Abstract
Background
There are scanty data on the occurrence of gastric tumours overexpressing human epidermal growth factor receptor 2 (HER2) in Africa.
Objective
To assess HER2 protein overexpression in gastric and gastroesophageal junction adenocarcinoma (GGEAC) samples from a single centre in Zambia.
Methodology
This was a cross-sectional study of formalin-fixed paraffin-embedded blocks with GGEAC. Prepared slides were first stained with Haematoxylin and Eosin and then evaluated for HER2 protein overexpression by immunohistochemistry.
Results
A total of 57 gastric tissues were stained and evaluated for HER2 overexpression. Thirteen (23%) showed overexpression, 41/57 (72%) had negative and 3/57 (5%) had equivocal staining. The equivocal cases were excluded from the final analysis. Of the remaining 54 tissues, 28 (52%) were from females, and 26 (48%) were from males. The mean age was 59 years (SD 15 years). HER2 overexpression was highest in moderately differentiated tumours (p=0.0005). Intestinal type tumours had a higher occurrenc of HER2 overexpression than diffuse or mixed sub-types (p=0.0087). HER2 overexpression was not associated with age (p=0.27), sex (p=1.00) or anatomical location (p=1.00).
Conclusion
The occurrence of GGEAC HER2 overexpression in Zambian patients is similar to proportions reported elsewhere, and it is associated with moderately differentiated tumours of the intestinal type.
Keywords: Gastric and Gastroesophageal junction adenocarcinoma, Human Epidermal growth factor Receptor 2 overexpression, immunohistochemistry
Introduction
Gastric cancer is one of the leading causes of cancer-related deaths worldwide. There were over one million gastric cancer cases in 2018, with an estimated 783 000 deaths. 1 The estimated age-standardised incidence rate in Africa is between 2.6 and 5.4 per 100 000. 2 In Zambia, gastric cancer is the tenth most common cancer, with an estimated age-standardised incidence of 2.9 per 100 000 and 3.4 per 100 000 in women and men respectively. 2
The most common type of gastric cancer is adenocarcinoma,3 which can either be located within the stomach or at the gastroesophageal junction referred to as gastric and gastroesophageal junction adenocarcinomas (GGEAC). GGEAC sub-types can be diffuse, intestinal or mixed Lauren classification). 4 Intestinal type adenocarcinoma is characterised by cohesive cells that form gland-like structures. Tumour cells that lack cell-to-cell interactions and infiltrate the stroma as single cells or small subgroups of non-cohesive, scattered tumour cells characterise diffuse adenocarcinomas. The mixed types have both the intestinal and diffuse characteristics.
A subset of advanced GGEAC overexpress HER2 protein, and these are aggressive, exhibit resistance to conventional chemotherapy and have poor outcomes. HER2 protein is a tyrosine kinase receptor, which is encoded by the HER2 proto-oncogene located on chromosome 17q21. HER2 is a member of the epidermal growth factor receptors (EGFRs) family, which is composed of four members, namely HER1, HER2, HER3 and HER4.5 Tumours with HER2 overexpression respond to targeted therapy with the anti-HER2 antibody trastuzumab and this treatment is associated with improved survival. 6 HER2 overexpression varies in different regions of the world with reported high expression in India (44%) 7 and low expression in China (6.9%). 8 In another study, Van Cutsem et al, reported similar proportions of HER2 overexpression from Europe (23.6%) and Asia (23.9%) but slightly lower from Central /South America (16.1%). 9 HER2 overexpression rates were higher in intestinal (31.8%) than diffuse (6.1%) type gastric cancer.
Data on the occurrence of gastric tumours with HER2 overexpression in Africa are scanty. A study done in Egypt on eighty-five tumour tissue samples from patients with gastric cancer revealed a HER2 positivity proportion of 27%. 10 It was expressed more in intestinal and mixed-type, adenocarcinoma and moderately differentiated tumours. 10 In a Kenyan descriptive cross-sectional study of sixty-six biopsy and resected specimens of histologically diagnosed gastric cancers, HER2 overexpression rate was found to be 42%. In this study, HER2 overexpression was higher in intestinal than diffuse-type cancers, and it was not associated with gender, age or anatomical site. 11
The prevalence of HER2 overexpression in GGE-AC in Zambia is not known. A retrospective audit of endoscopy records done in Zambia showed evidence of an increase in gastric carcinoma diagnosis, particularly in people below the age of sixty years. 12 Despite the poor GGEAC outcomes in Zambia, 13 patients with advanced disease are treated with chemotherapy and radiotherapy without the option of targeted immunotherapy. The lack of knowledge about the prevalence of HER2 overexpression in GGEAC hinders any prospects of targeted therapies that have the potential of improving patient outcomes. In this study, we therefore, assessed HER2 protein overexpression in patients with GGEAC. The University of Zambia Biomedical Research Ethics committee reference number 005-06-18 approved this study.
Methods
This was a cross-sectional study to evaluate HER2 overexpression in archived formalin-fixed paraffin-embedded (FFPE) tissue samples in patients diagnosed with GGEAC in the Department of Pathology, University Teaching Hospital (UTH), Lusaka, Zambia. All FFPE tissue blocks with a histological diagnosis of GGEAC from January 2015 to June 2018 were evaluated. We excluded sample blocks with inadequate tissue and extensive crush/ mechanical distortion.
Data collection plan and tools
Socio-demographic and clinical data were obtained from the data-intensive system and applications (a laboratory data collection system) and endoscopy reports. Clinical data (age, gender and tumour location) and pathological parameters (histological type and grade) were entered in standardised collection sheets. Retrieved FFPE tissue blocks were processed according to the standard operating procedure at the UTH pathology laboratory. Briefly, FFPE tissue blocks were sectioned, mounted on slides, stained with Hematoxylin and Eosin and read to confirm the initial diagnosis. Tissues with adenocarcinoma were classified histologically according to Lauren classification (intestinal, diffuse or mixed type). Tumours were graded as well differentiated if they exhibited more than 95% glands (Grade I). Moderately differentiated tumours had 50 to 95% glandular pattern (Grade II), while poorly differentiated ones have a predominantly solid pattern with less than 50% glands (Grade III). Immunohistochemistry for detection of HER2 followed strict adherence to the manufacturer's instructions using Dako rabbit polyclonal antibody Dako rbbit polyclonal antibody (RbAHuC-erbB2), Santa Clara, USA. Briefly, antigen retrieval was done using citrate buffer at pH 6.0 in a pressure cooker at high temperature for 30 minutes. Endogenous peroxidase blocking was done with 3% hydrogen peroxidase. The antibody was used at a dilution of 1:600.
Analysis and interpretation for the presence of HER2 overexpression
HER2 scoring was done according to the Hoffmann scoring criteria 14 (Table 1). Those whose score was either 0 or 1+ were negative, the ones with 2+ were equivocal, and the ones with 3+ were positive or showed HER2 overexpression. We excluded specimens with equivocal results in the final analysis. Four independent pathologists (CK, CM, FM and PJ) did the analysis and interpretation of stained slides. For each of the slides, a score reported by three or more of the pathologists was recorded as the final score. In case of a discordant outcome (less than three similar scores), the score reported by PJ was taken as the tiebreaker as he was the most experienced pathologist. The kappa-statistic measure of interrater agreement between each of the pathologists was as shown in Table 2.
Table 1.
Hoffmann scoring criteria for HER2 14
| Score | Interpretation | Criteria for GGEAC |
| 0 | Negative | No staining or membrane staining in clusters of <5 tumour cells. |
| 1+ | Negative | Cluster(s) of at least five cohesive tumour cells with weak (generally visible early at X400 magnification) complete, basolateral or lateral membrane staining, irrespective of tumour volume percentage. |
| 2+ | Equivocal | Cluster(s) of at least five cohesive tumour cells with moderate (generally visible at X100- X200 magnification complete, basolateral membrane staining, irrespective of tumour volume percentage. |
| 3+ | Positive | Cluster(s) of at least five cohesive tumour cells with strong (generally visible at X25- X50 magnification) complete, basolateral or lateral membrane, staining irrespective of tumour volume percentage. |
Table 2.
Interrater agreement between pathologists' interpretation of immunohistochemically stained slides for HER2
| Pathologist | CM | FM | PJ |
| CK | Kappa- 0.62 Agreement- 82% |
Kappa- 0.54 Agreement- 71% |
Kappa- 0.75 Agreement- 86% |
| CM | - | Kappa- 0.48 Agreement- 65% |
Kappa- 0.50 Agreement- 73% |
| FM | - | - | Kappa- 0.43 Agreement- 62% |
Statistical analysis
Continuous variables were normally distributed and therefore summarised as mean, standard deviation and range. Categorical variables were summarised as frequencies and percentages. The Kappa statistics were employed to assess interrater agreement. Fisher's exact test was used to determine the significance of the association between HER2 overexpression and clinicopathological parameters. Results were then presented as odds ratios with 95% confidence intervals. A two-sided p-value of <0.05 was considered statistically significant. The data were analysed using STATA 15 (College Station, TX, USA).
Results
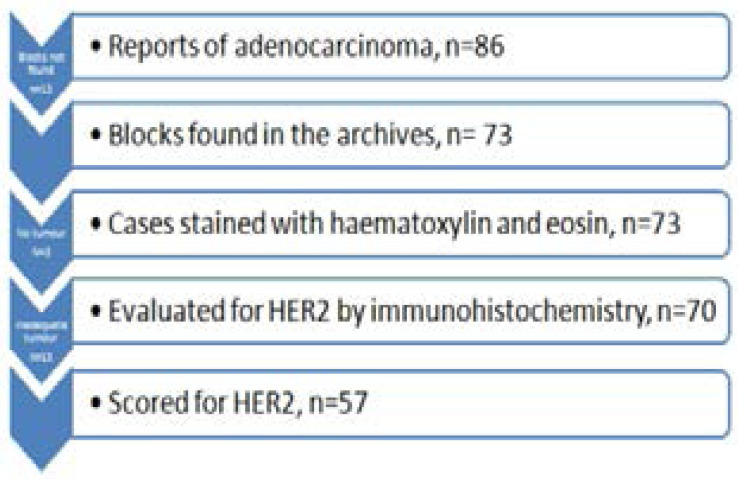
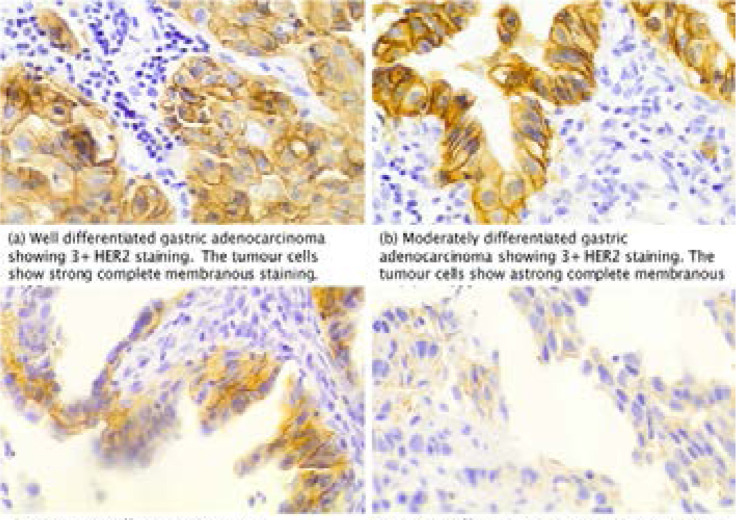
The laboratory electronic database had records for 86 gastric and gastroesophageal junction adenocarcinomas (GGEAC) for the period under study (Figure 1). Seventy-three blocks were found in the archives and stained with Hematoxylin and Eosin. From these, 16 were excluded on account of inadequate tissue; a lack of tumour in the sections or extensive fragmentation rendering histologic interpretation impossible. Therefore, 57 samples were of good enough quality to allow for adequate immunohistological scoring. Of these, 55 cases were endoscopic biopsies, and 2 were gastric resections. Immunostaining for HER2 protein resulted in 13/57 (23%) positives (3+ or HER2 overexpression), 41/57 (72%) negatives (0 or 1+) and 3/57 (5%) equivocal (2+) results. Figure 2 shows representative images of HER2 expression in the variable grades of gastric and gastroesophageal adenocarcinoma.
Comparison of HER2 positive and negative patients
Demographic and histologic characteristics were compared between HER2 positive and negative tumours. For these analyses, equivocal specimens were excluded (n=3). Of the 54 specimens included, 28 (52%) were from females, and 26 (48%) were males. The mean age was 59 years (SD 15 years). Nine (17%) of the patients were below the age of 45 years. Of the 47 specimens whose anatomical location was knon, 4 (9%) were from the gastroesophageal junction, and the rest from gastric regions; 7 (15%) fundus, 20 (43%) body and 16 (34%) antrum. There was no association between HER2 overexpression and age (p=0.27), sex (p=1.00) or anatomical location of the tumour (p=1.00), (Table 3). Thirty-three 33 (61%) of the tumours were of intestinal, 16 (30%) diffuse and 5 (9%) mixed sub-types. Ninety-two percent of the tumours with HER2 over-expression were of the intestinal, 1 (8%) diffuse and none were of the mixed subtype. These differences were statistically significant, p=0.009 (Table 3). Poorly differentiated tumours were most common among those negative for HER2 staining, with only one being exhibiting overexpression. Similarly, this difference was statistically significant, p=0.0005, (Table 3).
Table 3.
Patient and tumour characteristics for samples with either positive or negative HER2 staining
| Patient or tumour characteristics |
HER2 negative n=41 n (%) |
HER2 Positive n=13 n (%) |
95% Confidence Interval |
p-value |
|
Sex Male Female |
20 (49) 21 (51) |
6 (46) 7 (54) |
0.2–4.4 |
1.00 |
|
Age (years) Below 30 30–44 45–59 Above 60 |
0 (0) 8 (20) 14 (34) 19 (46) |
0 (0) 1 (8) 4 (30) 8 (62) |
- |
0.27 |
|
Site (n=47) Gastroesophageal junction Gastric (fundus, body and antrum) |
3 (75) 31 (72) |
1 (25) 12 (28) |
0.02–12 |
1.00 |
|
Histologic type Intestinal Diffuse Indeterminate |
21 (51) 15 (37) 5 (12) |
12 (92) 1 (8) 0 (0) |
- |
0.009 |
|
Tumour Differentiation Well Moderately Poorly |
2 (5) 14 (34) 25 (61) |
3 (23) 9 (69) 1 (8) |
- |
0.0005 |
Discussion
This study was undertaken to evaluate the prevalence of HER2 overexpression in patients with GGEAC presenting to the University Teaching Hospital in Lusaka, Zambia. Many GGEAC present with advanced disease and delayed referral is one of the major contributing factors. 15 In this study, there were slightly more females than males, but method of sampling does not allow for generalisation of the results to the Zambian population. Close to a quarter of the tumours examined in this study had HER2 overexpression. HER2 overexpression was variable depending on the histologic type and tumour differentiation. There was no association between HER2 overexpression and sex, age or anatomical location.
The proportion of HER2 positive GGEAC found in this study was less than that reported from Kenya, 11 another sub-Saharan African country. The Kenyan investigators had relatively more resection specimens while most of ours were endoscopic biopsies. Wand et al, reported a good concordance between biopsy and resection specimens for HER2 overexprssion in gastric cancer 16 therefore, specimen type is unlikely to be the reason for the observed difference. Our results were similar to those reported form Europe 17 and Egypt. 10 However, endoscopic biopsies do not allow for assessment of intra-tumoral HER2 heterogeneity. Intra-tumoral heterogeneity has been postulated as one of the factors that potentially influence therapeutic response of HER2 positive tumours. 18, 19
Anatomical site of the tumour was not associated with HER2 overexpression in this study, but the small sample size could have limited this. Other studies did, however, report similar findings.8, 20 Most of the tumours in this study were from the gastric region, predominantly distal portion. Available literature shows that distal gastric cancers are commoner in developing countries, among blacks of low socio-economic groups and are associated with Helicobacter pylori infection. 21
HER2 overexpression was reported to be positively associated with intestinal-type adenocarcinoma but inversely associated with E-cadherin mutations. 22 E-cadherin mutations are typical for diffuse gastric. 23 Here, we present similar findings. There is therefore, need to further investigate the selective overexpression of HER2 in intestinal-type gastric cancer.
Histological reporting of tumour differentiation is of limited clinical utility with no implications on treatment choices. Linking tumour differentiation grades to HER2 overexpression might be an avenue to improve its relevance. Information linking gastric tumour differentiation and HER2 overexpression have shown variable results with some studies showing an association,24,25, 26 while others have not. 20, 27
The anti-HER2 antibody trastuzumab, when added to chemotherapy, improves the survival of patients with HER2 positive GGEAC. 6 Trastuzumab is available in Zambia and patients with HER2 positive breast cancers receive this treatment. Gastric cancer patient outcomes in Zambia are very poor. 13 With the absence of data on the prevalence of HER2 overexpression in GGEAC currently, patients are not given the option of trastuzumab therapy. Results from this study have shown the need to test for HER2 overexpression in GGEAC in Zambia routinely. Incorporation of HER2 testing in the routine care of GGEAC patients and subsequent treatment with trastuzumab will contribute towards a better outcome for this sub-group of gastric cancer patients.
One limitation of this study was the small number of cases analysed due to the inability to retrieve all cases, and some blocks with inadequate tissue for analysis. Clinical data was incomplete for some cases. Being retrospective, we could not control for cold ischemic and fixation time. Lastly, we could not run fluorescent in-situ hybridisation on the three equivocal cases due to inadequate resources.
Conclusion
HER2 overexpression in GGEAC is associated with intestinal-type and moderately differentiated tumours. There is a need to routinely test for HER2 overexpression in Zambian patients as its occurrence is similar to proportions reported elsewhere.
Acknowledgements
We acknowledge the contribution of Drs Chibamba Mumba and Francis Musonda towards scoring of HER2 immunostained gastric cancer slides.
Author contribution
CK, PJ and VK formulated and designed the study. CK and VK collected the samples. IM prepared histologic slides. CK and PK evaluated gastric tissue prepared on slides. CK and VK conducted the data analysis. All authors contributed towards writing the manuscript.
Funding
The research reported in this publication was supported by the Fogarty International Center of the United States National Institutes of Health (NIH) under award number D43 TW009744. The U.S Civilian Research & Development Foundation (CRDF Global) provided additional funding award number DAA3-16-62699-1. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH or CRDF Global.
conflict of interest
None declared.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBO-CAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer; 2018. [24th January 2020]. Available from: https://gco.iarc.fr/today. [Google Scholar]
- 3.Casamayor M, Morlock R, Maeda H, Ajani J. Targeted literature review of the global burden of gastric cancer. Ecancermedicalscience. 2018 Nov 26;12:883. doi: 10.3332/ecancer.2018.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waldum HL, Fossmark R. Types of Gastric Carcinomas. Int J Mol Sci. 2018 Dec 18;19(12) doi: 10.3390/ijms19124109. pii: E4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madani SH, Rahmati A, Payandeh M. Survey of Her2-neu expression and its correlation with histology of gastric carcinoma and gastroesophageal junction adenocarcinoma. Asian Pac J Cancer Prev. 2015;16(17):7755–7758.8. doi: 10.7314/apjcp.2015.16.17.7755. [DOI] [PubMed] [Google Scholar]
- 6.Sawaki A, Ohashi Y, Omuro Y, Satoh T, Hamamoto Y, Boku N, et al. Efficacy of trastuzumab in Japanese patients with HER2-positive advanced gastric or gastroesophageal junction cancer: a subgroup analysis of the Trastuzumab for Gastric Cancer (ToGA) study. Gastric Cancer. 2012 Jul;15(3):313–322. doi: 10.1007/s10120-011-0118-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sekaran A, Kandagaddala RS, Darisetty S, Lakhtakia S, Ayyagari S, Rao GV, et al. HER2 expression in gastric cancer in Indian population—an immunohistochemistry and fluorescence in situ hybridisation study. Indian Journal of Gastroenterology. 2012 Jun 1;31(3):106–110. doi: 10.1007/s12664-012-0214-0. [DOI] [PubMed] [Google Scholar]
- 8.Yan SY, Hu Y, Fan JG, Tao GQ, Lu YM, Cai X, et al. Clinicopathologic significance of HER-2/neu protein expression and gene amplification in gastric carcinoma. World Journal of Gastroenterology: WJG. 2011 Mar 21;17(11):1501. doi: 10.3748/wjg.v17.i11.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015 Jul 1;18(3):476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadi AA, El Hindawi A, Hareedy A, Khalil H, Al Ashiry R, Elia S, et al. Her2/neu Protein Expression and Oncogene Amplification in Gastric Carcinoma with Clinico-Pathological Correlation in Egyptian Patients. Open access. Macedonian Journal of Medical Sciences. 2016 Dec 15;4(4):535. doi: 10.3889/oamjms.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussein AA, Emily R, Omulo TM, Ndaguatha PL. HER2/Neu Protein Over-Expression in Patients with Gastric and Gastro-Esophageal Junction Carcinoma Seen at Kenyatta National Hospital, Kenya. J Carcinog Mutagen. 2014;5(186):2. [Google Scholar]
- 12.Kayamba V, Sinkala E, Mwanamakondo S, Soko R, Kawimbe B, Amadi B, et al. Trends in upper gastrointestinal diagnosis over four decades in Lusaka, Zambia: a retrospective analysis of endoscopic findings. BMC Gastroenterology. 2015 Dec;15(1):127. doi: 10.1186/s12876-015-0353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asombang AW, Kayamba V, Turner-Moss E, Banda L, Colditz G, Mudenda V, et al. Gastric malignancy survival in Zambia, Southern Africa: A two year follow up study. Medical Journal of Zambia. 2014;41(1):13–18. [PMC free article] [PubMed] [Google Scholar]
- 14.Hechtman J.F, Polydorides A.D. HER2/neu gene amplification and protein overexpression in gastric and gastroesophageal junction adenocarcinoma: a review of histopathology, diagnostic testing, and clinical implications. Archives of Pathology & Laboratory Medicine. 2012;136(6):691–697. doi: 10.5858/arpa.2011-0168-RS. [DOI] [PubMed] [Google Scholar]
- 15.Kayamba V, Kelly P. Delayed referral for diagnostic endoscopy is a contributing factor to late gastric cancer diagnosis in Zambia. Health Press Zambia Bull. 2019;3(2):14–19. [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Hsieh ET, Henry P, Hanna W, Streutker CJ, Grin A. Matched biopsy and resection specimens of gastric and gastroesophageal adenocarcinoma show high concordance in HER2 status. Hum Pathol. 2014 May;45(5):970–975. doi: 10.1016/j.humpath.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Lordick F, Bang YJ, Kang YK, Reyes DO, Manikhas GM, Shen L, et al. 3541 POSTER HER2-positive advanced gastric cancer: similar HER2-positivity levels to breast cancer. European Journal of Cancer Supplements. 2007 Sep 1;5(4):272. [Google Scholar]
- 18.Wakatsuk T, Yamamoto N, Sano T, Chin K, Kawachi H, Takahari D, Ogura M, Ichimura T, Nakayama I, Osumi H, Matsushima T, Suenaga M, Shinozaki E, Hiki N, Ishikawa Y, Yamaguchi K. Clinical impact of intratumoral HER2 heterogeneity on trastuzumab efficacy in patients with HER2-positive gastric cancer. J Gastroenterol. 2018 Nov;53(11):1186–1195. doi: 10.1007/s00535-018-1464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsina M, Gullo I, Carneiro F. Intratumoral heterogeneity in gastric cancer: a new challenge to face. Ann Oncol. 2017 May 1;28(5):912–913. doi: 10.1093/annonc/mdx134. [DOI] [PubMed] [Google Scholar]
- 20.Sekaran A, Kandagaddala RS, Darisetty S, Lakhtakia S, Ayyagari S, Rao GV, et al. HER2 expression in gastric cancer in Indian population—an immunohistochemistry and fluorescence in situ hybridisation study. Indian Journal of Gastroenterology. 2012 Jun 1;31(3):106–110. doi: 10.1007/s12664-012-0214-0. [DOI] [PubMed] [Google Scholar]
- 21.Crew KD, Neugut AI. Epidemiology of gastric cancer. World Journal of Gastroenterology: WJG. 2006 Jan 21;12(3):354. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, et al. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Applied immunohistochemistry & Molecular Morphology: AIMM. 2012 Jan;20(1):13. doi: 10.1097/PAI.0b013e31821c821c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenoy S. CDH1 (E-Cadherin) Mutation and Gastric Cancer: Genetics, Molecular Mechanisms and Guidelines for Management. Cancer Manag Res. 2019 Dec 13;11:10477–10486. doi: 10.2147/CMAR.S208818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajagopal I, Niveditha SR, Sahadev R, Nagappa PK, Rajendra SG. HER 2 Expression in Gastric and Gastro-esophageal Junction (GEJ/span>) Adenocarcinomas. J Clin Diagn Res. 2015 Mar;9(3):EC06–EC10. doi: 10.7860/JCDR/2015/12581.5630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farzand S, Siddique T, Saba K, Bukhari MH. Frequency of HER2/neu overexpression in adenocarcinoma of the gastrointestinal system. World Journal of Gastroenterology: WJG. 2014 May 21;20(19):5889. doi: 10.3748/wjg.v20.i19.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan L, Ying J, Lu N. HER2 expression and relevant clinicopathological features in gastric and gastroesophageal junction adenocarcinoma in a Chinese population. Diagnostic pathology. 2013 Dec;8(1):76. doi: 10.1186/1746-1596-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aditi R, Aarathi R, Pradeep R, Hemalatha L, Akshatha C, Amar K. HER2 Expression in Gastric Adenocarcinoma— a Study in a Tertiary Care Centre in South India. Indian Journal of Surgical Oncology. 2016 Mar 1;7(1):18–24. doi: 10.1007/s13193-015-0436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]