Abstract
Background
Vaginal discharge syndrome (VDS) is a common clinical diagnosis during pregnancy in Botswana; it is treated with broad-spectrum antibiotics using a syndromic approach. We evaluated associations between the syndromic management of VDS and adverse birth outcomes.
Methods
The Tsepamo Study performs birth outcomes surveillance at government hospitals throughout Botswana. Obstetric record data collected from August 2014 to March 2019 were analyzed. Chi-square tests were conducted to compare proportions of maternal characteristics and infant outcomes. To avoid immortal time bias, all analyses were conducted among women who presented to care before 24 weeks gestation, with VDS categorized as present or absent by 24 weeks gestation. Log-binomial regression models were generated to determine associations between treated VDS and infant outcomes.
Results
VDS was diagnosed in 36 731 (30.7%) pregnant women, of whom 33 328 (90.7%) received antibiotics. Adjusted analyses yielded a harmful association between treated VDS and very preterm delivery (adjusted risk ratio, 1.11; 95% CI, 1.02–1.21). This association remained when restricting to women with VDS who received the recommended antibiotic treatment regimen. Sensitivity analyses produced nonsignificant associations when women with treated VDS were compared with women without VDS who received antibiotics for other indications.
Conclusions
A clinical diagnosis of VDS is common among pregnant women in Botswana, and the majority receive antibiotics in pregnancy. Although analyses of VDS occurring later in pregnancy are precluded by immortal time bias, a modest association between treated VDS and very preterm delivery was observed among women diagnosed with VDS by 24 weeks gestation.
Keywords: antibiotics, infant, pregnancy, vaginal discharge
Untreated sexually transmitted infections (STIs) during pregnancy are associated with an increased risk of adverse birth outcomes, including spontaneous abortion, preterm delivery, and stillbirth [1–5]. A lack of available laboratory tests for STIs in many resource-limited settings led to the adoption of the World Health Organization (WHO) syndromic management strategy in the 1990s [6–8]. This symptom-based treatment strategy is less expensive than laboratory testing and can lead to faster treatment, but it is neither sensitive nor specific and leads to high levels of antibiotic use among pregnant women [1, 6, 9, 10].
Botswana adopted the WHO recommendations for a symptom-driven approach to the diagnosis and treatment of STIs in pregnancy in 1992 [10]. Women who report symptoms of vaginal discharge syndrome (VDS) in pregnancy are empirically treated with antibiotics to cover common STIs (Chlamydia trachomatis, Neisseria gonorrhea, Trichomonas vaginalis) and bacterial vaginosis; syphilis is tested for and treated separately [11–13]. These recommendations are based on the high prevalence of STIs reported among pregnant women in Botswana, including chlamydia (8%–10%), gonorrhea (1.5%–3%), trichomonas vaginalis (5%–19%), and bacterial vaginosis (38%) [6, 14].
STIs during pregnancy have been associated with adverse birth outcomes that may be improved with antibiotics [15, 16]. While a randomized controlled trial in Uganda reported that receiving treatment for symptomatic and asymptomatic cases of STIs resulted in lower rates of adverse birth outcomes compared with syndromic STI treatment, the effects of VDS and syndromic treatment with antibiotics on adverse birth outcomes are less understood [17]. Concerns regarding antibiotic use in pregnancy relate to unknown teratogenicity, antimicrobial resistance, and microbiome effects [18–21], but favorable birth outcomes have also been reported in the setting of treating asymptomatic bacteriuria [22, 23]. This study utilized data from a large birth outcomes surveillance program (the Tsepamo Study) to evaluate associations between the syndromic treatment of VDS and adverse birth outcomes. We also highlight the potential for immortal time bias when addressing this research question.
METHODS
Study Population
We performed a secondary analysis of data from the Tsepamo Study, which captured birth outcomes at up to 18 maternity sites throughout Botswana. Before July 2018, data collection occurred at 8 government maternity wards (~45% of all births in Botswana) [24–26]. In 2018, the number of sites expanded to 18 maternity wards (~72% of all births in Botswana) [26, 27]. Data were extracted from obstetric records if births occurred within 1 of the study sites and at a gestational age of ≥24 weeks.
Data Extraction
Maternal obstetric records were reviewed at the time of discharge following delivery [24–27]. Extracted data included demographic information, maternal medical history, antenatal care information, inpatient care during labor and delivery, medical diagnoses made in pregnancy, prescribed antibiotic treatment, adverse birth outcomes, maternal HIV status, and HIV treatment information. Infant information included gestational age, birth weight, sex, and admission to the neonatal unit. With the exception of anemia (defined as hemoglobin ≤10 g/dL) and hypertension (defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg), all diagnoses were recorded by the treating physician or midwife. Maternal HIV status was determined by HIV test result documented in the maternity cards. Women with HIV who started antiretroviral therapy (ART) before the calculated date of the start of pregnancy were considered ART exposed from conception, and those who started ART after the start of pregnancy were considered ART started during pregnancy.
Vaginal Discharge Syndrome and Antibiotic Use
Botswana antenatal care (ANC) guidelines recommend that all pregnant women attend ANC visits every month until 28 weeks and additional ANC visits every 2 weeks until 36 weeks. After this period, pregnant women are recommended to attend weekly ANC visits until delivery [28]. Botswana ANC guidelines also recommend that pregnant women who attend ANC be screened for VDS using the WHO recommendation of syndromic approach criteria [28], and those with vaginal discharge alone or with lower abdominal pain are diagnosed with VDS (cervical infections are included in this definition) [13]. All women with a clinical documentation of VDS in the antenatal record were considered to have VDS for this analysis. Syphilis, herpes, genital sores/ulcers, genital warts, and candidiasis were diagnosed separately from VDS. According to Botswana STI treatment guidelines, the recommended treatment for VDS during the study period included ceftriaxone (250-mg intramuscular injection once), metronidazole (400 mg 3 times daily by mouth for 7 days, or 2 g once), and either erythromycin (before June 2016, 500 mg 4 times daily by mouth for 7 days) or azithromycin (1 g by mouth once) [13]. Women who were prescribed antibiotics for VDS and were noted to receive the prescribed course at least 1 time in pregnancy were considered “antibiotic exposed” regardless of the number of courses of antibiotics.
Adverse Birth Outcomes
Individual adverse birth outcomes were stillbirth, neonatal death, preterm delivery (PTD), very preterm delivery (VPTD), small for gestational age (SGA), and very small for gestational age (VSGA). “Any adverse birth outcome” consisted of births that resulted in PTD, SGA, stillbirth, or neonatal death. “Any severe adverse outcome” was defined as births that resulted in VPTD, stillbirth, VSGA, or neonatal death. Preterm delivery was classified as births at <37 weeks gestational age and very preterm delivery as births at <32 weeks gestational age. The Intergrowth-21 norms were used to define gestational age for infants born from 24 to 42 weeks gestational age [29, 30]. SGA was defined as infants who weighed less than the 10th percentile birth weight by gestational age. VSGA was defined as infants who weighed less than the third percentile birth weight by gestational age. Fetal death with Apgar scores of 0 at 1 and 5 minutes were considered stillbirths, and infant death before leaving the hospital within 28 days of delivery was classified as neonatal death. Health care providers used the last menstrual period to estimate the gestational age at the first antenatal clinic visit, and prenatal ultrasonography was used when available [24–26]. Fundal height was used when there was uncertainty in determining the gestational age by other means.
Statistical Analyses of VDS (and Exclusions to Avoid Immortal Time and Indication Bias)
For descriptive analyses, proportions of demographic factors were compared between women diagnosed with VDS and women without a VDS diagnosis any time during pregnancy using chi-square tests. However, for all comparative analyses, only women presenting to care by 24 weeks of pregnancy (the first time at which an outcome could occur in the data set) were included, and VDS status was determined at that baseline (VDS diagnosis needed to occur before 24 weeks of pregnancy). This exclusion prevented immortal time bias, which would otherwise occur if VDS status were assigned after a possible birth outcome, thus allowing “immortal time” to accrue before this assignment for the VDS group. In addition, because the vast majority of women with VDS received 1 or more antibiotics per national guidelines, we could not compare use or nonuse of antibiotics for VDS. Untreated women with VDS were considered “off guidelines” and potentially had more mild symptoms, and to avoid this indication bias, we only included women with antibiotic-treated VDS in most analyses.
Selected covariates included maternal age, education, occupation, parity, marital status, and antiretroviral therapy at conception. These covariates were chosen based on subject matter knowledge. A Wilcoxon rank-sum test was conducted to compare the median and interquartile range of maternal age between the 2 groups. Log-binomial regression models were used to obtain risk ratio estimates with 95% CIs for the primary adjusted analyses and sensitivity analyses. P values were computed using 2-sided tests with an α = .05 significance level. All statistical analyses were performed using SAS University Edition software (SAS Institute, Cary, NC, USA).
Patient Consent
The Health Research and Development Committee in Botswana and the institutional review board of Harvard T. H. Chan School of Public Health in Boston, Massachusetts, provided institutional approval for this study.
RESULTS
Maternal Characteristics
Between August 2014 and March 2019, 119 478 women delivered at the sites under surveillance. VDS was diagnosed among 36 731 (30.7%) of all pregnant women. The median maternal age (interquartile range [IQR]) for women with VDS was 25 (21–31) years compared with 27 (22–32) years for women without a VDS diagnosis (P < .0001) (Table 1). Women diagnosed with VDS were more likely to have secondary/tertiary education (93.3% vs 88.8%; P < .0001), to have a salaried occupation (35.4% vs 32.5%; P < .0001), to be single (88.9% vs 84.9%; P < .0001), and to be primiparous (42.6% vs 36.5%; P < .0001) compared with women not diagnosed with VDS. The proportions of women with HIV were similar between the 2 groups (24.6% vs 23.9%; P = .09). Women diagnosed with VDS were more likely to receive antibiotics for any indication during the first trimester compared with women who were not diagnosed with VDS (14.3% vs 1.1%; P < .0001).
Table 1.
Maternal Characteristics by VDS Diagnosis During Pregnancy
| Characteristics | VDS | No VDS | P Value |
|---|---|---|---|
| (n = 36 731), No. (%) | (n = 82 747), No. (%) | ||
| Maternal age, median (IQR),a y | 25 (21–31) | 27 (22–32) | <.0001 |
| Education | <.0001 | ||
| Primary/none | 2101 (5.7) | 6690 (8.1) | |
| Secondary/tertiary education | 34 253 (93.3) | 73 444 (88.8) | |
| Missing | 377 (1.0) | 2613 (3.1) | |
| Occupation | <.0001 | ||
| Nonsalaried/housewife | 19 787 (53.9) | 46 554 (56.3) | |
| Student | 2901 (7.9) | 5070 (6.1) | |
| Salaried | 12 992 (35.4) | 26 907 (32.5) | |
| Missing | 1051 (2.9) | 4216 (5.1) | |
| Marital status | <.0001 | ||
| Single | 32 653 (88.9) | 70 293 (84.9) | |
| Married | 3256 (8.9) | 9213 (11.1) | |
| Divorced/widowed | 104 (0.3) | 256 (0.3) | |
| Missing | 617 (1.7) | 2985 (3.6) | |
| Nationality | <.0001 | ||
| Botswana citizen | 35 965 (97.9) | 79 206 (95.7) | |
| Other | 708 (1.9) | 3190 (3.8) | |
| Missing | 58 (0.16) | 368 (0.42) | |
| Parity | <.0001 | ||
| Primiparous | 15 640 (42.6) | 30 213 (36.5) | |
| Multiparous 2–4 births | 18 626 (50.7) | 43 892 (53.0) | |
| Grand multiparous ≥5 births | 2432 (6.6) | 8260 (10.0) | |
| Missing | 33 (0.09) | 382 (0.5) | |
| HIV status | .09 | ||
| Positive | 9034 (24.6) | 19 802 (23.9) | |
| Negative | 27 618 (75.2) | 62 073 (75.0) | |
| Unknown | 79 (0.22) | 872 (1.1) | |
| Anemia during pregnancyb | .01 | ||
| Yes | 6745 (18.4) | 12 880 (15.6) | |
| No | 19 659 (53.5) | 39 172 (47.3) | |
| Missing | 10 327 (28.1) | 30 695 (37.1) | |
| Clinical visits, median (IQR) | 9 (6–10) | 8 (6–10) | <.0001 |
| Any antibiotics | <.0001 | ||
| Yes | 33 339 (90.8) | 12 658 (15.3) | |
| No | 3383 (9.2) | 65 408 (79.0) | |
| Missing | 9 (0.02) | 4681 (5.7) | |
| Timing of first antibiotic use | <.0001 | ||
| First trimester | 5240 (14.3) | 885 (1.1) | |
| Second trimester | 20 053 (54.6) | 6049 (7.3) | |
| Third trimester | 7388 (20.1) | 5277 (6.4) | |
| No antibiotic use | 3383 (9.2) | 65 408 (79.0) | |
| Missing | 667 (1.8) | 5128 (6.5) |
Percentages may not add to 100% due to rounding. Chi-square tests were used for comparing proportions.
Abbreviations: IQR, interquartile range; VDS, vaginal discharge syndrome.
aWilcoxon rank-sum test was used to compare median ages.
bAnemia was defined as hemoglobin measurement ≤10 g/dL.
Antibiotic Use
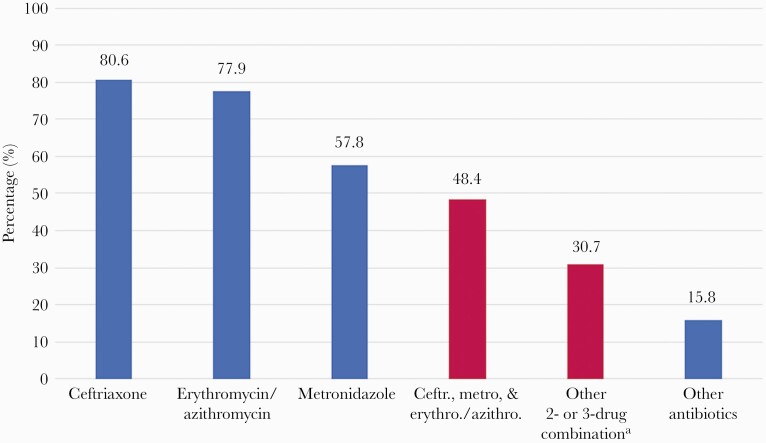
Antibiotics were prescribed in 45 997 (38.5%) of all pregnancies, including in 33 339 (90.8%) of the 36 731 women diagnosed with VDS. VDS therefore accounted for 72.5% of all antibiotic use in pregnancy. Among women diagnosed with VDS during pregnancy, 29 609 (80.6%) received any ceftriaxone, 21 226 (57.8%) received any metronidazole, and 28 613 (77.9%) received either any azithromycin or any erythromycin (Figure 1). Among the women diagnosed with VDS, 17 761 (48.4%) received a combination of ceftriaxone, metronidazole, and either azithromycin or erythromycin, according to the Botswana guidelines for syndromic management. Among women who were diagnosed with VDS before 24 weeks (n = 20 425), 18 546 (90.8%) received any ceftriaxone, 11 927 (58.4%) received any metronidazole, 17 667 (86.5%) received any azithromycin/erythromycin, and 9919 (48.6%) received the recommended combined treatment regimen.
Figure 1.
Proportions of any specific antibiotic used among women with VDS. aOther combinations included erythromycin/azithromycin and ceftriaxone (n = 8721, 23.7%), erythromycin/azithromycin and metronidazole (n = 1129, 3.1%), and ceftriaxone and metronidazole (n = 1450, 3.9%). There were 9 women with VDS who were missing antibiotics information (0.02%). Abbreviation: VDS, vaginal discharge syndrome.
VDS and Adverse Birth Outcomes
Among 91 470 women who presented to care before 24 weeks gestation, 20 425 (22.3%) received syndromic treatment for VDS by 24 weeks and 71 045 (77.7%) did not; the proportion of women with PTD (15.6% vs 15.5%), VPTD (3.7% vs 3.5%), stillbirth (2.2% vs 2.3%), neonatal death (1.2% vs 1.3%), VSGA (6.0% vs 6.2%), any adverse birth outcome (30.1% vs 30.2%), and any severe adverse birth outcome (5.4% vs 5.3%) did not differ significantly for these groups (all P > .05) (Table 2). In the unadjusted log-binomial regression models, VDS demonstrated an apparent nonsignificant association on each adverse birth outcome (Table 2). When adjusted for potential confounders, the risk ratios did not change appreciatively, with the exception of VPTD, which was positively associated with VPTD (adjusted risk ratio [aRR], 1.11; 95% CI, 1.02–1.21). When stratified by HIV status, the adjusted association of treated VDS with stillbirth among women with HIV was borderline protective (aRR, 0.80; 95% CI, 0.65–0.99). However, this association may be due to chance.
Table 2.
Proportions and Log-Binomial Regression Models for the Association Between VDS Diagnosis and Treatment and Adverse Birth Outcomes Among Women Presenting to Care at <24 Weeks Gestation (n = 91 470)
| Adverse Birth Outcome | VDS Treatment | No VDS Treatment | RR | aRRa |
|---|---|---|---|---|
| (n = 71 045), No. (%) | (95% CI) | (95% CI) | ||
| (n = 20 425), No. (%) | ||||
| Preterm delivery | 3185 (15.6) | 10 987 (15.5) | 1.01 (0.97–1.05) | 1.02 (0.99–1.06) |
| Very preterm delivery | 765 (3.7) | 2470 (3.5) | 1.08 (0.99–1.17) | 1.11 (1.02–1.21) |
| Stillbirth | 441 (2.2) | 1640 (2.3) | 0.94 (0.84–1.04) | 0.98 (0.88–1.09) |
| Neonatal death | 250 (1.2) | 898 (1.3) | 0.97 (0.84–1.11) | 0.97 (0.84–1.13) |
| SGA | 3305 (16.2) | 11 628 (16.4) | 0.99 (0.95–1.02) | 0.99 (0.95–1.03) |
| VSGA | 1233 (6.0) | 4402 (6.2) | 0.97 (0.91–1.03) | 0.99 (0.93–1.05) |
| Any adverse birth outcomeb | 6152 (30.1) | 21 433 (30.2) | 1.00 (0.98–1.02) | 1.00 (0.98–1.03) |
| Any severe adverse birth outcomec | 1106 (5.4) | 3792 (5.3) | 1.01 (0.95–1.08) | 1.04 (0.97–1.11) |
Abbreviations: aRR, adjusted risk ratio; RR, risk ratio; SGA, small for gestational age (<10th percentile of birth weight by gestational age); VDS, vaginal discharge syndrome; VSGA, very small for gestational age (<third percentile of birth weight by gestational age).
aModels were adjusted for maternal age, education, occupation, parity, marital status, nationality, and antiretroviral therapy at conception.
bAny adverse outcome includes preterm delivery (<37 weeks gestational age), small for gestational age (<10th percentile of birth weight by gestational age), neonatal death (infant death <28 days), and stillbirth (summed Apgar score of 0).
cAny severe adverse outcome includes very preterm delivery (<32 weeks), very small for gestational age (<third percentile of birth weight by gestational age), neonatal death, and stillbirth.
Sensitivity Analyses
We performed 3 sensitivity analyses to better explore the relationship between the syndromic management of VDS and adverse birth outcomes. The first sensitivity analysis included women who received antibiotics for VDS before 24 weeks (n = 20 425) and women who received antibiotics for other indications before 24 weeks (n = 5093) (Table 3). When adjusting for potential confounders, all associations were nonsignificant. The second sensitivity analysis excluded women who were diagnosed with VDS but did not receive antibiotics during pregnancy (n = 2635) from the comparator group, and this did not impact the main results; similar to the results in Table 2, the adjusted association with VPTD was harmful (aRR, 1.12; 95% CI, 1.02–1.22). The third sensitivity analysis restricted the study population to women who received the recommended 3-drug combined treatment for VDS (n = 9919) and compared them with all others (n = 81 551). The adjusted results remained robust, with the association with VPTD being similar to the prior sensitivity analyses and the primary analysis (aRR, 1.13; 95% CI, 1.01–1.27).
Table 3.
Crude and Adjusted Log-Binomial Regression Models for the Association Between VDS With Antibiotics at <24 Weeks Gestation (n = 20 425) vs Antibiotics for Other Indications at <24 Weeks Gestation (n = 5093)
| Adverse Birth Outcomes | RR | aRRa |
|---|---|---|
| (95% CI) | (95% CI) | |
| Preterm | 0.93 (0.87–0.99) | 0.94 (0.87–1.02) |
| Very preterm | 0.93 (0.80–1.08) | 0.98 (0.83–1.15) |
| Stillbirth | 0.76 (0.63–0.92) | 0.83 (0.68–1.00) |
| Neonatal death | 0.92 (0.71–1.21) | 0.92 (0.70–1.22) |
| SGA | 1.00 (0.93–1.07) | 1.02 (0.94–1.10) |
| VSGA | 0.94 (0.84–1.06) | 0.99 (0.87–1.12) |
| Any adverse birth outcomeb | 0.96 (0.92–1.01) | 0.98 (0.92–1.03) |
| Any severe birth outcomec | 0.87 (0.77–0.98) | 0.91 (0.80–1.03) |
Abbreviations: aRR, adjusted risk ratio; RR, risk ratio; SGA, small for gestational age (<10th percentile of birth weight by gestational age); VDS, vaginal discharge syndrome; VSGA, very small for gestational age (<third percentile of birth weight by gestational age).
aModels were adjusted for maternal age, education, occupation, parity, marital status, nationality, and antiretroviral therapy at conception.
bAny adverse outcome includes preterm delivery (<37 weeks gestational age), small for gestational age (<10th percentile of birth weight by gestational age), neonatal death (infant death <28 days), and stillbirth (summed Apgar score of 0).
cAny severe adverse outcome includes very preterm delivery (<32 weeks), very small for gestational age (<third percentile of birth weight by gestational age), neonatal death, and stillbirth.
DISCUSSION
In the largest birth outcomes surveillance study in Africa, we found a diagnosis of VD in a very high percentage of pregnancies (30.7%), with most episodes (90.8%) syndromically treated with 1 or more antibiotics. Immortal time bias limited the ability of this study to completely explore the relationship between late-onset VDS and adverse birth outcomes. However, the diagnosis and treatment of VDS before 24 weeks of pregnancy was modestly associated with VPTD in adjusted analyses.
The high proportion of pregnancies in Botswana with a VDS diagnosis is consistent with data from other Sub-Saharan African countries. In a study conducted in South Africa, 30% of the participants were diagnosed with VDS according the WHO criteria [31]. Moreover, in a randomized trial conducted in Uganda among pregnant women, 17% of the participants in the intervention and treatment groups presented with vaginal discharge [17]. In a study conducted among pregnant women in Kenya, 20% of the study population presented with or reported the occurrence of abnormal vaginal discharge [15].
We hypothesized that treatment with antibiotics may mitigate the harmful effect of VDS, but we could not separate the effect of antibiotic treatment from the presence of VDS because of the high proportion of treated VDS in our cohort (and the possibility of indication bias for the small proportion with untreated VDS). However, our findings of largely similar outcomes in the treated VDS group compared with all other women may support a benefit from antibiotics and, at a minimum, indicated no harmful effect of first or second trimester antibiotic use on birth outcomes. These considerations suggest that prescribing antibiotics by a syndromic approach may offer benefits, particularly in regions where diagnostic testing is unavailable or unaffordable. However, our study was not designed to collect information on all risks related to antibiotic use, including antimicrobial resistance, allergic reactions, and microbiome disruption. In addition, the inability to evaluate third trimester associations between treated VDS and inability to study untreated VDS limit the generalizability of our findings to VDS that is diagnosed and treated by 24 weeks of pregnancy.
Perhaps the best indicator in our study that treated VDS had minimal impact on adverse birth outcomes was the sensitivity analysis comparing treated VDS with those who received antibiotics for any other reason, with no differences in outcomes. This analysis suggests that VDS (as compared with other illnesses requiring antibiotics) did not add risk in the setting of antibiotic treatment. Prior studies have found harmful associations between STIs during pregnancy and adverse birth outcomes that may be mitigated by antibiotic use [16, 32, 33]. A recent meta-analysis found a harmful association between chlamydia infection during pregnancy and PTD [34]. Moreover, a study in Kenya demonstrated harmful associations between chlamydia, gonorrhea, and PTD, and also reported that vaginal discharge among women without an STI was associated with PTD [15]. However, a recent study by Burdette et al. found that regardless of the time to treatment for chlamydia or gonorrhea during pregnancy, these infections were positively associated with PTD and VPTD, while additional studies found that treatment for STIs before 20 weeks reduces the risk of PTD and proposed that treating STIs before 24 weeks may reduce the risk for PTD [15, 35, 36].
The strengths of our study included a large and representative sample size of pregnant women diagnosed with VDS, detailed information regarding antibiotic use and adverse birth outcomes, and a conservative analysis approach that minimized bias. However, this study was limited by potential misclassification of STI-related vaginal discharge due to untreated and asymptomatic cases, in addition to the unavailability of diagnostic testing and urine cultures for STIs and bacterial vaginosis, and a lack of surveillance for group B streptococcus. Additionally, this study could not account for the severity of the VDS cases within the analyses, could not compare treated vs untreated VDS, and could not exclude the possibility that antibiotic use was associated with higher severity. Anemia was considered a potential confounder and a possible effect mediator, as chronic infections may be associated with anemia; for this reason, and for missingness, it was not included in the log-binomial regression models [37]. Our analysis was conducted using observational data (no randomized trial of this question would be possible), raising the possibility of unmeasured confounding from factors such as additional indicators for antibiotic use during pregnancy and antibiotic availability. Additional limitations included that only 48.4% of women with VDS received the 3-drug antibiotic combination recommended by Botswana guidelines, we could not distinguish between the antibiotics prescribed and antibiotic use, and we were unable to tease out the potential impact of individual vs combination antibiotic use on outcomes as part of this analysis.
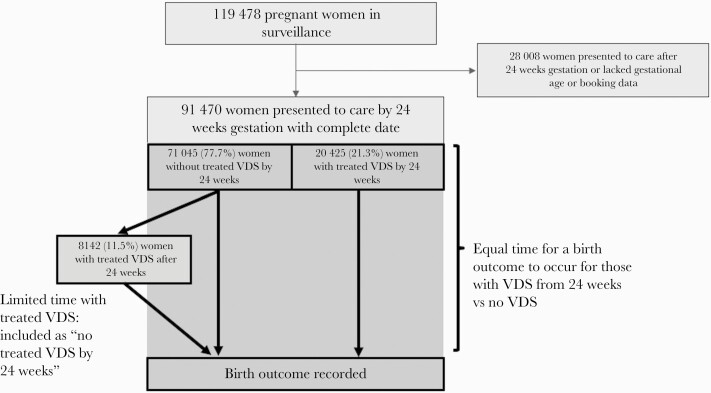
Finally, our analysis was limited by concern for immortal time bias, which is of concern for any exposure classification that can change during the period when an outcome might occur, and we believe this potential bias is worth highlighting for similar research studies where new diagnoses are made in pregnancy. Our efforts to mitigate this bias led to the restriction of the study population to women who presented to care before 24 weeks and restriction of VDS to being diagnosed by this time point (Figure 2). Although this restriction reduced the generalizability of our findings, it was necessary. When we attempted an unrestricted analysis, we observed an apparent protective effect from VDS and antibiotic use for most adverse birth outcomes, because more time to experience such events was shifted to the non-VDS group (data not shown).
Figure 2.
Flowchart of women presenting to care by 24 weeks gestation and illustration of immortal time bias. Abbreviation: VDS, vaginal discharge syndrome.
In sum, we observed a high proportion of pregnancies in Botswana that were complicated by VDS, accounting for the vast majority of antibiotic use in pregnancy. Our study identified a modest association between treated VDS and VPTD and found no harmful associations related to antibiotic use before 24 weeks gestation and adverse birth outcomes. We could neither support nor refute the current syndromic approach for providing antibiotics to women diagnosed with VDS in regions where diagnostic testing is not available. Our study also highlights the difficulty in designing either a randomized or observational study that can adequately address this question in late pregnancy, indicating a need for modeling or other novel approaches to decision science in this field.
Acknowledgments
We would like to acknowledge the hospital staff and maternity nurses in Botswana, the Botswana Ministry of Health, the staff at the Botswana Harvard Partnership, and the research assistants Cynthia Dube, Keemenao Mosala, Tsaone Gaonakala, Gosego Legase, Onkabetse Mokgosi, Rosemary Moremi, Shally Morgan, Edith Moseki, Tshephang Motlotlegi, Mmapula Ofhentse, Daphne Lekorwe, Kebabonye Rabasiako, Naledi Kamanga, Bathoba Mabiletsa, Nametsegang Tshosa, Annah Kgannyeng, Patricia Mophuthegi, Thabologo Baitsemi, Tshegofatso Sebetso, Tsholofelo Maswabi, Pricilla Mashona, Seele Mafokate, Kealeboga Mmokele, Obakeng Makalane, and Masego Kgafela.
Financial support. This study was supported by National Institutes of Health grants R01HD080471 and R01HD095766.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Adachi K, Nielsen-Saines K, Klausner JD. Chlamydia trachomatis infection in pregnancy: the global challenge of preventing adverse pregnancy and infant outcomes in Sub-Saharan Africa and Asia. Biomed Res Int 2016; 2016:9315757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gencay M, Koskiniemi M, Ammälä P, et al. Chlamydia trachomatis seropositivity is associated both with stillbirth and preterm delivery. APMIS 2000; 108:584–8. [DOI] [PubMed] [Google Scholar]
- 3. Ryan GM Jr, Abdella TN, McNeeley SG, et al. Chlamydia trachomatis infection in pregnancy and effect of treatment on outcome. Am J Obstet Gynecol 1990; 162:34–9. [DOI] [PubMed] [Google Scholar]
- 4. Gottlieb SL, Low N, Newman LM, et al. Toward global prevention of sexually transmitted infections (STIs): the need for STI vaccines. Vaccine 2014; 32:1527–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldenberg RL, Culhane JF, Johnson DC. Maternal infection and adverse fetal and neonatal outcomes. Clin Perinatol 2005; 32:523–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Romoren M, Sundby J, Velauthapillai M, et al. Chlamydia and gonorrhoea in pregnant Batswana women: time to discard the syndromic approach? BMC Infect Dis 2007; 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. UNAIDS. The public health approach to STD control. 1998. Available at: https://www.who.int/hiv/pub/sti/en/stdcontrol_en.pdf. Accessed 26 October 2020.
- 8. World Health Organization. Sexually transmitted and other reproductive tract infections. A guide to essential practice. 2005. Available at: https://www.who.int/reproductivehealth/publications/rtis/9241592656/en/. Accessed 26 October 2020.
- 9. Maitri S, Shetal D, Sangita VP, et al. Validation of vaginal discharge syndrome among pregnant women attending obstetric clinic, in the tertiary hospital of Western India. Indian J Sex Transm Dis AIDS 2014; 35:118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Paz-Bailey G, Rahman M, Chen C, et al. Changes in the etiology of sexually transmitted diseases in Botswana between 1993 and 2002: implications for the clinical management of genital ulcer disease. Clin Infect Dis 2005; 41:1304–12. [DOI] [PubMed] [Google Scholar]
- 11.Botswana Ministry of Health. Botswana Second Generation HIV Antenatal Sentinel Surveillance Technical Report. Gaborone, Botswana: Botswana Ministry of Health; 2009. [Google Scholar]
- 12.Botswana Ministry of Health. The Essential Health Package for Botswana. Gaborone, Botswana: Botswana Ministry of Health; 2010. [Google Scholar]
- 13.Botswana Ministry of Health. Management of Sexually Transmitted Infections: Reference Manual for Health Care Workers. Gaborone, Botswana: Botswana Ministry of Health; 2018. [Google Scholar]
- 14. Wynn A, Ramogola-Masire D, Gaolebale P, et al. Acceptability and feasibility of sexually transmitted infection testing and treatment among pregnant women in Gaborone, Botswana, 2015. BioMed Res Int 2016; 2016:1251238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ravindran J, Richardson B, Kinuthia J, et al. Chlamydia, gonorrhea, and incident HIV infection during pregnancy predict preterm birth despite treatment. J Infect Dis. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cohen I, Veille JC, Calkins BM. Improved pregnancy outcome following successful treatment of chlamydial infection. JAMA 1990; 263:3160–3. [PubMed] [Google Scholar]
- 17. Gray RH, Wabwire-Mangen F, Kigozi G, et al. Randomized trial of presumptive sexually transmitted disease therapy during pregnancy in Rakai, Uganda. Am J Obstet Gynecol 2001; 185:1209–17. [DOI] [PubMed] [Google Scholar]
- 18. Wright AJ, Unger S, Coleman BL, et al. Maternal antibiotic exposure and risk of antibiotic resistance in neonatal early-onset sepsis: a case-cohort study. Pediatr Infect Dis J 2012; 31:1206–8. [DOI] [PubMed] [Google Scholar]
- 19. Muanda FT, Sheehy O, Bérard A. Use of antibiotics during pregnancy and the risk of major congenital malformations: a population based cohort study. Br J Clin Pharmacol 2017; 83:2557–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramasethu J, Kawakita T. Antibiotic stewardship in perinatal and neonatal care. Semin Fetal Neonatal Med 2017; 22:278–83. [DOI] [PubMed] [Google Scholar]
- 21. Bookstaver PB, Bland CM, Griffin B, et al. A review of antibiotic use in pregnancy. Pharmacotherapy 2015; 35:1052–62. [DOI] [PubMed] [Google Scholar]
- 22. Sarah M, Ruridh AM, Ronald LF. The role of antimicrobial treatment during pregnancy on the neonatal gut microbiome and the development of atopy, asthma, allergy and obesity in childhood. Expert Opin on Drug Saf 2019; 18:173–85. [DOI] [PubMed] [Google Scholar]
- 23. Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2019; CD000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zash R, Souda S, Chen JY, et al. Reassuring birth outcomes with tenofovir/emtricitabine/efavirenz used for prevention of mother-to-child transmission of HIV in Botswana. J Acquir Immune Defic Syndr 2016; 71:428–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zash R, Jacobson DL, Diseko M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr 2017; 171:e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zash R, Jacobson DL, Diseko M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet 2018; 6:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019; 381:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botswana Ministry of Health. Guidelines for Antenatal Care and the Management of Obstetric Emergencies and Prevention of Mother-to-Child Transmission of HIV. Gaborone, Botswana: Botswana Ministry of Health; 2005. [Google Scholar]
- 29. Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) . International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet 2014; 384:857–68. [DOI] [PubMed] [Google Scholar]
- 30. Villar J, Giuliani F, Fenton TR, et al. ; INTERGROWTH-21st Consortium . INTERGROWTH-21st very preterm size at birth reference charts. Lancet 2016; 387:844–5. [DOI] [PubMed] [Google Scholar]
- 31. van der Eem L, Dubbink JH, Struthers HE, et al. Evaluation of syndromic management guidelines for treatment of sexually transmitted infections in South African women. Trop Med Int Health 2016; 21:1138–46. [DOI] [PubMed] [Google Scholar]
- 32. Crider KS, Cleves MA, Reefhuis J, et al. Antibacterial medication use during pregnancy and risk of birth defects: National Birth Defects Prevention Study. Arch Pediatr Adolesc Med 2009; 163:978–85. [DOI] [PubMed] [Google Scholar]
- 33. Rastogi S, Das B, Salhan S, Mittal A. Effect of treatment for Chlamydia trachomatis during pregnancy. Int J Gynaecol Obstet 2003; 80:129–37. [DOI] [PubMed] [Google Scholar]
- 34. He W, Jin Y, Zhu H, et al. Effect of Chlamydia trachomatis on adverse pregnancy outcomes: a meta-analysis. Arch Gynecol Obstet 2020; 302:553–67. [DOI] [PubMed] [Google Scholar]
- 35. Burdette E, Young M, Dude C, et al. Association of delayed treatment of chlamydial infection and gonorrhea in pregnancy and preterm birth, sexually transmitted diseases. Sex Transm Dis. In press. [DOI] [PubMed]
- 36. Folger AT. Maternal Chlamydia trachomatis infections and preterm birth: the impact of early detection and eradication during pregnancy. Matern Child Health J 2014; 18:1795–802. [DOI] [PubMed] [Google Scholar]
- 37. Subbaraman R, Devaleenal B, Selvamuthu P, et al. Factors associated with anaemia in HIV-infected individuals in Southern India. Int J STD AIDS 2009; 20:489–92. [DOI] [PMC free article] [PubMed] [Google Scholar]