Abstract
BACKGROUND
Previous systematic reviews have indicated that exercise-based cardiac rehabilitation (ExCR) for patients with heart failure (HF) has a beneficial effect on health-related quality-of-life (HRQoL) and exercise capacity. However, there is uncertainty regarding potential differential effects of ExCR across HF patient subgroups.
OBJECTIVES
The authors sought to undertake an individual participant data (IPD) meta-analysis to: 1) assess the impact of ExCR on HRQoL and exercise capacity in patients with HF; and 2) investigate differential effects of ExCR according to a range of patient characteristics: age, sex, ethnicity, New York Heart Association functional class, ischemic etiology, ejection fraction, and exercise capacity.
METHODS
A single dataset was produced, comprising randomized trials where ExCR (delivered for 3 weeks or more) was compared with a no exercise control group. Each trial provided IPD on HRQoL or exercise capacity (or both), with follow-up of 6 months or more. One- and 2-stage meta-analysis models were used to investigate the effect of ExCR overall and the interactions between ExCR and participant characteristics.
RESULTS
IPD was obtained from 13 trials for 3,990 patients, predominantly (97%) with reduced ejection fraction HF. Compared with the control group, there was a statistically significant difference in favor of ExCR for HRQoL and exercise capacity. At 12-month follow-up, improvements were seen in 6-min walk test (mean 21.0 m; 95% confidence interval: 1.57 to 40.4 m; p = 0.034) and Minnesota Living With HF score (mean improvement 5.9; 95% confidence interval: 1.0 to 10.9; p = 0.018). No consistent evidence was found of differential intervention effects across patient subgroups.
CONCLUSIONS
These results, based on an IPD meta-analysis of randomized trials, confirm the benefit of ExCR on HRQoL and exercise capacity and support the Class I recommendation of current international clinical guidelines that ExCR should be offered to all HF patients. (Exercise Training for Chronic Heart Failure [ExTraMATCH II]: protocol for an individual participant data meta-analysis; PROSPERO: international database of systematic reviews CRD42014007170).
Keywords: exercise capacity, heart failure, MLHFQ, QoL, quality-of-life, rehabilitation
Heart failure (HF) is a major public health problem with substantial morbidity and mortality and is a burden to patients and health systems (1). Whereas survival after HF diagnosis has improved, prognosis remains poor; 30% to 40% of patients die within a year of diagnosis (2). Patients living with HF experience marked reductions in their exercise capacity, which has detrimental effects on their health-related quality-of-life (HRQoL).
With increasing numbers of people living longer with symptomatic HF, the effectiveness and accessibility of health services for HF patients have never been more important. Exercise-based cardiac rehabilitation (ExCR) is widely recommended in clinical guidelines as integral to the comprehensive care of HF patients (3–7). ExCR is a process by which patients, in partnership with health professionals, are encouraged and supported to achieve and maintain optimal physical health (3). In addition to exercise training, it is now accepted that ExCR programs should be comprehensive and include education and psychological care, as well as including advice on health and lifestyle behavior change (3,4).
Systematic reviews and trial-level data meta-analyses have shown ExCR offers important health benefits for HF patients compared with control patients (8–10). On the basis of data from 26 randomized trials with a median follow-up of 12.4 months, Uddin et al. (9) reported a mean improvement in peak oxygen uptake (peak Vo2) of 2.79 ml/kg/min (95% confidence interval [CI]: 2.05 to 3.53 ml/kg/min) following ExCR. The 2014 Cochrane review reported a clinically important improvement across 13 random controlled trials in disease-specific HRQoL as assessed by the Minnesota Living With Heart Failure Questionnaire (MLHFQ) up to 12-month follow-up (mean score −5.8 points; 95% CI: −9.2 to −2.4 points) compared with control patients (8). Using meta-regression analysis, these meta-analyses found no association between trial-level patient characteristics (age, sex, ejection fraction) and ExCR on either exercise capacity or HRQoL. However, such analyses are highly prone to study-level confounding (ecological fallacy) and should be interpreted with great caution. Uncertainty, therefore, remains as to whether there are differential effects of ExCR on exercise capacity and HRQoL across HF patient subgroups (11). Individual participant data (IPD) meta-analysis is increasingly being recognized as the gold standard approach for assessing intervention subgroup effects (11,12). Although a previous IPD meta-analysis (ExTraMATCH [Exercise Training Meta-Analysis of Trials in Chronic Heart Failure]) reported the impact of ExCR on clinical events (death and hospitalization), it did not consider the outcomes of exercise capacity or HRQoL (13).
Using IPD meta-analysis, this ExTraMATCH II study aimed to assess the impact of ExCR on HRQoL and exercise capacity, and to investigate differential effects of ExCR across subgroups of patients with HF.
METHODS
This study was conducted and reported in accordance with the Preferred Reporting Items for a Systematic Review and Meta-analysis of Individual Participant Data (PRISMA-IPD) statement and current guidance on the use of IPD (14,15). Our full study protocol has been published elsewhere and is registered on the PROSPERO database of systematic review protocols (CRD42014007170) (16,17). The clinical events results have been published elsewhere (18).
SEARCH STRATEGY AND SELECTION CRITERIA.
Trials were identified from the original ExTraMATCH IPD meta-analysis carried out in 2004 and updated with trials identified in the 2014 Cochrane systematic review of ExCR for HF (8,13). The Cochrane review searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library, EMBASE, MEDLINE, CINAHL, PsycINFO, and the NHS Centre for Reviews and Dissemination (CRD). Conference proceedings were searched on Web of Science. Trial registers (Controlled-trials.com and Clinicaltrials.gov) and reference lists of all eligible trials and identified systematic reviews were also checked. No language limitations were imposed. Details of the search strategy used are reported elsewhere (16,17).
Trials were included if they met the following criteria: 1) randomized trials of adult patients (18 years of age and older) with a diagnosis of HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF) based on objective assessment of left ventricular ejection fraction and clinical findings; 2) ExCR intervention that delivered an aerobic exercise training component involving the lower limbs, lasting a minimum of 3 weeks, either alone or as part of a comprehensive cardiac rehabilitation program (which may also include health education and/or a psychological intervention); 3) a comparator arm that did not prescribe an exercise intervention; 4) a minimum follow-up of 6 months; and 5) and a sample size of more than 50 (to ensure that the logistical effort in obtaining, cleaning, and organizing the data was commensurate with the contribution of the dataset to the analysis) (19,20).
DATA MANAGEMENT.
Principal investigators of studies were invited by e-mail to participate in this IPD meta-analysis and share their anonymized trial data. Patients in the clinical trials providing data gave their consent on entry to the original clinical trial. All included datasets had ethical approval and consent from their sponsors; they were not required to seek additional ethical approval for the inclusion of their data in this analysis. The complete list of all requested variables and details on collaboration with principal investigators are reported in the study protocol (8). Data from each trial were checked on range, extreme values, internal consistency, missing values, and consistency with published reports. Trial investigators were contacted about data discrepancies or missing information. Each anonymized dataset was saved in its original format and then converted and combined into 1 overall master dataset. All files were stored on a secure, password-protected computer server managed and in accordance with the data management standard operating procedures of Exeter Clinical Trials Unit, a U.K. Clinical Research Collaboration (UKCRC) registered clinical trials unit. Access to data at all stages of cleaning and analysis was restricted to the Exeter research team (R.S.T., S.W., F.C.W., and O.C.).
SPECIFICATION OF OUTCOMES, SUBGROUPS, AND RISK OF BIAS ASSESSMENT.
HRQoL and exercise capacity data were obtained from trial investigators at the patient level. HRQoL was recorded as 1 of 3 validated measures: 1) Minnesota Living with Heart Failure Questionnaire (MLHFQ) (21); 2) Kansas City Cardiomyopathy Questionnaire (22); and 3) Guyatt Chronic Heart Failure scale (23). The first analysis was performed using only MLHFQ data; the second analysis used a standardized score calculated from any of the 3 aforementioned measures. Because MLHFQ reports higher HRQoL as a lower score, the scales of the Kansas City Cardiomyopathy Questionnaire and Guyatt Heart Failure score (which report higher HRQoL as a higher score) were reversed before standardizing so that the directionality would be the same as MLHFQ. Therefore, for both the MLHFQ score and standardized HRQoL score, an improvement in HRQoL is shown by a reduction in the overall score.
Exercise capacity was recorded as 1 of 4 validated exercise capacity measures: 1) peak Vo2 (ml/kg/min); 2) distance (meters) walked in a 6-min walk test (6MWT); 3) distance (meters) walked in an incremental shuttle walk test; and 4) cycle ergometer Watts. Two of these measures, peak Vo2 and 6MWT, were analyzed as separate outcomes. A third outcome, a standardized exercise capacity score for patients with any validated exercise capacity measure, was also analyzed. The large HF-ACTION (Exercise Training Program to Improve Clinical Outcomes in Individuals With Congestive Heart Failure) trial (24) provided data on both peak Vo2 and 6MWT, and was included in all analyses, with the peak Vo2 measure taking precedence for the standardized exercise capacity score.
We also sought IPD on the following pre-defined subgroups: age, sex, ejection fraction (HFpEF [≥45% ejection fraction] vs. HFrEF [<45% ejection fraction]), New York Heart Association (NYHA) functional class, HF etiology (ischemic vs. nonischemic), ethnicity (white vs. nonwhite), and baseline exercise capacity. Study quality and risk of bias were assessed using the TESTEX quality assessment tool (25).
STATISTICAL ANALYSIS.
A detailed statistical analysis plan was prepared (available from the authors). All analyses were carried out according to the principle of intention to treat (i.e., patients analyzed as randomized) and included all patients providing the data required for each model. All 1-stage and 2-stage analyses used random effects models as the overall dataset is likely to include a high degree of clinical heterogeneity across the individual trials due to differences in population, exercise-based rehabilitation intervention, and comparator intervention (26). All results are reported as a between-group mean difference (ExCR-control) with a 95% CI and p value.
The primary analyses comprised 1-stage and 2-stage IPD meta-analyses carried out at 2 follow-up times: 6 and 12 months. For all analyses, we used the observation at, or closest before, the analysis time. Using this criterion, more trials had available data at 12-month follow-up than at 6-month follow-up. Therefore, we have regarded the 12-month data analyses as being the primary analyses. The results at 12-month follow-up are reported ahead of the 6-month results in order to optimize the number of trials included.
One-stage IPD models used a hierarchical random effects regression model, adjusted for the baseline value of the outcome measure. We ran a series of models to estimate the overall treatment effect and to investigate potential interactions between ExCR and pre-defined patient subgroups (age, sex, left ventricular ejection fraction [<45% or ≥45%], heart failure etiology [ischemic vs. nonischemic], NYHA functional class [I/II vs. III/IV], and baseline exercise capacity [16,17]). Each model investigated 1 interaction effect only. We used 2-stage random effects models as a sensitivity analysis to estimate the effect of ExCR. The τ2 and I2 statistics were reported alongside the associated p value for the results of the main analyses.
The secondary analyses used a random effects hierarchical model that took account of the repeated measurement of the outcome (HRQoL or exercise capacity) over the duration of each trial. These models used outcome data at all available time points. Adjustments for baseline values of the outcome measure were made; no other covariates were included in the model. This model included a time by treatment interaction term.
To test the robustness of the primary analyses, prespecified sensitivity analyses were carried out. First, each primary analysis was repeated after exclusion of the largest trial, the HF-ACTION study (24). Second, aggregate data from studies that did not provide IPD was added and the impact on meta-analysis conclusions assessed. We checked for potential small-study bias by assessing funnel plot asymmetry and using the Egger test (27). Additional plots of the results of the 1-stage IPD meta-analysis models, stratified by patient characteristics, are presented in order to give the reader a visual representation of the differential effect of ExCR in each subgroup. All analyses were undertaken using Stata version 14.2 software (Stata-Corp, College Station, Texas).
RESULTS
SELECTION AND INCLUSION OF STUDIES.
Of the 23 trials identified either in the ExTraMATCH IPD meta-analysis (13) or the 2014 Cochrane systematic review of ExCR for HF (8,16), we were unable to include data from 3 trials (n = 355): for 2 trials, data were no longer available (28,29), and the investigators of the third trial could not be contacted (30).
Of the 20 trials remaining, 1 trial (31) was excluded due to an overlap between patients included in another identified trial (32). Thirteen studies provided anonymized IPD for analysis of HRQoL and exercise capacity outcomes (24,32–43). Published trial-level data were available for an additional 5 trials for each of the HRQoL (28,29,44–46) and exercise capacity analyses (28–30,44,45). In addition to comparing usual care to an intervention arm of usual care plus ExCR, Gary et al. (35) also compared the effects of cognitive behavior therapy to cognitive behavior therapy plus ExCR. For the purpose of analysis from this point forward, this will be described as 1 trial providing 2 comparators and be analyzed as separate trials from this point forward.
For the HRQoL analysis, 9 trials (including 10 comparator groups) provided data for 3,000 patients (1,496 ExCR, 1,504 control) with a median follow-up of 33 weeks (24,34,35,38–43). For the exercise capacity analysis, 13 trials (14 comparator groups) provided 3,332 patients (1,662 ExCR, 1,670 control) with a median follow-up of 26 weeks (24,32–43). Figure 1 summarizes the study selection process.
FIGURE 1. PRISMA-IPD Flow Diagram.
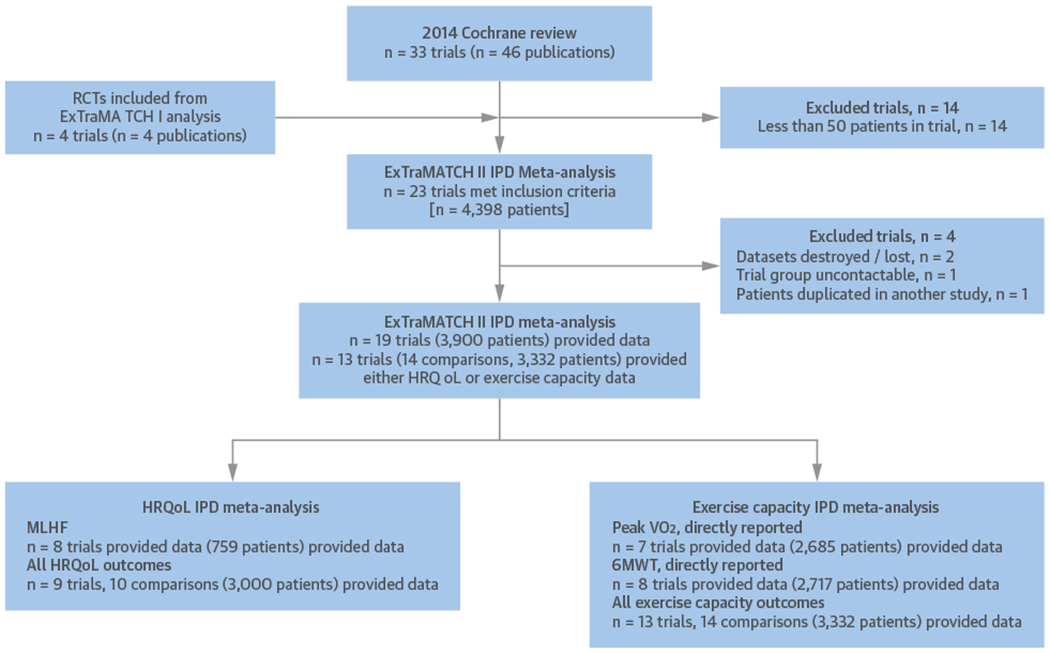
A PRISMA-IPD (Preferred Reporting Items for Systematic Reviews and Meta-Analyses of individual Participant Data) flow diagram to show selection and synthesis of ExTraMATCH (Exercise Training for Chronic Heart Failure) II study data. 6MWT = 6-min walk test; HRQoL = health-related quality-of-life; MLHF = Minnesota Living with Heart Failure Questionnaire; peak Vo2 = peak oxygen uptake; RCT = randomized controlled trial.
STUDY, PATIENT, AND TRIAL CHARACTERISTICS.
Patient baseline characteristics were well balanced between ExCR and control patients (Table 1). The majority of patients were male (73%) with a mean age of 61 years. The mean baseline left-ventricular ejection fraction was 27%; fewer than 3% of patients had preserved ejection fraction heart failure (defined as ejection fraction >45%). Most patients were in NYHA functional class II (62%) or III (36%). Studies were published between 2000 and 2012 across Europe and North America. Sample size ranged from 50 to 2,130 patients. All trials evaluated an aerobic exercise intervention; 4 also included resistance training (34,38,40,41). Four trials (5 comparators) were conducted in an exclusively home-based setting (34,35,38,43); all other trials delivered ExCR in a center-based setting. The “dose” of exercise training varied across studies; average session duration ranged from 15 to 60 min (including warmup and cooldown); minimum number of sessions per week was 2, with a maximum of 7; exercise intensity equivalent ranged from 40% to 70% peak Vo2; and the duration of intervention ranged from 4 to 120 weeks (Table 2).
TABLE 1.
Baseline Characteristics of Patients
| ExCR (n = 1,662) |
Control (n = 1,670) |
All (N = 3,332) |
|
|---|---|---|---|
| Age, yrs | 60.9 ± 13.2 | 61.2 ± 13.5 | 61.1 ± 13.4 |
| Sex | |||
| Male | 1,187 (71.4) | 1,237 (74.1) | 2,424 (72.8) |
| Female | 475 (28.6) | 433 (25.9) | 908 (27.3) |
| Baseline ejection fraction, % | 27.0 ± 8.8 | 26.9 ± 8.7 | 26.9 ± 8.8 |
| HFrEF <45% | 1,721 (96.8) | 1,744 (97.5) | 3,465 (97.1) |
| HFpEF ≥45% | 57 (3.2) | 45 (2.5) | 102 (2.9) |
| NYHA functional status | |||
| Class I | 20 (1.2) | 25 (1.5) | 45 (1.4) |
| Class II | 1,002 (61.2) | 1,032 (62.8) | 2,034 (62.0) |
| Class III | 597 (36.5) | 569 (34.6) | 1,166 (35.5) |
| Class IV | 19 (1.2) | 18 (1.1) | 37 (1.1) |
| Etiology | |||
| Ischemic | 892 (54.9) | 884 (54.1) | 1,776 (54.5) |
| Nonischemic | 732 (45.1) | 750 (45.9) | 1,482 (45.5) |
| Ethnicity | |||
| White | 1,085 (69.3) | 1,117 (70.9) | 2,202 (70.1) |
| Nonwhite | 480 (30.7) | 458 (29.1) | 938 (30.0) |
| MLHFQ | 35.6 ± 23.7 | 33.6 ± 25.6 | 34.6 ± 24.7 |
| Peak Vo2, ml/kg/min | 15.0 ± 4.5 | 15.1 ± 4.7 | 15.0 ± 4.6 |
| 6MWT, m | 362.6 ± 109.3 | 362.5 ± 112.1 | 362.6 ± 110.7 |
Values are mean ± SD or n (%).
6MWT = 6-min walk test; EF = ejection fraction; ExCR = exercise-based cardiac rehabilitation; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; MLHFQ = Minnesota Living With Heart Failure Questionnaire; NYHA = New York Heart Association; Vo2 = oxygen uptake.
TABLE 2.
Characteristics of Included Studies and Interventions (14 Comparator Studies)
| Study characteristics | |
|---|---|
| Publication year | |
| 1990 to 1999 | 0 (0) |
| 2000 to 2009 | 9 (64) |
| 2010 to 2012 | 5(36) |
| Unpublished | 0 (0) |
| Main study location | |
| Europe | 9 (64) |
| North America* | 5(36) |
| Single-study center | |
| Single | 10 (71) |
| Multiple | 4(29) |
| Sample size | |
| 0 to 99 | 8 (57) |
| 100 to 999 | 5(36) |
| ≥1,000 | 1 (7) |
| Duration of latest follow-up, weeks | |
| HRQoL outcomes | 33 (26-104) |
| Exercise capacity outcomes | 26 (9-520) |
| Intervention characteristics | |
| Intervention type | |
| Exercise-only programs | 9 (64) |
| Comprehensive programs | 5(36) |
| Type of exercise | |
| Aerobic exercise only | 10 (71) |
| Aerobic plus resistance training | 4(29) |
| Dose of intervention | |
| Duration of intervention, weeks | 24 (4-120) |
| Frequency, sessions per week | 3 (2-7) |
| Length of exercise session, min | 30 (15-60) |
| Exercise intensity, range | 40% to 70% peak Vo2 11 to 15 Borg rating |
| Setting | |
| Center-based only | 9 (64) |
| Home-based only | 5(36) |
Values are n (%), median (range), or range.
HF-ACTION study (24) was categorized as North America but was also delivered to a small number of patients in France.
HRQoL = health-related quality-of-life; peak Vo2 = peak oxygen uptake.
QUALITY OF INCLUDED TRIALS.
The overall quality of included trials was judged to be moderate to good, with a median TESTEX (25) score of 11 (range 9 to 14) of a maximum score of 15 (Online Table 1). The criteria of allocation concealment and physical activity monitoring in the control groups were met in only 2 (24,38) and 3 studies (24,34,42), respectively. The other TESTEX criteria were each met in at least 50% of trials.
EFFECT OF INTERVENTION ON OUTCOMES.
One-stage meta-analysis showed a significant improvement in HRQoL for those on the ExCR intervention compared with control, as assessed by the MLHFQ, at 12-month follow-up: (mean improvement 5.9; 95% CI: 1.0 to 10.9; p = 0.018; τ2 = 77; I2 = 88%) (Online Table 2) and standardized HRQoL score (mean improvement 0.20 SD units; 95% CI: 0.03 to 0.37; p = 0.020; τ2 = 0.07; I2 = 85%) (Online Table 3). Similar results were seen at 6-month follow-up. Two-stage meta-analysis results were comparable and are presented graphically for 12-month follow-up (Central Illustration, Online Figure 3) and 6-month follow-up (Figure 2).
CENTRAL ILLUSTRATION. Exercise-Based Heart Failure Rehabilitation: Health-Related Quality-of-Life and Exercise Capacity at 12 Months.
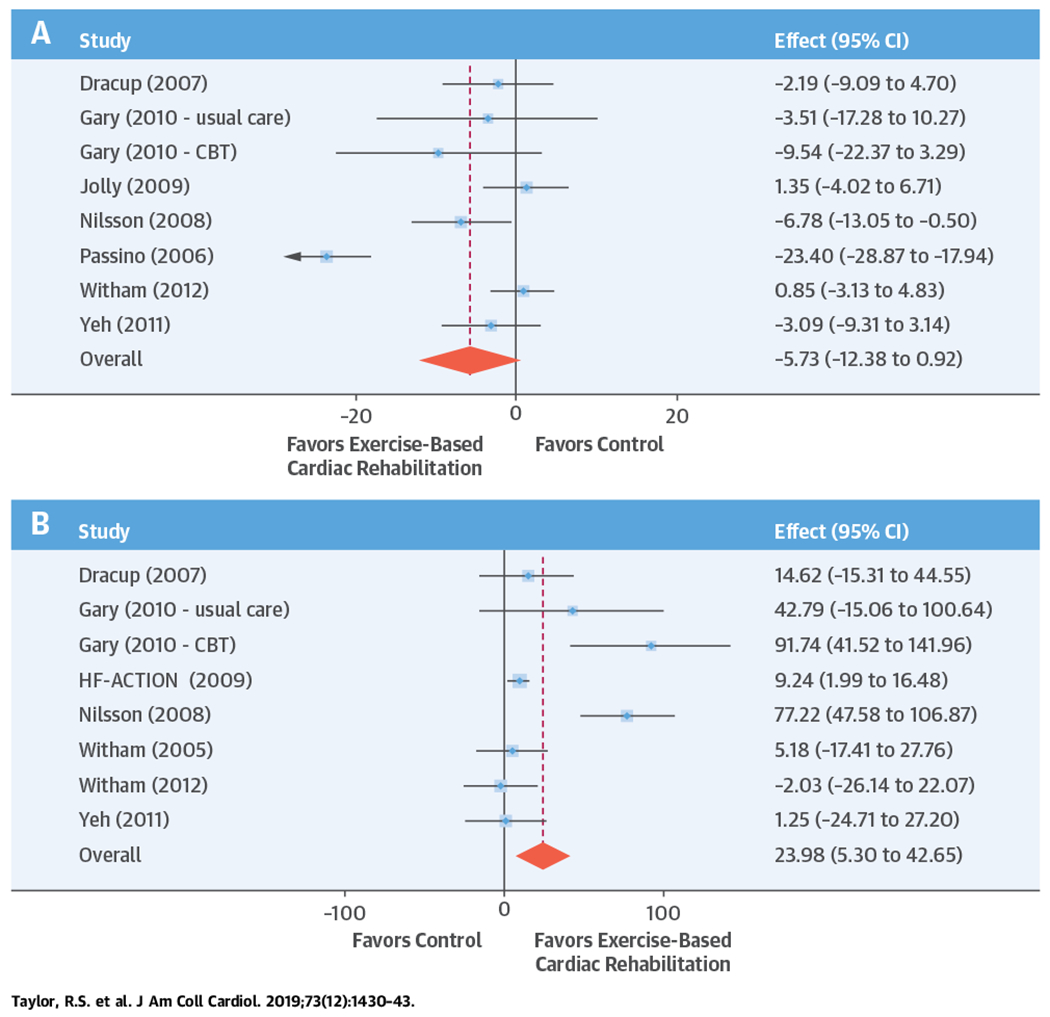
A forest plot from the 2-stage individual participant data meta-analysis model to (A) Minnesota Living with Heart Failure Questionnaire and (B) 6-min walk test, directly reported. CBT = cognitive behavioral therapy; CI = confidence interval; HF-ACTION = Exercise Training Program to Improve Clinical Outcomes in Individuals With Congestive Heart Failure.
FIGURE 2. Effect of ExCR on HRQoL and Exercise Capacity at 6 Months: 2-Stage IPD Meta-Analysis.
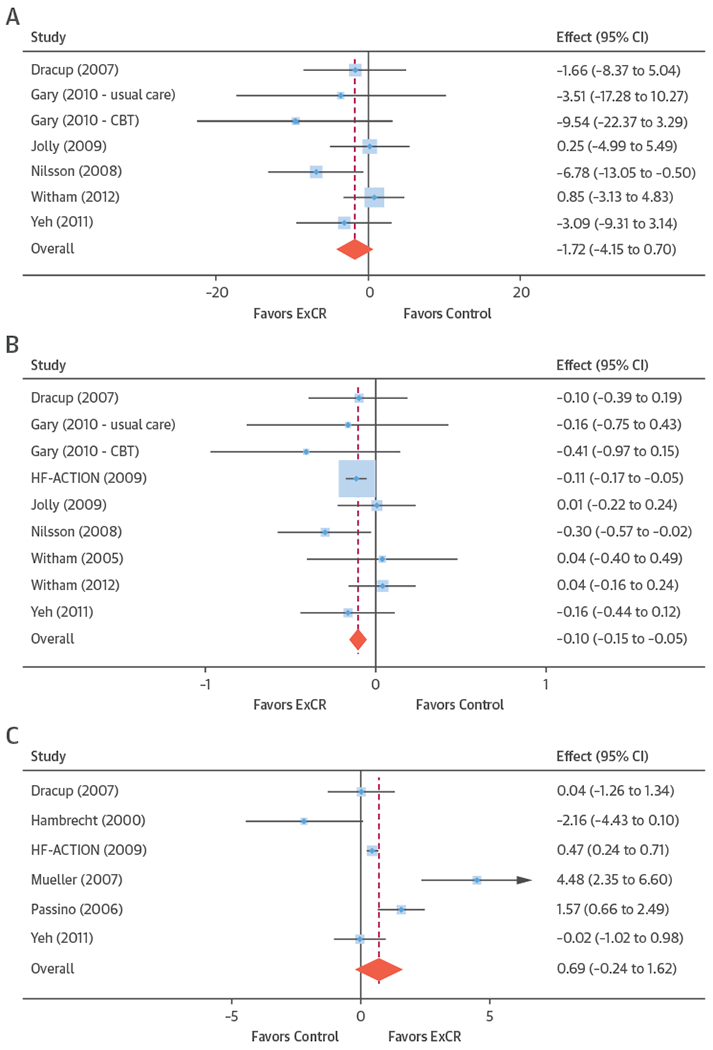
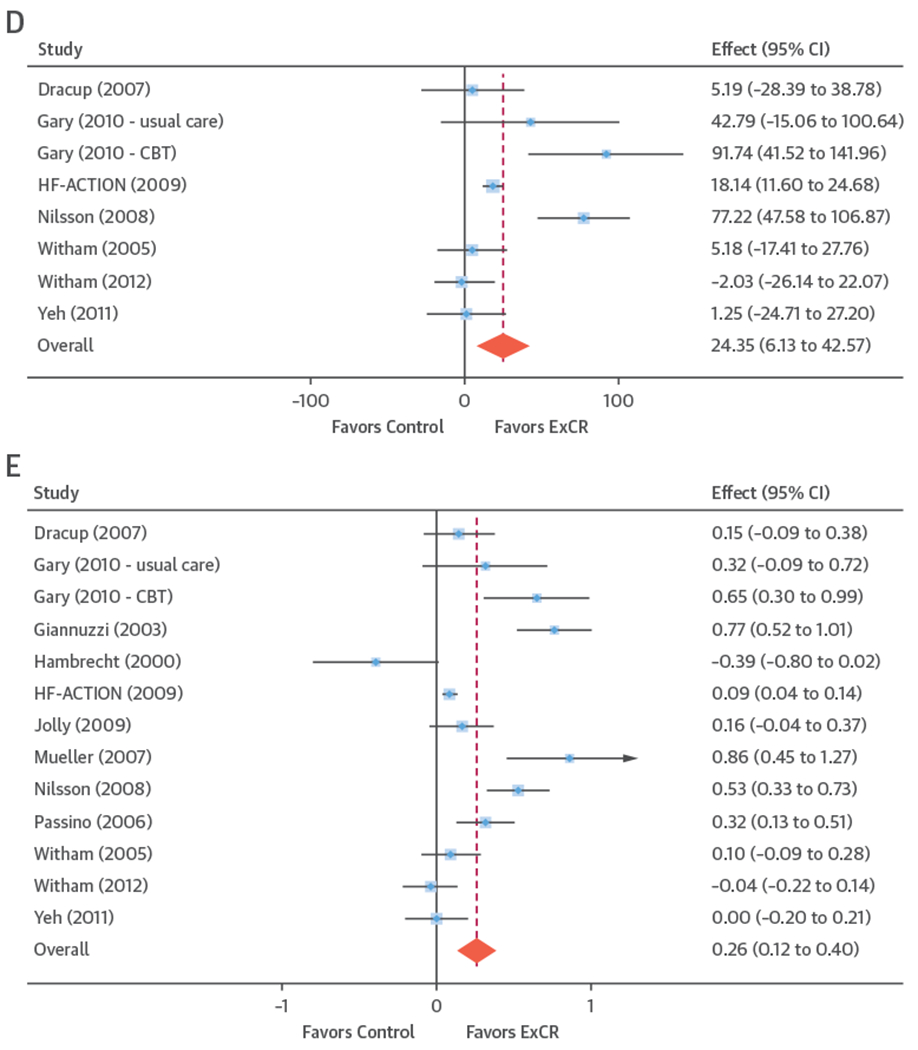
The blue circle is centered on the point estimate of the effect of ExCR in each trial, with the horizontal line showing the 95% confidence interval (CI) of this estimate. An arrow to either the left or right shows that the CI extends beyond the area shown in the forest plot. The size of the blue square around the point estimate is proportional to the weight that the individual trial contributes to the meta-analysis. The diamond and vertical red line show the overall estimate of the effect of ExCR in the 2-stage meta-analysis. (A) Minnesota Living with Heart Failure Questionnaire (MLHFQ). (B) All HRQoL measures (standardized score). (C) Peak Vo2, directly reported. (D) 6MWT, directly reported. (E) All exercise capacity measures (standardized score). CBT = cognitive behavioral therapy; ExCR = exercise-based cardiac rehabilitation; HF-ACTION = Exercise Training Program to Improve Clinical Outcomes in Individuals With Congestive Heart Failure; other abbreviations as in Figure 1.
Compared with control, treatment effects from the 1-stage meta-analysis at 12-month follow-up showed a statistically significant improvement with ExCR in exercise capacity as assessed by 6MWT (mean difference: 21.0 m; 95% CI: 1.6 to 40.4; p = 0.034; τ2 = 491; I2 = 78%) (Online Table 4) and standardized exercise capacity score (mean difference 0.27 SD units; 95% CI: 0.11 to 0.43; p = 0.001; τ2 = 0.08; I2 = 91%) (Online Table 5). No significant difference in peak Vo2 at 12 months was observed: 1.01 (95% CI: −0.42 to 2.44; p = 0.168; τ2 = 2.17; I2 = 94%) (Online Table 6).
In the repeated measures analyses for each HRQoL and exercise capacity outcome, a significant interaction between ExCR and time was observed (Online Figure 1). In sensitivity analyses, the results of the analyses excluding the HF-ACTION study, were broadly consistent with the overall results (Online Tables 3 to 6). Similar results were found with the addition of the trial-level aggregate data to the 2-stage model at 12-month follow-up.
There was no evidence of significant small study bias for the 5 outcomes studied (Online Figure 2).
DIFFERENTIAL EFFECTS ACROSS SUBGROUPS.
Analyses revealed no consistent interaction between the effect of ExCR and the pre-defined subgroups sex, ejection fraction, NYHA functional class, HF etiology, ethnicity, and baseline exercise capacity for either HRQoL or exercise capacity (Figure 3, Online Tables 2 to 6).
FIGURE 3. Effect of ExCR on HRQoL and Exercise Capacity Across Patient Subgroups 12 Months.
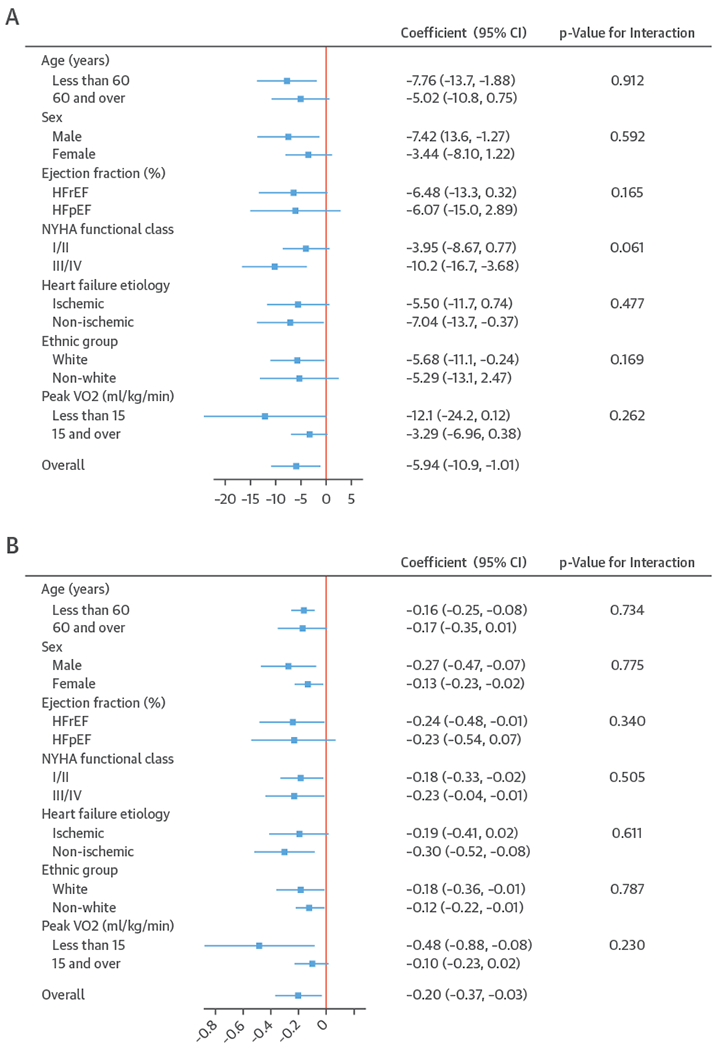
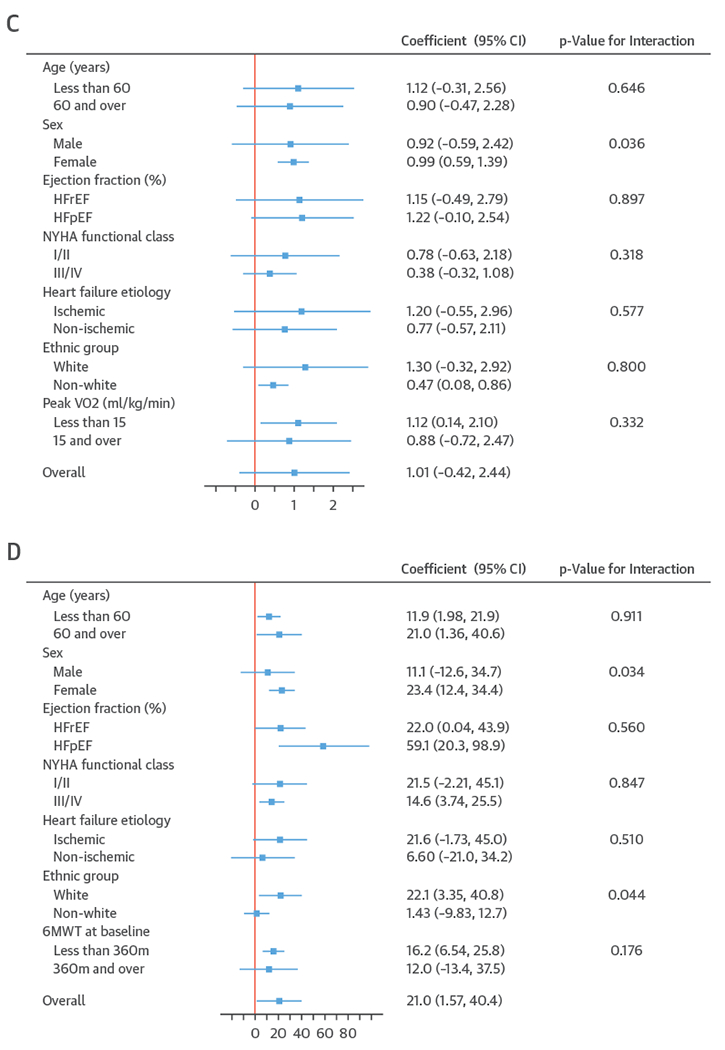
A plot to show the effect size for health-related quality-of-life outcomes (A and B) and exercise capacity outcomes (C and D), stratified by patient characteristics. All results are reported as a between group mean difference (ExCR-control) with a 95% confidence interval (CI) from 1-stage meta-analyses carried out by strata. The p values given are from the interaction tests in the main 1-stage meta-analysis. (A) Minnesota Living with Heart Failure Questionnaire (MLHFQ). (B) All HRQoL measures (standardized score). (C) Peak Vo2, directly reported. (D) 6MWT, directly reported. Abbreviations as in Figure 1.
A differential effect of ExCR across ages was observed in the standardized HRQoL score analysis at 6-month follow-up, with a differential reduction in HRQoL in the ExCR group compared with the control group (i.e., an increase in standardized HRQoL score) as age increased (0.006 SD units, 95% CI: 0.002 to 0.011; p = 0.006) (Online Table 3). To put this into context, based on an SD of 24 for MLHFQ score, this equates to a mean increase of 1.4 in MLHFQ score (i.e., a reduction in HRQoL) for an increase of 10 years in patient age, in the ExCR group compared with the control group.
Interaction analyses for the 1-stage model at 12 months showed differential effects of ExCR by sex, with women showing greater benefit from ExCR than men for each of peak Vo2 (0.57 ml/kg/min; 95% CI: 0.04 to 1.11 ml/kg/min; p = 0.036) (Online Table 6) and 6MWT (14.9 m; 95% CI: 1.2 to 28.7 m; p = 0.034) (Online Table 4). Differential effects of ExCR were also seen between ethnic groups (Online Table 4); white patients showed a greater improvement with ExCR in 6MWT distance compared with nonwhite patients: 14.2 m (95% CI: 0.40 to 28.0; p = 0.044).
DISCUSSION
We undertook an IPD meta-analysis to assess the impact of ExCR on exercise capacity and HRQoL in patients with HF. Analyses of data from 13 trials in 3990 randomized patients, predominantly (97%) with reduced ejection fraction HF, showed some evidence that ExCR improves both exercise capacity and HRQoL compared with no exercise control 12-month follow-up, with weaker evidence for a treatment effect at 6-month follow-up. The magnitude of the treatment effect of ExCR on MLHFQ score observed at 12-month follow-up was not only statistically significant, but also clinically important (47), with a mean between-group difference of >5 points, favoring the ExCR group. Also, there was an increase of ≥16 m in the 6MWT in the ExCR group, which may also be clinically significant (48). Interaction analyses showed that younger patients responded better to ExCR in terms of improved HRQoL; women and white patients had a better exercise capacity response. However, the interactions between ExCR and age, sex, and ethnicity were not consistent across health outcomes, different analyses, and time points. The findings should therefore be considered hypothesis generating.
We believe this to be the first IPD meta-analysis to assess the impact of ExCR on HRQoL and exercise capacity outcomes for patients with HF. The observed beneficial effects of ExCR on these outcomes are broadly consistent with previous trial-level (aggregate data) meta-analyses (8–10,49). The improvement (reduction) in MLHFQ score was similar to that reported by the 2014 Cochrane meta-analysis (5.8; 95% CI: 2.4 to 9.2) (8). The improvements in exercise capacity outcomes observed in our analyses were lower than those seen in trial-level meta-analyses (6MWT 41.1 m; 95% CI: 16.7 to 53.6 m [31]; peak Vo2 2.79 ml/kg/min; 95% CI: 2.05 to 3.53 ml/kg/min) (9). We found no consistent evidence of HF patient subgroup effects, in accord with trial-level meta-regression analyses (8,9). Within trial subgroup analyses from the HF-ACTION trial found no differential effect of ExCR on HRQoL across patient characteristics (50). A post hoc analysis of the same trial cohort reported a significant interaction between ExCR and ethnic group with regard to 6MWT distance at 3-month follow-up (adjusted p = 0.02), with mean improvement compared with control of 26 m (95% CI: 18 to 34 m) in white HF patients versus 11 m (95% CI: 0 to 21 m) in black HF patients, in the same direction as the current study (51).
STUDY LIMITATIONS.
IPD meta-analysis has a number of strengths relative to traditional trial-level meta-analysis, including: reduction in ecological biases; the ability to check and transform data to common scores or measures; consistent methods of analysis across trials, and improved power to detect overall and subgroup effects. In this study, we used a 1-stage meta-analysis approach to compare the outcomes between ExCR and control groups across all included trials. This approach adjusts the between-group comparisons of outcomes at follow-up for the baseline outcome score; this is important here as many of the included studies were small and therefore subject to chance differences in baseline score. Given these considerable advantages, meta-analyses that are based on IPD have been called the gold standard of systematic review (12).
An increasingly recognized challenge of IPD meta-analysis is that of obtaining IPD from study investigators (15,52). A recent systematic review across a total of 122 IPD meta-analyses found the average meta-analysis located only 61% (95% CI: 46% to 74%) of eligible datasets (53). In this study, we were able to retrieve patient data for all 13 trials with exercise capacity data; HRQoL data were available in 9 of 13 (69%) trials for 89% (2,970 of 3,332) of participants. Although our level of data retrieval compares favorably with this recent systematic review, we recognize that incomplete data capture is a limitation of our study, which may have introduced bias to our HRQoL analyses. Furthermore, we observed high levels of statistical heterogeneity for the outcomes of MLHFQ and 6MWT, likely to be due to the variation in population and intervention characteristics across the individual trials. Reassuringly, the inclusion of published results of trials for which no IPD were available did not change main effects. Due to limited published data on patient characteristics, we were unable to perform any sensitivity analyses using subgroup data.
Further important limitations of this analysis were the small number of patients with HFpEF that contributed to this analysis and the lack of data on patient-level ExCR dose. We did not have patient-level data on ExCR dose received, so we were unable to explore the effect of patient adherence to the rehabilitation program, or duration, frequency, or intensity of ExCR undertaken by an individual patient. Trials that include larger proportions of patients with HFpEF would enable us to address the question of whether ExCR has a differential effect in such patients compared to those with HFrEF. Improved reporting of patient-level data on adherence to ExCR will enable the investigation of any dose-response effect of ExCR. With regard to generalizability and application to clinical practice, the average age of participants in this study was 61 years, whereas the average age of HF patients in practice is approximately 10 years older (54).
CONCLUSIONS
Provision of ExCR to patients with HFrEF produces clinically important benefits in HRQoL and exercise capacity. Although we did observe some differences in the treatment effect of ExCR with age, sex, and ethnicity, these subgroup effects were not consistent across outcomes, time points, and analyses; hence, our findings do not endorse limiting ExCR interventions to subgroups of HF patients. However, due to the low numbers of women and nonwhite patients participating in ExCR, the ExTraMATCH II study would support the increasing representation of these groups. These results, based on an IPD meta-analysis of randomized trials, support the Class I recommendation of current international clinical guidelines that ExCR should be offered to all HF patients and the need to improve current poor uptake of ExCR in this population. Future data collection in this field requires a consensus on the definition, collection, and reporting of core outcomes, including a defined minimum standardized set of outcomes that should be measured and reported in all clinical trials in specific areas of health or health care (55). Additionally, we call for capture of data on patient-level adherence to exercise training during the ExCR intervention period. Future trials should be extended to include more women, older patients, and more patients with HFpEF, as well as patients with comorbid conditions. More generally, the research community should continue to implement policies that encourage primary study authors to make their datasets available, either by depositing their datasets in publicly available repositories or sharing with IPD meta-analysis collaborations when directly requested.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS:
Meta-analysis of data from previous studies suggests that exercise-based cardiac rehabilitation improves health-related quality-of-life and exercise capacity in patients with heart failure, irrespective of patient characteristics.
TRANSLATIONAL OUTLOOK:
Future trials should evaluate the effect of exercise-based cardiac rehabilitation in contemporary populations of patients with heart failure.
ACKNOWLEDGMENTS
The authors acknowledge the contribution of patient data from Dr. Rebecca Gary, Dr. Rainer Hambrecht, the late Dr. Romualdo Belardinelli, and the late Dr. Pantaleo Giannuzzi. The authors thank Tim Eames, Exeter Clinical Trials Unit, for his advice and support on data management for this study.
This study presents independent research funded by the National Institute for Health Research Health Technology Assessment Programme (NIHR-HTA 15/80/30). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health and Social Care. The funders peer review process informed the study protocol. The sponsor of the study had no role in data interpretation, or writing of the report. Prof. Taylor and Dr. Dalal are co-chief investigators and Prof. Jolly a co-investigator on an NIHR-funded program grant (RP-PG-1210-12004). Prof. Jolly is funded in part by NIHR CLAHRC West Midlands. Dr. Coats has received personal fees from Actimed, AstraZeneca, Faraday, Gore, Impulse Dynamics, Menarini, Novartis, Nutricia, Resmed, Respicardia, Servier, Stealth Peptides, Verona, and Vifor. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- 6MWT
6-min walk test
- CI
confidence interval
- ExCR
exercise-based cardiac rehabilitation
- HF
heart failure
- HFpEF
heart failure with preserved ejection fraction
- HFrEF
heart failure with reduced ejection fraction
- HRQoL
health-related quality-of-life
- IPD
individual participant (or patient) data
- MLHFQ
Minnesota Living With Heart Failure Questionnaire
- NYHA
New York Heart Association
- peak Vo2
peak oxygen uptake
Footnotes
APPENDIX For supplemental figures and tables, please see the online version of this paper.
REFERENCES
- 1.Braunwald E The war against heart failure: the Lancet lecture. Lancet 2015;385:812–24. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart 2007;93:1137–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.British Association for Cardiovascular Prevention and Rehabilitation. The BACPR Standards and Core Components for Cardiovascular Disease Prevention and Rehabilitation 2017. 3rd edition. Available at: http://www.bacpr.com/resources/6A7_BACR_Standards_and_Core_Components_2017.pdf. Accessed August 1, 2018.
- 4.Dalal HM, Doherty P, Taylor RS. Cardiac rehabilitation. BMJ 2015:351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: executive summary. a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:1495–539. [DOI] [PubMed] [Google Scholar]
- 6.Kondamudi N, Haykowsky M, Forman DE, Berry JD, Pandey A. Exercise training for prevention and treatment of heart failure. Prog Cardiovasc Dis 2017;60:115–20. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher GF, Landolfo C, Niebauer J, Ozemek C, Arena R, Lavie CJ. Promoting physical activity and exercise: JACC Health Promotion Series. J Am Coll Cardiol 2018;72:1622–39. [DOI] [PubMed] [Google Scholar]
- 8.Taylor RS, Sagar VA, Davies EJ, et al. Exercise-based rehabilitation for heart failure. Cochrane Database Syst Rev 2014;4:CD003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uddin J, Zwisler AD, Lewinter C, et al. Predictors of exercise capacity following exercise-based rehabilitation in patients with coronary heart disease and heart failure: a meta-regression analysis. Eur J Prev Cardiol 2016;23:683–93. [DOI] [PubMed] [Google Scholar]
- 10.Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med 2004;116:693–706. [DOI] [PubMed] [Google Scholar]
- 11.Thompson S, Higgins J. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–73. [DOI] [PubMed] [Google Scholar]
- 12.Chalmers I. The Cochrane Collaboration: preparing, maintaining, and disseminating systematic reviews of the effects of health care. Ann N Y Acad Sci 1993;703:156–65. [DOI] [PubMed] [Google Scholar]
- 13.ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004;328:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart LA, Clarke M, Rovers M, et al. Preferred reporting items for a systematic review and meta-analysis of individual participant data: the PRISMA-IPD statement. JAMA 2015;313: 1657–65. [DOI] [PubMed] [Google Scholar]
- 15.Tierney J, Vale C, Riley R, et al. Individual participant data (IPD) meta-analyses of randomised controlled trials: guidance on their use. PLoS Med 2015;12:e1001855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor R, Piepoli M, Smart N, et al. Exercise training for chronic heart failure (ExTraMATCH II): protocol for an individual participant data meta-analysis PROSPERO. 2014. Available at: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42014007170. Accessed August 1, 2018. [DOI] [PubMed]
- 17.Taylor RS, Piepoli MF, Smart N, et al. Exercise training for chronic heart failure (ExTraMATCH II): protocol for an individual participant data meta-analysis. Int J Cardiol 2014;174:683–7. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail 2018;20:1735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ 2010;340:c221. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ 2012;344:d7762. [DOI] [PubMed] [Google Scholar]
- 21.Bilbao A, Escobar A, García-Perez L, Navarro G, Quirós R. The Minnesota Living with Heart Failure Questionnaire: comparison of different factor structures. Health Qual Life Outcomes 2016;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Nogradi S, Halcrow S, Singer J, Sullivan MJJ, Fallen EL. Development and testing of a new measure of health status for clinical trials in heart failure. J Gen Intern Med 1989;4:101–7. [DOI] [PubMed] [Google Scholar]
- 24.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smart NA, Waldron M, Ismail H, et al. Validation of a new tool for the assessment of study quality and reporting in exercise training studies: TESTEX. Int J Evid Based Healthc 2015;13:9–18. [DOI] [PubMed] [Google Scholar]
- 26.Higgins J, Thompson S. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 27.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson PM, Cockburn J, Newton PJ, et al. Can a heart failure-specific cardiac rehabilitation program decrease hospitalizations and improve outcomes in high-risk patients? Eur J Prev Cardiol 2010;17:393–402. [DOI] [PubMed] [Google Scholar]
- 29.Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail 2005;7:411–7. [DOI] [PubMed] [Google Scholar]
- 30.Klecha A, Kawecka-Jaszcz K, Bacior B, et al. Physical training in patients with chronic heart failure of ischemic origin: effect on exercise capacity and left ventricular remodeling. Eur J Prev Cardiol 2007;14:85–91. [DOI] [PubMed] [Google Scholar]
- 31.Myers J, Goebbels U, Dzeikan G, et al. Exercise training and myocardial remodeling in patients with reduced ventricular function: one-year follow-up with magnetic resonance imaging. Am Heart J 2000;139 Part 1:252–61. [DOI] [PubMed] [Google Scholar]
- 32.Mueller L, Myers J, Kottman W, et al. Exercise capacity, physical activity patterns and outcomes six years after cardiac rehabilitation in patients with heart failure. Clin Rehabil 2007;21:923–31. [DOI] [PubMed] [Google Scholar]
- 33.Belardinelli R, Georgiou D, Cianci G, Purcaro A. 10-year exercise training in chronic heart failure: a randomized controlled trial. J Am Coll Cardiol 2012;60:1521–8. [DOI] [PubMed] [Google Scholar]
- 34.Dracup K, Evangelista LS, Hamilton MA, et al. Effects of a home-based exercise program on clinical outcomes in heart failure. Am Heart J 2007;154:877–83. [DOI] [PubMed] [Google Scholar]
- 35.Gary RA, Dunbar SB, Higgins MK, Musselman DL, Smith AL. Combined exercise and cognitive behavioral therapy improves outcomes in patients with heart failure. J Psychosom Res 2010;69:119–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giannuzzi P, Temporelli PL, Corra U, Tavazzi L. Antiremodeling effect of long-term exercise training in patients with stable chronic heart failure: results of the Exercise in Left Ventricular Dysfunction and Chronic Heart Failure (ELVD-CHF) trial. Circulation 2003;108:554–9. [DOI] [PubMed] [Google Scholar]
- 37.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA 2000;283: 3095–101. [DOI] [PubMed] [Google Scholar]
- 38.Jolly K, Taylor RS, Lip GY, et al. A randomized trial of the addition of home-based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM-CHF) study. Eur J Heart Fail 2009;11:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson BB, Westheim A, Risberg MA. Long-term effects of a group-based high-intensity aerobic interval-training program in patients with chronic heart failure. Am J Cardiol 2008;102: 1220–4. [DOI] [PubMed] [Google Scholar]
- 40.Witham MD, Gray JM, Argo IS, Johnston DW, Struthers AD, McMurdo ME. Effect of a seated exercise program to improve physical function and health status in frail patients > or = 70 years of age with heart failure. Am J Cardiol 2005;95: 1120–4. [DOI] [PubMed] [Google Scholar]
- 41.Witham MD, Fulton RL, Greig CA, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2012; 5:209–16. [DOI] [PubMed] [Google Scholar]
- 42.Yeh GY, McCarthy EP, Wayne PM, et al. Tai chi exercise in patients with chronic heart failure: a randomized clinical trial. Arch Intern Med 2011;171: 750–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passino C, Severino S, Poletti R, et al. Aerobic training decreases b-type natriuretic peptide expression and adrenergic activation in patients with heart failure. J Am Coll Cardiol 2006;47:1835–9. [DOI] [PubMed] [Google Scholar]
- 44.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173–82. [DOI] [PubMed] [Google Scholar]
- 45.McKelvie RS, Teo KK, Roberts R, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT). Am Heart J 2002;144:23–30. [DOI] [PubMed] [Google Scholar]
- 46.Willenheimer R, Rydberg E, Cline C, et al. Effects on quality of life, symptoms and daily activity 6 months after termination of an exercise training programme in heart failure patients. Int J Cardiol 2001;77:25–31. [DOI] [PubMed] [Google Scholar]
- 47.American Thoracic Society. Minnesota Living with Heart Failure Questionnaire 2004. Available at: http://qol.thoracic.org/sections/instruments/ko/pages/mlwhfq.html. Accessed August 1, 2018.
- 48.Bohannon RW, Crouch R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract 2017;23:377–81. [DOI] [PubMed] [Google Scholar]
- 49.Ciani O, Piepoli M, Smart N, et al. Validation of exercise capacity as a surrogate endpoint in exercise-based rehabilitation for heart failure: a meta-analysis of randomized controlled trials. J Am Coll Cardiol HF 2018;6:596–604. [DOI] [PubMed] [Google Scholar]
- 50.Flynn KE, Piña IL, Whellan DJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mentz RJ, Bittner V, Schulte PJ, et al. Race, exercise training, and outcomes in chronic heart failure: findings from Heart Failure-a Controlled Trial Investigating Outcomes in Exercise TraiNing (HF-ACTION). Am Heart J 2013;166: 488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart LA, Parmar MKB. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet 1993;341:418–22. [DOI] [PubMed] [Google Scholar]
- 53.Polanin JR. Efforts to retrieve individual participant data sets for use in a meta-analysis result in moderate data sharing but many data sets remain missing. J Clin Epidemiol 2018;98: 157–9. [DOI] [PubMed] [Google Scholar]
- 54.Cowie MR, Wood DA, Coats AJ, et al. Incidence and aetiology of heart failure; a population-based study. Eur Heart J 1999;20:421–8. [DOI] [PubMed] [Google Scholar]
- 55.Williamson PR, Altman DG, Blazeby JM, et al. Developing core outcome sets for clinical trials: issues to consider. Trials 2012;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


