Abstract
Lomonosov-2 supercomputer is used to search for new organic compounds that can suppress the replication of the SARS-CoV-2 coronavirus. The latter is responsible for the COVID-19 pandemic. Docking and a quantum-chemical semiempirical atomistic modeling method are used to find inhibitors of the SARS-CoV-2 papain-like protease, which is one of the key coronavirus enzymes responsible for its replication. The atomistic model of the papain-like protease of this coronavirus is based on the high-resolution structure deposited in the Protein Data Bank. The SOL docking program has been used for virtual screening of more than  low molecular weight molecules (ligands). Ligands with the highest protein-ligand binding energy, selected using the docking results, were subjected to quantum-chemical calculations. The latters are performed by the PM7 semiempirical method with the COSMO implicit solvent model using the MOPAC program. The enthalpy of protein-ligand binding is calculated for the best position of the ligand in the protein.
low molecular weight molecules (ligands). Ligands with the highest protein-ligand binding energy, selected using the docking results, were subjected to quantum-chemical calculations. The latters are performed by the PM7 semiempirical method with the COSMO implicit solvent model using the MOPAC program. The enthalpy of protein-ligand binding is calculated for the best position of the ligand in the protein. 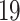 ligands were selected for experimental in vitro testing as candidates for papain-like protease inhibitors base on docking and quantum-chemical results. In case of experimental confirmation, these compounds may become the basis for direct-acting antiviral drugs for the SARS-CoV-2 coronavirus.
ligands were selected for experimental in vitro testing as candidates for papain-like protease inhibitors base on docking and quantum-chemical results. In case of experimental confirmation, these compounds may become the basis for direct-acting antiviral drugs for the SARS-CoV-2 coronavirus.
Keywords: drug discovery, docking, quantum chemistry, inhibitors, PLpro, SARS-CoV-2, COVID-19, CADD
INTRODUCTION
The COVID-19 pandemic caused by the SARS-CoV-2 coronavirus makes the development of drugs that directly suppress viral replication extremely urgent. Molecules of such drugs should bind to the active sites of the proteins (therapeutic targets) that are responsible for replication of the virus, block the functioning of these proteins, and thereby suppress virus replication. Computer-aided structure based drug design is the most efficient way to design and discover such molecules, called inhibitors [1, 2]. To use this technology, you need to know the therapeutic targets as well as their 3D structures. Currently, the molecular mechanisms of the SARS-CoV-2 replication life cycle are largely understood, the therapeutic targets are identified [3–5], and 3D structures of corresponding protein-ligand complexes are determined with high resolution and stores in Protein Data Bank (PDB) [6]. The PDB structures of the protein-ligand complexes are used to construct a full-atomic model of the corresponding target protein, which, in turn, is used to search for inhibitors of the target protein. The search is performed using docking programs that determine the position of the ligand (a molecule assumed to be an inhibitor) in the active site of the target protein and estimate the energy of the protein-ligand binding [7, 8]. The higher the binding energy, the more effective a drug can be created using such an inhibitor because a lower drug concentration should lead to the desired therapeutic effect.
Virtual screening of large databases of ligands using docking
helps identify ligands with high protein-ligand binding
energy [9]. The inhibitory activity of best binding ligands should
be tested experimentally using an in vitro protein-binding assay
where the so-called  concentration is determined. The
latter is the concentration of the inhibitor capable of
suppressing the target protein by
concentration is determined. The
latter is the concentration of the inhibitor capable of
suppressing the target protein by  . The experimentally
confirmed inhibitors, the so-called ‘‘hits’’, are subject to
further optimization using the chemical synthesis of new compounds
to increase their inhibitory activity, aqueous solubility and
other properties that a medicine should have. The discovery of new
inhibitors launches the entire drug development pipeline,
consisting of testing inhibitor’s ability to suppress viral
replication in cell cultures, preclinical studies in animal
models, and several phases of clinical studies in humans. Thus,
the development of inhibitors is a key step in the entire drug
development process that determines the success of new drug
discovery.
. The experimentally
confirmed inhibitors, the so-called ‘‘hits’’, are subject to
further optimization using the chemical synthesis of new compounds
to increase their inhibitory activity, aqueous solubility and
other properties that a medicine should have. The discovery of new
inhibitors launches the entire drug development pipeline,
consisting of testing inhibitor’s ability to suppress viral
replication in cell cultures, preclinical studies in animal
models, and several phases of clinical studies in humans. Thus,
the development of inhibitors is a key step in the entire drug
development process that determines the success of new drug
discovery.
Currently, there are no drugs that inhibit druggable targets of
the SARS-CoV-2 coronavirus, but many computational and
experimental efforts were made in the past year to find such
inhibitors among the existing and approved drug [10, 11]. Several
reversible and irreversible (covalent) low molecular weight
inhibitors of various targets have been found, mainly
RNA-dependent RNA protease and main protease ( ). However,
SAR-CoV-2 papain-like polymerase (
). However,
SAR-CoV-2 papain-like polymerase (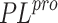 ) is also a
wide-recognized druggable target, but there are only a few
publications on its inhibitors with experimental confirmation [4,
10], and most of them have been identified among existing drugs.
) is also a
wide-recognized druggable target, but there are only a few
publications on its inhibitors with experimental confirmation [4,
10], and most of them have been identified among existing drugs.
We present here the results of virtual screening a database
containing more than  thousand structures of low molecular
weight organic molecules using our own SOL docking program.
The target protein is the SARS-CoV-2 papain-like protease. For
the ligands with best docking scores the binding enthalpy is
calculated using a quantum-chemical semiempirical method and
an implicit solvent model.
thousand structures of low molecular
weight organic molecules using our own SOL docking program.
The target protein is the SARS-CoV-2 papain-like protease. For
the ligands with best docking scores the binding enthalpy is
calculated using a quantum-chemical semiempirical method and
an implicit solvent model.  compounds have been selected for
the further experimental in vitro validation on the base of best
docking scores as well as best binding enthalpy values. The same
approach and database were recently used to search for SARS-CoV-2
compounds have been selected for
the further experimental in vitro validation on the base of best
docking scores as well as best binding enthalpy values. The same
approach and database were recently used to search for SARS-CoV-2
 inhibitors [12], and later the inhibitory activity of
some of the identified compounds was experimentally confirmed.
inhibitors [12], and later the inhibitory activity of
some of the identified compounds was experimentally confirmed.
MODEL DESCRIPTION
To predict the activity of ligands against SARS-CoV-2 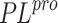 ,
we use structure-based virtual screening combined with
quantum-chemical refinement of docking results. For this, a
,
we use structure-based virtual screening combined with
quantum-chemical refinement of docking results. For this, a
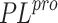 model is prepared using available structures of this
protein in the PDB. We focus on the
model is prepared using available structures of this
protein in the PDB. We focus on the  structures that
contain co-crystallized inhibitors, since the
structures that
contain co-crystallized inhibitors, since the  active
site has a flexible BL2 loop containing Tyr268, which changes its
conformation upon binding of the inhibitor [13]. In terms of
biochemistry,
active
site has a flexible BL2 loop containing Tyr268, which changes its
conformation upon binding of the inhibitor [13]. In terms of
biochemistry,  is a cysteine endoproteinase which
cleaves three viral proteins: nsp1, nsp2, and nsp3—from
polyproteins of SARS-CoV-2. Being cleaved, these proteins start
performing their function. Besides proteolysis of polyproteins
is a cysteine endoproteinase which
cleaves three viral proteins: nsp1, nsp2, and nsp3—from
polyproteins of SARS-CoV-2. Being cleaved, these proteins start
performing their function. Besides proteolysis of polyproteins
 also inhibits host innate immune responses helping the
virus evade immune recognition and elimination. The catalytic
triad of SARS-CoV-2
also inhibits host innate immune responses helping the
virus evade immune recognition and elimination. The catalytic
triad of SARS-CoV-2  consists of
consists of 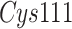 ,
, 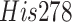 , and
, and
 .
.
Validation of the model should be performed using docking of known
non-covalent PLpro inhibitors. Low-molecular-weight organic
molecules subject to docking are prepared with considering
different protomers at pH 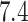 and conformers of macrocycles. The
virtual screening consists of two main stages: docking with
subsequent quantum-chemical calculations of the protein-ligand
binding enthalpy. The final selection of possible actives relies
upon three criteria: sufficiently negative docking score and
binding enthalpy, and visual observation of ligand positions in
the protein active site.
and conformers of macrocycles. The
virtual screening consists of two main stages: docking with
subsequent quantum-chemical calculations of the protein-ligand
binding enthalpy. The final selection of possible actives relies
upon three criteria: sufficiently negative docking score and
binding enthalpy, and visual observation of ligand positions in
the protein active site.
Protein Spatial Model
The success of computer screening largely depends on the initial
experimental structure of the protein used to prepare its complete
atomic spatial model suitable for modeling. To date, there are
only twelve SARS-CoV-2 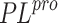 structures in the PDB. Of these,
five crystal structures are related to apo-form, i.e. without
ligands. Other
structures in the PDB. Of these,
five crystal structures are related to apo-form, i.e. without
ligands. Other 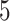 complexes contain covalent inhibitors. The
remaining two complexes, 7D7T and 7JRN, crystallized with
non-covalent SARS-CoV-2
complexes contain covalent inhibitors. The
remaining two complexes, 7D7T and 7JRN, crystallized with
non-covalent SARS-CoV-2 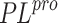 inhibitors, have many missed
atoms and poor
inhibitors, have many missed
atoms and poor 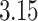 Å(7D7T) or satisfactory
Å(7D7T) or satisfactory
 Åresolution (7JRN). Only three PDB structures of
SARS-CoV-2
Åresolution (7JRN). Only three PDB structures of
SARS-CoV-2  have resolution better than
have resolution better than 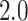 Å, and
only one of them is crystallized with an inhibitor (PDB ID:
6WX4) [14]. This complex (see Fig. 1) contains a peptide inhibitor
covalently bound to the catalytic cysteine (
Å, and
only one of them is crystallized with an inhibitor (PDB ID:
6WX4) [14]. This complex (see Fig. 1) contains a peptide inhibitor
covalently bound to the catalytic cysteine ( ) of
) of
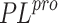 . For clarity, the
. For clarity, the  is represented in Fig. 1 by the solvent accessible surface covering this protein. Because
this complex has good resolution (
is represented in Fig. 1 by the solvent accessible surface covering this protein. Because
this complex has good resolution ( Å) and only few
terminus residues missed, we choose the 6WX4 for our design
efforts.
Å) and only few
terminus residues missed, we choose the 6WX4 for our design
efforts.
Fig. 1.

Crystallized complex SARS-CoV-2  with
inhibitor, PDB ID 6WX4.
with
inhibitor, PDB ID 6WX4.
Preparing  extracted from 6WX4 includes the following
steps. First, the covalent inhibitor, the VIR251 tetrapeptide
bound to the
extracted from 6WX4 includes the following
steps. First, the covalent inhibitor, the VIR251 tetrapeptide
bound to the  sulfur atom, is removed. Next, protonation
of the protein atoms at pH
sulfur atom, is removed. Next, protonation
of the protein atoms at pH 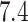 is performed using the APLITE
program [15]. The addition of hydrogen atoms to the protein is
required because PDB structures usually have no hydrogen atoms at
all. After that, the broken covalent bond of the sulfur atom is
saturated by a hydrogen atom, and
is performed using the APLITE
program [15]. The addition of hydrogen atoms to the protein is
required because PDB structures usually have no hydrogen atoms at
all. After that, the broken covalent bond of the sulfur atom is
saturated by a hydrogen atom, and 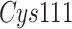 is restored to the
state before the covalent inhibitor is bound. Finally, the
positions of the
is restored to the
state before the covalent inhibitor is bound. Finally, the
positions of the  atoms are optimized using the MMFF94
force field to eliminate possible changes caused by the binding of
the covalent inhibitor. In this study, we are not targeting the
pocket next to
atoms are optimized using the MMFF94
force field to eliminate possible changes caused by the binding of
the covalent inhibitor. In this study, we are not targeting the
pocket next to  , as it is very narrow and appears to only
be appropriate for covalent inhibitors. Instead, we are looking
for inhibitors that can bind next to the catalytic site, including
, as it is very narrow and appears to only
be appropriate for covalent inhibitors. Instead, we are looking
for inhibitors that can bind next to the catalytic site, including
 , which is responsible for the cleavage of a substrate
peptide bond, namely in the
, which is responsible for the cleavage of a substrate
peptide bond, namely in the  and
and 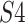 subpockets located at
the entrance to the catalytic pocket. The non-covalent SARS-CoV-2
subpockets located at
the entrance to the catalytic pocket. The non-covalent SARS-CoV-2
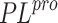 inhibitors bind to these particular pockets in the 7D7T
and 7JRN complexes.
inhibitors bind to these particular pockets in the 7D7T
and 7JRN complexes.
Due to the high similarity between the SARS-CoV-2 and SARS-CoV-1
coronaviruses (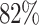 gene sequence identity), we perform
cross-docking validation of non-covalent SARS-CoV-1
gene sequence identity), we perform
cross-docking validation of non-covalent SARS-CoV-1 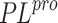 inhibitors to verify the reproducibility of experimental
conformations of the inhibitors with our
inhibitors to verify the reproducibility of experimental
conformations of the inhibitors with our  model. The
obtained values of the scoring function of the docking program,
corresponding to the docked positions of such inhibitors, estimate
the threshold separating inhibitors from inactive ligands during
virtual screening. To assess accuracy of cross-docking, we
superimpose atoms of the 6WX4 complex onto atoms of two complexes,
3MJ5 [16] and 4OW0 [17], of SARS-CoV-1
model. The
obtained values of the scoring function of the docking program,
corresponding to the docked positions of such inhibitors, estimate
the threshold separating inhibitors from inactive ligands during
virtual screening. To assess accuracy of cross-docking, we
superimpose atoms of the 6WX4 complex onto atoms of two complexes,
3MJ5 [16] and 4OW0 [17], of SARS-CoV-1  with
non-covalent inhibitors. The superimposed inhibitors become
‘‘quasi-native’’ as if they are crystallized with the protein of
the 6WX4 complex. This procedure allows one to estimate
cross-docking accuracy in terms of RMSD values calculated between
the ‘‘quasi-native’’ ligand position and a predicted docked
position. RMSD values lower than
with
non-covalent inhibitors. The superimposed inhibitors become
‘‘quasi-native’’ as if they are crystallized with the protein of
the 6WX4 complex. This procedure allows one to estimate
cross-docking accuracy in terms of RMSD values calculated between
the ‘‘quasi-native’’ ligand position and a predicted docked
position. RMSD values lower than 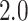 Åare indicators of
successful cross-docking and confirm applicability of the model
for screening. The results of cross-docking for our
Åare indicators of
successful cross-docking and confirm applicability of the model
for screening. The results of cross-docking for our  model are presented in Table 1.
model are presented in Table 1.
Table 1.
Results of cross-docking for the model of  prepared from the 6WX4 complex
prepared from the 6WX4 complex
| PDB ID | Resolution, Å | Ligand ID |
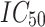 , ,  M M |
SOL score, kcal/mol | RMSD, Å |
|---|---|---|---|---|---|
| 3MJ5 | 2.63 | GRM | 0.32 |
 4.45 4.45 |
1.95 |
| 4OW0 | 2.1 | S88 | 0.15 |
 4.39 4.39 |
3.45 |
As shown in Table 1, docking with the prepared SARS-CoV-2
 model reproduces the bioactive conformation reasonably
well for one of the two known non-covalent inhibitors of
SARS-CoV-1
model reproduces the bioactive conformation reasonably
well for one of the two known non-covalent inhibitors of
SARS-CoV-1 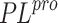 . In the case of the 4OW0 complex, the reason
for the failed docking may be that this inhibitor is bound to the
SARS-CoV-1
. In the case of the 4OW0 complex, the reason
for the failed docking may be that this inhibitor is bound to the
SARS-CoV-1 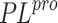 and its activity against SARS-CoV-2
and its activity against SARS-CoV-2
 is unknown. Based on this validation and limited
available data on known inhibitors, we used the model based on
6WX4 for our further virtual screening. Taking into account
extremely high worldwide scientific activity in the search for
inhibitors of SARS-CoV-2 target proteins, new structures of
SARS-CoV-2
is unknown. Based on this validation and limited
available data on known inhibitors, we used the model based on
6WX4 for our further virtual screening. Taking into account
extremely high worldwide scientific activity in the search for
inhibitors of SARS-CoV-2 target proteins, new structures of
SARS-CoV-2  complexes with non-covalent inhibitors will
appear soon in the Protein Data Bank, and they should be used for
further validation of the constructed here model and possibly its
modification.
complexes with non-covalent inhibitors will
appear soon in the Protein Data Bank, and they should be used for
further validation of the constructed here model and possibly its
modification.
Database and Ligand 3D Structure Preparation
In this study, the chemical library of Voronezh State University
(VGU) is selected for in silico screening. This library consists
of around 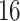 thousand off-the-shelf compounds having drug-like
and lead-like properties. The library has been successfully
exploited in several drug design projects in which kinase
inhibitors, thrombin inhibitors, factor Xa/XIa inhibitors, plant
growth stimulants were predicted and experimentally confirmed.
thousand off-the-shelf compounds having drug-like
and lead-like properties. The library has been successfully
exploited in several drug design projects in which kinase
inhibitors, thrombin inhibitors, factor Xa/XIa inhibitors, plant
growth stimulants were predicted and experimentally confirmed.
Preparation of the VGU library for docking includes two steps:
protonation at pH 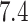 and conversion into 3D format. The first
step is performed with Chemaxon’s pKa module [18] capable of
predicting distribution of different charged microspecies for an
organic molecule at given pH. More technically, it calculates pKa
values of the molecule based on its partial charge distribution,
polarizability and inner H-bonds. In this study, a few charged
species for a ligand molecule are considered in our virtual
screening with keeping those which are at least
and conversion into 3D format. The first
step is performed with Chemaxon’s pKa module [18] capable of
predicting distribution of different charged microspecies for an
organic molecule at given pH. More technically, it calculates pKa
values of the molecule based on its partial charge distribution,
polarizability and inner H-bonds. In this study, a few charged
species for a ligand molecule are considered in our virtual
screening with keeping those which are at least  present at
a physiological pH. The second step, computation of 3D geometry,
is conducted by OpenBabel [19] with additional generation of
conformers for macrocycles (limit—no more than three conformers
for each charge state of molecule). The total size of the VGU
library prepared for screening is
present at
a physiological pH. The second step, computation of 3D geometry,
is conducted by OpenBabel [19] with additional generation of
conformers for macrocycles (limit—no more than three conformers
for each charge state of molecule). The total size of the VGU
library prepared for screening is  three-dimensional
molecular structures.
three-dimensional
molecular structures.
SOL Docking Program
SOL [2, 7, 20–22] is a classic docking program using a genetic algorithm for global optimization, and a pre-calculated grid of potentials describing the interactions of ligand atoms with a protein within the MMFF94 force field [23]. The target energy function undergoing global optimization is the sum of the energy of the ligand in the field of the protein and the ligand internal stress energy, the latter is also calculated within the MMFF94 force field. The desolvation effect upon ligand binding is taken into account in a simplified form of the generalized Born implicit solvent model. The best ligand position in the protein active site corresponds to the lowest value of the target energy function. This ligand position is found using a genetic algorithm that transforms an initial random population of ligand positions in a protein (a population of individuals) through a series of successive generations of the population, keeping the population size constant. The transition from the previous generation to the next is carried out by selecting individuals (ligand positions) with lowest values of the target energy function using the operations of crossover, mutations, elitism and niching [2, 20–22]. The position of the ligand in the protein active site with the lowest target energy function in the last generation is the solution to the problem of global optimization.
The default values for the most important parameters of the
genetic algorithm, population size and number of generations, are
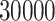 and
and 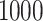 , respectively. Each independent run of this
algorithm requires from
, respectively. Each independent run of this
algorithm requires from 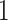 to a couple of dozens of minutes on
one computing core using the default parameters. The running time
of the algorithm depends both on the size of the ligand and on the
complexity of the active center of the protein. The Fig. 2 shows
two graphs of the dependence of the average time of SOL docking on
the number of ligand atoms for two different SARS-CoV-2 target
proteins
to a couple of dozens of minutes on
one computing core using the default parameters. The running time
of the algorithm depends both on the size of the ligand and on the
complexity of the active center of the protein. The Fig. 2 shows
two graphs of the dependence of the average time of SOL docking on
the number of ligand atoms for two different SARS-CoV-2 target
proteins 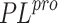 and
and  [12].
[12].
Fig. 2.

Dependence of the average SOL docking time on the number
of ligand atoms for two different SARS-CoV-2 target proteins
 and
and  .
.
The complexity of the ligand (the number of rotating groups) does
not affect the duration of the SOL calculations. The heuristic
nature of the genetic algorithm requires confirmation of
reliability of the obtained solution to the global optimization
problem. For this, 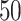 (by default) independent runs of the
algorithm from independent initial random populations are
performed and
(by default) independent runs of the
algorithm from independent initial random populations are
performed and 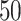 independent solutions, best ligand positions,
are obtained. These
independent solutions, best ligand positions,
are obtained. These 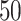 ligand positions are clustered, each
cluster contains ligand positions that differ from each other by
RMSD <
ligand positions are clustered, each
cluster contains ligand positions that differ from each other by
RMSD < 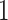 Å, where RMSD is the root-mean-square deviation
between two ligand positions calculated for all corresponding
ligand atoms. Docking reliability is high, if the population of
the cluster #
Å, where RMSD is the root-mean-square deviation
between two ligand positions calculated for all corresponding
ligand atoms. Docking reliability is high, if the population of
the cluster #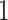 containing the ligand positions with the lowest
values of the target energy function is high enough. When
screening large database of ligands, we select for further
evaluation only ligands with a sufficiently negative docking score
and with a population of the first cluster of at least
containing the ligand positions with the lowest
values of the target energy function is high enough. When
screening large database of ligands, we select for further
evaluation only ligands with a sufficiently negative docking score
and with a population of the first cluster of at least 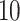 . The
docking score estimates the energy of protein-ligand binding. A
virtual screening of a large database of ligands was performed on
the Lomonosov-2 supercomputer of Lomonosov Moscow State
University [24].
. The
docking score estimates the energy of protein-ligand binding. A
virtual screening of a large database of ligands was performed on
the Lomonosov-2 supercomputer of Lomonosov Moscow State
University [24].
Protein-ligand Binding Enthalpy
After virtual screening using docking, best selected ligands are
subjected to quantum-chemical calculations. The enthalpy of
protein-ligand binding  is calculated
using following equation:
is calculated
using following equation:
 |
1 |
where 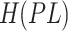 ,
,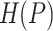 ,
,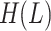 are values of the heat of formation of
the protein-ligand complex, protein and ligand, respectively.
These values are calculated by the MOPAC program [25] using the
PM7 semiempirical quantum-chemical method [26] and the COSMO
solvent model [27]. The PM7 method is a state-of-the-art
semiempirical method with dispersion and hydrogen and halogen bond
corrections, parameterized on an unprecedentedly broad molecular
data calculated using rigorous ab initio
quantum-chemical methods. Dispersion and hydrogen bonds are
extremely important for the correct description of intermolecular
as well as intramolecular interactions. The MOZYME module [28, 29]
implemented in MOPAC makes it possible to calculate such large
molecular systems as protein-ligand complexes containing many
thousands of atoms. The choice of the PM7 method with the COSMO
solvent model for calculating energies is also made because this
energy function demonstrates high docking accuracy [30–33].
are values of the heat of formation of
the protein-ligand complex, protein and ligand, respectively.
These values are calculated by the MOPAC program [25] using the
PM7 semiempirical quantum-chemical method [26] and the COSMO
solvent model [27]. The PM7 method is a state-of-the-art
semiempirical method with dispersion and hydrogen and halogen bond
corrections, parameterized on an unprecedentedly broad molecular
data calculated using rigorous ab initio
quantum-chemical methods. Dispersion and hydrogen bonds are
extremely important for the correct description of intermolecular
as well as intramolecular interactions. The MOZYME module [28, 29]
implemented in MOPAC makes it possible to calculate such large
molecular systems as protein-ligand complexes containing many
thousands of atoms. The choice of the PM7 method with the COSMO
solvent model for calculating energies is also made because this
energy function demonstrates high docking accuracy [30–33].
The 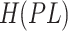 is calculated as follows. The position of the ligand
in the active site of the protein, found during docking, is used
as a starting point for local optimization of the PM7 energy of
the protein-ligand complex in vacuum using the
limited-memory Broyden–Fletcher–Goldfarb–Shanno method in
MOPAC. The Cartesian coordinates of all ligand atoms are varied
while the positions of all protein atoms are unchanged. In the
optimized geometry, the energy is calculated using PM7 again, but
with COSMO solvent. The heat of formation of the unbound ligand
is calculated as follows. The position of the ligand
in the active site of the protein, found during docking, is used
as a starting point for local optimization of the PM7 energy of
the protein-ligand complex in vacuum using the
limited-memory Broyden–Fletcher–Goldfarb–Shanno method in
MOPAC. The Cartesian coordinates of all ligand atoms are varied
while the positions of all protein atoms are unchanged. In the
optimized geometry, the energy is calculated using PM7 again, but
with COSMO solvent. The heat of formation of the unbound ligand
 is found using the following procedure. Several low energy
ligand conformations are generated using the Open Babel
program [19], and then the energies of these conformations are
optimized by the PM7 method in vacuum by changing the Cartesian
coordinates of all ligand atoms, the heats of formation of the
optimized conformations are recalculated using the COSMO solvent
model, and the most negative of them is used in the equation (1)
as
is found using the following procedure. Several low energy
ligand conformations are generated using the Open Babel
program [19], and then the energies of these conformations are
optimized by the PM7 method in vacuum by changing the Cartesian
coordinates of all ligand atoms, the heats of formation of the
optimized conformations are recalculated using the COSMO solvent
model, and the most negative of them is used in the equation (1)
as  . The heat of formation of the unbound protein is
calculated by PM7+COSMO without energy optimization for the same
protein conformation used in docking.
. The heat of formation of the unbound protein is
calculated by PM7+COSMO without energy optimization for the same
protein conformation used in docking.
RESULTS AND DISCUSSION
After virtual screening of all molecular structures of the VGU
library only  ligands demonstrated reasonable values of the
SOL docking score <
ligands demonstrated reasonable values of the
SOL docking score < 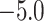 kcal/mol. Only for these ligands the
enthalpy of protein-ligand binding is calculated. As a result,
kcal/mol. Only for these ligands the
enthalpy of protein-ligand binding is calculated. As a result,
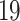 compounds have been selected for the experimental testing.
They are presented in Table 2. Two-dimensional structures of
representatives of these compounds are shown in Fig. 2, and their
analogues are listed in Table 2. SOL score is the estimation of
the free energy of protein-ligand binding calculated by the SOL
docking program.
compounds have been selected for the experimental testing.
They are presented in Table 2. Two-dimensional structures of
representatives of these compounds are shown in Fig. 2, and their
analogues are listed in Table 2. SOL score is the estimation of
the free energy of protein-ligand binding calculated by the SOL
docking program.  is the enthalpy of
protein-ligand binding calculated by the equation (1).
is the enthalpy of
protein-ligand binding calculated by the equation (1).
Table 2.
Organic compounds candidates to become inhibitors of the
SARS-CoV-2 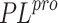 selected from the VGU database
selected from the VGU database
| Name of VGU compound | SOL score, kcal/mol |
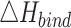 by PM7, kcal/mol by PM7, kcal/mol |
Structure |
|---|---|---|---|
| 200513 |
 6.81 6.81 |
 65.24 65.24 |
Analog of 119636 |
| 119636 |
 6.73 6.73 |
 63.02 63.02 |
See Fig. 3 |
| 201630 |
 6.59 6.59 |
 61.98 61.98 |
Analog of 119636 |
| 114162 |
 5.73 5.73 |
 60.43 60.43 |
Analog of 119636 |
| 201174 |
 6.23 6.23 |
 59.85 59.85 |
Analog of 114162 |
| 225718 |
 5.78 5.78 |
 53.41 53.41 |
Analog of 114162 |
| 100545 |
 5.51 5.51 |
 46.04 46.04 |
Analog of 114162 |
| 15630 |
 5.64 5.64 |
 45.69 45.69 |
See Fig. 3 |
| 40858 |
 5.66 5.66 |
 45.54 45.54 |
See Fig. 3 |
| 15108 |
 5.51 5.51 |
 42.78 42.78 |
See Fig. 3 |
| 39544 |
 5.81 5.81 |
 41.94 41.94 |
See Fig. 3 |
| 214136 |
 5.88 5.88 |
 37.84 37.84 |
See Fig. 3 |
| 127620 |
 5.96 5.96 |
 58.81 58.81 |
Analog of 40858 |
| 114161 |
 5.74 5.74 |
 58.43 58.43 |
See Fig. 3 |
| 40863 |
 5.80 5.80 |
 34.67 34.67 |
Analog of 40858 |
| 17513 |
 5.79 5.79 |
 34.55 34.55 |
See Fig. 3 |
| 214132 |
 6.03 6.03 |
 34.01 34.01 |
Analog of 214136 |
| 33372 |
 5.65 5.65 |
 25.98 25.98 |
See Fig. 3 |
| 16189 |
 5.87 5.87 |
 25.01 25.01 |
See Fig. 3 |
As can be seen in Fig. 3, the selected molecules do not contain
warhead groups. These are reactive groups of atoms that are
capable of forming covalent bonds with some protein residues. This
highlights that we have mainly found non-covalent inhibitors of
SARS-CoV-2  here. One exception is 17513 which
is a derivative of dithiocarboxylic acid and its mercapto part can
serve as a reactive group.
here. One exception is 17513 which
is a derivative of dithiocarboxylic acid and its mercapto part can
serve as a reactive group.
Fig. 3.
Structures of compounds selected as  inhibitors
for in vitro experimental test (see Table 2).
inhibitors
for in vitro experimental test (see Table 2).
The known non-covalent inhibitors of SARS-CoV  and
SARS-CoV-2
and
SARS-CoV-2  , for which crystal complexes are resolved,
are related to derivatives of naphthalenylmethylpiperidine. This
moiety is not found among our top compounds. However, the common
pharmacophore pattern resembling naphthalenylmethylpiperidine "—
a positively charged nitrogen in proximity with an aromatic
fragment can be found for 15108, 16189,
39544, 40858, 40863, 214132,
214136, 225718. This finding underpins the
importance of negatively charged
, for which crystal complexes are resolved,
are related to derivatives of naphthalenylmethylpiperidine. This
moiety is not found among our top compounds. However, the common
pharmacophore pattern resembling naphthalenylmethylpiperidine "—
a positively charged nitrogen in proximity with an aromatic
fragment can be found for 15108, 16189,
39544, 40858, 40863, 214132,
214136, 225718. This finding underpins the
importance of negatively charged  and a few aromatic
residues (
and a few aromatic
residues ( ,
,  ,
, 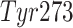 ) for ligand binding. We can
also note that the benzodioxole fragment found for one of known
inhibitors of SARS-CoV
) for ligand binding. We can
also note that the benzodioxole fragment found for one of known
inhibitors of SARS-CoV 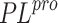 (the native ligand from 3MJ5) is
also present in 15108.
(the native ligand from 3MJ5) is
also present in 15108.
CONCLUSIONS
The virtual screening of a drug-like database of organic compounds is performed using a two-step procedure. First, the SOL program is used to select most promising ligands on the base of their SOL score. Second, the enthalpy of protein-ligand binding is computed for these selected ligand using the quantum-chemical semiempirical PM7 method with the COSMO implicit solvent model. The most promising candidates 19 compounds have been submitted for experimental determination of their inhibitory activity against the SARS-CoV-2 papain-like protease.
FUNDING
The work is supported by Russian Ministry of Science and Higher Education, agreement no. 075-15-2019-1621. The research is carried out using the equipment of the shared research facilities of HPC computing resources at Lomonosov Moscow State University.
Footnotes
(Submitted by E. E. Tyrtyshnikov)
Contributor Information
A. V. Sulimov, Email: sulimovv@mail.ru
I. S. Ilin, Email: ilyinivan2711@gmail.com
D. C. Kutov, Email: dk@dimonta.com
N. V. Stolpovskaya, Email: gusnv@yandex.ru
Kh. S. Shikhaliev, Email: shikh1961@yandex.ru
V. B. Sulimov, Email: vladimir.sulimov@gmail.com
REFERENCES
- 1.Sadovnichii V. A., Sulimov V. B. Supercomputing Technologies in Science, Education, and Industry. Moscow: Mosk. Gos. Univ.; 2009. [Google Scholar]
- 2.Sulimov V. B., Sulimov A. V. Docking: Molecular Modeling for Drug Discovery. Moscow: AINTELL; 2017. [Google Scholar]
- 3.Gil C., Ginex T., Maestro I., Nozal V., Barrado-Gil L., Cuesta-Geijo M. A., Urquiza J., Ramirez D., Alonso C., Campillo N. E., Martinez A. COVID-19: Drug targets and potential treatments. J. Med. Chem. 2020;63:12359–12386. doi: 10.1021/acs.jmedchem.0c00606. [DOI] [PubMed] [Google Scholar]
- 4.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.L. R. Silva, P. F. da Silva Santos-Junior, J. de Andrade Brandao, L. Anderson, E. J. Bassi, J. Xavier de Araujo-Junior, S. H. Cardoso, and E. F. da Silva-Junior, ‘‘Druggable targets from coronaviruses for designing new antiviral drugs,’’ Bioorg. Med. Chem. 28, 115745 (2020). [DOI] [PMC free article] [PubMed]
- 6.Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H., Shindyalov I. N., Bourne P. E. The protein data bank. Nucl. Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sulimov V. B., Kutov D. C., Sulimov A. V. Advances in docking. Curr. Med. Chem. 2019;26:7555–7580. doi: 10.2174/0929867325666180904115000. [DOI] [PubMed] [Google Scholar]
- 8.Sulimov V. B., Kutov D. C., Taschilova A. S., Ilin I. S., Tyrtyshnikov E. E., Sulimov A. V. Docking paradigm in drug design. Curr. Top. Med. Chem. 2021;21:1–47. doi: 10.2174/1568026620666201207095626. [DOI] [PubMed] [Google Scholar]
- 9.Sulimov A. V., Kutov D. C., Sulimov V. B. Supercomputer docking. Supercomput. Front. Innov. 2019;6:26–50. [Google Scholar]
- 10.Y. Wu, Z. Li, Y.-S. Zhao, Y.-Y. Huang, M.-Y. Jiang, and H.-B. Luo, ‘‘Therapeutic targets and potential agents for the treatment of COVID-19,’’ Med. Res. Rev. (2021). [DOI] [PubMed]
- 11.Choudhry N., Zhao X., Xu D., Zanin M., Chen W., Yang Z., Chen J. Chinese therapeutic strategy for fighting COVID-19 and potential small-molecule inhibitors against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J. Med. Chem. 2020;63:13205–13227. doi: 10.1021/acs.jmedchem.0c00626. [DOI] [PubMed] [Google Scholar]
- 12.Sulimov A. V., Kutov D. C., Taschilova A. S., Ilin I. S., Stolpovskaya N. V., Shikhaliev K. S., Sulimov V. B. In search of non-covalent inhibitors of SARS-CoV-2 main protease: Computer aided drug design using docking and quantum chemistry. Supercomput. Front. Innov. 2020;7:41–56. [Google Scholar]
- 13.R. Cannalire, C. Cerchia, A. R. Beccari, F. S. di Leva, and V. Summa, ‘‘Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: State of the art and future opportunities,’’ J. Med. Chem. (2020). [DOI] [PMC free article] [PubMed]
- 14.W. Rut, Z. Lv, M. Zmudzinski, S. Patchett, D. Nayak, S. J. Snipas, F. El Oualid, T. T. Huang, M. Bekes, M. Drag, and S. K. Olsen, ‘‘Activity profiling and crystal structures of inhibitor-bound SARS-CoV-2 papain-like protease: A framework for anti-COVID-19 drug design,’’ Sci. Adv. 6 (42), eabd4596 (2020). [DOI] [PMC free article] [PubMed]
- 15.Kutov D. C., Katkova E. V., Kondakova O. A., Sulimov A. V., Sulimov V. B. “Influence of the method of hydrogen atoms incorporation into the target protein on the protein-ligand binding energy,” Vestn. Yuzh.-Ural. Univ. Ser. Mat. Model. Programm. 2017;10:94–107. [Google Scholar]
- 16.Ghosh A. K., Takayama J., Rao K. V., Ratia K., Chaudhuri R., Mulhearn D. C., Lee H., Nichols D. B., Baliji S., Baker S. C., Johnson M. E., Mesecar A. D. Severe acute respiratory syndrome coronavirus papain-like novel protease inhibitors: Design, synthesis, protein-ligand X-ray structure and biological evaluation. J. Med. Chem. 2010;53:4968–4979. doi: 10.1021/jm1004489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baez-Santos Y. M., Barraza S. J., Wilson M. W., Agius M. P., Mielech A. M., Davis N. M., Baker S. C., Larsen S. D., Mesecar A. D. X-ray structural and biological evaluation of a series of potent and highly selective inhibitors of human coronavirus papain-like proteases. J. Med. Chem. 2014;57:2393–2412. doi: 10.1021/jm401712t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ChemAxonsoftware. http://www.chemaxon.com. Accessed 2021.
- 19.O’Boyle N. M., Banck M., James C. A., Morley C., Vandermeersch T., Hutchison G. R. Open Babel: An open chemical toolbox. J. Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romanov A. N., Kondakova O. A., Grigoriev F. V., Sulimov A. V., Luschekina S. V., Martynov Y. B., Sulimov V. B. The SOL docking package for computer-aided drug design. Chisl. Metody Programmir. 2008;9:213–233. [Google Scholar]
- 21.Sulimov A. V., Kutov D. C., Oferkin I. V., Katkova E. V., Sulimov V. B. Application of the docking program SOL for CSAR benchmark. J. Chem. Inf. Model. 2013;53:1946–1956. doi: 10.1021/ci400094h. [DOI] [PubMed] [Google Scholar]
- 22.Sulimov V. B., Ilin I. S., Kutov D. C., Sulimov A. V. “Development of docking programs for Lomonosov supercomputer,” J. Turk. Chem. Soc. Sect. A: Chem. 2020;7:259–276. [Google Scholar]
- 23.Halgren T. A. Merck molecular force field. J. Comput. Chem. 1996;17:490–641. doi: 10.1002/(SICI)1096-987X(199604)17:5/6<490::AID-JCC1>3.0.CO;2-P. [DOI] [Google Scholar]
- 24.Voevodin V. V., Antonov A. S., Nikitenko D. A., Shvets P. A., Sobolev S. I., Sidorov I. Y., Stefanov K. S., Voevodin V. V., Zhumatiy S. A. Supercomputer Lomonosov-2: Large scale, deep monitoring and fine analytics for the user community. Supercomput. Front. Innov. 2019;6:4–11. [Google Scholar]
- 25.J. J. P. Stewart, Stewart Computational Chemistry. MOPAC2016 (Colorado Springs, CO, 2016).
-
26.Stewart J. J. Optimization of parameters for semiempirical methods
 J. Mol. Model. 2013;19:1–32. doi: 10.1007/s00894-012-1667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
J. Mol. Model. 2013;19:1–32. doi: 10.1007/s00894-012-1667-x. [DOI] [PMC free article] [PubMed] [Google Scholar] - 27.Klamt A., Schuurmann G. COSMO: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 1993;2:799–805. doi: 10.1039/P29930000799. [DOI] [Google Scholar]
- 28.Stewart J. J. P. Application of localized molecular orbitals to the solution of semiempirical self-consistent field equations. Int. J. Quantum Chem. 1996;58:133–146. doi: 10.1002/(SICI)1097-461X(1996)58:2<133::AID-QUA2>3.0.CO;2-Z. [DOI] [Google Scholar]
- 29.Bikadi Z., Hazai E. Application of the PM6 semi-empirical method to modeling proteins enhances docking accuracy of AutoDock. J. Cheminform. 2009;1:15. doi: 10.1186/1758-2946-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.I. V. Oferkin, E. V. Katkova, A. V. Sulimov, D. C. Kutov, S. I. Sobolev, V. V. Voevodin, and V. B. Sulimov, ‘‘Evaluation of docking target functions by the comprehensive investigation of protein-ligand energy minima,’’ Adv. Bioinform. 2015, 126858 (2015). [DOI] [PMC free article] [PubMed]
- 31.Pecina A., Meier R., Fanfrlik J., Lepsik M., Rezac J., Hobza P., Baldauf C. The SQM/COSMO filter: Reliable native pose identification based on the quantum-mechanical description of protein-ligand interactions and implicit COSMO solvation. Chem. Commun. 2016;52:3312–3315. doi: 10.1039/C5CC09499B. [DOI] [PubMed] [Google Scholar]
- 32.Sulimov A. V., Kutov D. C., Katkova E. V., Ilin I. S., Sulimov V. B. New generation of docking programs: Supercomputer validation of force fields and quantum-chemical methods for docking. J. Mol. Graph. Model. 2017;78:139–147. doi: 10.1016/j.jmgm.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 33.A. V. Sulimov, D. C. Kutov, A. K. Gribkova, I. S. Ilin, A. S. Tashchilova, and V. B. Sulimov, ‘‘Search for approaches to supercomputer quantum-chemical docking,’’ in Proceedings of the 5th Russian Supercomputer Days, RuSCDays 2019, Ed. by V. Voevodin and S. Sobolev (Springer, Cham, 2019), pp. 363–378.



