a chronic, inflammatory condition causing a substantial burden to patients and caregivers. SHR0302 is an oral, highly selective, Janus kinase 1 inhibitor under investigation for inflammatory skin diseases.
Objective
The aim of this study was to investigate the efficacy and safety of SHR0302 in Chinese patients with moderate to severe atopic dermatitis.
Design and Setting
A randomized, double-blind, placebo-controlled, multicenter, phase II trial was conducted in China between October 2019 and August 2020.
Participants
Patients (n = 105) aged 18–75 years with moderate to severe dermatitis and nonresponsive or intolerant to topical or conventional systemic treatments were included.
Interventions
Patients were randomly assigned in a ratio of 1:1:1 to receive SHR0302 4 mg once daily, SHR0302 8 mg once daily, or placebo for 12 weeks.
Main Outcome Measures
The primary efficacy endpoint was the proportion of patients achieving Investigator’s Global Assessment (IGA) response (IGA of 0 [clear] or 1 [almost clear] with improvement of ≥2 grades) at week 12. Secondary efficacy assessments included Eczema Area and Severity Index (EASI) and pruritus Numerical Rating Scale (NRS) scores.
Results
At week 12, IGA response was achieved in nine patients (25.7%; 90% confidence interval [CI] 13.6–37.9%; p = 0.022) in the SHR0302 4 mg group, 19 patients (54.3%; 90% CI 40.4–68.1%; p < 0.001) in the SHR0302 8 mg group, and two patients (5.7%; 90% CI 0.0–12.2%) in the placebo group. EASI75 was achieved in 51.4% (p = 0.013), 74.3% (p < 0.001), and 22.9% of patients in the SHR0302 4 mg, SHR0302 8 mg, and placebo groups, respectively, while an NRS ≥3-point improvement occurred in 65.7% (p < 0.001), 74.3% (p < 0.001), and 22.9% of patients, respectively. Treatment-emergent adverse events were reported in 60.0%, 68.6%, and 51.4% of patients in the SHR0302 4 mg, SHR0302 8 mg, and placebo groups, respectively. The adverse events were mild in most cases. Three serious adverse events were reported, all being worsening of atopic dermatitis. No serious infection was reported.
Conclusions and Relevance
Oral SHR0302 was effective and well tolerated in Chinese adult patients with moderate to severe atopic dermatitis.
Trial Registration
ClinicalTrials.gov identifier: NCT04162899; URL: https://clinicaltrials.gov/. Date first registered: 14 November 2019.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-021-00627-2.
Key Points
| This 12-week, randomized, phase II trial reports the efficacy and safety of SHR0302 (a highly selective Janus kinase 1 inhibitor) in 105 patients with moderate to severe atopic dermatitis. |
| Overall, 54.3%/25.7% of patients receiving SHR0302 8 mg/4 mg once daily achieved an Investigator’s Global Assessment response, compared with 5.7% receiving placebo (p < 0.001 and p = 0.022, respectively). Furthermore, 74.3%/51.4% of patients receiving SHR0302 8 mg/4 mg once daily achieved a ≥75% improvement from baseline in the Eczema Area and Severity Index, compared with 22.9% receiving placebo (p < 0.001 and p = 0.013, respectively). Treatment-emergent adverse events were reported in 60.0%, 68.6%, and 51.4% of patients in the SHR0302 4 mg, SHR0302 8 mg, and placebo groups, respectively, and were mild adverse events in most cases. |
| SHR0302 was effective and well-tolerated in adults with moderate to severe atopic dermatitis. |
Introduction
Atopic dermatitis (AD) is a chronic, inflammatory skin condition with a relapsing and remitting course, characterized by eczematous lesions with intense pruritus [1, 2]. The prevalence of AD ranges from 10 to 20% in children and 2 to 10% in adults worldwide [3–5]. An estimated 7.3% of adults in the US have a diagnosis of AD [6], and an epidemiological study in China reported the incidence of AD at 7.8% [7]. Symptoms in patients with moderate to severe AD encompass sleep disturbance, anxiety and depression, impaired quality of life, and increased comorbidities [8, 9]. The adverse physical and psychological effects of AD produce a substantial burden on patients, caregivers, and society, with significant healthcare cost implications [8–14].
The treatment of AD remains a challenge [5]. For patients with moderate to severe AD, systemic immunosuppressive agents are often mandatory [5, 15–18]; however, the immunosuppressive agents have modest efficacy and are associated with considerable adverse events (AEs) that limit their long-term use [5, 16, 19]. The interleukin (IL)-4 receptor inhibitor dupilumab was recently approved in several countries for the treatment of moderate to severe AD; while this introduction brings a new treatment option, there remain opportunities for improvements in efficacy in terms of eczema severity and onset of pruritus relief [20, 21]. There continues to be a need for new treatment therapies in patients with moderate to severe AD.
The mechanism of AD includes skin-barrier defects and immune dysregulation [22]. A number of cytokine signaling pathways are involved in the development of AD, including IL-4, IL-5, IL-13, IL-31, and interferon (IFN)-γ [23]. Janus kinase (JAK) is a nonreceptor tyrosine kinase (TYK) that serves as an important signal transducer for these cytokines [24]. The JAK family includes JAK1, JAK2, JAK3, and TYK2. Blocking of the JAK/STAT pathway has the potential to inhibit the pathophysiological processes of AD [25]. A number of topical [26–28] and systemic [29–33] JAK inhibitors, with differing JAK inhibition characteristics, are undergoing investigation to assess their efficacy and safety in moderate to severe AD.
SHR0302 is a highly selective JAK1 inhibitor that modulates signaling by IL-4, IL-5, IL-13, IFN-γ, and other cytokine pathways involved in the pathogenesis of AD, while sparing inhibition of JAK2 to minimize the potential risks of neutropenia and anemia [34]. SHR0302 is well absorbed after oral administration, with a median time to reach maximum concentration of 1.0 h and a half-life of 8.33–9.87 h that increases slightly with dosage. Pharmacodynamic evidence supports a once daily dosing regimen.
The objective of this phase II study was to evaluate the efficacy and safety of oral SHR0302 in adult patients with moderate to severe AD. SHR0302 doses of 4 mg and 8 mg once daily were selected based on the data from completed phase I studies in healthy volunteers, as well as an ongoing dose-ranging phase II study investigating once daily doses ranging from 0.5 to 8 mg in patients with rheumatoid arthritis (NCT03254966).
Methods
Study Design and Participants
This randomized, double-blind, placebo-controlled, multicenter, phase II trial was conducted between 24 October 2019 and 31 August 2020 at 23 centers in China (NCT04162899) [see Trial Protocol in the electronic supplementary material (ESM)]. The study included up to 4-week screening, 12-week treatment, and 2-week follow-up periods (ESM Fig. S1). This treatment period was selected based on the clinical study experience that 12–16 weeks are sufficient to observe clinical effects from JAK inhibitors in AD [30–33].
The study was conducted in compliance with the Declaration of Helsinki [35], the International Conference on Harmonization Good Clinical Practice guidelines, and all local regulatory requirements. The research was approved by the Institutional Review Board and Independent Ethics Committee of the Peking University People's Hospital and participating study centers. All patients provided written informed consent and could withdraw from the study at any time at their own request or at the investigator’s or study sponsor’s discretion.
The diagnosis of AD was confirmed at screening and baseline visits as moderate to severe, defined by an Eczema Area and Severity Index (EASI) score ≥12, body surface area (BSA) affected of ≥10%, and Investigator's Global Assessment (IGA) score of ≥3. Eligible patients were males or females aged 18–75 years with a history of AD for at least 1 year at the time of screening and who were nonresponsive and/or intolerant to one or more of the following treatments for at least 4 weeks: topical corticosteroids and/or calcineurin inhibitors; systemic corticosteroids and/or phototherapy; and cyclosporine and/or other immunosuppressants, such as methotrexate, mycophenolate mofetil, and azathioprine.
Concomitant medications permitted during the study included oral antihistamines, nonsteroidal anti-inflammatory agents, topical or oral antibiotics required for secondary skin infections, and nonmedication moisturizers. Rescue treatments (both topical and systemic) were not allowed in this study.
Key exclusion criteria included the use of systemic glucocorticoids, cyclosporine, and other immunosuppressants; phosphodiesterase inhibitors; and phototherapy within 4 weeks or topical treatments that might affect AD within 2 weeks prior to the first dose of study drug. Subjects who had previously received JAK inhibitors or biologic agents (such as dupilumab) for AD were also excluded. Detailed eligibility criteria are provided in the ESM.
Randomization and Masking
Patients were randomly assigned to receive SHR0302 4 mg once daily, SHR0302 8 mg once daily, or placebo in a ratio of 1:1:1 using a computer-generated randomization table. Double-blind methodology was adopted for study drugs taken during the 12-week treatment period, ensuring that patients, investigators, and sponsors were blinded to study treatment.
Endpoints
The primary efficacy endpoint was the proportion of patients who achieved an IGA response at week 12 [36], defined as an IGA score of 0 (clear) or 1 (almost clear) with an improvement of ≥2 grades from baseline.
Secondary efficacy endpoints included the proportion of patients who achieved an IGA response at weeks 1, 4, and 8; change in EASI score from baseline, and the proportion of patients whose EASI scores improved by ≥50%, ≥75%, and ≥90% at weeks 1, 4, 8, and 12; change in pruritus Numerical Rating Scale (NRS) score from baseline, and the proportion of patients whose NRS improved by ≥3 points (NRS-3) at weeks 1, 4, 8, and 12; change in Scoring Atopic Dermatitis (SCORAD) index score from baseline, and the proportion of patients whose SCORAD score improved by ≥50%, ≥75%, and ≥90% at weeks 1, 4, 8, and 12; and change in the Dermatology Life Quality Index (DLQI) from baseline at weeks 1, 4, 8, and 12. Changes in immunoglobulin E (IgE) and absolute eosinophil count from baseline were also assessed at weeks 1, 4, 8, and 12 to investigate the effects of SHR0302 on these putative biomarkers of AD [1, 37].
Safety endpoints throughout the study comprised monitoring of AEs (including severity and relationship to study drug), clinical laboratory tests, vital signs, and electrocardiography.
Statistical Analysis
A sample size of 105 patients was determined to provide 82% power to detect a 20% difference in the primary endpoint between at least one active drug group and the placebo group, assuming a 6% response rate in the placebo group. Using Hochberg's incremental procedure, the overall type I error rate was maintained at the level of 0.1 (two-sided).
The analysis of primary efficacy was performed on the full analysis set, which included all patients who were randomized and received at least one dose of study drug. Safety was assessed in the safety analysis set, which included all patients who received at least one dose of study drug.
Chi-square testing was adopted in the primary efficacy analysis to detect whether each active drug group was superior to placebo; differences in response rate between the active treatment and placebo groups and their 90% confidence intervals (CIs) were estimated, and p-values in testing for difference were reported. The descriptive summary of primary efficacy endpoints was based on nonmissing data. Patients who withdrew prematurely from the study for any reason were considered nonresponders. For binary secondary endpoints, the same methodology was used as for the primary analysis; the mixed-effects model for repeated measures was applied for continuous endpoints. Descriptive statistics were used to analyze AEs reported during the treatment period. AEs were coded by system organ class and preferred terms according to the Medical Dictionary for Regulatory Activities (MedDRA; version 23.0).
All statistical analyses were completed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Patients
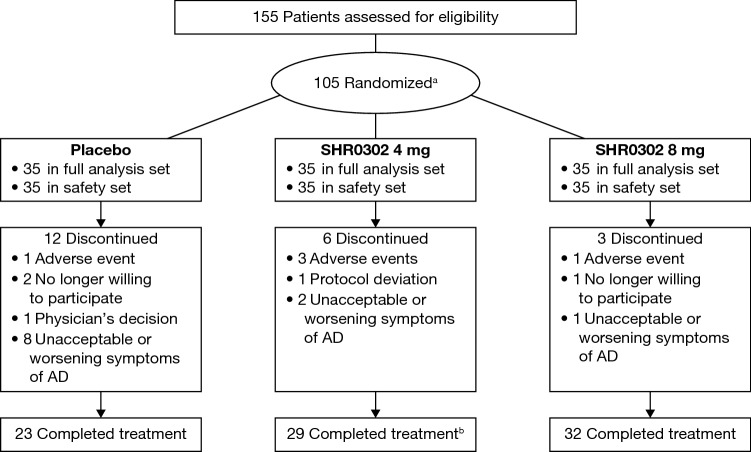
A total of 105 patients were randomly assigned to the SHR0302 4 mg (n = 35), SHR0302 8 mg (n = 35), and placebo groups (n = 35) (Fig. 1). Eighty-four patients (80.0%) completed treatment; however, six (17.1%) patients in the SHR0302 4 mg group, three (8.6%) patients in the SHR0302 8 mg group, and 12 (34.3%) patients in the placebo group withdrew from the study. The main reason for withdrawal was worsening of AD (two [5.7%] patients in the SHR0302 4 mg group, one [2.9%] patient in the SHR0302 8 mg group, and eight [22.9%] patients in the placebo group). One patient in the SHR0302 4 mg group completed treatment but was lost to follow-up.
Fig. 1.
CONSORT diagram. a The primary reason for screening failure was the potential presence of Mycobacterium tuberculosis infection (n = 21). b One patient completed treatment but was lost to follow-up. AD atopic dermatitis, CONSORT Consolidated Standards of Reporting Trials
Demographics and baseline characteristics were balanced across the treatment arms (Table 1). The median age in the overall patient population was 31.0 years (range 18–72 years), median body height was 169.0 cm (152.0–188.0 cm), and median body weight was 67.5 kg (39.5–143.0 kg). Median duration of AD was 7.4 years (range 1.0–40.4 years). Median (range) baseline EASI score was 24.4 (12.2–63.0) points, median BSA was 51.0% (10.0–98.0%), and median NRS was 8.0 (1–10) points. IGA score was 3 (moderate), 4 (severe), or 5 (extremely severe) in 57 (54.3%), 41 (39.0%), and 7 (6.7%) patients, respectively.
Table 1.
Demographics and general baseline characteristics (full analysis set)
| SHR0302 4 mg [n = 35] |
SHR0302 8 mg [n = 35] |
Placebo [n = 35] |
|
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 38.5 (14.8) | 35.2 (14.8) | 30.3 (12.6) |
| Median (min, max) | 37.0 (18, 7) | 31.0 (19, 7) | 25.0 (18, 6) |
| Sex | |||
| Male [n (%)] | 20 (57.1) | 23 (65.7) | 26 (74.3) |
| Race | |||
| Asian [n (%)] | 35 (100.0) | 35 (100.0) | 35 (100.0) |
| Body height, cm | |||
| Mean (SD) | 167.9 (7.6) | 168.9 (8.5) | 170.5 (7.6) |
| Median (min, max) | 167.0 (154.0, 187.0) | 169.0 (152.0, 188.0) | 172.0 (153.0, 188.0) |
| Weight, kg | |||
| Mean (SD) | 70.0 (17.0) | 69.7 (19.4) | 69.0 (14.6) |
| Median (min, max) | 67.8 (43.5, 115.0) | 67.5 (39.5, 143.0) | 67.0 (47.0, 104.0) |
| BMI, kg/m2 | |||
| Mean (SD) | 24.6 (4.9) | 24.2 (5.0) | 23.6 (4.1) |
| Median (min, max) | 23.7 (17.5, 41.7) | 23.1 (17.1, 40.5) | 22.8 (15.8, 33.6) |
| AD disease duration, years | |||
| Mean (SD) | 10.9 (10.2) | 9.0 (8.5) | 8.7 (7.6) |
| Median (min, max) | 7.7 (1.1, 40.4) | 6.9 (1.0, 35.3) | 7.2 (1.5, 33.7) |
| EASI | |||
| Mean (SD) | 30.5 (15.7) | 25.4 (11.3) | 28.2 (12.1) |
| Median (min, max) | 23.8 (12.4, 63.0) | 22.9 (12.2, 62.9) | 27.8 (12.3, 59.5) |
| BSA affected, % | |||
| Mean (SD) | 52.4 (23.0) | 49.8 (19.6) | 52.0 (23.5) |
| Median (min, max) | 50.0 (15.0, 90.0) | 51.0 (18.0, 90.0) | 52.0 (10.0, 98.0) |
| IGA grade [n (%)] | |||
| 3: Moderate | 15 (42.9) | 20 (57.1) | 22 (62.9) |
| 4: Severe | 16 (45.7) | 14 (40.0) | 11 (31.4) |
| 5: Extremely severe | 4 (11.4) | 1 (2.9) | 2 (5.7) |
| SCORAD score | |||
| Mean (SD) | 52.4 (23.0) | 19.6 (51.0) | 23.5 (52.0) |
| Median (min, max) | 50 (15, 90) | 51 (18, 90) | 52 (10, 98) |
| Pruritus NRS | |||
| Mean (SD) | 7.9 (1.7) | 7.1 (1.8) | 7.3 (2.3) |
| Median (min, max) | 8.0 (4, 10) | 8.0 (3, 10) | 8.0 (1, 10) |
| DLQI score | |||
| Mean (SD) | 16.3 (7.7) | 15.6 (7.0) | 15.3 (7.2) |
| Median (min, max) | 17.0 (2, 30) | 13.0 (5, 29) | 15.0 (4, 30) |
| Immunoglobulin E, IU/mL | |||
| Mean (SD) | 1519.8 (2026.3) | 1055.8 (1081.6) | 2771.1 (8765.1) |
| Median (min, max) | 1205.2 (8.8, 11,700.0) | 500.0 (2.7, 5000.0) | 855.0 (8.78, 52,000.0) |
AD atopic dermatitis, BMI body mass index, BSA body surface area, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, IGA Investigator’s Global Assessment, max maximum, min minimum, NRS Numerical Rating Scale, SCORAD Scoring Atopic Dermatitis, SD standard deviation
Efficacy
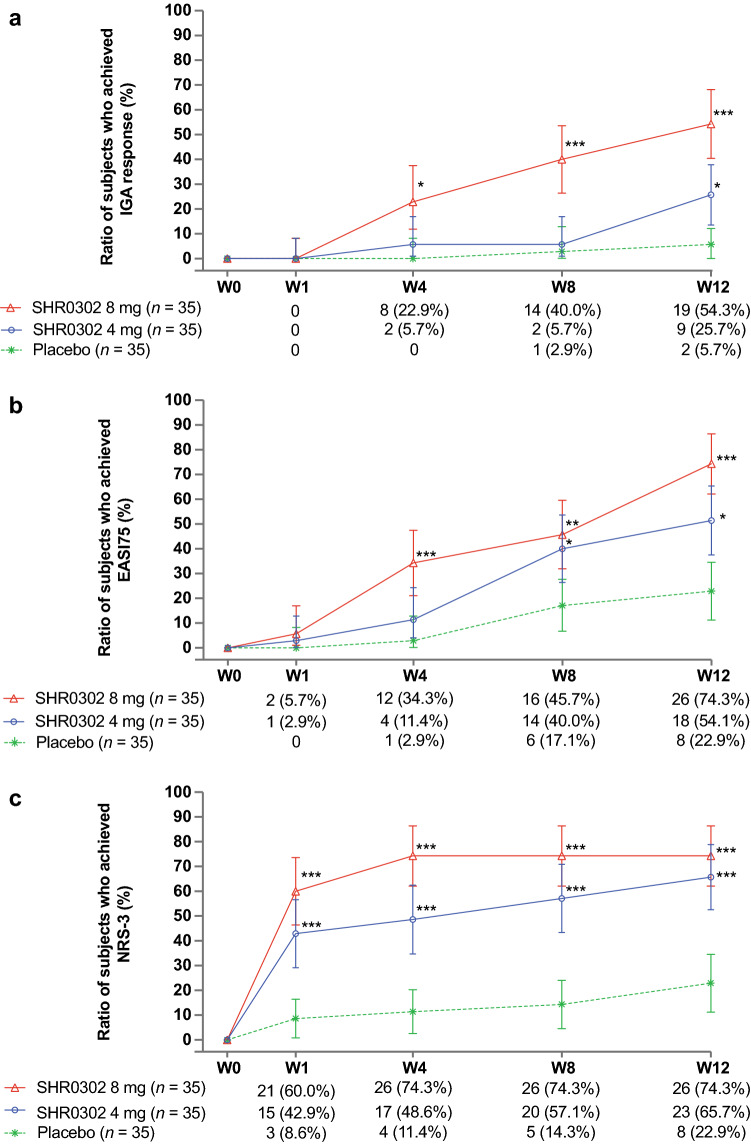
IGA response at week 12 was achieved in nine (25.7%) patients in the SHR0302 4 mg group, 19 (54.3%) patients in the SHR0302 8 mg group, and two (5.7%) patients in the placebo group (Table 2; Fig. 2a). Differences from placebo were 20.0% (90% CI 6.2–33.8%; p = 0.022) for the 4 mg group and 48.6% (90% CI 33.3–63.9%; p < 0.001) for the 8 mg group. The proportions of patients achieving an IGA response increased from weeks 1 to 12 in both the SHR0302 4 mg and 8 mg groups. Differences between the SHR0302 8 mg and placebo groups were statistically significant at weeks 4, 8, and 12 (p = 0.005, p < 0.001, and p < 0.001, respectively), while differences between the SHR0302 4 mg and placebo groups were statistically significant at week 12 only (p = 0.022).
Table 2.
Efficacy endpoints at week 12 (full analysis set)
| SHR0302 4 mg [n = 35] | SHR0302 8 mg [n = 35] | Placebo [n = 35] | |
|---|---|---|---|
| IGA responsea | 9 (25.7%) | 19 (54.3%) | 2 (5.7%) |
| 90% CI | 13.6%, 37.9%i | 40.4%, 68.1%i | 0.0%, 12.2%i |
| Compared with the placebo group | |||
| Difference | 20.0% | 48.6% | |
| 90% CI | 6.2%, 33.8%i | 33.3%, 63.9%i | |
| p-Value | 0.022i | <0.001i | |
| EASI score ≥50% (EASI50)b | 24 (68.6%) | 29 (82.9%) | 15 (42.9%) |
| Compared with the placebo group | |||
| Difference | 25.7% | 40.0% | |
| 90% CI | 6.9%, 44.6%i | 22.7%, 57.3%i | |
| p-Value | 0.030i | <0.001i | |
| EASI score ≥75% (EASI75)c | 18 (51.4%) | 26 (74.3%) | 8 (22.9%) |
| Compared with the placebo group | |||
| Difference | 28.6% | 51.4% | |
| 90% CI | 10.4%, 46.7%i | 34.6%, 68.3%i | |
| p-Value | 0.013i | <0.001i | |
| EASI score ≥90% (EASI90)d | 11 (31.4%) | 16 (45.7%) | 3 (8.6%) |
| Compared with the placebo group | |||
| Difference | 22.9% | 37.1% | |
| 90% CI | 7.8%, 37.9%i | 21.3%, 53.0%i | |
| p-Value | 0.017i | <0.001i | |
| NRS score ≥3 (NRS-3)e | 23 (65.7%) | 26 (74.3%) | 8 (22.9%) |
| Compared with the placebo group | |||
| Difference | 42.9% | 51.4% | |
| 90% CI | 25.2%, 60.5%i | 34.6%, 68.3%i | |
| p-Value | <0.001i | <0.001i | |
| SCORAD score ≥50% (SCORAD50)f | 17 (48.6%) | 25 (71.4%) | 8 (22.9%) |
| Compared with the placebo group | |||
| Difference | 25.7% | 48.6% | |
| 90% CI | 7.6%, 43.9%i | 31.4%, 65.7%i | |
| p-Value | 0.025i | <0.001i | |
| SCORAD score ≥75% (SCORAD75)g | 10 (28.6%) | 12 (34.3%) | 3 (8.6%) |
| Compared with the placebo group | |||
| Difference | 20.0% | 25.7% | |
| 90% CI | 5.2%, 34.8%i | 10.4%, 41.0%i | |
| p-Value | 0.031i | 0.009i | |
| SCORAD score ≥90% (SCORAD90)h | 2 (5.7%) | 3 (8.6%) | 0 |
| Compared with the placebo group | |||
| Difference | 5.7% | 8.6% | |
| 90% CI | −2.1%, 16.9%j | 0.3%, 20.7j | |
| p-Value | 0.493j | 0.239j |
CI confidence interval, EASI Eczema Area and Severity Index, FAS full analysis set, IGA Investigator’s Global Assessment, NRS Numerical Rating Scale, SCORAD Scoring Atopic Dermatitis
aIGA response was defined as an IGA score of 0/1 (complete or almost complete clearance of skin lesions) with an improvement in IGA score by ≥2 from baseline. The following situations would be regarded as nonresponding: (1) IGA 2/3/4/5; (2) IGA score of 0/1, but an improvement in IGA score of <2 from baseline; (3) missing visits; (4) early withdrawal from the treatment
bEASI50: EASI score improved by ≥50% from baseline
cEASI75: EASI score improved by ≥75% from baseline
dEASI90: EASI score improved by ≥90% from baseline.
The following situations would be considered as nonresponding to EASI50 (the same rules applied to EASI75/EASI90): (1) EASI score improved by <50% from baseline; (2) missing visits; (3) early withdrawal from the treatment
eTwo patients in the placebo group had a baseline NRS of <3, therefore these patients would not show an improvement of ≥3 from baseline; the other two treatment groups had no patients with a baseline NRS of <3. The denominator of each treatment group was the number of patients in the FAS of each treatment group
fSCORAD50: SCORAD score improved by ≥50% from baseline
gSCORAD75: SCORAD score improved by ≥75% from baseline
hSCORAD90: SCORAD score improved by ≥90% from baseline
The following situations would be considered as nonresponding to SCORAD50 (the same rules applied to SCORAD75/SCORAD90): (1) SCORAD score improved by <50% from baseline; (2) missing visits; (3) early withdrawal from the treatment
Both remote visits and delayed visits were included in the analysis
iThe confidence interval and p-value were analyzed using the normal approximation method
jThe confidence interval and p-value were analyzed using Fisher's exact test
The efficacy analysis was carried out using Hochberg's incremental test
Fig. 2.
Line plots for proportions of patients who achieved a IGA response, b EASI75, and c NRS-3 (FAS). Two subjects in the placebo group had baseline NRS <3, therefore these two subjects would not show an improvement of ≥3 from baseline. The denominator of each treatment group was the number of subjects in the FAS of each treatment group. EASI Eczema Area and Severity Index, FAS full analysis set, IGA Investigator’s Global Assessment, NRS Numerical Rating Scale, W week. *p < 0.05, **p < 0.01, ***p < 0.001
Percentage changes in EASI score from baseline at weeks 1, 4, 8, and 12 were −28.0%, −52.5%, −58.2%, and −66.0%, respectively, in the SHR0302 4 mg group; −40.4%, −63.9%, −73.4%, and −78.8% in the SHR0302 8 mg group; and −10.5%, −17.9%, −35.6%, and −34.5% in the placebo group (ESM Fig. S2). Least square means (LSM) differences in percentage change in EASI score from baseline were statistically significant at all time points in the SHR0302 4 mg group (−17.5% [90% CI −28.1% to −7.0%; p = 0.007], −34.6% [90% CI −48.7% to −20.6%; p < 0.001], −22.5% [90% CI −40.5% to −4.6%; p = 0.040], and −31.5% [90% CI −52.6% to −10.4%; p = 0.015], respectively) and SHR0302 8 mg group (−30.0% [90% CI −40.2% to −19.8%; p < 0.001], −46.0% [90% CI −59.6% to −32.3%; p < 0.001], −37.7% [90% CI −55.2% to −20.3%; p < 0.001], and −44.3% [90% CI −64.8% to −23.8%; p < 0.001], respectively) versus the placebo group (ESM Fig. S2).
The proportions of patients whose EASI scores improved by ≥50%, ≥75%, and ≥90% (EASI50, EASI75, and EASI90) from baseline at each time point are shown in Table 2 and Fig. 2b (EASI75). The SHR0302 4 mg group showed statistically significant differences versus placebo in EASI50 at week 4, EASI50/75 at week 8, and EASI50/75/90 at week 12. The SHR0302 8 mg group showed statistically significant differences versus placebo in EASI50 at week 1, EASI50/75 at week 4, EASI50/75/90 at week 8, and EASI50/75/90 at week 12.
LSM percentage changes in NRS score from baseline at weeks 1, 4, 8, and 12 were −36.0%, −41.5%, −49.2%, and −55.9%, respectively, in the SHR0302 4 mg group; −51.6%, −56.4%, −61.7%, and −61.4%, respectively, in the SHR0302 8 mg group; and −8.9%, −4.5%, −20.6%, and −26.7%, respectively, in the placebo group. LSM differences in percentage change in NRS from baseline were statistically significant at all time points in the SHR0302 4 mg group (−27.1% [90% CI −38.0% to −16.2%; p < 0.001], −37.0% [90% CI −52.0 to −21.9; p < 0.001], −28.6% [90% CI −42.6 to −14.5; p = 0.001], and −29.2% [90% CI −44.7 to −13.6; p = 0.002], respectively) and SHR0302 8 mg group (–42.6% [90% CI −53.1 to −32.1; p < 0.001], −51.9% [90% CI −66.5 to −37.3; p < 0.001], −41.1% [90% CI −54.7 to −27.5; p < 0.001], and −34.7% [90% CI −49.8 to −19.6; p < 0.001], respectively) compared with the placebo group (ESM Fig. S3).
The proportions of patients with NRS ≥3 are shown in Fig. 2c; additional analyses on the proportions of patients whose NRS improved by ≥4 points (NRS-4) are shown in Fig. S4 in the ESM. Both the 4 mg and 8 mg SHR0302 groups showed statistically significant differences versus placebo in the proportions of patients with NRS ≥3 (p < 0.001, weeks 1, 4, 8, and 12) and NRS ≥4 (p < 0.017, week 1; p = 0.003, week 4; p < 0.001, weeks 8 and 12).
LSM changes in SCORAD score from baseline at weeks 1, 4, 8, and 12 were −16.6, −28.8, −30.5, and −33.5, respectively, in the SHR0302 4 mg group; −22.4, −32.1, −34.4, and −39.2, respectively, in the SHR0302 8 mg group; and −5.3, −8.1, −15.9, and −20.1, respectively, in the placebo group (ESM Fig. S5). LSM differences in change in SCORAD score from baseline were statistically significant at all time points in the SHR0302 4 mg group (−11.3 [90% CI −16.0 to −6.6; p < 0.001], −20.7 [90% CI −27.4 to −14.1; p < 0.001], −14.5 [90% CI −22.5 to −6.5; p = 0.003], and −13.5 [90% CI −22.5 to −4.4; p = 0.015], respectively) and SHR0302 8 mg group (−17.2 [90% CI −21.7 to −12.6; p < 0.001], −24.0 [90% CI −30.4 to −17.5; p < 0.001), −18.5 [90% CI −26.3 to −10.7; p < 0.001], and −19.1 [90% CI −27.9 to −10.3; p < 0.001], respectively) versus placebo (ESM Fig. S5).
The proportions of patients whose SCORAD score improved by ≥50%, ≥75%, and ≥90% (SCORAD50, SCORAD75, and SCORAD90) from baseline at week 12 are shown in Table 2. The SHR0302 4 mg group showed statistically significant differences versus placebo in SCORAD50 at weeks 4, 8, and 12, and SCORAD75 at week 12 (p < 0.001, 0.005, 0.025, 0.031, respectively). The SHR0302 8 mg group showed statistically significant differences versus placebo in SCORAD50 at weeks 4, 8, and 12, and SCORAD75 at weeks 8 and 12 (p < 0.001, p < 0.001, and p < 0.001, 0.006, and 0.009, respectively).
LSM changes in DLQI from baseline at weeks 1, 4, 8, and 12 were −3.4, −6.7, −7.0, and −6.0, respectively, in the SHR0302 4 mg group; −5.8, −8.8, −9.4, and 9.3 in the SHR0302 8 mg group; and −1.1, −0.5, −4.2, and −4.6 in the placebo group (ESM Fig. S6). LSM differences in change in DLQI from baseline at weeks 1, 4, 8, and 12 between the SHR0302 4 mg and placebo groups were −2.3 (90% CI −4.1 to −0.6; p < 0.031), −6.1 (90% CI −8.3 to −3.9; p < 0.001), −2.8 (90% CI −5.0 to −0.6; p = 0.037), and −1.3 (90% CI −4.1 to 1.4; p = 0.424), respectively. LSM differences in the change in DLQI from baseline at weeks 1, 4, 8, and 12 between the SHR0302 8 mg and placebo groups were −4.7 (90% CI −6.4 to −3.0; p < 0.001), −8.3 (90% CI −10.4 to −6.2; p < 0.001), −5.3 (90% CI −7.4 to −3.1; p < 0.001), and −4.7 (90% CI −7.4 to −2.0; p < 0.004).
Analysis results for IgE and absolute eosinophil count changes from baseline are shown in ESM Part 2. There was no clear relationship between IgE or eosinophil count change and relief in symptoms.
Safety
A total of 63/105 (60.0%) patients experienced 138 treatment-emergent AEs (TEAEs) during the study. Overall, 21 (60.0%) patients in the SHR0302 4 mg group, 24 (68.6%) patients in the SHR0302 8 mg group, and 18 (51.4%) patients in the placebo group reported TEAEs of any causality (Table 3). TEAEs in any active drug group with an incidence >5% and higher than placebo were blood creatine phosphokinase increased (n = 2 patients, SHR0302 8 mg), blood pressure increased (n = 2, SHR0302 8 mg), upper respiratory tract infection (n = 2, SHR0302 4 mg), folliculitis (n = 2, SHR0302 8 mg), urinary tract infection (n = 2, SHR0302 8 mg), hyperlipidemia (n = 3, SHR0302 4 mg; n = 2, SHR0302 8 mg), hyperuricemia (n = 2, SHR0302 4 mg; n = 3, SHR0302 8 mg), hypertriglyceridemia (n = 2, SHR0302 4 mg), hypercholesterolemia (n = 2, SHR0302 8 mg), headache (n = 2, SHR0302 8 mg), AD (n = 2, SHR0302 4 mg), and leukocytosis (n = 3, SHR0302 4 mg). No serious infections were reported. Herpes zoster TEAEs were reported in two patients, one each in the SHR0302 4 mg and SHR0302 8 mg groups; no cases were reported in the placebo group. Herpes simplex/herpes virus infection TEAEs were reported in three patients, one each in the SHR0302 4 mg, SHR0302 8 mg, and placebo groups. One case of Kaposi's varicelliform eruption was reported in the SHR0302 4 mg group. There were no cases of malignant neoplasm, thrombosis, liver injury, or death.
Table 3.
Summary of adverse events (safety set)
| SHR0302 4 mg [n = 35] | SHR0302 8 mg [n = 35] | Placebo [n = 35] | ||||
|---|---|---|---|---|---|---|
| No. of cases (%) | No. of events | No. of cases (%) | No. of events | No. of cases (%) | No. of events | |
| All AEs | 21 (60.0) | 43 | 24 (68.6) | 62 | 18 (51.4) | 33 |
| AEs during screening | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAEs | 21 (60.0) | 43 | 24 (68.6) | 62 | 18 (51.4) | 33 |
| Moderate/severe TEAE | 3 (8.6) | 4 | 0 | 0 | 1 (2.9) | 4 |
| Serious TEAE | 2 (5.7) | 2 | 1 (2.9) | 1 | 0 | 0 |
| TEAE leading to death | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAE affecting the administration of the study drug | 3 (8.6) | 3 | 2 (5.7) | 2 | 1 (2.9) | 1 |
| TEAE resulting in study withdrawal | 3 (8.6) | 3 | 1 (2.9) | 1 | 1 (2.9) | 1 |
| Preferred term, frequency ≥5% in any group | ||||||
| Tri-iodothyronine free increased | 2 (5.7) | 1 (2.9) | 4 (11.4) | |||
| Transaminases increased | 2 (5.7) | 1 (2.9) | 3 (8.6) | |||
| Blood creatine phosphokinase increased | 1 (2.9) | 2 (5.7) | 0 | |||
| Blood pressure increased | 0 | 2 (5.7) | 0 | |||
| Weight decreased | 0 | 0 | 2 (5.7) | |||
| Upper respiratory tract infection | 2 (5.7) | 1 (2.9) | 0 | |||
| Folliculitis | 0 | 2 (5.7) | 0 | |||
| Urinary tract infection | 0 | 2 (5.7) | 0 | |||
| Hyperlipidemia | 3 (8.6) | 2 (5.7) | 0 | |||
| Hyperuricemia | 2 (5.7) | 3 (8.6) | 0 | |||
| Hypertriglyceridemia | 2 (5.7) | 1 (2.9) | 1 (2.9) | |||
| Hypercholesterolemia | 0 | 2 (5.7) | 1 (2.9) | |||
| Headache | 0 | 2 (5.7) | 0 | |||
| Atopic dermatitis | 2 (5.7) | 1 (2.9) | 0 | |||
| Leukocytosis | 3 (8.6) | 0 | 0 | |||
AEs adverse events, TEAEs treatment-emergent adverse events
Drug-related TEAEs had the highest incidence in the SHR0302 8 mg group with 14 (40.0%, n = 26 events) patients, followed by the placebo group with 10 (28.6%, n = 19) patients and the SHR0302 4 mg group with eight (22.9%, n = 14) patients. Drug-related TEAEs resulted in drug discontinuation in two patients (n = 1, ‘dizziness’, SHR0302 4 mg group; n = 1, ‘dermatitis atopic’, SHR0302 8 mg group) and drug interruption in one patient (‘herpes zoster’, SHR0302 8 mg group).
A total of three (2.9%, n = 3 events) patients experienced serious AEs (SAEs), all of which were ‘worsening of AD’, including two moderate cases in the SHR0302 4 mg group (considered by the investigator unlikely to be related to study treatment) and one mild case in the SHR0302 8 mg group (considered possibly related to study treatment). All three patients with SAEs recovered.
There were no clinically significant changes in hematology parameters, laboratory test results, urinalysis, vital signs, or electrocardiograms.
Discussion
This phase II trial shows that adults with moderate to severe AD achieved significant improvements in clinical symptoms, including pruritus, and improvement of skin lesions following oral administration of SHR0302 4 mg and 8 mg. The overall efficacy of SHR0302 at both doses was superior to placebo, with the 8 mg group showing greater efficacy than the 4 mg group.
Disease severity measured by IGA showed progressive improvements during SHR0302 treatment over 12 weeks, with significant differences between the SHR0302 8 mg and placebo groups at weeks 4, 8, and 12, and between the SHR0302 4 mg and placebo groups at week 12. EASI assessments of eczema severity showed significant improvement during SHR0302 treatment versus placebo at weeks 1, 4, 8, and 12 in both the 4 mg and 8 mg dose groups. AD severity measured by SCORAD score and pruritus measured by NRS similarly demonstrated significant improvements at all time points with both SHR0302 doses versus placebo. Achieving an improvement in pruritus by week 1 represents a notable benefit for patients with moderate to severe AD, in whom itch represents a central and debilitating symptom associated with difficult-to-control scratching, superimposed skin inflammation and infection, sleep disturbance, functional impairment and mental distress, and feelings of anxiety and depression [12, 26, 38, 39]. Finally, quality of life measured by DLQI was significantly improved by SHR0302 at all time points during the study except one.
By study end at week 12, all efficacy endpoints were consistent in demonstrating greater improvements with SHR0302 than placebo. For IGA response in particular, there were indications that a plateau had not been reached and that additional improvements in outcome could be expected from continued treatment beyond 12 weeks. A phase III study is planned to investigate this potential trend, which will assess efficacy outcomes using additional time points.
SHR0302 at both the 4 mg and 8 mg doses demonstrated acceptable tolerability and safety. TEAEs were mild in most cases and were associated with low rates of treatment discontinuation, while the three SAEs observed were all related to activities of the primary disease. Infection rates appeared to show a dose-related relationship, but no other dose-related association to TEAEs was evident in these descriptive analyses.
The improvements in symptoms during SHR0302 treatment showed no clear relationship to changes in serum IgE and absolute eosinophil counts, suggesting that the efficacy of SHR0302 is independent of effects on these biomarkers of AD.
These data provide further evidence for the use of JAK inhibition in the treatment of moderate to severe AD, in support of previous JAK inhibitor trials and the recommendations in recent Chinese guidelines [40]. Direct comparison of the outcomes between SHR0302 and other JAK inhibitors is restricted by the limited study durations, small sample sizes, diverse patient populations, and different outcome measures adopted in these phase II or III trials. However, SHR0302 has demonstrated an efficacy that is broadly consistent with other JAK1 inhibitors under investigation for the treatment of AD.
Selective JAK1 inhibitors, such as SHR0302, offer the potential advantage of reduced AEs compared with JAK inhibitors that additionally target JAK2, JAK3, or TYK2 [41]. Evidence from the current study suggests that the incidences of AEs associated with SHR0302 are consistent with other JAK inhibitors [30–33]. Overall incidences of SAEs are reported to be low for JAK inhibitors in the treatment of AD, although potentially increased rates of thromboembolic, cardiovascular, and hematological events have been described for oral JAK inhibitors in other dermatologic conditions [42]. No evidence of thromboembolic, cardiovascular, or hematological abnormalities associated with SHR0302 was observed in our study.
This study is limited to the investigation of Chinese patients with moderate to severe AD. Additional study limitations are the small sample size and short treatment duration, similar to other phase II trials of JAK inhibitors. Finally, the concurrent coronavirus disease 2019 (COVID-19) pandemic impacted on-site visits and treatment duration for a limited number of patients, but is not considered to have altered the validity or interpretation of the study results.
Conclusions
This phase II study of orally administered SHR0302 4 mg and 8 mg provided results consistent with the pharmacological properties of SHR0302, a highly selective JAK1 inhibitor for once daily administration. SHR0302 demonstrated potential as an effective and well-tolerated treatment for adult patients with moderate to severe AD, which will be further investigated in a phase III study. Based on these phase II trial data, the Chinese National Medical Products Administration has granted a breakthrough therapy designation for SHR0302 in AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgments
The authors wish to acknowledge other investigators in this study: Weifeng Yao, Tianjin Academy of Traditional Chinese Medicine Affiliated Hospital; Yunlu Yao, Shanghai Skin Disease Hospital, Tongji University School of Medicine; Shasha Fan, Zhejiang Provincial People's Hospital, People's Hospital of Hangzhou Medical College; Zhenlan Wu, The First Affiliated Hospital of Fujian Medical University; Jianji Wan, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences; Aiyuan Guo, The Third Xiangya Hospital, Central South University; Chen Jian, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine; Jin Peng, Xinqiao Hospital, Third Military Medical University (Army Medical University); Qing Zhang, The Second Xiangya Hospital, Central South University; Shu Ping Guo and Hongye Liu, The First Hospital of Shanxi Medical University; Hongzhong Jin and Chang Shu, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences; Hong Fang and Jianjun Qiao, The First Affiliated Hospital of Zhejiang University School of Medicine; Tao Juan and Song Zhang, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology; Yi Zhao and Dian Chen, Beijing Tsinghua Changgung Hospital; Wei Zhu and Chen Zhao, Xuanwu Hospital of Capital Medical University; Jie Li, Xiangya Hospital, Central South University; Chunlei Zhang and Xueyan Lu, The Third Hospital of Peking University; Qing Guo and Yijin Luo, Sun Yat-sen Memorial Hospital, Sun Yat-sen University; Xinghua Gao and Li Zhang, The First Affiliated Hospital of China Medical University; Qihong Qian and Sun Ren, The First Affiliated Hospital of Soochow University; Shoumin Zhang and Hongwei Liu, Henan Provincial People's Hospital; Ningning Dang and Guojing Qin, Jinan Central Hospital. The authors would also like to thank all patients who participated in this study. Editorial assistance was provided by Bill Wolvey at Parexel, with funding from Reistone Biopharma Co., Ltd.
Declarations
Funding
This study was funded by Reistone Biopharma Co., Ltd.
Conflicts of interest
Aik Han Goh, Rongjun Liu, and Zhiguo Zhang are employees of Reistone Biopharma Co., Ltd. Yan Zhao, Litao Zhang, Yangfeng Ding, Xiaohua Tao, Chao Ji, Xiuqin Dong, Jianyun Lu, Liming Wu, Rupeng Wang, Qianjin Lu, and Jianzhong Zhang declare they have no conflicts of interest.
Role of Funder/Sponsor
This study was designed, funded, and managed by Reistone Biopharma Co., Ltd and was conducted by investigators contracted by and under the direction of the sponsor. Data collection, management, and analysis were conducted by Reistone Biopharma Co., Ltd. Confidentiality agreements were established between the authors and the sponsor. All authors participated in the interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication with no participation by or financial compensation from the sponsor. Medical writing and editorial support were provided by Parexel and funded by Reistone.
Availability of Data and Material
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Data Access, Responsibility, and Analysis
Jianzhong Zhang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics approval
This research was approved by the Institutional Review Board and Independent Ethics Committee of the Peking University People's Hospital and the participating study centers. The study was conducted in compliance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines, as well as all local regulatory requirements.
Consent to participate
All patients provided written informed consent and could withdraw from the study at any time at their own request or at the investigator’s or study sponsor’s discretion.
Consent for publish
Not applicable.
Code Availability
Not applicable.
Author contributions
Drafting of the manuscript, and acquisition, analysis, or interpretation of data: YZ. Acquisition, analysis, or interpretation of data: LZ, YD, XT, CJ, XD, JL, LW, RW, and QL. Concept and design, and statistical analysis: AHG, RL, and ZZ. Acquisition, analysis, or interpretation of data; critical revision of the manuscript for important intellectual content; supervision: JZ.
Contributor Information
Yan Zhao, Email: zhaoyan_2021@126.com.
Litao Zhang, Email: zhanglitao@medmail.com.cn.
Yangfeng Ding, Email: dingyangfeng@aliyun.com.
Xiaohua Tao, Email: 13505811700@163.com.
Chao Ji, Email: surpassing_ji@aliyun.com.
Xiuqin Dong, Email: dxq1996@163.com.
Jianyun Lu, Email: 1010739024@qq.com.
Liming Wu, Email: 18957118053@163.com.
Rupeng Wang, Email: wrp71@163.com.
Qianjin Lu, Email: qianlu5860@csu.edu.cn.
Aik Han Goh, Email: aik.goh@reistonebio.com.
Rongjun Liu, Email: roger.liu@reistonebio.com.
Zhiguo Zhang, Email: jeremy.zhang@reistonebio.com.
Jianzhong Zhang, Email: rmzjz@126.com.
References
- 1.Eichenfield LF, Tom WL, Chamlin SL, Feldman SR, Hanifin JM, Simpson EL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70(2):338–351. doi: 10.1016/j.jaad.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Ann Allergy Asthma Immunol. 2017;119(6):548–52.e3. doi: 10.1016/j.anai.2017.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg JI, Hanifin JM. Adult eczema prevalence and associations with asthma and other health and demographic factors: a US population-based study. J Allergy Clin Immunol. 2013;132(5):1132–1138. doi: 10.1016/j.jaci.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Williams H, Robertson C, Stewart A, Aït-Khaled N, Anabwani G, Anderson R, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103(1 Pt 1):125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 5.Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32(5):657–682. doi: 10.1111/jdv.14891. [DOI] [PubMed] [Google Scholar]
- 6.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic Dermatitis in America Study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Li LF, Zhao DY, Shen YW. Prevalence and clinical features of atopic dermatitis in China. Biomed Res Int. 2016;2016:2568301. doi: 10.1155/2016/2568301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. The burden of atopic dermatitis in US adults: health care resource utilization data from the 2013 National Health and Wellness Survey. J Am Acad Dermatol. 2018;78(1):54–61.e1. doi: 10.1016/j.jaad.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Eckert L, Gupta S, Gadkari A, Mahajan P, Gelfand JM. Burden of illness in adults with atopic dermatitis: analysis of National Health and Wellness Survey data from France, Germany, Italy, Spain, and the United Kingdom. J Am Acad Dermatol. 2019;81(1):187–195. doi: 10.1016/j.jaad.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 10.Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB, Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi: 10.1111/j.1525-1470.2005.22303.x. [DOI] [PubMed] [Google Scholar]
- 11.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Rønnstad ATM, Halling-Overgaard AS, Hamann CR, Skov L, Egeberg A, Thyssen JP. Association of atopic dermatitis with depression, anxiety, and suicidal ideation in children and adults: a systematic review and meta-analysis. J Am Acad Dermatol. 2018;79(3):448–56.e30. doi: 10.1016/j.jaad.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 13.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Symptoms and diagnosis of anxiety and depression in atopic dermatitis in U.S. adults. Br J Dermatol. 2019;181(3):554–565. doi: 10.1111/bjd.17683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Atopic Dermatitis Working Group, Immunology Group Chinese Society of Dermatology. Guideline for diagnosis and treatment of atopic dermatitis in China (2020). Chinese Journal of Dermatology. 2020;53(2):81-8.
- 16.Boguniewicz M, Alexis AF, Beck LA, Block J, Eichenfield LF, Fonacier L, et al. Expert perspectives on management of moderate-to-severe atopic dermatitis: a multidisciplinary consensus addressing current and emerging therapies. J Allergy Clin Immunol Pract. 2017;5(6):1519–1531. doi: 10.1016/j.jaip.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Calzavara Pinton P, Cristaudo A, Foti C, Canonica GW, Balato N, Costanzo A, et al. Diagnosis and management of moderate to severe adult atopic dermatitis: a Consensus by the Italian Society of Dermatology and Venereology (SIDeMaST), the Italian Association of Hospital Dermatologists (ADOI), the Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC), and the Italian Society of Allergological, Environmental and Occupational Dermatology (SIDAPA). G Ital Dermatol Venereol. 2018;153(2):133-45. 10.23736/s0392-0488.17.05892-8. [DOI] [PubMed]
- 18.Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–132. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71(2):327–349. doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hendricks AJ, Lio PA, Shi VY. Management recommendations for dupilumab partial and non-durable responders in atopic dermatitis. Am J Clin Dermatol. 2019;20(4):565–569. doi: 10.1007/s40257-019-00436-8. [DOI] [PubMed] [Google Scholar]
- 21.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375(24):2335–2348. doi: 10.1056/NEJMoa1610020. [DOI] [PubMed] [Google Scholar]
- 22.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242(1):233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandt EB, Sivaprasad U. Th2 cytokines and atopic dermatitis. J Clin Cell Immunol. 2011;2(3). 10.4172/2155-9899.1000110. [DOI] [PMC free article] [PubMed]
- 24.Singh R, Heron CE, Ghamrawi RI, Strowd LC, Feldman SR. Emerging role of Janus kinase inhibitors for the treatment of atopic dermatitis. Immunotargets Ther. 2020;9:255–272. doi: 10.2147/itt.s229667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotter DG, Schairer D, Eichenfield L. Emerging therapies for atopic dermatitis: JAK inhibitors. J Am Acad Dermatol. 2018;78(3 Suppl 1):S53–S62. doi: 10.1016/j.jaad.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Bissonnette R, Papp KA, Poulin Y, Gooderham M, Raman M, Mallbris L, et al. Topical tofacitinib for atopic dermatitis: a phase IIa randomized trial. Br J Dermatol. 2016;175(5):902–911. doi: 10.1111/bjd.14871. [DOI] [PubMed] [Google Scholar]
- 27.Kim BS, Howell MD, Sun K, Papp K, Nasir A, Kuligowski ME. Treatment of atopic dermatitis with ruxolitinib cream (JAK1/JAK2 inhibitor) or triamcinolone cream. J Allergy Clin Immunol. 2020;145(2):572–582. doi: 10.1016/j.jaci.2019.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82(4):823–831. doi: 10.1016/j.jaad.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Bissonnette R, Maari C, Forman S, Bhatia N, Lee M, Fowler J, et al. The oral Janus kinase/spleen tyrosine kinase inhibitor ASN002 demonstrates efficacy and improves associated systemic inflammation in patients with moderate-to-severe atopic dermatitis: results from a randomized double-blind placebo-controlled study. Br J Dermatol. 2019;181(4):733–742. doi: 10.1111/bjd.17932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guttman-Yassky E, Thaçi D, Pangan AL, Hong HC, Papp KA, Reich K, et al. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145(3):877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156(8):863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183(2):242–255. doi: 10.1111/bjd.18898. [DOI] [PubMed] [Google Scholar]
- 33.Simpson EL, Sinclair R, Forman S, Wollenberg A, Aschoff R, Cork M, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266. doi: 10.1016/s0140-6736(20)30732-7. [DOI] [PubMed] [Google Scholar]
- 34.Babon JJ, Lucet IS, Murphy JM, Nicola NA, Varghese LN. The molecular regulation of Janus kinase (JAK) activation. Biochem J. 2014;462(1):1–13. doi: 10.1042/bj20140712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 36.Gooderham MJ, Bissonnette R, Grewal P, Lansang P, Papp KA, Hong CH. Approach to the assessment and management of adult patients with atopic dermatitis: a consensus document. Section II: tools for assessing the severity of atopic dermatitis. J Cutan Med Surg. 2018;22(1 Suppl):10s–s16. doi: 10.1177/1203475418803628. [DOI] [PubMed] [Google Scholar]
- 37.Paller AS, Kabashima K, Bieber T. Therapeutic pipeline for atopic dermatitis: end of the drought? J Allergy Clin Immunol. 2017;140(3):633–643. doi: 10.1016/j.jaci.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Jeon C, Yan D, Nakamura M, Sekhon S, Bhutani T, Berger T, et al. Frequency and management of sleep disturbance in adults with atopic dermatitis: a systematic review. Dermatol Ther (Heidelb). 2017;7(3):349–364. doi: 10.1007/s13555-017-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapur S, Watson W, Carr S. Atopic dermatitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):52. doi: 10.1186/s13223-018-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atopic Dermatitis Working Group, Immunology Group, Chinese Society of Dermatology. Guideline for diagnosis and treatment of atopic dermatitis in China. Chinese Journal of Dermatology. 2020;53(2):81-8.
- 41.Nezamololama N, Fieldhouse K, Metzger K, Gooderham M. Emerging systemic JAK inhibitors in the treatment of atopic dermatitis: a review of abrocitinib, baricitinib, and upadacitinib. Drugs Context. 2020;9:2020-8-5. 10.7573/dic.2020-8-5. [DOI] [PMC free article] [PubMed]
- 42.He H, Guttman-Yassky E. JAK inhibitors for atopic dermatitis: an update. Am J Clin Dermatol. 2019;20(2):181–192. doi: 10.1007/s40257-018-0413-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.