Abstract
Objectives:
The objective of this study was to examine the effects of traumatic brain injury (TBI) and age on facial emotion recognition abilities in adults. Age and TBI were expected to have negative effects on emotion recognition and a TBI by age interaction was hypothesized such that older adults with TBI would have the lowest emotion recognition scores.
Methods:
A prospective cohort study was conducted. Participants were 26 adults with moderate-severe TBI (13 older and 13 younger) and 26 uninjured peers matched for age, sex, and education. Emotion recognition was measured using the Emotion Recognition Task, which is comprised of dynamically morphed facial expressions of the six basic emotions, presented at different intensity levels.
Results:
TBI and older age were associated with poorer recognition of both subtle and intense expressions, but only for expressions of anger and sadness. There was no interaction of age and TBI.
Conclusions:
Results add to the growing evidence of emotion recognition impairments after TBI, particularly for select negative emotions, and extend this finding to adults over the age of 60. Further research is needed to better understand social cognitive effects of TBI across the adult lifespan.
Keywords: Traumatic brain injury, social cognition, affect recognition, aging, speech-language pathology
Introduction
Traumatic brain injury (TBI) is a serious public health concern in the U.S. and worldwide.1–4 Adults over age 65 years are among those most at risk for TBI,3; 5 and with an aging U.S. population,6 the TBI incidence among older adults has increased in recent years.3 The high incidence of TBI in older adults contrasts with the sparse literature on their outcomes, particularly outcomes related to social functioning. TBI is a risk factor for negative social outcomes across the adult lifespan, including poor social integration,7–10 loss of relationships,10–12 and difficulty establishing new friendships.8; 13 These social problems are particularly concerning for older adults with TBI, who are especially vulnerable to the negative health effects associated with social isolation. 14; 15
While social outcomes after TBI are influenced by many factors,16; 17 there is growing evidence that social cognitive skills are critical contributors, particularly the ability to recognize emotions in others’ facial expressions. In previous studies, facial emotion recognition impairments contributed to negative social outcomes of younger adults with TBI,18; 19 were significantly associated with reduced social and occupational integration,20 and were associated with reports of having fewer friends.21
Facial emotion recognition impairments are common in adults with TBI22; 23 and have been shown to persist in the chronic stages of recovery.24; 25 These impairments have been documented using presentations of static images,19; 20; 25–27 dynamic video displays,20; 28 and dynamic audio-visual displays.18; 20; 29; 30
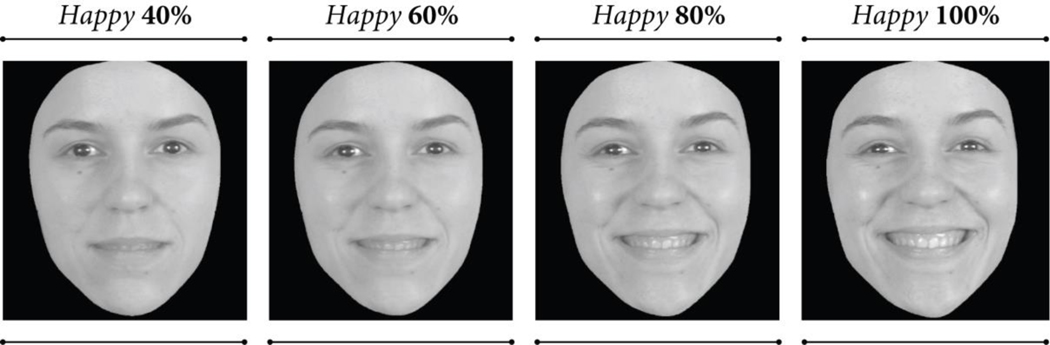
TBI can impair recognition of basic and complex emotions,21; 24 and there is evidence that negative emotions are especially vulnerable,31; 32 though findings are mixed (for discussion see ref. 28). For example, McDonald and Flanagan29 reported TBI-related impairments in recognition of both postive and negative emotions in a sample of adults with severe TBI, whereas Rigon and colleagues33 found that young and middle-aged adults with TBI had lower scores only for certain negative emotions (sadness, anger, and disgust). Findings from Rigon, et al.33 and others26; 28 further suggest that emotion recognition is not only mediated by emotion type, but also expression intensity. Rosenberg and colleagues28 for example, examined expression intensity as a factor in facial emotion recogonition of adults with TBI using the Emotion Recognition Task34 which is comprised of images morphed to vary from low intensity (≤ 40% of “full blown” intensity) to high intensity (≥ 80% maximum expression intensity), see Figure 1. Results revealed a complex interaction between TBI history, emotion type, and intensity such that individuals with TBI identified high intensity expressions of happiness as accurately as their uninjured peers, but were impaired in their recognition of low intensity happy expressions. For other emotions (fear, surprise) however, the TBI group was impaired only on high-intensity expressions, and for yet other emotions (anger, disgust), recognition was impaired regardless of intensity level, suggesting they did not benefit from increased intensity to the same degree as uninjured peers for certain emotions.28
Figure 1.
While facial emotion recognition impairments have been well documented in the TBI literature, this work has primarily focused on young to middle-aged adults.20; 25; 26; 29; 33 Given the growing incidence and prevalence of TBI among adults over age 65, and the high costs of social limitations in this population, knowledge about the social abilities of older adults with TBI is important to inform their rehabilitation. Similar cognitive problems, in general, have been reported in younger and older adults with TBI,35–39 so older adults might be expected to have similar emotion recognition impairments as younger individuals. Beyond the effects of TBI, however, emotion recognition is also affected by typical aging.40–42 Age-related declines in emotion recognition have been reported using presentations of static images41; 43–45 and dynamic audio and audio-visual displays,40; 42; 46 and a 2008 meta-analysis indicated that typically aging older adults had significantly poorer facial emotion recognition than younger individuals, particularly for expressions of anger, sadness, fear, and, to a lesser degree, happiness.44
It is clear that older adults are at risk for emotion recognition impairments due to their increased risk for TBI and their age. We do not know whether emotion recognition deficits in older adults with TBI are prevalent and if such deficits are similar in nature to those reported in the younger TBI and typically aging literature. Without this knowledge, we lack benchmarks for older adults’ emotion recognition after TBI and have limited information to guide appropriate assessment and treatment practices for adults with TBI across the lifespan.
As a first attempt to address these knowledge gaps, we conducted a preliminary prospective cohort study of older and younger adults who were aging typically or with TBI, to examine the effects of both TBI and age on facial emotion recognition performance. We measured emotion recognition ability with the Emotion Recognition Task,47 which is comprised of short dynamic clips of actors portraying six basic emotions: happiness, anger, sadness, fear, surprise, and disgust. Each item begins by showing an actor with a neutral expression, then his or her face is morphed to depict each emotion at increasing levels of intensity (40% of maximum intensity to 100% intensity). The use of dynamic clips and inclusion of subtle facial emotion displays gives the Emotion Recognition Task greater ecological validity than frequently used static image tasks, and allows for a nuanced examination of possible interactions between participant and stimuli characteristics.28; 33; 47
Hypotheses
Based on robust evidence that both TBI and age are risk factors for emotion recognition impairment, we expected these preliminary data to show that older participants and those with TBI would have poorer emotion recognition, as measured by the Emotion Recognition Task, than younger and uninjured participants. Further, it has been suggested that TBI alters the typical cognitive aging trajectory, resulting in greater cognitive decline with age48–51 though see refs. 52–54. Applying this hypothesis to facial emotion recognition, we expected a TBI by age interaction such that aging would have a greater negative effect on our TBI group’s emotion recognition than that of our uninjured comparison group. Based on evidence from the younger adult TBI literature, we also predicted that TBI and age effects would be specific to negative emotions. In addition, previous findings indicate adults with TBI have superior emotion recognition accuracy for intense versus subtle expressions,26; 28 thus we expected TBI effects to be specific to subtle expressions.
Method
Participants
Participants were 13 older adults with moderate-severe TBI (age 60 years or older) and three comparison groups: 1) younger adults (< age 35 years) with moderate-severe TBI; 2) older adults with no history of TBI; and 3) younger adults with no TBI history. Each comparison group was matched to the TBI Older group for sex (8 males, 5 females) and years of education (F(3,51) = 0.16, p = 0.92, ηp2 = 0.01), and the Older and Younger groups, respectively, were matched for age (Older: t(24) = 0.14, p = 0.89, d = 0.06; Younger: t(24) = 0.34, p = 0.74, d = 0.13). The groups of older and younger adults with TBI did not differ significantly in months post-injury, after correcting for unequal variances (t(13.13) = 0.58, p= 0.57, d = 0.27) (See Table 1). Available information regarding individual participants’ cause of injury and resulting neuropathology are shown in Tables 2 and 3. Older and younger participants with TBI were not matched for cause of injury. It is noted that while the majority of younger adults with TBI were injured in motor vehicles accidents, most of the older participants were injured as the result of various types of falls. This age-related difference in injury mechanism is commonly reported in the older adult TBI literature 55–57 and is considered in our interpretation of study results. Neuropathology within and across groups was variable, yet all participants were categorized has having a moderate-severe TBI as defined by nationally accepted criteria56.
Table 1.
Demographic and time-post injury data for each participant group.
| TBI Older | TBI Younger | Uninjured Older | Uninjured Younger | ||
|---|---|---|---|---|---|
| Age (Months) | Mean (SD) | 784.77 (44.02) | 312.00 (39.62) | 782.00 (56.65) | 305.92 (51.02) |
| Range | 727.00–868.00 | 234.00–398.00 | 716.00–876.00 | 216.00–390.00 | |
| Years Education | Mean (SD) | 15.00 (2.94) | 15.00 (2.45) | 14.69 (1.89) | 15.31 (1.38) |
| Range | 12.00 – 20.00 | 12.00 – 20.00 | 12.00 – 18.00 | 13.00 – 18.00 | |
| Recruitment Site | Wisconsin Iowa |
2 11 |
10 3 |
4 9 |
9 4 |
| Months Post-Injury | Mean (SD) | 108.85 (166.41) | 81.62 (36.10) | ||
| Range | 8.00–541.00 | 18.00–133.00 | |||
Table 2.
Injury and neuropathology data of older participants with TBI.
| Participant | Injury Cause | GCS | Neuropathology |
|---|---|---|---|
| Older TBI 1 | Fall from ladder | 5 | Subarachnoid hemorrhage |
| Older TBI 2 | Bicycle accident | Unknown | Medical records unavailable |
| Older TBI 3 | Fall from standing | Unknown | Subarachnoid hemorrhage |
| Older TBI 4 | Fall from standing | 10 | Bilateral frontal and temporal lobe contusions, subarachnoid hemorrhage within bilateral Sylvian cisterns |
| Older TBI 5 | Fall down stairs | Unknown | Medical records unavailable but participant confirms acute neurosurgery |
| Older TBI 6 | MVA | Unknown | Obstructive hydrocephalus |
| Older TBI 7 | Skiing accident | Unknown | No neuroimaging available, but participant confirms acute neurosurgery |
| Older TBI 8 | Fall | Unknown | Subarachnoid hemorrhage along left precentral sulcus |
| Older TBI 9 | Head struck by heavy object | 14 | Subarachnoid hemorrhage |
| Older TBI 10 | Fall from atop vehicle | 15 | Intracerebral hemorrhage, right frontal contusion |
| Older TBI 11 | Fall from horse | 9 | Medical records unavailable |
| Older TBI 12 | Fall from standing | 3 | Bilateral frontal contusions and subarachnoid hemorrhage |
| Older TBI 13 | Fall from standing | Unknown | Subdural hematoma with focal left frontal lesion |
Note: GCS = Glasgow coma scale score; MVA = motor vehicle accident
Table 3.
Injury and neuropathology data of younger participants with TBI.
| Participant | Injury Cause | GCS | Neuropathology |
|---|---|---|---|
| Younger TBI 1 | Skiing accident | Unknown | Medical records unavailable, but participant confirmed acute neurosurgery |
| Younger TBI 2 | MVA | 3 | Left lateral and right medial parietal hemorrhages, orbital frontal contusion |
| Younger TBI 3 | MVA | 3 | Diffuse cerebral hemorrhages, more severe on right than left |
| Younger TBI 4 | MVA | 6T | Diffuse axonal injury, left frontal contusion, subarachnoid hemorrhage, subcortical lesions including to midbrain and thalamus |
| Younger TBI 5 | MVA | 6 | Diffuse axonal injury, intraventricular hemorrhage, interparenchymal hemorrhages, including to insula and near junction of putamen and thalamus |
| Younger TBI 6 | MVA | 3T | Diffuse axonal injury, left thalamic hematoma, hemorrhage near left pons, contusion to deep posterior frontal lobes and anterior limb of right internal capsule |
| Younger TBI 7 | MVA | 8 | Left subdural and epidural hematomas, bilateral frontal lobe hemorrhages |
| Younger TBI 8 | MVA | 9 | Medical records unavailable |
| Younger TBI 9 | Fall | 8 | Left subdural hematoma, left frontal hemorrhagic contusion |
| Younger TBI 10 | Skiing accident | 3 | Neuroimaging records unavailable |
| Younger TBI 11 | Fall | Unknown | Medical records unavailable |
| Younger TBI 12 | MVA | Unknown | Medical records unavailable |
| Younger TBI 13 | Fall from about 3 feet | Unknown | Subdural hematoma |
Note: GCS = Glasgow coma scale score; MVA = motor vehicle accident
Participants were recruited through community contacts in Madison, Wisconsin and Iowa City, Iowa as part of a larger study on social cognition and communication after TBI. Inclusion criteria included English as a primary language, no history of neurological disease or injury affecting the brain (pre-morbidly for participants with TBI), no history of language and/or learning disability (pre-morbidly for participants with TBI), normal or corrected to normal near vision, and a Western Aphasia Battery aphasia quotient higher than 93.8.58 Participants with TBI had a moderate-severe TBI as defined by nationally accepted criteria59 and were at least 6 months post injury and out of posttraumatic amnesia.
Procedures
After giving informed consent, participants provided information about their education, employment, and injury histories through a structured interview with a research assistant. Study tasks were embedded in the protocol for the larger study, which was completed in two, two-hour testing sessions. Task order was randomized for each participant and participants were tested individually in a quiet testing room.
Measures to Characterize the Sample.
To facilitate comparison of findings from the present study to previously published data, participants completed a series of tasks recommended by the Common Data Elements Committee for TBI research.60 The California Verbal Learning Test-II (CVLT)61 was used to assess immediate recall (CVLT-IR), short-delay verbal recall (CVLT-SR), and long-delay recall (CVLT-LR). The number of words recalled for each condition was compared across groups. The Trail Making Test (TMT)62 was used to assess executive functioning and the times to complete TMT Part A and TMT Part B were analyzed. The Symbol Search and Coding subtests of the Wechsler Adult Intelligence Scale, 4th Edition (WAIS-IV)63 were administered as measures of information processing speed. The total number of items completed for the Symbol Search and Coding subtests was analyzed.
Emotion Recognition Task. 34; 47
In this task, participants view faces that morph from a neutral expression to one of six basic emotions: afraid, angry, disgusted, happy, sad, and surprised. We used the short version of the task, which includes morphs from neutral to four levels of emotion intensity: 40%, 60%, 80%, and 100%. At each intensity level, 24 trials were presented, four from each emotion category (two presented by a male actor and two by a female actor), for a total of 96 items. Item presentation order is fixed with incremental increases from lowest to highest intensity, to preclude participants using examples from higher intensity items to improve performance on lower intensity items. For each trial, participants were presented with the morph and six response choices listed to the right of the image. Participants were asked to choose “the word that best describes what the person is feeling” by clicking the appropriate label with a computer mouse. Dependent variables were number of correct responses for each emotion, intensity level, emotion type at each intensity level, and total number of correct responses across all items.
Analysis
Analyses were completed using IBM© SPSS© version 23.64 Two-way analyses of variance (ANOVA) were conducted for each Common Data Elements subtest. TBI status and age group (older vs. younger) were the within-subjects variables and raw performance scores were the dependent variables. Post-hoc 1-way ANOVAs were conducted to delineate significant interactions. Data distributions were examined for the presence of outliers prior to completing each analysis. Outliers were defined as data points falling 1.5 times the interquartile range above the third quartile or below the first quartile.
Following the analysis of Rigon, et al.33 we used a mixed-effects repeated-measures ANOVA to test for main effects of TBI (adults with TBI vs. no history of TBI) and age (older vs. younger), and interactions among group, age, emotion type (anger, disgust, fearfulness, happiness, sadness, and surprise) and intensity level (40%, 60%, 80%, 100%) on Emotion Recognition Task scores. TBI and age were between-subjects variables and emotion type and intensity level were repeated, within-subjects factors. Planned independent samples t-tests were conducted for significant interactions among within- and between-subjects variables and 1-way repeated-measures ANOVA and paired-samples t-tests were conducted for each emotion to examine interaction between the two within-subjects variables (emotion type and intensity level). Bonferroni correction was applied for multiple comparisons. Effect sizes were calculated as partial eta squared (ηp2) values for ANOVA and Cohen’s d values were calculated for post-hoc group comparisons.
Results
Measures to Characterize the Sample
Descriptive data of each group’s scores on Common Data Elements tasks are shown in Table 4.
Table 4.
Common Data Elements test scores for each participant group (Mean (Standard Deviation)).
| TBI Older | TBI Younger | Uninjured Older | Uninjured Younger | ||
|---|---|---|---|---|---|
| CVLT -Immediate Recall (total words) | Outliers included | 49.38 (13.90) | 48.15 (13.20) | 53.62 (5.69) | 60.00 (7.48) |
| Outliers excluded | 49.38 (13.90) | 48.15 (13.20) | 53.62 (5.69) | 61.50 (5.40) | |
| CVLT Short Delay Recall (total words) | Outliers included | 10.00 (3.85) | 8.46 (4.54) | 10.92 (2.18) | 12.85 (2.41) |
| Outliers excluded | 10.00 (3.85) | 7.90 (3.38) | 10.02 (2.18) | 13.73 (1.10) | |
| CVLT Long Delay Recall (total words) | Outliers included | 10.85 (4.00) | 8.38 (4.17) | 11.77 (2.49) | 13.77 (2.39) |
| Outliers excluded | 10.85 (4.00) | 8.38 (4.17) | 11.77 (2.49) | 14.90 (0.74) | |
| TMT Part A (seconds) | Outliers included | 31.75 (7.34) | 26.69 (10.03) | 28.23 (6.93) | 22.31 (7.63) |
| Outliers excluded | 31.75 (7.34) | 24.67 (7.18) | 28.23 (6.93) | 20.83 (5.72) | |
| TMT Part B (seconds) | Outliers included | 79.70 (21.31) | 58.54 (27.81) | 76.54 (47.95) | 53.15 (30.94) |
| Outliers excluded | 79.70 (21.31) | 58.54 (27.81) | 57.82 (15.19) | 49.17 (15.90) | |
| WAIS-IV Symbol Search (total items completed) | No Outliers | 27.00 (5.57) | 32.31 (10.65) | 30.15 (8.75) | 34.15 (8.74) |
| WAIS-IV Coding (total items completed) | No Outliers | 55.77 (14.61) | 68.23 (19.47) | 62.54 (14.13) | 75.08 (20.45) |
Note: CVLT = California Verbal Learning Test; TMT = Trail Making Test; WAIS-IV = Wechsler Adult Intelligence Scale, 4th Edition
CVLT Immediate Recall.
Inspection of CVLT Immediate Recall scores indicated the presence of one outlier in the younger uninjured group. Inclusion of this data point did not change the results of the 2-way ANOVA so results of the full data set are reported here. There was a significant TBI effect on immediate recall, F(1, 48) = 7.37, p = .01, ηp2 = 0.13, with participants with TBI recalling fewer words than participants with no history of TBI. There was not a significant age effect on immediate recall, F(1, 48) = .76, p = .39, ηp2 = 0.02 and the TBI by age interaction was not significant, F(1, 48) = 1.65, p = .21, ηp2 = 0.03.
CVLT Short-delay Recall.
There were five outliers identified via inspection of short-delay recall scores, three in the younger TBI group and two in the younger uninjured group. With outliers included in the analysis there was a significant TBI effect (TBI < uninjured), F(1, 48) = 7.96, p = .01, ηp2 = 0.14. There was not a significant effect of age on short-delay recall, F(1, 48) = 0.04, p = .84, ηp2 < 0.01 and the TBI by age interaction was not significant, F(1, 48) = 3.39, p = .07, ηp2 = 0.07. With outliers excluded however, there was a significant TBI by age interaction, F(1, 43) = 8.57, p = .01, ηp2 = 0.17. Post-hoc one-way ANOVAs indicated that while older and younger participants with TBI scored similarly, F(1, 21) = 1.86, p = .19, ηp2 = 0.08, younger adults with no history of TBI recalled significantly more words after a short delay than did older adults without TBI F(1, 22) = 14.92, p < .01, ηp2 = 0.40.
CVLT Long-delay Recall.
There were three outliers in the uninjured younger group’s long-delay recall scores. Results were similar with and without outliers included so, for clarity, only results of the full data set are reported here. There was a significant TBI effect on long-delay recall, F(1, 48) = 11.42, p < .01, ηp2 = 0.19 and the TBI by age interaction was significant, F(1, 48) = 5.72, p = .02, ηp2 = 0.11. There was not a significant effect of age, F(1, 48) = 0.46, p = .81, ηp2 < 0.01. Post-hoc analyses indicated that the older and younger participants with TBI recalled a similar number of words, F(1, 24) = 2.36, p = .14, ηp2 = 0.09, but within the group of participants without a history of TBI, younger participants recalled significantly more words than older participants after a long delay, F(1, 24) = 4.38, p = .05, ηp2 = 0.15.
Trail Making Test Part A.
One older participant with TBI was unable to complete the Trails Part A task. Examination of the data revealed two outliers, one in the younger TBI group and one in the younger uninjured group. Results were similar with and without outliers included so results of the analysis on the full data set are reported here. Results indicated that there was a significant age effect on Trails Part A completion times, F(1, 47) = 5.87, p = .02, ηp2 = 0.11, with older participants taking longer to complete the task. TBI status, F(1, 47) = 3.04, p = .09, ηp2 = 0.06 did not have a significant effect on completion times and the TBI by age interaction was not significant, F(1, 47) = 0.04, p = .85, ηp2 < 0.01.
Trail Making Test Part B.
Three older adults with TBI failed to complete Part B of the Trail Making Test. One of these three also did not complete Part A. Further, three outliers were identified in the data from participants without TBI, two from older participants and one from a younger participant. With the outliers included, it was found that older participants, as whole, took significantly longer than younger participants to compete the Trails B task, F(1, 45) = 5.84, p = .02, ηp2 = 0.12. TBI did not have a significant effect on completion times, F(1, 45) = 0.22, p = .65, ηp2 = 0.01 and the TBI by age interaction was not significant, F(1, 45) = 0.02, p = .91, ηp2 < 0.01. When the analysis was repeated with outliers excluded however, there was a significant TBI effect (TBI < injured), F(1, 42) = 6.33, p = .02, ηp2 = 0.13 in addition to the significant effect of age, F(1, 42) = 5.76, p = .02, ηp2 = 0.12. The TBI by age interaction was not significant, F(1, 42) = 1.01, p = .32, ηp2 = 0.02.
Symbol Search.
There were not significant effects of TBI, F(1,48) = 1.10, p = .30, ηp2 = .02 or age, F(1,48) = 3.81, p = .06, ηp2 = .07) on information processing speed, as measured by the Symbol Search task, and the TBI by age interaction was not significant, F(1,48) = 0.75, p = .79, ηp2 = .002).
Coding.
There was a significant effect of age on information processing speed as measured by the Coding subtest of the WAIS-IV, F(1, 48) = 6.71, p = .01, ηp2 = 0.11. There was not a significant effect of TBI on Coding speed, F(1, 48) = 1.99, p = .17, ηp2 = 0.04 and there was not a significant TBI by age interaction, F(1, 48) = 0.02, p = .99, ηp2 < 0.01.
Emotion Recognition
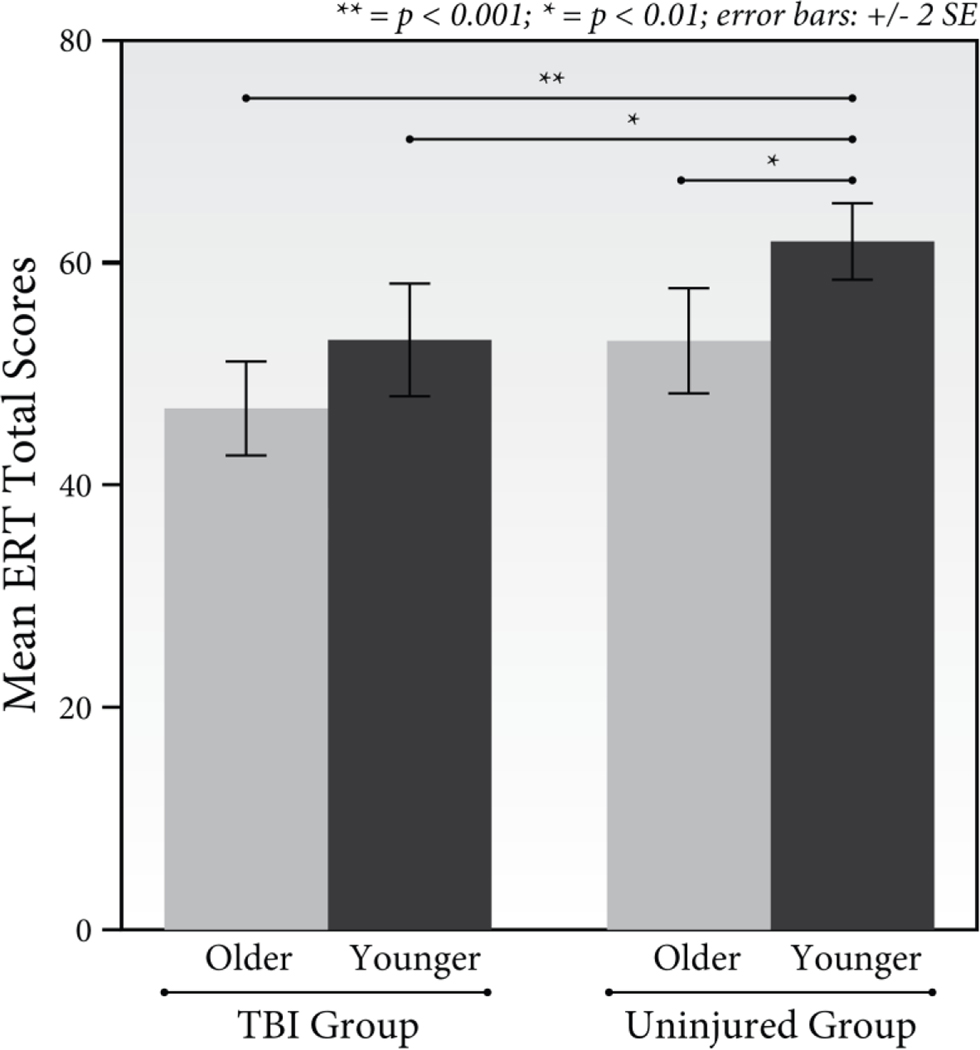
Emotion recognition data are summarized in Table 5. Results of the mixed effects repeated-measures ANOVA revealed that there was not a significant 4-way interaction between TBI, age, emotion type, and intensity, F(15, 720) = 1.13, p = .32, np2 = 0.02. Results also indicated that there were not significant three-way interactions (TBI x Age x Emotion, F(5, 240) = 1.17, p = .33, np2 = 0.02; TBI x Age x Intensity, F(3, 144) = 1.50, p = .22 . np2 = 0.03; TBI x Intensity x Emotion, F(15, 720) = 1.29, p = .20, np2 = .03; Age x Intensity x Emotion, F(15, 720) = 0.84, p = .64, np2 = 0.02). The predicted interaction between TBI and age was not significant F(1, 48) = 0.01, p = .95, np2 < .001, however as hypothesized, there were significant interactions between TBI and emotion type, F(5, 240) = 2.50, p = .03, np2 = 0.05 and TBI and intensity level, F(3,144) = 5.53, p = .001, np2 = 0.10. There was also a significant interaction between emotion type and intensity level, F(15, 720) = 2.47, p = .002, np2 = .05. Significant interactions were not found between age and emotion type, F(5, 240) = 2.01, p = .08, np2 = .04, nor age and intensity level, F(3, 144) = 0.91, p = .44, np2 = 0.02. There were significant main effects of TBI, F(1,48) = 18.85, p = .001, np2 = 0.28, and age F(1,48) = 7.60, p = .008, np2 = 0.14; with higher overall emotion recognition scores for the uninjured and younger adult groups. Planned comparisons of the four groups’ Emotion Recognition Task total scores revealed that while older adults with TBI had the lowest average scores (see Figure 2), after adjusting for multiple comparisons, their scores were only significantly lower than those of the younger uninjured group, (t(24) = −5.52, p < .001, d = −2.18), and not those of younger participants with TBI, (t(24) = −1.87, p = .07, d = −0.74), nor those of the older uninjured group, (t(24) = −1.92, p = .07, d = −0.75). The group of younger uninjured participants also scored marginally higher on the Emotion Recognition Task than did younger participants with TBI (t(24) = 2.89, p = .008, d = 1.16) and those in the older injured group (t(24) = −3.06, p = .005, d = 1.21). There were also significant main effects of emotion type, F(5, 240) = 122.08, p < .001, np2 = 0.72 and intensity level, F(3,144) = 71.60, p < .001, np2 = 0.60 (see Table 5).
Table 5.
Emotion Recognition Task scores of each participant group for each emotion type and intensity level tested (Mean (Standard Deviation)).
| Emotion | Intensity | TBI Older | TBI Younger | Uninjured Older | Uninjured Younger | All Groups |
|---|---|---|---|---|---|---|
| Anger | 40% | 1.31 (1.18) | 2.31 (0.86) | 2.38 (1.04) | 2.92 (0.95) | 10.63 (3.12) |
| 60% | 2.23 (1.30) | 2.62 (0.65) | 3.00 (0.91) | 3.15 (1.07) | ||
| 80% | 2.62 (1.12) | 2.62 (0.96) | 3.08 (0.64) | 3.08 (0.86) | ||
| 100% | 2.38 (0.96) | 2.54 (1.13) | 2.92 (0.95) | 3.38 (0.65) | ||
| Disgust | 40% | 1.46 (0.97) | 2.23 (1.17) | 2.15 (1.21) | 2.23 (1.24) | 10.46 (3.16) |
| 60% | 2.46 (0.78) | 2.31 (0.95) | 2.69 (1.38) | 3.08 (0.95) | ||
| 80% | 2.62 (1.19) | 2.92 (0.76) | 3.15 (1.07) | 3.46 (0.66) | ||
| 100% | 2.54 (1.33) | 2.31 (1.03) | 3.00 (1.35) | 3.23 (0.73) | ||
| Fear | 40% | 0.62 (0.87) | 0.62 (0.87) | 0.92 (0.86) | 1.08 (0.76) | 4.31 (2.65) |
| 60% | 0.69 (0.95) | 0.69 (0.95) | 0.85 (0.80) | 0.77 (0.93) | ||
| 80% | 0.69 (1.03) | 1.23 (1.17) | 1.77 (1.48) | 1.15 (0.80) | ||
| 100% | 1.54 (0.97) | 1.08 (1.26) | 1.77(1.42) | 1.77 (1.24) | ||
| Happiness | 40% | 3.00 (0.82) | 3.54 (0.52) | 3.54 (0.66) | 3.46 (0.66) | 15.13 (1.17) |
| 60% | 3.92 (0.28) | 3.92 (0.28) | 3.77 (0.44) | 3.92 (0.28) | ||
| 80% | 3.92 (0.28) | 4.00 (0.00) | 3.92 (0.28) | 3.92 (0.28 | ||
| 100% | 3.85 (0.38) | 4.00 (0.00) | 4.00 (0.00) | 3.85 (0.38) | ||
| Sadness | 40% | 0.69 (1.03) | 0.69 (0.95) | 0.92 (0.76) | 1.46 (0.88) | 6.65 (3.44) |
| 60% | 1.23 (1.01) | 1.38 (1.04) | 0.92 (1.12) | 2.46 (1.05) | ||
| 80% | 1.69 (1.18) | 2.08 (1.26) | 1.69 (1.25) | 2.85 (0.69) | ||
| 100% | 1.08 (0.86) | 1. 69 (1.11) | 2.46 (1.13) | 3.31 (0.63) | ||
| Surprise | 40% | 1.15 (0.99) | 1.69 (1.03) | 1.23 (0.83) | 1.62 (0.87) | 7.50 (2.87) |
| 60% | 1.69 (1.25) | 2.23 (0.83) | 1.85 (1.07) | 1.62 (1.04) | ||
| 80% | 1.85 (1.46) | 2.23 (1.09) | 2.08 (0.95) | 2.23 (1.17) | ||
| 100% | 1.62 (0.96) | 1.92 (0.86) | 2.31 (1.11) | 2.69 (0.95) | ||
| All Emotions | 40% | 10.81 (2.88) | ||||
| 60% | 13.37 (2.72) | |||||
| 80% | 15.21 (2.77) | |||||
| 100% | 15.31 (2.30) | |||||
| ERT Total Scores | 46.85 (7.60) | 53.00 (9.19) | 52.92 (8.52) | 61.85 (6.18) |
Note: ERT = Emotion Recognition Task
Figure 2.
Mean Emotion Recognition Task total scores for each participant group.
Recognition Accuracy Across Emotion Types after TBI
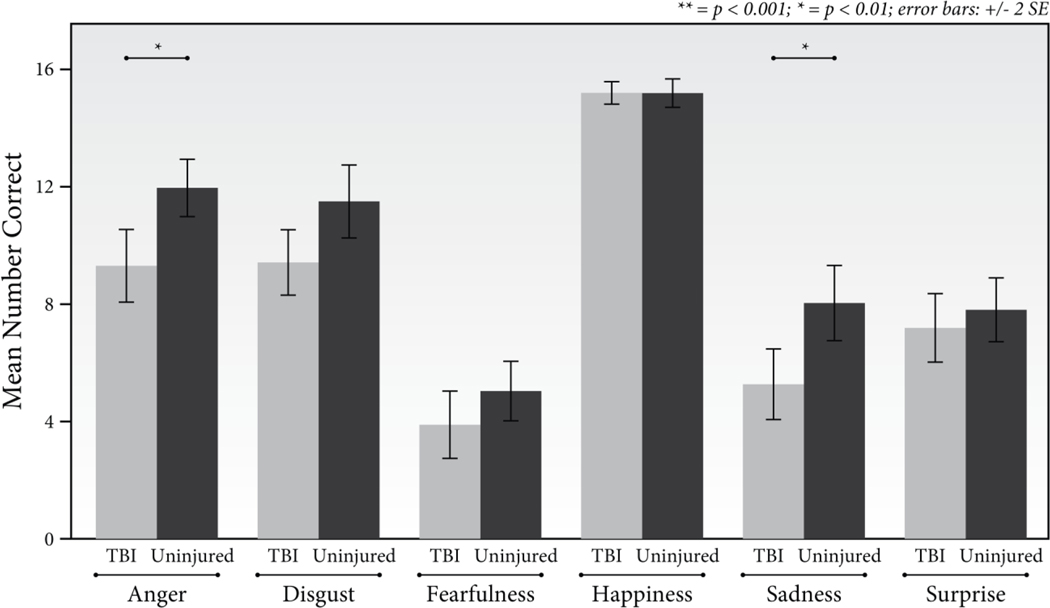
To examine the TBI by emotion type interaction, we collapsed across age groups due to the lack of a significant interaction between age, TBI, and emotion type. As shown in Figure 3, results indicated that after correcting for multiple comparisons (α = .05/6 = .008) participants with TBI scored significantly lower than the uninjured group on items featuring expressions of anger (t(50) = −3.36, p = .001, d = −0.94) and sadness (t(50) = −3.15, p = .003, d = −0.87). The groups had similar scores for disgust (t(50) = −2.49, p = .02, d = −0.69), fear (t(50) = −2.05, p = .05, −0.57, d = −0.09), happiness, (t(50) = −.35, p = .73), and surprise, (t(50) = −.77, p = .44, d = −0.22).
Figure 3.
Mean number of items correctly identified by the TBI and uninjured groups for each emotion type.
Recognition Accuracy Across Intensity Levels
As shown in Table 6, analysis of the TBI by intensity level interaction indicated that after correcting for multiple comparisons (α = .05/4 = .01) the TBI group (collapsed across age groups) identified fewer items correctly than the combined group of older and younger uninjured participants in the 40%, 80%, and 100% intensity levels. The two groups scored similarly the 60% intensity condition.
Table 6.
Comparison of TBI and uninjured groups’ Emotion Recognition Task scores for each intensity level.
| Intensity Level | TBI Group Mean (SD) | Uninjured Group Mean (SD) | Test statistic, p-value, effect size |
|---|---|---|---|
| 40% | 9.65 (3.07) | 11.96 (2.18) | t(50) = −3.12, p = .003, d = −0.87 |
| 60% | 12.73 (2.22) | 13.88 (3.13) | t(50) = −1.53, p = .13, d = −0.42 |
| 80% | 14.23 (2.72) | 16.12 (2.55) | t(50) = −2.58, p = .01, d = −0.72 |
| 100% | 13.31 (2.65) | 16.08 (3.36) | t(50) = −3.30, p = .002, d = −0.92 |
The Influence of Intensity Across Emotions
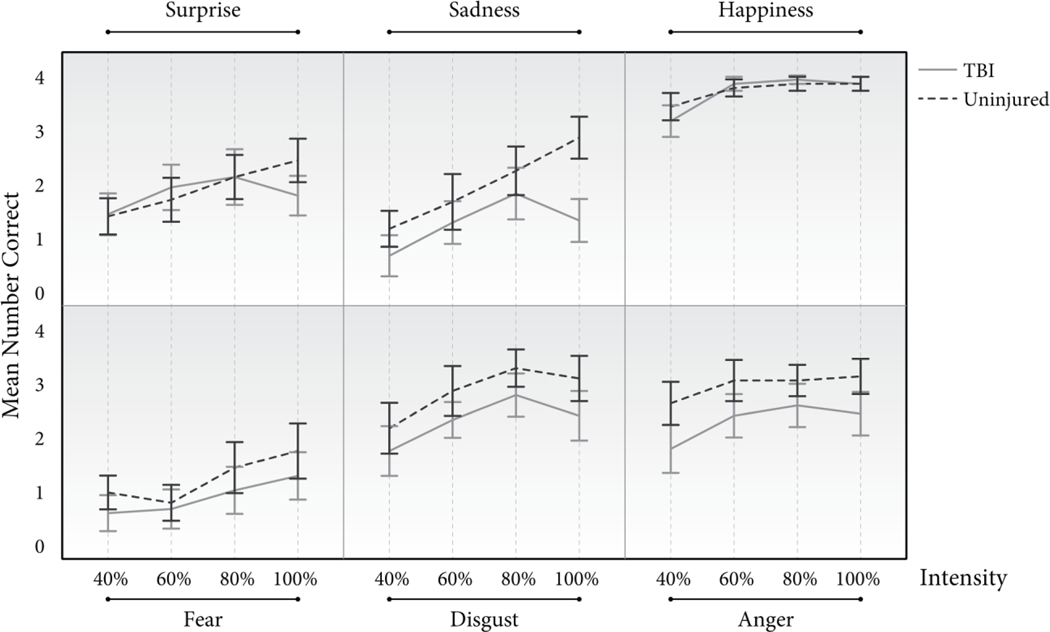
After collapsing data across TBI and age, inspection of the data for each emotion at each intensity level indicated that the data were not normally distributed therefore separate Friedman tests with post-hoc Wilcoxon Signed-Ranks tests were conducted to test for intensity effects for each emotion. Bonferroni correction was applied for post-hoc comparisons between intensity levels (α = .05/6 = .008). As shown in Figure 4, expression intensity affected recognition accuracy for each emotion type tested (Anger: χ2(3) = 14.24, p = .003; Disgust: χ2(3) = 29.83, p < .001; Fear: χ2(3) = 14.216.54, p = .001; Happiness: χ2(3) = 61.46, p < .001; Sadness: χ2(3) = 47.89, p < .001; Surprise: χ2(3) = 12.91, p = .01). Test statistics for post-hoc comparisons of performance on each intensity level for each emotion type are available in the supplemental materials. Post-hoc examination of “angry” items revealed that accuracy improved significantly once expression intensity reached at least 60% (40% vs. 60% intensity, Z = −3.03, p = .002), and no significant benefit was gained from increased intensity after 60%. Similar patterns were found for items featuring expressions of disgust (Z = −3.00, p = .003) and happiness (Z = −4.44, p < .001). Post-hoc testing of scores for items featuring “sad” expressions revealed a similar pattern to those previously described (Z = −3.30, p = .001), however participants also improved as the intensity increased from 60% to 80% (Z = −3.13, p = .002) and from 60% to 100% (Z = −3.25, p = .001). Examination of scores for fearful expressions indicated that participants needed a higher intensity to improve their accuracy than for the previously described emotions, as their median scores for 40% and 60% were similar (Z = −0.45, p = .65). Participants’ more accurately recognized fearful expressions as intensity increased from 60% to 80% (Z = −3.02, p = .003) of maximum intensity and from 40% to 100% (Z = −3.11, p = .002). Testing of expressions of surprise indicated that like results for expressions of fear, median accuracy did not improve from 40% to 60% (Z = −2.38, p = .02), however participants’ recognition accuracy did improve when they were presented with surprised expressions of at least 80% maximum intensity (40% vs. 80% intensity, Z = −3.37, p = .001).
Figure 4.
Mean number of items correctly identified by the TBI and uninjured groups for each emotion and intensity level, collapsed across age.
Discussion
Emotion recognition impairments are common in individuals with TBI and can hinder recovery of social functioning. This study represents an early effort to address the dearth of knowledge related to emotion recognition abilities of the growing population of older adults with TBI. Results of this preliminary investigation suggested that consistent with our hypotheses, and similar to previous findings, moderate-severe TBI and older age were both associated with poorer emotion recognition, as measured by the Emotion Recognition Task.
Our findings replicate our previous work33 and demonstrate that participants with TBI had lower emotion recognition scores overall, with greater group differences for certain negative emotions. We found in the combined sample of older and younger adults that individuals with TBI identified expressions of sadness and anger less accurately than uninjured participants. These findings were consistent with those of Rigon et al.33 except our TBI and uninjured comparison groups scored similarly on items featuring disgusted expressions while Rigon and colleagues33 reported that recognition of disgust was negatively affected by TBI. Findings regarding impairments in recognizing facial expressions of disgust are mixed in the aging literature40; 44, although the factors leading to these mixed results are not clear and warrant further investigation the population of older adults with TBI.
In addition to our hypotheses regarding the interaction between TBI and emotion type, we also expected older participants and participants with TBI to have poorer emotion recognition when facial expressions were very subtle. These hypotheses were not supported as we found that our combined TBI sample (older and younger participants) was less accurate on both subtle and intense conditions and no interaction was found between age and intensity level. The finding that adults with TBI may be impaired in recognizing both subtle expressions and intense, more prototypical emotion displays differs from those of Spell and Frank,26 who reported TBI-related impairments in recognizing only intense expressions. Our findings however, are line with those of Rigon, et al.33 and Rosenberg, et al.28 which suggested that adults with TBI have difficulty recognizing others’ very subtle emotional displays and don’t seem to benefit from increased intensity to the same degree as their uninjured peers. Both Rosenberg28 and Rigon33 and their colleagues however, found interactions between TBI, emotion, and intensity, while we did not find such an interaction. We had also expected an interaction between TBI, age and intensity such that older adults with TBI would be particularly impaired in their recognition of subtle displays. It is possible that our limited sample size as well as characteristics of our sample, namely the high average cognitive abilities of our older TBI group contributed to our ability to detect such relationships.
There exists a gap in our knowledge base regarding the effects of TBI on social cognition across the adult lifespan. The current study provides some of the first data on social cognition of older adults with TBI. In contrast to our prediction, we did not find an age by TBI interaction, although the older TBI group had the lowest Emotion Recognition Task performance. While sample size and characteristics may have contributed to this outcome (see below), these data offer a starting point for future investigations and an opportunity to integrate findings with work from the cognitive aging literature. For example, according to the Socioemotional Selectivity Theory of Aging65 healthy adults become more selective in their social efforts and relationships with age, in attempt to optimize positive experiences and limit negative interactions.65; 66 Support for the Socioemotional Selectivity Theory of Aging comes from findings that later in life, healthy adults prioritize meaningful, positive social experiences and as a result show a bias toward positive information and poorer recognition of negative emotions with age.40; 41; 43; 66 It is unclear how TBI across the adult lifespan may interfere, if at all, with these biases and at which levels of social and emotional processing (e.g., facial affect recognition, conversational interactional). For example, is it possible that impairments in the identification of expressions of certain negative emotions could be interpreted as evidence of such a bias toward positive facial emotions across the population of adults with TBI. The older adults in our sample were injured during adulthood, and perhaps had already begun to develop social information processing strategies that favor positive stimuli. This example interpretation is speculative, but highlights the interdisciplinary connections that are ripe for future study. More nuanced investigation is needed to evaluate the characteristics of individual emotion expressions that might lead to certain emotions being “easier” for older adults with TBI to recognize than others.28 Replication with larger samples that allow for more complex statistical analysis would inform not only a more complete understanding of the social cognitive effects of TBI on older adults, but could also inform potential treatment targets.
Limitations and Future Directions
While our findings add preliminary information about emotion recognition of older adults with TBI, study limitations must be considered. We had predicted that for older adults with TBI, the combined effects of age and TBI would result in lower emotion recognition scores than those of younger adults with TBI and uninjured age-matched participants. This hypothesis was not supported in this sample. The group of older adults with TBI had the lowest average emotion recognition scores of our four groups, but only differed significantly from the younger uninjured group. Study characteristics might have limited our ability to detect differences between our older TBI group and the groups of typically aging older adults and younger adults with TBI. First, while each of our group comparisons on overall Emotion Recognition Task scores were in the predicted direction (i.e. younger adults with and without TBI and older uninjured adults > older adults with TBI), our individual groups were small. Although we found significant age and TBI effects in the sample as a whole, we may have lacked sufficient power to detect differences between individual groups. Second, our ability to detect group differences was also affected by the large within-group variability evident in the older and younger TBI groups’ Emotion Recognition Task scores. The variability observed in our TBI groups likely reflects the heterogeneity that characterizes the TBI population and is consistent with Babbage and colleagues’ 2011 meta-analysis,22 which showed that emotion recognition was significantly more variable within samples of adults with TBI than in comparison groups.
Our study sample also had limitations. Despite restricting our TBI sample to individuals with moderate-severe injuries, the older and younger TBI groups might have differed in terms of injury characteristics. As noted previously, the majority of the older adults with TBI in our sample were injured as the result of a fall, while motor vehicle accidents were the most common cause of injury among younger adults. Differences in injury mechanism between the younger and older groups could suggest that the younger adult group might have sustained more severe injuries -- and as a result more severe cognitive impairments -- due to the higher velocity nature of motor vehicle accidents versus falls. If this was indeed the case with our sample, it is possible that age effects within the TBI group were masked by greater injury-related cognitive impairments among the younger versus older participants. Conclusions about possible group differences in the severity and cognitive effects of TBI based on differences in injury mechanisms alone must be made with caution, however. TBI has been shown to affect older and younger adults differently; with less positive outcomes reported in older adults, even when older adults have been judged to have less severe injuries resulting from lower velocity events.55–57 For example, Susman and colleagues 57 examined data from a large trauma registry and found that older adults with TBI had higher mortality rates and poorer functional outcomes than younger adults with TBI, even though older adults tended to have less severe injuries and higher neurologic function at admission than younger adults. Salottolo and colleagues 55 also examined a large group of adults with TBI and also found that older adults had poorer outcomes after TBI, including higher mortality rates, than younger adults, despite being more likely to be injured in a fall, and having better neurologic functioning upon admission. These authors described age-related physiological (e.g., decreased inflammation response to injury) and anatomical (age-related cerebral atrophy) changes that could result in blunted or delayed effects of TBI among older adults, which could lead to underestimation of injury severity among older individuals. 55 Like in our sample, both Susman 57 Salottolo 55 and their colleagues reported that falls were the most common cause of injury in older adults, while motor vehicle accidents were most common among younger adults, indicating that older adults might have worse TBI-related outcomes than younger adults even when their injuries were caused by lower velocity events. This study is an early attempt to understand how these age-related differences after TBI might affect social cognitive functioning. While our findings suggest that older adults are at risk for emotion recognition impairments, even after sustaining low-velocity moderate-severe TBI, further research is needed to delineate the interaction between injury characteristics, age, and social cognitive outcomes.
An additional limitation of this study was its relatively young older adult groups. Adults over age 60 have been shown to have poorer emotion recognition than younger adults, however further age-related declines have been reported between age 60 and age 80.42 Additional studies that include adults with TBI into and beyond their seventh decade will provide needed information about social cognitive abilities across the lifespan, information that is much needed as TBI is increasingly viewed as a chronic health condition.67
Further, the population of adults with TBI is highly variable. This was also true within and between our older and younger TBI groups. We were particularly interested in the interacting effects of TBI and age, and thus our groups’ variability in the age at injury and time-post injury must be considered. While our participants were injured as adults, social functioning develops across the lifespan41; 66 and thus, outcomes in social functioning might be expected to be different for individuals injured during young adulthood versus at age 70. Social outcomes might also be expected to differ depending on the time-post injury, as individuals might experience greater recovery as their injuries become more remote. Alternately, however, individuals with TBI may experience social isolation, which could result in poorer social functioning as time-post injury increases.
Another important next step in understanding the social cognition of older adults with TBI is to examine performance on more ecologically valid tasks. Task consideration may be especially important for future investigations of emotion recognition of older adults with TBI because while there is evidence that younger adults with TBI do more poorly on tasks with rich contextual cues,20 the typically aging literature suggests that with age we rely more heavily on contextual cues to interpret social information.41; 68; 69 Additional work using more naturalistic measures of social cue perception and interpretation are needed to delineate the interaction between effects of age and TBI for older adults.
As the number of older adults with TBI increases, understanding the interacting effects of injury and age is critical to providing individualized rehabilitation to those affected. Social cognitive impairments have been well documented in the younger adult TBI literature and findings from this study begin to extend this work to older adults. Further studies on this older population will be critical to understanding and caring for individuals with TBI across the lifespan.
Supplementary Material
Acknowledgements
The authors thank Dr. Erica Richmond and the student researchers on the Social Building Blocks Project for their assistance with data collection and scoring.
Declaration of interest
This manuscript was prepared in part at the William S. Middleton Memorial Veterans Hospital in Madison, WI; GRECC manuscript No. 003-2019. The views and content expressed in this article are solely the responsibility of the authors and do not necessarily reflect the position, policy, or official views of the Department of Veteran Affairs or the U.S. government. This work was supported by NICHD/NCMRR grant R01 HD071089. The authors have no conflicts of interest to report.
References
- 1.Ao BT, Tobias M, Ameratunga S, McPherson K, Theadom A, Dowell A, Starkey N, Jones K, Barker-Collo S, Brown P et al. 2015. Burden of traumatic brain injury in new zealand: Incidence, prevalence and disability-adjusted life years. Neuroepidemiology. 44(4):255–261. [DOI] [PubMed] [Google Scholar]
- 2.Miekisiak G, Czyz M, Tykocki T, Kaczmarczyk J, Zaluski R, Latka D. 2016. Traumatic brain injury in poland from 2009–2012: A national study on incidence. Brain Injury. 30(1):79–82. [DOI] [PubMed] [Google Scholar]
- 3.Taylor CA, Bell JM, Breiding MJ, Xu L. 2017. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths -- united states, 2007 and 2013. MMWR Surveillance Summaries. 66(9):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roozenbeek B, Maas AI, Menon DK. 2013. Changing patterns in the epidemiology of traumatic brain injury. Nature Reviews Neurology. 9(4):231–236. [DOI] [PubMed] [Google Scholar]
- 5.Faul M, Xu L, Wald MM, Coronado VG. 2010. Traumatic brain injury in the united states: Emergency department visits, hospitalization,s and deaths. Atlanta, GA: Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. [Google Scholar]
- 6.Ortman JM, Velkoff VA, Hogan H. 2014. An aging nation: The older population in the united states. Washington, DC: US Census Bureau.25–1140. [Google Scholar]
- 7.Galski T, Tompkins C, Johnston MV. 1998. Competence in discourse as a measure of social integration and quality of life in persons with traumatic brain injury. Brain Injury. 12(9):769–782. [DOI] [PubMed] [Google Scholar]
- 8.Morton MV, Wehman P. 1995. Psychosocial and emotional sequelae of individuals with traumatic brain injury: A literature review and recommendations. Brain Injury. 9(1):81–92. [DOI] [PubMed] [Google Scholar]
- 9.Oddy M, Coughlan T, Tyerman A, Jenkins D. 1985. Social adjustment after closed head injury: A further follow-up seven years after injury. Journal of Neurology, Neurosurgery & Psychiatry. 48(6):564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomsen IV. 1984. Late outcome of very severe blunt head trauma: A 10–15 year second follow-up. Journal of Neurology, Neurosurgery, and Psychiatry. 47(3):260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoofien D, Gilboa A, Vakil E, Donovick PJ. 2001. Traumatic brain injury (tbi) 10?20 years later: A comprehensive outcome study of psychiatric symptomatology, cognitive abilities and psychosocial functioning. Brain Injury. 15(3):189–209. [DOI] [PubMed] [Google Scholar]
- 12.Oddy M, Humphrey M. 1980. Social recovery during the year following severe head injury. Journal of Neurology, Neurosurgery and Psychiatry. 43(9):798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weddell R, Oddy M, Jenkins D. 1980. Social adjustment after rehabilitation: A two year follow-up of patients with severe head injury. Psychological Medicine. 10(2):257–263. [DOI] [PubMed] [Google Scholar]
- 14.Cornwell EY, Waite LJ. 2009. Social disconnectedness, perceived isolation, and health among older adults. Journal of Health and Social Behavior. 50(1):31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomaka J, Thompson S, Palacios R. 2006. The relation of social isolation, loneliness, and social support to disease outcomes among the elderly. Journal of Aging and Health. 18(3):359–384. [DOI] [PubMed] [Google Scholar]
- 16.Beauchamp M, Catroppa C, Godfrey C, Morse S, Rosenfeld JV, Anderson V. 2011. Selective changes in executive functioning ten years after severe childhood traumatic brain injury. Developmental Neuropsychology. 36(5):578–595. [DOI] [PubMed] [Google Scholar]
- 17.Yeates KO, Bigler ED, Dennis M, Gerhardt CA, Rubin KH, Stancin T, Taylor HG, Vannatta K. 2007. Social outcomes in childhood brain disorder: A heuristic integration of social neuroscience and developmental psychology. Psychological Bulletin. 133(3):535–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watts AJ, Douglas JM. 2006. Interpreting facial expression and communication competence following severe traumatic brain injury. Aphasiology. 20(8):707–722. [Google Scholar]
- 19.Spikman JM, Milders M, Visser-Keizer AC, Westerhof-Evers HJ, Herben-Dekker M, van der Naalt J. 2013. Deficits in facial emotion recognition indicate behavioral changes and impaired self-awareness after moderate to severe traumatic brain injury. PLOS ONE. 8(6):e65581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knox L, Douglas J. 2009. Long-term ability to interpret facial expression after traumatic brain injury and its relation to social integration. Brain and Cognition. 69(2):442–449.18951674 [Google Scholar]
- 21.Rosenberg H, McDonald S, Rosenberg J, Frederick Westbrook R. 2016. Amused, flirting or simply baffled? Is recognition of all emotions affected by traumatic brain injury? Journal of Neuropsychology. [DOI] [PubMed] [Google Scholar]
- 22.Babbage DR, Yim J, Zupan B, Neumann D, Tomita MR, Willer B. 2011. Meta-analysis of facial affect recognition difficulties after traumatic brain injury. Neuropsychology. 25(3):277–285. [DOI] [PubMed] [Google Scholar]
- 23.Biszak AM, Babbage DR. 2014. Facial affect recognition difficulties in traumatic brain injury rehabilitation services. Brain Injury. 28(1):97–104. [DOI] [PubMed] [Google Scholar]
- 24.Ietswaart M, Milders M, Crawford JR, Currie D, Scott CL. 2008. Longitudinal aspects of emotion recognition in patients with traumatic brain injury. Neuropsychologia. 46(1):148–159. [DOI] [PubMed] [Google Scholar]
- 25.Milders M, Ietswaart M, Crawford JR, Currie D. 2008. Social behavior following traumatic brain injury and its association with emotion recognition, understanding of intentions, and cognitive flexibility. Journal of the International Neuropsychological Society. 14:318–326. [DOI] [PubMed] [Google Scholar]
- 26.Spell LA, Frank E. 2000. Recognition of nonverbal communication of affect following traumatic brain injury. Journal of Nonverbal Behavior. 24(4):285–300. [Google Scholar]
- 27.Zupan B, Neumann D. 2014. Affect recognition in traumatic brain injury: Responses to unimodal and multimodal media. The Journal of Head Trauma Rehabilitation. 29(4):E1–E12. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg H, McDonald S, Dethier M, Kessels RPC, Westbrook RF. 2014. Facial emotion recognition deficits following moderate–severe traumatic brain injury (tbi): Re-examining the valence effect and the role of emotion intensity. Journal of the International Neuropsychological Society. 20(10):994–1003. [DOI] [PubMed] [Google Scholar]
- 29.McDonald S, Flanagan S. 2004. Social perception deficits after traumatic brain injury: Interaction between emotion recognition, mentalizing ability, and social communication. Neuropsychology. 18(3):572–579. [DOI] [PubMed] [Google Scholar]
- 30.McDonald S, Saunders JC. 2005. Differential impairment in recognition of emotion across different media in people with severe traumatic brain injury. Journal of the International Neuropsychological Society. 11(4):392–399. [DOI] [PubMed] [Google Scholar]
- 31.Croker V, McDonald S. 2005. Recognition of emotion from facial expression following traumatic brain injury. Brain Injury. 19(10):787–799. [DOI] [PubMed] [Google Scholar]
- 32.Milders M, Fuchs S, Crawford JR. 2003. Neuropsychological impairments and changes in emotional and social behaviour following severe traumatic brain injury. Journal of Clinical and Experimental Neuropsychology. 25(2):157–172. [DOI] [PubMed] [Google Scholar]
- 33.Rigon A, Turkstra L, Mutlu B, Duff M. 2016. The female advantage: Sex as a possible protective factor against emotion recognition impairment following traumatic brain injury. Cognitive, Affective, & Behavioral Neuroscience. 16(5):866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Montagne B, Kessels RP, De Haan EH, Perrett DI. 2007. The emotion recognition task: A paradigm to measure the perception of facial emotional expressions at different intensities. Perceptual and Motor Skills. 104(2):589–598. [DOI] [PubMed] [Google Scholar]
- 35.Draper K, Ponsford J. 2008. Cognitive functioning ten years following traumatic brain injury and rehabilitation. Neuropsychology. 22(5):618–625. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein FC, Levin HS. 2001. Cognitive outcome after mild and moderate traumatic brain injury in older adults. Journal of Clinical and Experimental Neuropsychology. 23(6):739–753. [DOI] [PubMed] [Google Scholar]
- 37.Mathias JL, Wheaton P. 2007. Changes in attention and information-processing speed following severe traumatic brain injury: A meta-analytic review. Neuropsychology. 21(2):212–223. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy MRT, Wozniak JR, Muetzel RL, Mueller BA, Chiou H-H, Pantekoek K, Lim KO. 2009. White matter and neurocognitive changes in adults with chronic traumatic brain injury. Journal of the International Neuropsychological Society. 15(1):130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sendroy-Terrill M, Whiteneck GG, Brooks CA. 2010. Aging with traumatic brain injury: Cross-sectional follow-up of people receiving inpatient rehabilitation over more than 3 decades. Archives of Physical Medicine and Rehabilitation. 91(3):489–497. [DOI] [PubMed] [Google Scholar]
- 40.Sarabia-Cobo MC, Navas MJ, Ellgring H, García-Rodríguez B. 2016. Skilful communication: Emotional facial expressions recognition in very old adults. International Journal of Nursing Studies. 54:104–111. [DOI] [PubMed] [Google Scholar]
- 41.Sze JA, Goodkind MS, Gyurak A, Levenson RW. 2012. Aging and emotion recognition: Not just a losing matter. Psychology and Aging. 27(4):940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.West JT, Horning SM, Klebe KJ, Foster SM, Cornwell RE, Perrett D, Burt DM, Davis HP. 2012. Age effects on emotion recognition in facial displays: From 20 to 89 years of age. Experimental Aging Research. 38(2):146–168. [DOI] [PubMed] [Google Scholar]
- 43.Ma Z, Li J, Niu Y, Yu J, Yang L. 2013. Age differences in emotion recognition between chinese younger and older adults. The Psychological Record. 63:629–640. [Google Scholar]
- 44.Ruffman T, Henry JD, Livingstone V, Philips LH. 2008. A meta-analytic review of emotion recognition and aging: Implications for neuropsychological models of aging. Neuroscience and Biobehavioral Reviews. 32:863–881. [DOI] [PubMed] [Google Scholar]
- 45.Sullivan S, Ruffman T. 2004. Emotion recognition deficits in the elderly. International Journal of Neuroscience. 114(3):403–432. [DOI] [PubMed] [Google Scholar]
- 46.Lambrecht L, Kreifelts B, Wildgruber D. 2012. Age-related decrease in recognition of emotional facial and prosodic expressions. Emotion. 12(3):529–539. [DOI] [PubMed] [Google Scholar]
- 47.Kessels RP, Montagne B, Hendriks AW, Perrett DI, de Haan EH. 2013. Assessment of perception of morphed facial expressions using the emotion recognition task: Normative data from healthy participants aged 8–75. Journal of Neuropsychology. 8(1): 75–93. [DOI] [PubMed] [Google Scholar]
- 48.Merkley TL, Larson MJ, Bigler ED, Good DA, Perlstein WM. 2013. Structural and functional changes of the cingulate gyrus following traumatic brain injury: Relation to attention and executive skills. Journal of the International Neuropsychological Society. 19(8):899–910. [DOI] [PubMed] [Google Scholar]
- 49.Wood RL. 2017. Accelerated cognitive aging following severe traumatic brain injury: A review. Brain Injury. 31(10):1270–1278. [DOI] [PubMed] [Google Scholar]
- 50.Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. 2012. Cognitive decline in older adults with a history of traumatic brain injury. The Lancet Neurology. 11(12):1103–1112. [DOI] [PubMed] [Google Scholar]
- 51.Smith C. 2013. Review: The long‐term consequences of microglial activation following acute traumatic brain injury. Neuropathology and Applied Neurobiology. 39(1):35–44. [DOI] [PubMed] [Google Scholar]
- 52.Corkin S, Rosen TJ, Sullivan EV, Clegg RA. 1989. Penetrating head injury in young adulthood exacerbates cognitive decline in later years. Journal of Neuroscience. 9(11):3876–3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. 2006. Longitudinal cognitive changes in traumatic brain injury: A 30-year follow-up study. Neurology. 66(2):187–192. [DOI] [PubMed] [Google Scholar]
- 54.Senathi-Raja D, Ponsford J, Schönberger M. 2010. Impact of age on long-term cognitive function after traumatic brain injury. Neuropsychology. 24(3):336. [DOI] [PubMed] [Google Scholar]
- 55.Salottolo K, Levy AS, Slone DS, Mains CW, Bar-Or D. 2014. The effect of age on glasgow coma scale score in patients with traumatic brain injury. JAMA surgery. 149(7):727–734. [DOI] [PubMed] [Google Scholar]
- 56.Mosenthal AC, Livingston DH, Lavery RF, Knudson MM, Lee S, Morabito D, Manley GT, Nathens A, Jurkovich G, Hoyt DB et al. 2004. The effect of age on functional outcome in mild traumatic brain injury: 6-month report of a prospective multicenter trial. Journal of Trauma and Acute Care Surgery. 56(5):1042–1048. [DOI] [PubMed] [Google Scholar]
- 57.Susman M, DiRusso SM, Sullivan T, Risucci D, Nealon P, Cuff S, Haider A, Benzil D. 2002. Traumatic brain injury in the elderly: Increased mortality and worse functional outcome at discharge despite lower injury severity. Journal of Trauma and Acute Care Surgery. 53(2):219–224. [DOI] [PubMed] [Google Scholar]
- 58.Shewan CM, Kertesz A. 1980. Reliability and validity characteristics of the western aphasia battery (wab). The Journal of Speech and Hearing Disorders. 45(3):308–324. [DOI] [PubMed] [Google Scholar]
- 59.Malec JF, Brown AW, Leibson CL, Flaada JT, Mandrekar JN, Diehl NN, Perkins PK. 2007. The mayo classification system for traumatic brain injury severity. Journal of Neurotrauma. 24(9):1417–1424. [DOI] [PubMed] [Google Scholar]
- 60.Wilde EA, Whiteneck GG, Bogner J, Bushnik T, Cifu DX, Dikmen S, French L, Giancino JT, Hart T, Malec JF et al. 2010. Recommendations for the use of common outcome measures in truamatic brain injury research. Archives of Physical Medicine and Rehabilitation. 91:1650–1660.e1616. [DOI] [PubMed] [Google Scholar]
- 61.Delis DC, Freeland J, Kramer JH, Kaplan E. 1988. Integrating clinical assessment with cognitive neuroscience: Construct validation of the california verbal learning test. Journal of Consulting and Clinical Psychology. 56(1):123–130. [DOI] [PubMed] [Google Scholar]
- 62.Gordon NG. 1972. The trail making test in neuropsychological diagnosis. Journal of Clinical Psychology. 28(2):167–169. [DOI] [PubMed] [Google Scholar]
- 63.Wechsler D. 2008. Wechsler Adult Intelligence Scale. San Antonio, TX: Pearson. [Google Scholar]
- 64.IBM. 2015. SPSS Statistics for Macintosh. 23.0.0.0 ed.: IBM. [Google Scholar]
- 65.Carstensen LL, Isaacowitz DM, Charles ST. 1999. Taking time seriously: A theory of socioemotional selectivity. American Psychologist. 54(3):165–181. [DOI] [PubMed] [Google Scholar]
- 66.Carstensen LL, Mikels JA. 2005. At the intersection of emotion and cognition: Aging and the positivity effect. Current Directions in Psychological Science. 14(3):117–121. [Google Scholar]
- 67.Masel BE, DeWitt DS. 2010. Traumatic brain injury: A disease process, not an event. J Neurotrauma. 27(8):1529–1540. [DOI] [PubMed] [Google Scholar]
- 68.Morrow DG, Ridolfo HE, Menard WE, Sanborn A, Stine-Morrow EA, Magnor C, Herman L, Teller T, Bryant D. 2003. Environmental support promotes expertise-based mitigation of age differences on pilot communication tasks. Psychology and Aging. 18(2):268. [DOI] [PubMed] [Google Scholar]
- 69.Noh SR, Isaacowitz DM. 2013. Emotional faces in context: Age differences in recognition accuracy and scanning patterns. Emotion. 13(2):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.