Abstract
Background
Clinical studies have demonstrated inferior cure rates when metronidazole (MTZ) is used to treat Clostridioides difficile infection (CDI). We hypothesized that a newly identified, heme-inducible form of reduced MTZ susceptibility in C. difficile leads to higher odds of initial clinical failure in patients with CDI treated with MTZ.
Methods
This multicenter cohort study included adults diagnosed with CDI between 2017 and 2018. C. difficile isolated from stool samples underwent agar dilution MTZ susceptibility testing with incorporation of fresh heme. Blinded investigators reviewed medical records for initial clinical failure and other relevant clinical variables. Classification and regression tree (CART) analysis was used to identify the MTZ minimum inhibitory concentration (MIC) breakpoint that was predictive of initial clinical failure. Results were confirmed using univariate and multivariable logistic regression analyses to account for potential confounders.
Results
Of the 356 patients included, 72% received MTZ-based therapy and 27% experienced initial clinical failure. CART analysis identified an MTZ MIC ≥1 µg/mL above which patients had a higher rate of initial clinical failure. MTZ MICs ranged from 0.25 to 8 µg/mL (MIC50/90 = 0.25/2 µg/mL), and approximately 18% of isolates had MTZ MICs ≥1 µg/mL. In multivariable analysis, an MTZ MIC ≥1 µg/mL was an independent predictor of initial clinical failure in patients receiving an MTZ-based treatment regimen (odds ratio, 2.27 [95% confidence interval, 1.18–4.34]).
Conclusions
Using a reproducible method to determine C. difficile MICs to MTZ, a breakpoint of ≥1 µg/mL identified patients at higher risk of initial clinical failure.
Keywords: antibiotic resistance, antimicrobial resistance, Clostridium difficile, heme, susceptibility testing
A newly identified and heme-inducible form of metronidazole nonsusceptibility in Clostridioides difficile correlates with increased odds of initial clinical failure.
Clostridioides difficile infection (CDI) is a major public health threat in the United States, causing 450 000 infections and 15 000–30 000 associated deaths annually [1–3]. Despite a lack of US Food and Drug Administration approval for CDI, metronidazole (MTZ) has been a mainstay in the treatment of CDI since the 1970s [4–6]. However, recent randomized controlled trials (RCTs) demonstrated higher rates of clinical failure among those treated with MTZ [7–10], eventually culminating in the recommendation from national guidelines to reserve MTZ for instances when fidaxomicin and vancomycin are unavailable [6].
The reasons for worse outcomes following MTZ treatment are unclear, although the leading hypothesis centers on suboptimal pharmacokinetic/pharmacodynamic properties of MTZ in the colon. Mean MTZ fecal concentrations, which serve as a surrogate for colonic concentrations, range from <0.25 to 9.5 µg/g with observed decreases in drug concentration as diarrhea and inflammation resolve [11, 12]. Furthermore, MTZ minimum inhibitory concentrations (MICs) have been gradually increasing in recent decades and are consistently higher in the ribotype (RT) 027 epidemic strain than in nonepidemic strains [13–15]. With poor bioavailability of MTZ in feces and an increase in MTZ MICs, it is plausible that increased MICs to MTZ may be contributing to treatment failures.
Although the Clinical and Laboratory Standards Institute (CLSI) has established a breakpoint of ≥32 µg/mL to define MTZ resistance and the European Committee on Antimicrobial Susceptibility Testing uses an epidemiologic cutoff value of >2 µg/mL to denote reduced susceptibility to MTZ, neither have been informed by clinical outcomes data in patients with CDI [16, 17]. Additionally, C. difficile susceptibility testing is not routinely done; thus, reports of clinical outcomes associated with MIC are lacking. Further complicating matters, studies show that MTZ-resistant phenotypes are unstable [18–20], MICs are not reproducible when using standard susceptibility testing methods [13, 21–23], and MTZ resistance appears to be heterogenous [24–26]. This has presented a quandary in determining the correlation between reduced susceptibility to MTZ and clinical outcomes.
A recent discovery by our research group demonstrated that fresh heme incorporation in agar medium was able to reproducibly detect MTZ resistance in C. difficile [27]. While the central mechanism for heme-associated resistance is unclear, this observation has also been independently reported by another research group [28]. This provided the opportunity to evaluate clinical outcomes of patients with CDI treated with MTZ-based compared to non-MTZ-based regimens. We hypothesized that reduced susceptibility identified via this new method would be associated with higher rates of initial clinical failure.
METHODS
Study Population
This was a multicenter, cohort study of adults admitted to 1 of 14 hospitals, including 2 university-affiliated tertiary care referral centers, in Houston, Texas. Patients with a C. difficile–positive stool specimen collected between September 2017 and April 2018 were included. This study was approved by the Committee for the Protection of Research Subjects at the University of Houston (CPHS 000128).
Sample Collection and Ribotyping
Leftover stool samples from patients diagnosed with CDI as a part of routine clinical care were collected and brought to a centralized research laboratory at the University of Houston for further testing. All sites used a multiplex nucleic acid amplification test (NAAT) to test stool for C. difficile toxin B genes. Patients testing positive for gastrointestinal pathogens in addition to C. difficile were excluded. Stool samples were enriched using brain-heart infusion (BHI) agar, then plated onto cefoxitin-cycloserine-fructose agar plates and incubated under strict anaerobic conditions for 48–72 hours. Fluorescent ribotyping was performed as previously described [29].
MTZ Susceptibility
MTZ MICs were measured via agar dilution as described previously [27]. In brief, a C. difficile innocula of approximately 105 colony-forming units/mL was used on BHI agar plates that were supplemented with hemin (5 µg/mL) and protected from light. Agars contained doubling dilutions of MTZ from 0.25 to 32 µg/mL. Another set of BHI agar plates with doubling dilutions of MTZ were prepared but without heme supplementation to detect strains that exhibited heme-associated reduced susceptibility.
Definitions
Treatment regimens were categorized as MTZ-based if the patient received ≥1 dose of MTZ, alone or in combination with other antibiotic therapy (fidaxomicin or vancomycin), within 48 hours of CDI diagnosis as recorded in the electronic medication administration record. Non-MTZ-based regimens included receipt of ≥1 dose of vancomycin or fidaxomicin and no concomitant MTZ. CDI severity was assigned as defined by the 2017 Infectious Diseases Society of America and Society for Healthcare Epidemiology of America CDI guideline and utilizing hemodynamic instability, white blood cell count, and serum creatinine values collected within 24 hours of diagnostic stool specimen collection [6]. This study used a composite definition of “severe” disease that incorporated both “severe” and “fulminant” CDI. Patients were categorized as having “healthcare facility–onset” disease if the diagnostic stool specimen was collected from an inpatient location >3 days after admission in accordance with Centers for Disease Control and Prevention definitions [30].
Outcomes
The primary endpoint was initial clinical failure, which was defined as the presence of CDI-specific symptoms on day 6 of treatment or later, a change in CDI therapy due to lack of patient response before day 7, and/or CDI-contributable mortality within the first 7 days of treatment [10]. Patient electronic medical records (EMRs) were retrospectively reviewed by clinicians blinded to ribotype and MTZ MIC to determine patient outcome. If investigators were unable to determine initial clinical failure following a thorough review of the EMR, the patient was assumed to have experienced a successful treatment outcome.
Statistical Analysis
Classification and regression tree (CART) analysis was used to identify the MTZ MIC breakpoint predictive of initial clinical failure using the method suggested by Gumbo et al [31]. CART analysis uses a machine learning algorithm and binary recursive partitioning to evaluate all MIC values and identifies the best breakpoint for classifying patients with or without initial clinical failure. The Gini criterion was used to split the node into a suggested breakpoint by maximizing the receiver operating characteristic curve (ROC). A 10-fold validation was performed on the results. In the cross-validation, the dataset was randomly split into a learning and test database 10 times and CART analysis was performed each time. The ROC score for the test sample was reported.
For baseline characteristic comparison, binary and categorical variables were compared using χ 2 or Fisher exact tests and continuous variables were compared using the Student t test or Wilcoxon rank-sum test, depending on the data distribution. For the primary endpoint analysis, the cohort was stratified by treatment regimen: MTZ-based and non-MTZ-based. To account for potential confounders, a multivariable logistic regression model was built that included the CART-derived MTZ MIC breakpoint and any identified confounders. Variables with a P value < .2 from the univariate analyses were chosen as initial candidates for the multivariable model. Then, a stepwise backwards elimination procedure was performed by which variables with a P > .05 were removed 1 at a time. All variables with a P < .05 were included in the final model and defined as statistically significant. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All statistical analyses were performed using Minitab version 20.1.3 (Minitab LLC, State College, Pennsylvania), SPSS version 27.0.0.0 (IBM Corporation, Armonk, New York) or Stata version 15.1 (StataCorp LLC, College Station, Texas).
RESULTS
Patient Characteristics
The study cohort comprised 356 patients, who were majority white (62%) and female (59%) (Table 1). Around half (n = 194 [55%]) of the cohort had severe (n = 189) or fulminant (n = 5) CDI. Most patients (n = 255 [72%]) received MTZ-based treatment regimens, including 138 (54%) patients receiving MTZ monotherapy and 117 (46%) who received MTZ in combination with either fidaxomicin or vancomycin. Of the 101 patients receiving non-MTZ-based regimens, 94 (93%) received vancomycin monotherapy, 4 (4%) received fidaxomicin monotherapy, and 3 (3%) received both vancomycin and fidaxomicin. Although those receiving MTZ-based regimens less often had healthcare facility–onset disease (41% vs 54%; P = .02), rates of severe/fulminant disease were similar between the 2 cohorts.
Table 1.
Patient Demographics, Comorbidities, Laboratory Parameters, and Disease Characteristics
| Characteristic | Overall (N = 356) | MTZ-Based Treatment (n = 255) | Non-MTZ-Based Treatment (n = 101) | P Value |
|---|---|---|---|---|
| Age, y, mean (SD) | 64.3 (17.0) | 65.0 (17.0) | 62.6 (17.1) | .89 |
| Female sex | 209 (58.7) | 147 (57.6) | 62 (61.4) | .52 |
| Race/ethnicity | .57 | |||
| White, non-Hispanic | 222 (62.4) | 162 (63.5) | 60 (59.4) | |
| Black, non-Hispanic | 67 (18.8) | 48 (18.8) | 19 (18.8) | |
| Hispanic | 43 (12.1) | 29 (11.4) | 14 (13.9) | |
| Other/not reported | 24 (6.7) | 16 (6.3) | 8 (7.9) | |
| CCI, median (IQR) | 2 (1–4) | 2 (1–3) | 2 (1–4) | .36 |
| WBC count, cells/μL, mean (SD) | 13 300 (10 500) | 14 100 (11 700) | 11 300 (5800) | .43 |
| Albumin, g/dL, mean (SD) | 3.0 (0.7) | 3.0 (0.7) | 2.9 (0.8) | .16 |
| Severe/fulminant CDIa | 194 (54.5) | 138 (54.1) | 56 (55.4) | .82 |
| ICU admission within 48 h | 54 (15.2) | 38 (14.9) | 16 (15.8) | .82 |
| RT 027 infectionb | 48 (15.2) | 40 (17.5) | 8 (9.1) | .06 |
| HO-CDIa | 158 (44.4) | 104 (40.8) | 54 (53.5) | .02 |
Data are presented as No. (%) unless otherwise indicated. Bolded values indicate those deemed significant with a P value < .05.
Abbreviations: CCI, Charlson Comorbidity Index; CDI, Clostridioides difficile infection; HO-CDI, healthcare facility–onset Clostridioides difficile infection; IQR, interquartile range; MTZ, metronidazole; RT, ribotype; SD, standard deviation; WBC, white blood cell.
aAs defined per Infectious Diseases Society of America 2017 guidelines.
bShown as percentage of 316 isolates with ribotyping completed (228 MTZ-based treatment, 88 non-MTZ-based treatment).
C. difficile Strain Characteristics
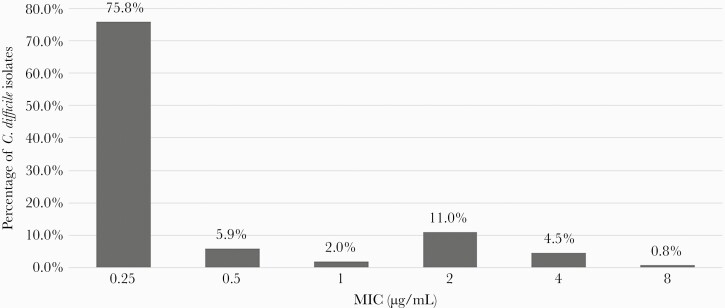
Overall, MTZ MICs ranged from 0.25 to 8 µg/mL, and the MIC required to inhibit grow of 50% of organisms (MIC50) was 0.25 µg/mL while an MIC of 2 ug/mL was required to inhibit growth of 90% of the organisms (MIC90). (Figure 1). The CART analysis (Figure 2) showed that an MTZ MIC ≥1 µg/mL was the optimal breakpoint to identify patients at higher risk of initial clinical failure (ROC score, 0.53 [95% CI, .47–.61]; misclassification cost, 0.85). Furthermore, a CART analysis limited to patients receiving MTZ-based regimens also identified MTZ MIC ≥1 µg/mL as the optimal breakpoint to identify patients at higher risk of initial clinical failure (ROC score, 0.56 [95% CI, .48–.64]; misclassification cost, 0.83). A total of 65 (18%) isolates had MTZ MICs ≥1 µg/mL and were classified as having reduced MTZ susceptibility. All strains demonstrating reduced MTZ susceptibility had at least a 4-fold increase in MIC in the presence of heme; MICs were 0.25–0.5 µg/mL without heme compared to 1–8 µg/mL with heme. Ribotyping results were obtainable for 316 (89%) isolates, of which 59 (19%) were RT 014-020, 48 (15%) were RT 027, and 35 (11%) were RT 106. The proportion of infections caused by RT 027 was not significantly different between those receiving MTZ-based regimens (n = 40/228 [15%]) and those receiving non-MTZ-based regimens (n = 8/88 [9%]) (P = .06). The majority of strains with an MTZ MIC ≥1 µg/mL were RT 027 (n = 45/65 [69%]).
Figure 1.
Clostridioides difficile metronidazole minimum inhibitory concentration (MIC) distribution measured using the agar dilution method with incorporation of fresh heme.
Figure 2.
Classification and regression tree (CART) analysis for initial clinical failure stratified by metronidazole (MTZ) minimum inhibitory concentration (MIC). The primary node, based on initial clinical failure, indicates that the MIC above which therapy fails is ≥1.0 µg/mL.
Clinical Outcomes
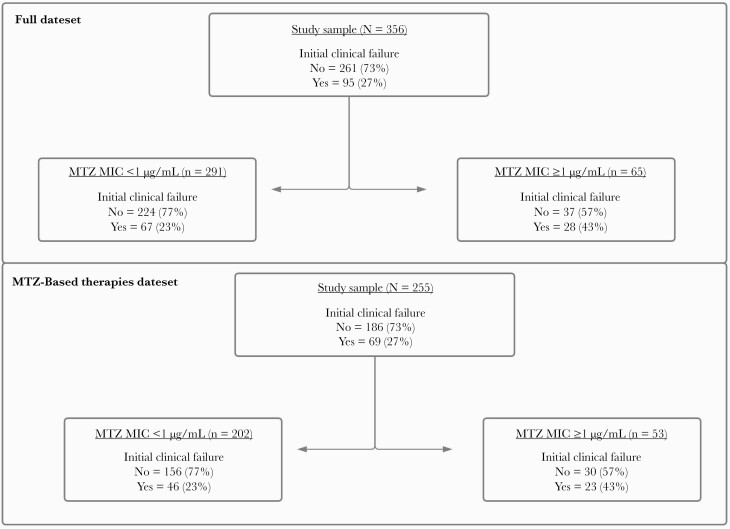
When analyzing the entire cohort, 95 (27%) patients experienced initial clinical failure: 39 (41%) had continued CDI-specific symptoms on or after day 6 of treatment, 21 (22%) required a change in CDI therapy due to a lack of response before day 7, 5 (5%) experienced CDI-contributable mortality within 7 days of treatment initiation, and 30 (32%) met the definition of initial clinical failure through 2 or more criteria. Overall, 14 (15%) patients experienced 7-day, CDI-contributable mortality. When stratified by the CART-derived MTZ MIC breakpoint, initial clinical failure occurred in 67 of the 291 (23%) patients infected with isolates with MTZ MICs <1 µg/mL and 28 of the 65 (43%) patients infected with isolates with MTZ MICs ≥1 µg/mL, regardless of treatment regimen (P = .001).
In the multivariable analysis, MTZ MIC ≥1 µg/mL was identified as an independent predictor of initial clinical failure regardless of treatment regimen (OR, 2.44 [95% CI, 1.36–4.36]) (Table 2). After stratifying based on MTZ receipt, MTZ MIC ≥1 µg/mL remained an independent predictor of initial clinical failure in those receiving MTZ-based treatment (OR, 2.27 [95% CI, 1.18–4.34]), but not in those receiving non-MTZ-based therapies (OR, 3.15 [95% CI, .83–11.9]) (Table 3). In those receiving MTZ-based treatment, severe/fulminant CDI was also associated with increased odds of initial clinical failure, while age was associated with initial clinical failure in those receiving non-MTZ-based treatment.
Table 2.
Univariate and Multivariable Analyses for Predictors of Initial Clinical Failure
| Predictor | Univariate | Multivariable | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| MTZ MIC ≥1 µg/mL | 2.48 (1.42–4.35) | .001 | 2.44 (1.36–4.36) | .003 |
| Age (per 1-year increase) | 0.99 (.97–1.00) | .007 | 0.98 (.97–.99) | .006 |
| CCI score (per 1-unit increase) | 1.13 (1.01–1.26) | .04 | 1.13 (1.01–1.28) | .04 |
| Severe/fulminant CDI | 1.67 (1.03–2.70) | .04 | 1.61 (.97–2.68) | .07 |
Bolded values indicate those deemed significant with a P value < .05.
Abbreviations: CCI, Charlson Comorbidity Index; CDI, Clostridioides difficile infection; CI, confidence interval; MIC, minimum inhibitory concentration; MTZ, metronidazole.
Table 3.
Univariate and Multivariable Analyses for Predictors of Initial Clinical Failure, Stratified by Treatment Regimen
| MTZ-Based Treatment Regimen (n = 255) | Non-MTZ-Based Treatment Regimen (n = 101) | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariable | Univariate | Multivariable | |||||
| Predictor | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| MTZ MIC ≥1 µg/mL | 2.60 (1.38–4.91) | .003 | 2.27 (1.18–4.34) | .014 | 2.18 (.63–7.55) | .22 | 3.15 (.83–11.94) | .09 |
| Age (per 1-year increase) | 0.99 (.98–1.01) | .49 | 0.99 (.97–1.00) | .15 | 0.97 (.94–.99) | .017 | 0.96 (.93–.99) | .004 |
| CCI score (per 1-unit increase) | 1.11 (.97–1.26) | .14 | 1.01 (.94–1.26) | .24 | 1.17 (.95–1.44) | .13 | 1.25 (.99–1.57) | .05 |
| Severe/fulminant CDI | 2.67 (1.47–4.84) | .001 | 2.56 (1.33–4.44) | .004 | 0.55 (.22–1.33) | .18 | 0.54 (.20–1.49) | .24 |
Bolded values indicate those deemed significant with a P value < .05.
Abbreviations: CCI, Charlson Comorbidity Index; CDI, Clostridioides difficile infection; CI, confidence interval; MIC, minimum inhibitory concentration; MTZ, metronidazole.
Discussion
Evidence over the past several decades has demonstrated a decreased clinical response rate to MTZ, leading major guidelines to remove it as a first-line treatment option for CDI [6]. A clear explanation for this decreased response has yet to be described; however, recent reports of increased MTZ resistance have emerged, albeit without clear clinical implications [32–34]. In this current study, an MTZ MIC breakpoint of ≥1 µg/mL was associated with an increased risk of initial clinical failure. To the best of our knowledge, this is the first report to demonstrate an association between reduced MTZ susceptibility and higher rates of treatment failure in patients with CDI treated with an MTZ-based treatment regimen.
Our study has several unique strengths allowing us to demonstrate these novel findings. First, the incorporation of fresh heme into our agar medium provided reproducible MICs to determine a resistance breakpoint, without which all isolates displayed an MTZ-susceptible phenotype. Only 1 other study incorporating fresh heme into their testing media has been conducted, and noted an 8- to 24-fold increase in MTZ MIC when strains were tested on BHI supplemented with hemin compared to BHI alone [28]. We found that almost one-fifth of samples from our multicenter cohort had MTZ MICs ≥1 µg/mL, implying that this phenomenon is not uncommon. It is particularly common among RT 027 isolates, which we omitted from our multivariate analyses due to multi-collinearity. Second, our sample size provided us with enough power to detect a difference in the rate of initial clinical failure between groups. Finally, the availability of clinical data allowed us to incorporate antibiotic treatment in the analysis to determine the effect of reduced MTZ MICs in patients treated with MTZ-based regimens.
Our findings contrast with those of 2 previous efforts to study the link between MTZ MICs and clinical outcomes, neither of which used fresh hemin to determine MIC values [19, 21]. The first study, conducted on patients diagnosed with CDI between 1982 and 1991, found a 2% clinical failure rate among 632 patients treated with MTZ [21]. Ten of these patients were compared to a control group of 20 patients successfully treated with MTZ, and their infecting isolates underwent susceptibility testing by E-test and agar dilution methods. No resistant isolates were identified and there was no difference in the mean (E-test) or geometric mean (agar dilution) MTZ MIC between those with clinical failure or success by either method. MTZ MICs are known to have increased since the 1980s [15, 35], which may account for the differences between our study findings. Notably, their reported failure rate of 2% is similar to the 5% rate seen in RCTs conducted before 2000 [36, 37]. The second prospective observational study, conducted on patients with CDI during the early 2000s, compared treatment outcomes between patients treated with MTZ vs vancomycin [19]. Of the 34 patients treated with MTZ, 10 (29%) had persistent symptoms requiring a change to vancomycin therapy. All 10 of the infecting isolates had MTZ MICs ≤0.75 µg/mL by E-test, leading the authors to attribute these MTZ failures to host factors and slower response times. In addition to the potential for E-tests to underestimate MICs in comparison to our methodology [13, 14, 23, 38, 39], their study was designed to test a different hypothesis and was limited by its small sample size. Since publication of these 2 prior studies, RCTs since 2000 have shown failure rates >20% [7, 10], which is similar to our failure rate of 27%. Recent epidemiologic surveys have shown that endemic strains, including RT 027, have higher rates of MTZ nonsusceptibility [15, 32, 40], which may be due to the dissemination of a plasmid encoding MTZ resistance [25]. As previously mentioned, 45 of the 48 (94%) RT 027 isolates identified in our study had an MTZ MIC ≥1 µg/mL. Thus, although the association between MTZ MIC and initial clinical failure is a novel finding, we believe that the increasing prevalence of reduced MTZ susceptibility, our ability to detect it using a new methodology, and our large sample size allowed us to observe a true difference in the rate of initial clinical failure among patients treated with MTZ-based regimens who were infected by strains with reduced MTZ susceptibility.
This study has several limitations. First, we did not measure fecal concentrations of MTZ, which are known to vary widely from undetectable to approximately 10 µg/g stool [11, 12]. Future studies will be needed to investigate the pharmacokinetic/pharmacodynamic drug exposure threshold associated with treatment failure. Second, our susceptibility testing was conducted using BHI agar instead of supplemented Brucella agar or broth as recommended by the CLSI, although recent comparisons of the 2 methods have demonstrated similar MIC results [27, 28]. Third, our primary outcome of initial clinical failure relied on accurate medical record documentation and investigator classification. Although misclassification bias is possible, we minimized this likelihood by blinding our investigators to C. difficile strain characteristics, including MTZ MIC, while collecting clinical outcomes and by conservatively assuming those without clear documentation of failure achieved clinical success. Notably, we chose only outcomes that were reliably documented in the EMR and did not assess CDI recurrence or mortality after hospital discharge. Future studies will be needed to evaluate the impact of reduced MTZ susceptibility on these important outcomes. Fourth, all patients were diagnosed by a NAAT and some may have been asymptomatically colonized with an alternative explanation for their symptoms. We attempted to minimize this possibility by excluding patients who tested positive for another gastrointestinal pathogen. Additionally, the overall initial clinical failure rate of 26% in patients treated with MTZ-based regimens in our study is comparable to those seen with MTZ in recent RCTs [7, 10]. Fifth, we observed that reduced MTZ susceptibility appeared to have an effect on response rates to other antibiotic therapies, albeit to a lesser extent. Although resistance to MTZ mainly involves altering drug activation, detoxifying reactive species, or repairing cellular damage, further research will be needed to better understand resistance mechanisms and their effect(s) on the host oxidative stress response underlying our findings [41]. Furthermore, an investigation of the genetic mechanism(s) underlying the reduced susceptibility identified here, including the potential presence of the pCD-METRO plasmid [25], will be an area of future study for our group. Given the difficulty of susceptibility testing in strictly anaerobic C. difficile, future research should seek to identify additional genetic marker(s) that can be more easily identified and serve as a surrogate for reduced MTZ susceptibility [28, 42]. Last, given the retrospective design, we cannot discount the possibility of unmeasured confounding variables. However, we did control for several relevant variables known to affect CDI outcomes.
In conclusion, this study represents the first to demonstrate higher initial clinical failure rates associated with reduced MTZ susceptibility in CDI. We observed more than doubled odds of initial clinical failure with MTZ MICs ≥1 µg/mL in patients receiving MTZ-based treatment regimens. As we observed MTZ MICs ≥1 µg/mL in 18% of our isolates, we believe this may be an increasingly common phenomenon and may help explain the declining response rates to MTZ in recent decades. Based on these findings, we recommend conducting more widespread testing for heme-inducible MTZ nonsusceptibility, as identification of such may aid in identifying patients who still may benefit from MTZ therapy.
Notes
Patient consent statement. This study was approved by the Committee for the Protection of Research Subjects at the University of Houston (CPHS 000128) and does not include factors necessitating patient consent. This study conforms to standards currently applied in the United States.
Disclaimer. The funders had no role in the study design, data collection, interpretation of the findings, or writing and submission of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (grant number R01AI139261).
Potential conflicts of interest. All authors: No reported conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
REFERENCES
- 1. Lessa FC, Mu Y, Bamberg WM, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med 2015; 372:825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall AJ, Curns AT, McDonald LC, et al. The roles of Clostridium difficile and norovirus among gastroenteritis-associated deaths in the United States, 1999–2007. Clin Infect Dis 2012; 55:216–23. [DOI] [PubMed] [Google Scholar]
- 3. Guh AY, Mu Y, Winston LG, et al. Emerging Infections Program Clostridioides difficile Infection Working Group. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N Engl J Med 2020; 382:1320–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cohen SH, Gerding DN, Johnson S, et al. Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31:431–55. [DOI] [PubMed] [Google Scholar]
- 5. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108:478–98; quiz 99. [DOI] [PubMed] [Google Scholar]
- 6. McDonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis 2018; 66:e.1–48. [DOI] [PubMed] [Google Scholar]
- 7. Johnson S, Louie TJ, Gerding DN, et al. Polymer Alternative for CDI Treatment (PACT) Investigators. Vancomycin, metronidazole, or tolevamer for Clostridium difficile infection: results from two multinational, randomized, controlled trials. Clin Infect Dis 2014; 59:345–54. [DOI] [PubMed] [Google Scholar]
- 8. Musher DM, Aslam S, Logan N, et al. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin Infect Dis 2005; 40:1586–90. [DOI] [PubMed] [Google Scholar]
- 9. Pepin J, Alary ME, Valiquette L, et al. Increasing risk of relapse after treatment of Clostridium difficile colitis in Quebec, Canada. Clin Infect Dis 2005; 40:1591–7. [DOI] [PubMed] [Google Scholar]
- 10. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile–associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45:302–7. [DOI] [PubMed] [Google Scholar]
- 11. Bolton RP, Culshaw MA. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 1986; 27:1169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Arabi Y, Dimock F, Burdon DW, et al. Influence of neomycin and metronidazole on colonic microflora of volunteers. J Antimicrob Chemother 1979; 5:531–7. [DOI] [PubMed] [Google Scholar]
- 13. Baines SD, O’Connor R, Freeman J, et al. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J Antimicrob Chemother 2008; 62:1046–52. [DOI] [PubMed] [Google Scholar]
- 14. Thorpe CM, McDermott LA, Tran MK, et al. U.S.-based national surveillance for fidaxomicin susceptibility of Clostridioides difficile–associated diarrheal isolates from 2013 to 2016. Antimicrob Agents Chemother 2019; 63:e00391-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah D, Dang MD, Hasbun R, et al. Clostridium difficile infection: update on emerging antibiotic treatment options and antibiotic resistance. Expert Rev Anti Infect Ther 2010; 8:555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: CLSI; 2020. [Google Scholar]
- 17. European Committee on Antimicrobial Susceptibility Testing. Data from the EUCAST MIC distribution website. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_11.0_Breakpoint_Tables.pdf. Accessed 26 November 2020.
- 18. Martin H, Willey B, Low DE, et al. Characterization of Clostridium difficile strains isolated from patients in Ontario, Canada, from 2004 to 2006. J Clin Microbiol 2008; 46:2999–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Al-Nassir WN, Sethi AK, Nerandzic MM, et al. Comparison of clinical and microbiological response to treatment of Clostridium difficile-associated disease with metronidazole and vancomycin. Clin Infect Dis 2008; 47:56–62. [DOI] [PubMed] [Google Scholar]
- 20. Indra A, Schmid D, Huhulescu S, et al. Characterization of clinical Clostridium difficile isolates by PCR ribotyping and detection of toxin genes in Austria, 2006–2007. J Med Microbiol 2008; 57:702–8. [DOI] [PubMed] [Google Scholar]
- 21. Sanchez JL, Gerding DN, Olson MM, Johnson S. Metronidazole susceptibility in Clostridium difficile isolates recovered from cases of C. difficile–associated disease treatment failures and successes. Anaerobe 1999; 5:201–4. [Google Scholar]
- 22. Moura I, Spigaglia P, Barbanti F, Mastrantonio P. Analysis of metronidazole susceptibility in different Clostridium difficile PCR ribotypes. J Antimicrob Chemother 2013; 68:362–5. [DOI] [PubMed] [Google Scholar]
- 23. Poilane I, Cruaud P, Torlotin JC, Collignon A. Comparison of the E test to the reference agar dilution method for antibiotic susceptibility testing of Clostridium difficile. Clin Microbiol Infect 2000; 6:155–6. [DOI] [PubMed] [Google Scholar]
- 24. Peláez T, Cercenado E, Alcalá L, et al. Metronidazole resistance in Clostridium difficile is heterogeneous. J Clin Microbiol 2008; 46:3028–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boekhoud IM, Hornung BVH, Sevilla E, et al. Plasmid-mediated metronidazole resistance in Clostridioides difficile. Nat Commun 2020; 11:598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dingsdag SA, Hunter N. Metronidazole: an update on metabolism, structure-cytotoxicity and resistance mechanisms. J Antimicrob Chemother 2018; 73:265–79. [DOI] [PubMed] [Google Scholar]
- 27. Wu X, Shen W-J, Deshpande A, et al. The integrity of heme is essential for reproducible detection of metronidazole-resistant Clostridioides difficile by agar dilution susceptibility tests [manuscript published online ahead of print 16 June 2021]. J Clin Microbiol 2021. doi:10.1128/JCM.00585-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boekhoud IM, Sidorov I, Nooij S, et al. COMBACTE-CDI Consortium. Haem is crucial for medium-dependent metronidazole resistance in clinical isolates of Clostridioides difficile. J Antimicrob Chemother 2021; 76:1731–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alam MJ, Anu A, Walk ST, Garey KW. Investigation of potentially pathogenic Clostridium difficile contamination in household environs. Anaerobe 2014; 27:31–3. [DOI] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. Clostridioides difficile infection (CDI) tracking. https://www.cdc.gov/hai/eip/cdiff-tracking.html#reports. Accessed 3 June 2021.
- 31. Gumbo T, Chigutsa E, Pasipanodya J, et al. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 2014; 69:2420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adler A, Miller-Roll T, Bradenstein R, et al. A national survey of the molecular epidemiology of Clostridium difficile in Israel: the dissemination of the ribotype 027 strain with reduced susceptibility to vancomycin and metronidazole. Diagn Microbiol Infect Dis 2015; 83:21–4. [DOI] [PubMed] [Google Scholar]
- 33. Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis 2015; 3:23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karlowsky JA, Adam HJ, Kosowan T, et al. PCR ribotyping and antimicrobial susceptibility testing of isolates of Clostridium difficile cultured from toxin-positive diarrheal stools of patients receiving medical care in Canadian hospitals: the Canadian Clostridium difficile Surveillance Study (CAN-DIFF) 2013–2015. Diagn Microbiol Infect Dis 2018; 91:105–11. [DOI] [PubMed] [Google Scholar]
- 35. Hecht DW, Galang MA, Sambol SP, et al. In vitro activities of 15 antimicrobial agents against 110 toxigenic Clostridium difficile clinical isolates collected from 1983 to 2004. Antimicrob Agents Chemother 2007; 51:2716–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Teasley DG, Gerding DN, Olson MM, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium-difficile–associated diarrhoea and colitis. Lancet 1983; 2:1043–6. [DOI] [PubMed] [Google Scholar]
- 37. Wenisch C, Parschalk B, Hasenhündl M, et al. Comparison of vancomycin, teicoplanin, metronidazole, and fusidic acid for the treatment of Clostridium difficile–associated diarrhea. Clin Infect Dis 1996; 22:813–8. [DOI] [PubMed] [Google Scholar]
- 38. Moura I, Spigaglia P, Barbanti F, Mastrantonio P. Analysis of metronidazole susceptibility in different Clostridium difficile PCR ribotypes. J Antimicrob Chemother 2013; 68:362–5. [DOI] [PubMed] [Google Scholar]
- 39. Aspevall O, Lundberg A, Burman LG, et al. Antimicrobial susceptibility pattern of Clostridium difficile and its relation to PCR ribotypes in a Swedish university hospital. Antimicrob Agents Chemother 2006; 50:1890–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Snydman DR, McDermott LA, Jacobus NV, et al. U.S.-based national sentinel surveillance study for the epidemiology of Clostridium difficile–associated diarrheal isolates and their susceptibility to fidaxomicin. Antimicrob Agents Chemother 2015; 59:6437–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Deshpande A, Wu X, Huo W, et al. Chromosomal resistance to metronidazole in Clostridioides difficile can be mediated by epistasis between iron homeostasis and oxidoreductases. Antimicrob Agents Chemother 2020; 64:e00415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhao H, Nickle DC, Zeng Z, et al. Global landscape of Clostridioides difficile phylogeography, antibiotic susceptibility, and toxin polymorphisms by post-hoc whole-genome sequencing from the MODIFY I/II studies. Infect Dis Ther 2021; 10:853–70. [DOI] [PMC free article] [PubMed] [Google Scholar]