TO THE EDITOR:
Chimeric antigen receptor (CAR)-modified T cells targeting the CD19 antigen are approved to treat relapsed and refractory B-cell malignancies.1-4 Despite durable objective responses, most patients experience acute toxicities such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).5 Although many cases of ICANS resolve with supportive measures, high-grade ICANS may result in status epilepticus, lasting neurologic deficits, cerebral edema,6 and death.7 Therefore, novel approaches for severe ICANS fill an urgent unmet need to enhance the use of adoptive cellular therapy.
T cells engineered with safety switches, like the inducible caspase-9 (iC9), have been explored clinically to mitigate graft-versus-host disease after haploidentical stem cell transplantation.8,9 When donor T lymphocytes that have been engineered to express the iC9 are exposed to the dimerizing drug rimiducid, such graft-versus-host disease can be abrogated by pharmacologically induced donor T-cell apoptosis. We report here the first use of rimiducid to abrogate CAR T-cell–associated neurotoxicity.
The case described here was enrolled in the dose-expansion cohort of a phase 1/2 trial (NCT03016377) to test the safety and efficacy of autologous T lymphocytes genetically modified to express CD19.CAR (encoding 4-1BB), a truncated low-affinity nerve growth factor receptor (ΔNGFR) (for selection and tracking purposes), and iC9, administered to adult patients with relapsed and refractory B-lymphoblastic leukemia. The study is approved by the University of North Carolina Institutional Review Board and Institutional Biosafety Committee. The subject (UNC 109; see supplemental Materials, available on the Blood Web site) provided written informed consent. The trial is described in further detail in supplemental Materials. After lymphodepletion (fludarabine and cyclophosphamide) and infusion of 1 × 106 CAR T cells per kilogram, toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 5)10 or American Society for Transplantation and Cellular Therapy consensus grading for CRS and ICANS (including the Immune effector Cell-associated Encephalopathy [ICE] score).11 CAR T-cell expansion in peripheral blood was monitored by quantitative polymerase chain reaction (qPCR). Neurologic monitoring included magnetic resonance imaging, continuous video electroencephalogram (vEEG), and serial optic nerve sheath measurements by ultrasound for surrogate intracranial pressure.12 Leukemia response was determined by bone marrow (BM) evaluation, cerebrospinal fluid analysis, and imaging of extramedullary leukemia at 4 and 8 weeks after CAR T-cell infusion.
A 26-year-old woman with B-cell acute lymphoblastic leukemia relapsed in the BM, mesentery, and peritoneum, but not cerebrospinal fluid, 3 years after haploidentical stem cell transplantation. After 2 unsuccessful salvage attempts, she enrolled in the CAR T-cell study. Levetiracetam 750 mg twice daily was the patient’s ongoing treatment of a well-controlled seizure disorder and was continued as seizure prophylaxis. One day after cell infusion, she developed grade 1 CRS, and, on day 7, mild cognitive changes indicated grade 1 ICANS (ICE score 8). She received dexamethasone and tocilizumab for ongoing CRS. Although her fever resolved, ICANS worsened to grade 3 the following day (ICE score 0), and to grade 4 two days later, characterized by stupor and ICE score 0. Continuous vEEG showed generalized 2.5 Hz δ activity with a triphasic morphology, which was clinically consistent with nonconvulsive status epilepticus (Figure 1). Neurologic examination and vEEG did not improve despite methylprednisolone 1000 mg daily for 2 doses. Magnetic resonance imaging of the brain on days 1 and 3 of ICANS demonstrated no new findings compared with prior imaging. Lumbar puncture was not performed as it was deemed unsafe due to bleeding risk and patient inability to cooperate. Despite resolution of seizures on lacosamide and levetiracetam for 24 hours, the patient was arousable only to vigorous stimuli and unable to participate in ICE scoring (score 0) consistent with grade 3 ICANS. Given the persistence of grade 3-4 ICANS for 72 hours despite standard care, rimiducid 0.4 mg/kg was given per protocol.
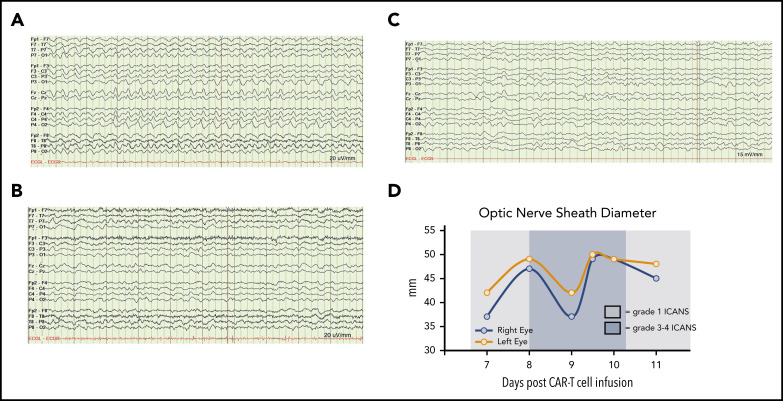
Figure 1.
Neurologic monitoring of case 1 during grade 3-4 ICANS. (A) Continuous vEEG showed generalized 2.5 Hz δ activity with a triphasic morphology. (B) Electrographic resolution of seizures on vEEG after empiric lorazepam. (C) Continued electrographic resolution of seizures after treatment with levetiracetam, lacosamide, and rimiducid. (D) Optic nerve sheath diameter tracings before and after rimiducid were within the normal range and not indicative of elevated intracranial pressure. A cutoff of >5.5 mm was used to indicate possibly elevated intracranial pressure.
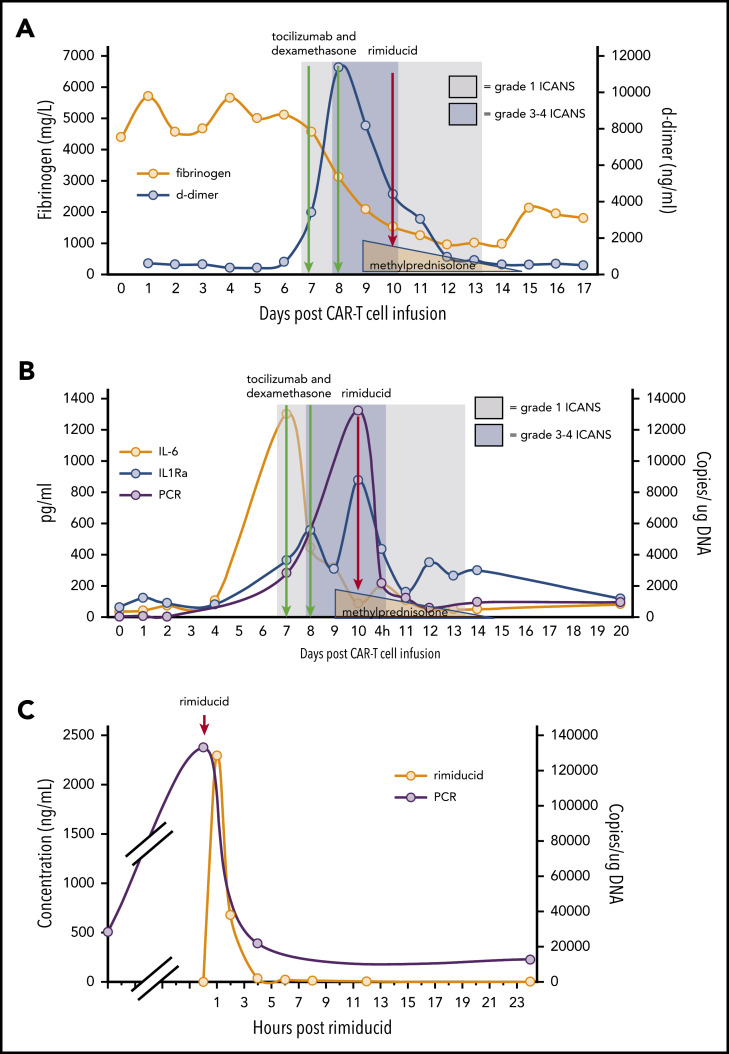
Within 12 hours of rimiducid administration, the ICANS grade improved from 3 to 1, with the ICE score improving from 0 to 7. ICANS fully resolved 4 days after rimiducid administration. The only adverse event possibly related to rimiducid was grade 2 bilirubin elevation that lasted for 3 days. Elevated D-dimer and hypofibrinogenemia, often seen with blood-brain-barrier endothelial disruption, normalized during recovery from ICANS (Figure 2A). After tocilizumab and corticosteroid doses, cytokine levels (Figure 2B) diminished, but the ICANS grade did not improve. Quantification of circulating CAR T cells by qPCR demonstrated 28 400 copies per microgram of DNA on day 7 post–cell infusion, during the CRS/ICANS event. At peak severity of ICANS, the copy number increased to over 130 000 copies per microgram of DNA despite corticosteroids. Within 4 hours of the administration of rimiducid and achievement of maximum rimiducid plasma concentration (Figure 2C), the transgene copy number diminished to 21 800 copies per microgram of DNA and to 12 400 and 5900 copies per microgram of DNA at 24 and 48 hours, respectively (Figure 2B). Low levels of transgene (3000 copies per microgram of DNA in blood and 1200 copies per microgram of DNA in marrow) remained detectable 18 days after rimiducid. At week 4 post–CAR T-cell infusion, BM lymphoblasts had diminished from 70% of marrow cellularity at baseline to rare blasts detected by immunohistochemistry in a hypocellular marrow. At week 8, there were no lymphoblasts detected by morphology or immunohistochemistry. Details of ancillary marrow studies are provided in supplemental Materials. At both 4- and 8-week time points, the bulk of fluorodeoxyglucose-avid tissue was diminished at prior sites of extramedullary leukemia on positron emission tomography/computed tomography scan.
Figure 2.
ICANS grade and measures of endothelial activation, cytokine levels, and qPCR for ΔNGFR relative to cell infusion, anticytokine therapy, and rimiducid administration. (A) Fibrinogen and D-dimer. (B) Cytokine levels and ΔNGFR qPCR. (C) Rimiducid pharmacokinetics and CAR T-cell kinetics by qPCR for ΔNGFR.
In conclusion, for the first time, we show that severe and steroid-refractory ICANS occurring after CD19-specific CAR T cells can be mitigated using rimiducid when CAR T cells have been engineered with the iC9 safety switch. Upon administration of rimiducid, there was abrupt improvement in clinical manifestations of ICANS, and elimination of >60% of the CAR T cells from the circulation within 4 hours and >90% within 24 hours.
The correlative studies highlight some of the critical pathogenic events that seem to promote and sustain ICANS. First, we saw that the reduced plasma levels of cytokines including interleukin 6 (IL-6) and IL1Rα after standard-of-care treatment did not alleviate the severity of ICANS. Second, administration of steroids did not immediately eliminate CAR T cells. In sharp contrast, the rapid reduction of circulating CAR T cells was achieved only after the administration of rimiducid. This evidence supports that the mechanism for neurologic complications post–CD19.CAR T-cell therapy may be independent of cytokine production.
Here, the course of severe ICANS and the peak of CAR T-cell expansion were shorter after rimiducid than observed for other patients in this trial with ICANS who did not require rimiducid (M.C.F. and B.S., unpublished data showing peak expansion of CAR-T cells at 4 days after dexamethasone and tocilizumab in subject 004 who developed ICANS that did not require rimiducid) and in previously reported patients treated with conventional therapy for ICANS.13,14 These comparisons suggest that the dramatic, immediate reductions in CAR T cells and resolution of neurologic findings were a consequence of rimiducid. Importantly, despite elimination of >90% of CAR T cells after rimiducid, we observed a clinically significant antileukemic response. Whether the residual CAR T cells promote antitumor surveillance remains unclear. However, because the proapoptotic effect of the iC9 is dose dependent, lower doses of rimiducid may prove beneficial in mitigating side effects without hampering efficacy, so that use of a full dose of rimiducid can be limited to cases of life-threatening toxicity.15 Such a dosing strategy could allow study of rimiducid without the confounding effects of high-dose corticosteroids and is integrated into the expansion cohort of this clinical trial. As on-target, off-tissue toxicity remains a major unknown when using novel CAR T cells, the inclusion of the iC9 switch has the potential to dramatically improve the safety of cellular immunotherapies.
Acknowledgments
Rimiducid was provided by Bellicum Pharmaceuticals. The authors acknowledge the patients as well as Paul Eldridge, Kathryn Mackay, Desirae Shelley, and the staff of the Lineberger Advanced Cellular Therapeutics Facility (University of North Carolina, Chapel Hill, NC).
This work was supported in part by Leukemia & Lymphoma Society Translational Research (TRP 6536-18) (B.S.) and The V Foundation (T2017-006) (B.S.). The production of CAR T cells was supported in part by the University Cancer Research Fund and by Bellicum Pharmaceuticals.
Footnotes
The datasets generated and analyzed in the present study are available from the corresponding author on reasonable request.
The online version of this article contains a data supplement.
Authorship
Contribution: M.C.F., B.S., J.S., and G.D. conceptualized the overall strategy, developed its clinical translation and implementation, and wrote the manuscript; M.C.F. is the principal investigator of the trial; M.C.F., N.G., and J.S. wrote the clinical protocol; K.M., J.S., and B.S. are the investigational new drug sponsors; J.W. and B.S. manufactured T cells and supervised analyses of clinical samples; A.I. performed statistical analyses; M.C.F., N.G., P.A., J.C., F.B.B., and R.S.H. enrolled patients in the protocol and/or managed the patients; W.L. and C.R. managed ICANS; C.C. and S.L. were the study coordinators, assisting with enrollment, sample acquisition, and data safety monitoring of patients; A.F. contributed to preclinical development of the iC9 safety switch and facilitated pharmacokinetic analyses; and all authors discussed and interpreted the results.
Conflict-of-interest disclosure: M.C.F. received research support from Bellicum Pharmaceuticals and Macrogenics, and consulting fees from Macrogenics and Daiichi Sankyo. B.S. received research support from Bellicum Pharmaceuticals, Bluebirdbio, Tessa Therapeutics, and Cell Medica, and consulting fees from Tessa Therapeutics. N.G. received research support from Genentech and consulting fees from Tessa Therapeutics, and participated in an advisory board for Kite. A.F. is employed at Bellicum Pharmaceuticals. J.S. received research support from Merck Inc, GlaxoSmithKline, and Carisma Therapeutics, and consulting fees from PIQUE Inc. G.D. received research support from Bellicum Pharmaceuticals and Tessa Therapeutics, and consulting fees from Tessa Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Matthew C. Foster, Lineberger Comprehensive Cancer Center, University of North Carolina, Department of Medicine, Hematology, CB#7305, Third Floor POB, 170 Manning Dr, Chapel Hill, NC 27599-7305; e-mail: mcfoster@med.unc.edu.
REFERENCES
- 1.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20(1):31-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuster SJ, Bishop MR, Tam CS, et al. ; JULIET Investigators . Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45-56. [DOI] [PubMed] [Google Scholar]
- 4.Wang M, Munoz J, Goy A, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turtle CJ, Hanafi LA, Berger C, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123-2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbasi J. Amid FDA approval filings, another CAR-T therapy patient death. JAMA. 2017;317(22):2271. [DOI] [PubMed] [Google Scholar]
- 8.Di Stasi A, Tey SK, Dotti G, et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365(18):1673-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, Dotti G, Krance RA, et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood. 2015;125(26):4103-4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), version 5. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed 3 November 2020.
- 11.Lee DW, Santomasso BD, Locke FL, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625-638. [DOI] [PubMed] [Google Scholar]
- 12.Dubourg J, Javouhey E, Geeraerts T, Messerer M, Kassai B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: a systematic review and meta-analysis. Intensive Care Med. 2011;37(7):1059-1068. [DOI] [PubMed] [Google Scholar]
- 13.Gust J, Hay KA, Hanafi LA, et al. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7(12):1404-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santomasso BD, Park JH, Salloum D, et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 2018;8(8):958-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaconu I, Ballard B, Zhang M, et al. Inducible caspase-9 selectively modulates the toxicities of CD19-specific chimeric antigen receptor-modified T cells. Mol Ther. 2017;25(3):580-592. [DOI] [PMC free article] [PubMed] [Google Scholar]