Abstract
Individuals with autism spectrum disorder (ASD) are significantly more likely to experience sensory over-responsivity (SOR) compared to neurotypical controls. SOR in autism has been shown to be related to atypical functional connectivity in the salience network (SN), a brain network thought to help direct attention to the most relevant stimuli in one’s environment. However, all studies to date which have examined the neurobiological basis of sensory processing in ASD have used primarily male samples so little is known about sex differences in the neural processing of sensory information. This study examined the relationship between SOR and resting-state functional connectivity in the SN for 37 males and 16 females with autism, ages 8–17 years. While there were no sex differences in parent-rated SOR symptoms, there were significant sex differences in how SOR related to SN connectivity. Relative to females with ASD, males with ASD showed a stronger association between SOR and increased connectivity between the salience and primary sensory networks, suggesting increased allocation to sensory information. Conversely, for females with ASD, SOR was more strongly related to increased connectivity between the SN and prefrontal cortex. Results suggest that the underlying mechanisms of SOR in ASD are sex specific, providing insight into the differences seen in the diagnosis rate and symptom profiles of males and females with ASD.
Keywords: autism spectrum disorder, resting-state fMRI, salience network, sensory, sex differences
Lay Summary:
Sensory over-responsivity (SOR) is common in autism. Most research on the neural basis of SOR has focused on males, so little is known about SOR or its neurobiology in females with autism spectrum disorder. Here despite no sex differences in SOR symptoms, we found sex differences in how SOR related to intrinsic connectivity in a salience detection network. Results show sex differences in the neural mechanisms underlying SOR and inform sex differences seen in diagnosis rates and symptom profiles in autism.
Introduction
Individuals with autism spectrum disorder (ASD) are commonly affected by atypical sensory processing, often expressed as sensory over-responsivity [SOR; e.g., Liss, Saulnier, Fein, & Kinsbourne, 2006; Ben-Sasson et al., 2009; Tavassoli, Miller, Schoen, Nielsen, & Baron-Cohen, 2014]. SOR is characterized as a heightened aversive response to sensory stimuli, such as loud noises, scratchy fabrics, or bright lights [Green et al., 2013, 2015; Kientz & Dunn, 1997; Klintwall et al., 2011]. It is estimated that at least 70% of individuals with ASD experience atypical sensory processing [Baker, Lane, Angley, & Young, 2008; Baranek, David, Poe, Stone, & Watson, 2006], and these symptoms have been found to correlate with autism-related social difficulties [Hilton et al., 2010; Taylor et al., 2018]. SOR is impairing; it has been shown to correlate with maladaptive behavior, impaired daily living skills [Baker et al., 2008] problem behaviors [O’Donnell, Deitz, Kartin, Nalty, & Dawson, 2012], sleep problems [Mazurek & Petroski, 2015] and anxiety [Green & Ben-Sasson, 2010; Hofmann & Bitran, 2007; Jerome & Liss, 2005]. The most recent edition of the Diagnostic and Statistical Manual of Mental Disorders (fifth edition, DSM-5; American Psychiatric Association, 2013) incorporated atypical sensory processing as a core feature of ASD, recognizing the importance of these symptoms to the ASD phenotype. Yet the neurobiological underpinnings of sensory issues in ASD are still vastly understudied.
Studies examining the neural basis of SOR have found that youth with ASD show increased activation and reduced habituation in the amygdala and sensory processing regions of the brain (primary auditory and somatosensory cortices) during exposure to mildly aversive sensory stimuli [Green et al., 2013, 2015, 2019]. Further, activity in these regions correlate with parent-reported SOR severity. The role of the amygdala in determining what is salient and what an individual should attend to [e.g., Zheng et al., 2017; Anderson & Phelps, 2001; Sander, Grafman, & Zalla, 2003] suggests that SOR may be related to an over-attribution of salience to extraneous sensory information. Indeed, sensory-evoked activity in the amygdala and primary somatosensory cortex is correlated with greater resting-state functional connectivity between these regions and the anterior insula [Green, Hernandez, Bookheimer, & Dapretto, 2016], the hub of an intrinsic brain network known as the salience network [SN; Seeley et al., 2007].
The SN plays a role in determining which of many internal and/or external stimuli require attention [Menon & Uddin, 2010; Seeley et al., 2007]. Atypical resting-state functional connectivity in the SN is well documented in ASD [e.g., Chen et al., 2017; Elton, Di Martino, Hazlett, & Gao, 2016; Uddin, 2015; von dem Hagen, Stoyanova, Baron-Cohen, & Calder, 2013], and has been shown to discriminate between ASD and TD participants [Uddin, Supekar, & Menon, 2013]. The anterior insula has been shown to be hypoactive in ASD individuals [for review, see Uddin & Menon, 2009; Di Martino et al., 2009], and decreased activity in this region relates to impairments in emotional awareness often seen in those with the disorder [Silani et al., 2008]. There is also evidence that the anterior insula is overactive in individuals with ASD during the processing of sensory information [Di Martino et al., 2009; Green et al., 2015]. Increased SN connectivity with the amygdala and primary auditory and somatosensory cortices is associated with increased behavioral symptoms of SOR in addition to hyperactivity in these regions during exposure to mildly aversive stimuli [Green et al., 2016], suggesting that altered salience attribution may be one of the predominant factors in SOR. This prior study, like most imaging studies examining autism, featured a predominantly male sample, however, leaving little information on how females with ASD process information.
Three to four males are diagnosed with ASD for every female diagnosed [e.g., Baio et al., 2018; Loomes, Hull, & Mandy, 2017], and this likely explains why most ASD studies only have a small fraction of females, if any [for review, see Lai, Baron-Cohen, & Buxbaum, 2015; Philip et al., 2012]. Nonetheless, the results of these studies are often generalized to all individuals with ASD, although they may not be accurately reflecting females with the diagnosis. There are several well-known theories explaining why more males than females are diagnosed with ASD. One theory is that there are female-specific protective factors that allow girls to tolerate more of an etiological load before reaching clinical thresholds for ASD [Robinson, Lichtenstein, Anckarsäter, Happé, & Ronald, 2013]. Another theory is that cognitive and behavioral differences seen in males and females with ASD may result in under-diagnosis of females [Frazier, Georgiades, Bishop, & Hardan, 2014; McFayden, Antezana, Albright, Muskett, & Scarpa, 2019]. A third theory suggests that females are underdiagnosed because of their ability to mask their symptoms. This “camouflage” hypothesis states that young girls with ASD may shield their social shortcomings more effectively than males because of how they interact with their peers, staying in close proximity to their classmates as they shift between different activities while ASD boys tend to draw more attention as they play alone [Dean, Harwood, & Kasari, 2017]. This can affect diagnosis rates when one is examining a disorder that relies on observations of behavioral symptoms [Rynkiewicz et al., 2016]. Even if males and females with ASD show similar social and behavioral impairments, parents tend to rate their daughters as being more affected than their sons [Holtmann, Bölte, & Poustka, 2007], potentially reflecting different expectations for females than males.
At the neural level, there is evidence that males and females on the autism spectrum display different patterns of brain activity and connectivity which in turn differ from those seen in neurotypical (NT) controls [for review, see Lai et al., 2017]. For example, females with ASD generally show hyperconnectivity compared to males (i.e., greater number of brain regions fluctuating together during rest and/or a higher degree of correlation between the brain regions fluctuating together), whereas in NT individuals the opposite pattern is evident [Alaerts, Swinnen, & Wenderoth, 2016]. However, results are mixed, and a recent study found no sex differences in SN connectivity in children and adolescents with ASD [Lawrence et al., 2020]. To our knowledge, few if any studies have examined sex differences in the associations between behavior and brain connectivity in ASD. Studies that have examined task-related brain activity in ASD have also found evidence of sex differences in neural activity underlying visuospatial [Beacher et al., 2012] and socially relevant processes [Coffman, Anderson, Naples, & McPartland, 2015]. It is possible that ASD results in atypical sex differences, such that females and males show different neural signatures and symptom profiles compared to each other and to what is observed in males and females in the NT population.
Given the prevalence of and impairment caused by sensory processing difficulties in ASD, and that girls as well as boys experience these difficulties [Moseley, Hitchiner, & Kirkby, 2018], more research is needed on sex differences in the neurobiology underlying SOR in autism. Some studies have even suggested that females with ASD show greater sensory processing difficulties compared to males with ASD [Kumazaki et al., 2015; Lai et al., 2011], furthering the need to examine SOR in this population. If there is a significant difference in the neurobiological mechanisms of SOR in females versus males, this could have an impact on many aspects of their functioning and potentially help explain differential manifestations of ASD at the behavioral level. Thus, this study aimed to examine sex differences in the relationship between SOR and resting-state functional connectivity of the SN in females versus males with ASD.
Methods
Participants
Participants were 53 (16F) youth and adolescents with ASD (mean age, 13.7 years; range, 8.2–17.9 years). Each participant had a full-scale IQ within the normal range according to the Weschler Abbreviated Scales of Intelligence [Wechsler, 1999]. Each had a formal autism diagnosis according to the Autism Diagnostic Interview—Revised [ADI-R, Lord, Rutter, & Le Couteur, 1994], and/or the Autism Diagnostic Observation Schedule—second edition [ADOS-2; Lord, Rutter, DiLavore, Risi, & Bishop, 2012], and clinical judgment. Females and males with ASD did not differ significantly on full-scale IQ (FSIQ-t[51] = −1.2, p = 0.26), age (t[51] = −1.5, p = 0.14), handedness (X2 [1, N = 53] = 0.9, p = 0.34) or mean relative or absolute motion during the scan (t[51] = 0.2, p = 0.84; t[51] = 1.8, p = 0.08; Table 1). Additionally, there were no significant differences in overall autism symptomatology, as assessed through the ADOS (t[50] = −1.2, p = 0.23) or parent report on the ADI-R for the Social Interaction (t[49] = −0.7, p = 0.45), Communication (t[49] = 0.3, p = 0.78), or Restrictive and Repetitive Behaviors (t[49] = 0.2, p = 0.88) subscales. The only difference in the ADI-R between males and females was on the fourth subscale, Abnormality of Development Evident at or Before 36 months, with ASD males showing more signs of autism at an earlier age compared to diagnosed females (t[49] = −2.2, p = 0.03). Of the original sample of 57 ASD participants, three (1 female, 2 males) were excluded due to motion, and 1 male was excluded due to an echo-planar imaging (EPI) artifact. Subjects with a mean absolute motion greater than 1 mm and mean relative motion greater than 0.25 mm were excluded from all analyses. All study procedures were approved by the University of California Los Angeles Institutional Review Board.
Table 1.
Descriptive Statistics
| Female mean (SD) | Male mean (SD) | Female versus male p values | |
|---|---|---|---|
| N | 16 | 37 | – |
| Age (years) | 12.84 (2.6) | 14.10 (2.9) | 0.14 |
| Full-scale IQ | 100.63 (19.2) | 106.76 (13.6) | 0.26 |
| Handedness (R/L) | 16/0 | 35/2 | 0.34 |
| Behavioral measures | |||
| ADOSa | 5.75 (1.6) | 6.33 (1.6) | 0.23 |
| ADI-Rb: Social Interaction | 17.67 (5.3) | 19.03 (5.9) | 0.45 |
| ADI-Rb: Communication | 16.60 (4.1) | 16.19 (4.9) | 0.78 |
| ADI-Rb: Restrictive/Repetitive Behaviors | 6.20 (2.3) | 6.08 (2.5) | 0.88 |
| ADI-Rb: Abnormality of Development | 2.67(1.2) | 3.53 (1.3) | 0.03* |
| SOR total score | 8.63 (6.5) | 9.59 (8.0) | 0.67 |
| SCARED total score | 25.44 (12.7) | 15.24 (10.2) | 0.003** |
| Scanner measures | |||
| Mean absolute motion (mm) | .50 (.2) | .40 (.2) | 0.08 |
| Mean relative motion (mm) | .13 (.1) | .13 (.1) | 0.84 |
| Components removed | 104.31 (33.5) | 113.86 (33.1) | 0.34 |
Note. Higher SOR/SCARED scores indicate higher symptom severity. N = 16 ASD females, 37 ASD males.
Abbreviations: ADI-R, Autism Diagnostic Interview—Revised; ADOS, Autism Diagnostic Observation Schedule; SCARED, Screen for Child Anxiety-Related Disorders; SOR, sensory over-responsivity.
N = 16F, 36 M.
N = 15F, 36 M.
p < 0.05;
p < 0.01;
p < 0.001.
Measures
The following questionnaires were completed by participant’s parents.
Sensory Over-Responsivity Inventory.
The Sensory Over-Responsivity Inventory [Schoen, Miller, & Green, 2008] is a checklist of sensations that one may find aversive and was used to determine SOR severity. Each participant’s SOR score was calculated by taking a count of the number of tactile, visual, and auditory items the parent endorsed as being bothersome for their child. The SOR total score used in this study was highly correlated with each of the modality subscales (with Auditory SOR: r = 0.93, p = 0.01; Visual SOR: r = 0.56, p = 0.01; Tactile SOR: r = 0.87, p = 0.01), thus the total score was used in analyses.
Screen for Child Anxiety-Related Disorders.
The Screen for Child Anxiety Related Disorders [SCARED; Birmaher et al., 1999] measures anxiety symptoms and categorizes them into different subscales in order to screen for specific anxiety disorders. The total score was used to examine general anxiety severity for each participant.
Magnetic Resonance Imaging Data Acquisition
Functional magnetic resonance imaging (fMRI) resting-state scans were completed on a Siemens Prisma 3 Tesla scanner with a 64-channel head coil. This scan was the first functional scan administered as part of a larger protocol, to ensure no contamination from task-based scans. Participants fixed their gaze on a white crosshair on a black background, presented using a pair of 800 × 640 resolution magnet-compatible 3D goggles under computer control (Resonance Technologies, Inc.). Scans were acquired using an EPI multiband acquisition lasting 8 min and covering the entire cerebral volume (repetition time (TR) = 720 ms, field-of-view (FOV) = 208 mm, echo time (TE) = 37 ms, flip angle = 52, in-plane voxel size = 2 mm2, 72 slices).
Data Preprocessing and Analysis
The fMRI data were analyzed using the FMRIB Software Library (FSL), Version 5.0.10 (www.fmrib.ox.ac.uk/fsl). The preprocessing pipeline included spatial smoothing (Gaussian kernel full width at half maximum = 5 mm), bandpass filtering (0.1 Hz > t > 0.01 Hz), and the regression of mean white matter, cerebrospinal fluid, and global signal times series. Independent Component Analysis—Automatic Removal of Motion Artifacts [Pruim, Mennes, Buitelaar, & Beckmann, 2015] was used to remove potential confounds resulting from head motion by regressing out single-subject components labeled as motion or noise. Each participant’s data was then registered to the MNI152 T1 2-mm template brain (12 degrees of freedom).
A fixed-effects model was run for each individual subject using FSL’s fMRI Expert Analysis Tool (FEAT, Version 6.0) before they were combined in a higher-level mixed-effects model to examine within- and between-group differences. Higher-level group analyses were conducted using FSL’s Local Analysis of Mixed Effects State (FLAME 1 + 2). Research examining the SN supports a right dominance, as hubs of the network have been shown to be more connected to regions on the right hemisphere compared to the left [Cauda et al., 2011; Seeley et al., 2007; Zhang et al., 2019] in addition to activating more in response to salient stimuli [Sridharan, Levitin, & Menon, 2008]. Therefore, a 5-mm sphere in the right anterior insula (rAI; Montreal Neurological Institute [MNI] coordinates 38, 26, −10) was used as the seed for the SN [Seeley et al., 2007]. Single-subject connectivity maps were created by isolating the time-series from this region in individual subject space and correlating it with the activity of every other voxel in the brain in order to find regions of synchronous activity. Fischer’s r-to-z transformation was then used to create z-statistic maps prior to conducting group-level analyses. All whole-brain contrasts were corrected for multiple comparisons using Gaussian random-field theory in FSL with a voxel-wise threshold of z > 2.3 and corrected cluster threshold of p < 0.05. To examine how SN connectivity related to SOR, SOR scores were entered as a bottom-up regressor in the whole-brain analysis. Anxiety symptoms were used as a covariate in these analyses because of their high comorbidity with SOR [Green & Ben-Sasson, 2010; Ben-Sasson et al., 2008], and their correlation with SOR in the ASD sample (r = 0.31, p = .05). While not a primary focus of this paper, we also show within-group and sex differences in SN connectivity in the supplement (Figure S1, Table S1).
Results
Behavioral
An independent samples t test showed that there were no significant sex differences in total SOR score (t[51] = −0.4, p = 0.67). Females with ASD had significantly higher anxiety compared to males with ASD (t[51] = 3.1, p = 0.003).
Connectivity
Sex differences in SOR correlations with SN connectivity.
In males with ASD, SOR was positively correlated with connectivity between the rAI and temporal regions involved in auditory and language processing, including the planum temporale, temporal pole, and temporal gyrus, as well as the left hippocampus, and inferior frontal gyrus. SOR was also negatively correlated with connectivity between the SN seed and the posterior cingulate, right thalamus, precuneus, and occipital regions of the brain.
In females with ASD, SOR was positively correlated with connectivity between the rAI and frontal regions, including the frontal pole, superior frontal gyrus (dorsal lateral prefrontal cortex, dlPFC), paracingulate (dorsal medial prefrontal cortex, dmPFC), and anterior cingulate cortex (ACC). In this group, SOR was negatively correlated with connectivity between the rAI and sensory-motor regions including precentral and postcentral gyri and auditory cortex. Additionally, SOR was negatively correlated with rAI connectivity with the insular/opercular cortex and additional higher-level sensory processing regions including the planum temporale, planum polare, and supramarginal gyrus.
Between-group contrasts demonstrated that, compared to females with ASD, males showed a more positive correlation between SOR and rAI connectivity with sensory regions including temporal cortex and postcentral gyrus. This group difference was accounted for both by a negative correlation with SOR in females as well as a positive correlation in males, although this was only visible at lower thresholds (i.e., z > 1.7), suggesting opposite effects in males compared to females. In contrast, compared to males with ASD, in females, SOR was more strongly related to connectivity between the rAI and dlPFC/dmPFC/ACC (Figure 1, Table 2).
Figure 1.
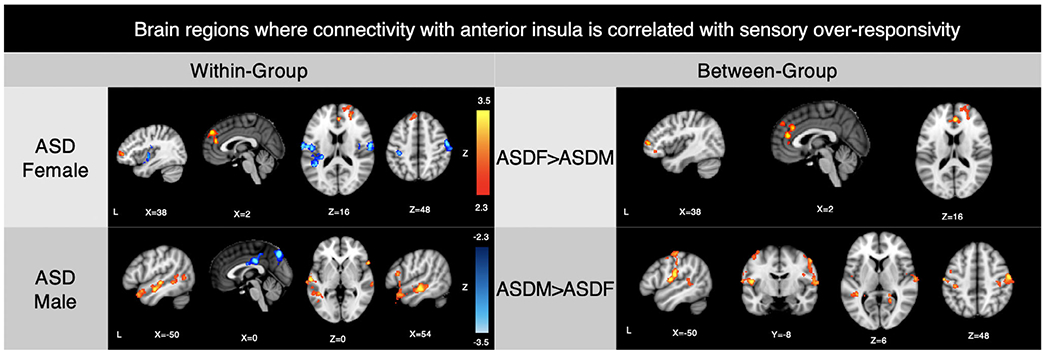
Within- and between-group results: Sex differences in salience network connectivity related to SOR in the autism group. Note. Within- and between-group contrasts thresholded at z > 2.3, corrected (p < 0.05). ASD, autism spectrum disorder; SOR, sensory over-responsivity
Table 2.
Montreal Neurological Institute (MNI) Coordinates for Brain Areas where Connectivity with the Right Anterior Insula (rAI) Correlated with Sensory Over-Responsivity (SOR)
| ASD female |
ASD male |
ASD female>ASD male |
ASD male>ASD female |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI peak (mm) | MNI peak (mm) | MNI peak (mm) | MNI peak (mm) | |||||||||||||||||
| Voxels | x | y | z | Max Z | Voxels | x | y | z | Max Z | Voxels | x | y | z | Max Z | Voxels | x | y | z | Max Z | |
| Superior frontal gyrus | 1266 | 4 | 48 | 38 | 3.65 | |||||||||||||||
| ACC/paracingulate/superior frontal gyrus | 2 | 42 | 20 | 3.61 | 582 | 4 | 40 | 16 | 4.04 | |||||||||||
| Middle frontal gyrus | 42 | 52 | 8 | 3.15 | 883 | 40 | 50 | 6 | 3.64 | |||||||||||
| Right OFC | 28 | 26 | −10 | 3.25 | ||||||||||||||||
| Right postcentral gyrus | 1373 | 52 | −24 | 60 | 7.7 | 1230 | 58 | −18 | 54 | 4.6 | ||||||||||
| Left operculum/insula | 1321 | −48 | −16 | 20 | 4.87 | 1068 | −48 | −16 | 20 | 4.3 | ||||||||||
| Left postcentral gyrus | −56 | −16 | 52 | 4.26 | ||||||||||||||||
| Lingual gyrus | 20 | −48 | −4 | 3.63 | ||||||||||||||||
| Left superior temporal gyrus | 1174 | −60 | −16 | −2 | 4.21 | −44 | −42 | 2 | 3.76 | |||||||||||
| Right superior temporal gyrus/middle temporal gyrus | 605 | 54 | −20 | −10 | 3.71 | |||||||||||||||
| Right planum polare | 747 | 48 | 26 | −22 | 4.26 | |||||||||||||||
| Left temporal fusiform gyrus | 643 | −42 | −38 | −18 | 4.07 | |||||||||||||||
| Precuneus | 1149 | −2 | −74 | 46 | 4.19 | |||||||||||||||
| Posterior cingulate gyrus | 525 | 2 | −26 | 32 | 4.27 | |||||||||||||||
Note. x, y, and z refer to the left–right, anterior–posterior, and inferior–superior dimensions, respectively; Z refers to the Z-score at those coordinates (local maxima or submaxima). Voxels indicates cluster size; coordinates in italics are local maxima within the same cluster as the coordinates above them. Within- and between-group analyses are cluster corrected for multiple comparisons, z > 2.3, p < .05. Within-group coordinates indicate either a positive correlation with SOR and functional connectivity with the right anterior insula seed or a negative correlation with SOR (in bold). Between-group coordinates indicate clusters where the one group had a significantly correlation between SOR and rAI connectivity with that region relative to the other group. Peak coordinates were found in similar regions when anxiety was not covaried, except for the frontal regions positively associated with SOR for ASD females (Figure S4).
Abbreviations: ASD, autism spectrum disorder; OFC, orbitofrontal cortex; ACC, anterior cingulate cortex.
Parameter estimates were extracted from regions where there were significant sex differences in SN connectivity as a function of SOR to illustrate the direction of effects and ensure that correlations were not driven by outliers (Figure 2). Finally, to ensure that these effects were due to real sex differences, rather than differences in power due to unequal male and female sample sizes, we reran these analyses with a subset of 16 males that were matched on age, SOR, anxiety, FSIQ, and motion to the original group of 37 (Table S2). Results from the original analysis were replicated and showed stronger within-group connectivity in the male sample (Figure S2, Table S3).
Figure 2.
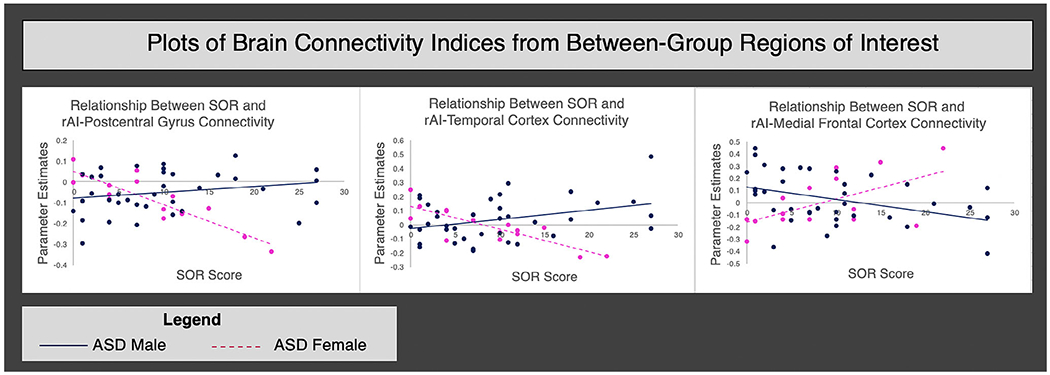
The relationship between sensory over-responsivity (SOR) severity and connectivity with the right anterior insula (rAI) for males and females with autism spectrum disorder (ASD). Note: The horizontal axis displays the SOR score and the vertical axis displays the parameter estimates extracted from areas where significant correlations between SOR severity and connectivity with the rAI were observed in the between-group contrasts
Discussion
In this study, we aimed to identify whether males and females with ASD differ in the neurobiology underlying sensory over-responsivity. To do so, we examined sex differences in SOR symptoms and the relationship between SOR and SN connectivity. We found that despite sharing similar behavioral profiles, males and females with ASD differ in how SN connectivity relates to SOR symptoms.
At the behavioral level, we found that males and females with ASD did not show sex differences in SOR symptom severity, which is consistent with a number of other studies [Mandy et al. 2012; Bitsika et al., 2018; Nguyen & Ronald, 2014]. Although one study [Kumazaki et al., 2015] reported greater sensory issues in females compared to males with ASD, these participants were younger than in the current study, suggesting a need to examine sex differences in sensory processing across development.
Importantly, although males and females with ASD did not differ in SOR symptomatology, they differed significantly in how SOR related to SN connectivity. Only in males with ASD, higher SOR was associated with greater SN connectivity with sensory processing regions and reduced connectivity with regions important for social processing such as the precuneus. Furthermore, in the current study, SOR was significantly more correlated with primary sensory cortices in males than in females. These results may reflect a lack of functional segregation between the salience and sensory networks in males with ASD, which is consistent with our previous findings of SOR associations with SN connectivity in a predominantly male ASD sample (Green et al., 2016). In addition, they are in line with prior reports, also in predominantly male samples, that SOR is associated with overactive brain responses to sensory stimuli in both sensory processing regions as well as regions implicated in salience and attention [i.e., amygdala and insula;Green et al., 2013, 2015]. These results support the theory that, at least in males with ASD, SOR may result from the mis-attribution of salience to external sensory stimuli.
Notably, SOR related quite differently to SN connectivity in ASD females compared to ASD males, suggesting that females with ASD may engage different neural networks in response to aversive sensory stimuli. SN connectivity with sensory cortices was actually correlated with reduced SOR in females. In contrast, increased SN connectivity with prefrontal regions including dmPFC, dlPFC, and ACC was associated with higher SOR in females with ASD.
The distinct relationships observed between SOR and SN connectivity in males versus females with ASD may reflect sex differences in how SOR and other sensory processing atypicalities are experienced, or in how SOR relates to other symptoms such as social functioning [Head, McGillivray, & Stokes, 2014; Lai et al., 2011]. Activity in medial prefrontal regions, including in the dorsal medial prefrontal cortex/anterior cingulate cortex and in dorsolateral prefrontal cortex, as seen in this study, as well as in more ventral regions has been implicated in emotional regulation [Ochsner, Silvers, & Buhle, 2012]. It is possible that females compared to males with ASD may extend more effort toward regulating negative emotions in relation to aversive sensory stimuli. This finding could indicate that girls with higher levels of SOR are working harder to regulate and reduce sensory-related behaviors in order to fit in better, as in the camouflage hypothesis [Dean et al., 2017]. This process of masking sensory symptoms may require prefrontal top-down regulation, and there is evidence to suggest that for women with autism, enhanced camouflaging is associated with increased activation in the prefrontal cortex during a self-representation task [Lai et al., 2018]. However, the prefrontal region found in Lai et al. was more ventral than that seen in this study, and more research is necessary to determine how and whether prefrontal down-regulation during sensory stimulation might be associated with camouflaging.
The dmPFC/ACC regions seen here to be related to SOR also overlap with regions associated with pain perception [Bräscher, Becker, Hoeppli, & Schweinhardt, 2016; Woo et al., 2017; Woo, Roy, Buhle, & Wager, 2015]. It is possible that SOR is more highly associated with the pain network for females with ASD and the somatosensory network in males with ASD. Finally, activity in the vmPFC and ACC has also been shown to relate to reward processing in autism, specifically in relation to the individual’s own restricted interests [Dichter et al., 2012]. Research on sensory subgroupings within ASD suggests that SOR and sensory seeking actually cluster together in those most severely affected by sensory processing challenges [Ausderau et al., 2014; Liss et al., 2006]. Accordingly, the observed greater SN connectivity with mPFC in ASD females might also be associated with sensory seeking and actually represent a higher reward value of sensory stimuli to this group. Future research should examine sex differences in the internal experience of different types of sensory stimuli and how these relate to distinct neural profiles in males and females with ASD.
Sensory processing difficulties can span across several domains, including SOR, sensory under responsivity, and sensory seeking [Ben-Sasson et al., 2009; Liss et al., 2006]. These atypical sensory processing symptoms often cluster together according to severity as opposed to sensory processing style [e.g., Ausderau et al., 2014; Elwin, Schröder, Ek, Wallsten, & Kjellin, 2017], such that individuals with autism often experience difficulties across domains and modalities. Given the high correlation between the SOR composite score used in this study with each of the subscales (auditory, tactile, visual), we decided to use the SOR composite for all analyses. The strongest correlation in scores was for the auditory and tactile subscales, possibly because these sections of the SOR Inventory contain more items than the visual subscale, or possibly because auditory and tactile are the most commonly cited modalities of over-responsivity in general [Schoen et al., 2008] and in autism [Mikkelsen, Wodka, Mostofsky, & Puts, 2016; Tavassoli et al., 2014]. Future research examining sensory processing should consider examining specific modalities of SOR in order to determine whether SOR in any particular domain has differential effects on brain response or behavior. Further, while we examined here the unique effect of SOR after controlling for anxiety symptoms, future research should examine the specificity of the relationship between SN connectivity and SOR in general, for example determining whether SN connectivity is predictive of SOR over and above autism symptom severity.
While this study did have a higher proportion of affected females compared to many other autism imaging studies, the female sample size was still relatively small, and future studies should aim to replicate these results with a larger sample size. Future studies should also examine atypical sensory processing using more robust sensory questionnaires and behavioral assessments across development in ASD in order to determine the stability and generalizability of sex differences in SOR symptomatology. If there are sex differences in sensory processing in young children with ASD that diminish with development, neuroimaging can provide insight into why this may occur.
Results inform potential avenues for sex-specific sensory therapies for youth with ASD. For example, a therapeutic approach that involves consciously thinking about one’s external world and engages prefrontal areas, such as cognitive behavioral therapy [Mason, Peters, Williams, & Kumari, 2017; van der Straten, Huyser, Wolters, Denys, & van Wingen, 2018], may be an effective treatment plan for females with ASD and SOR, as they may already be engaging prefrontal regions to regulate their responses to sensory stimuli. In males with ASD, meanwhile, SOR appears to be related to attributing increased salience to extraneous sensory stimuli, potentially at the cost of attention to key social stimuli and cues. Therefore, interventions that help to redirect attention either by reducing salience of extraneous sensory stimuli or increasing salience of social stimuli may be particularly helpful for males with SOR. There is some evidence for the effectiveness of this technique: Green, Hernandez, Bowman, Bookheimer, and Dapretto [2018] found that distracting sensory stimuli disrupted neural processing of social cues for primarily male ASD youth, and this effect was mitigated by instructing participants to direct their attention to key social cues. Additionally, if SOR is related to different internal experiences in females compared to males, such as a greater perception of pain versus an increase in the amount of effort and attention put in to processing sensory information, this too would inform targeted treatment approaches.
To the best of our knowledge, this study is the first to examine sex differences in brain connectivity as it relates to sensory symptoms in ASD. The observed sex differences in the associations between SN connectivity and SOR support the need for imaging studies in autism to examine sex as a moderator and indicate that prior findings from predominantly male samples cannot necessarily be generalized to females with autism. Research focused on the role of sensory processing and its relationship to social functioning should also keep in mind the need to study females and males separately.
Supplementary Material
Appendix S1: Supplementary Results
Table S1. Montreal Neurological Institute (MNI) coordinates for salience network maps: autism spectrum disorder group.
Table S2. Descriptive characteristics of sex-matched autism sample
Table S3. Montreal Neurological Institute (MNI) coordinates for brain areas where connectivity with the right anterior insula (rAI) correlated with sensory over-responsivity (SOR): matched sample
Figure S1. Within- and between-group results: sex differences in salience network connectivity in the ASD group
Figure S2. Within- and between-group results: sex differences in salience network connectivity related to SOR in sample-size-matched male and female groups
Figure S3. Within- and between-group results: Sex differences in salience network connectivity related to SOR, not covarying for anxiety
Acknowledgments
This work was supported by grants from the the Simons Foundation Autism Research Initiative (Grant Number 345389), National Institute of Child Health and Human Development (P50 HD055784), and the National Institute of Mental Health (R01MH100028; K08 MH112871). The authors were also supported by the following training grants/fellowships: a National Research Service Award postdoctoral fellowship to SG (F32 MH105167), a National Institute of Neurological Disorders and Stroke pre/post-doctoral training grant (F99 NS105206) to L. M. H., a National Institute of Neurological Disorders and Stroke institutional training grant (T32NS048004) a National Research Service Award predoctoral fellowship (F31MH110140) to K. E. L., a National Institutes of Mental Health institutional training grant (T32MH073517-12) and a American Academy of Child and Adolescent Psychiatry Pilot Research Award to E. T. W. and a fellowship from the Roche/ARCS Foundation Scholar Award Program in the Life Sciences to K. E. L. For generous support, the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project described was supported by Grant Numbers RR12169, RR13642, and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH); its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCR or NIH. The funding sources and organizations listed above had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. These research efforts were conducted in part under the auspices of The Help Group-UCLA Autism Research Alliance, which contributed to participant recruitment.
Footnotes
Conflict of interest
All authors declare that there is no conflict of interest.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Alaerts K, Swinnen SP, & Wenderoth N (2016). Sex differences in autism: A resting-state fMRI investigation of functional brain connectivity in males and females. Social Cognitive and Affective Neuroscience, 11(6), 1002–1016. 10.1093/scan/nsw027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Anderson AK, & Phelps EA (2001). Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature, 411(6835), 305–309. 10.1038/35077083 [DOI] [PubMed] [Google Scholar]
- Ausderau KK, Furlong M, Sideris J, Bulluck J, Little LM, Watson LR, … Baranek GT (2014). sensory subtypes in children with autism spectrum disorder: latent profile transition analysis using a national survey of sensory features. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(8), 935–944. 10.1111/jcpp.12219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of autism spectrum disorder among children aged 8 years autism and developmental disabilities monitoring network, 11 sites, United States, 2014. Morbidity and Mortality Weekly Report. Surveillance Summaries, 67(6), 1–23. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AEZ, Lane A, Angley MT, & Young RL (2008). The relationship between sensory processing patterns and behavioural responsiveness in autistic disorder: A pilot study. Journal of Autism and Developmental Disorders, 38(5), 867–875. 10.1007/s10803-007-0459-0 [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, & Watson LR (2006). Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 47(6), 591–601. 10.1111/j.14697610.2005.01546.x [DOI] [PubMed] [Google Scholar]
- Beacher FDCC, Radulescu E, Minati L, Baron-Cohen S, Lombardo MV, Lai M-C, … Critchley HD (2012). Sex differences and autism: Brain function during verbal fluency and mental rotation. PloS One, 7(6), e38355. 10.1371/journal.pone.0038355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sasson A, Cermak SA, Orsmond GI, Tager-Flusberg H, Kadlec MB, & Carter AS (2008). Sensory clusters of tod-dlers with autism spectrum disorders: Differences in affective symptoms. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 49(8), 817–825. 10.1111/j.1469-7610.2008.01899.x [DOI] [PubMed] [Google Scholar]
- Ben-Sasson A, Hen L, Fluss R, Cermak SA, Engel-Yeger B, & Gal E (2009). A meta analysis of sensory modulation symptoms in individuals with autism spectrum disorders. Journal of Autism and Developmental Disorders, 39(1), 1–11. 10.1007/s10803-008-0593-3 [DOI] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, & Baugher M (1999). Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): A replication study. Journal of the American Academy of Child and Adolescent Psychiatry, 38(10), 1230–1236. 10.1097/00004583-199910000-00011 [DOI] [PubMed] [Google Scholar]
- Bitsika V, Sharpley CF, Mills R (2018). Sex differences in sensory features between boys and girls with autism spectrum disorder. Research in Autism Spectrum Disorders, 51, 49–55. 10.1016/j.rasd.2018.04.002 [DOI] [Google Scholar]
- Bräscher A-K, Becker S, Hoeppli M-E, & Schweinhardt P (2016). Different brain circuitries mediating controllable and uncontrollable pain. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 36(18), 5013–5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, & Vercelli A (2011). Functional connectivity of the insula in the resting brain. Neuroimage, 55(1), 8–23. 10.1016/j.neuroimage.2010.11.049 [DOI] [PubMed] [Google Scholar]
- Chen H, Uddin L, Duan X, Zheng J, Long Z, ZHang Y, … Chen H (2017). Shared atypical default mode and salience network functional connectivity between autism and schizophrenia. Autism Research, 10(11), 1776–1786. 10.1002/aur.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman MC, Anderson LC, Naples AJ, & McPartland JC (2015). Sex differences in social perception in children with ASD. Journal of Autism and Developmental Disorders, 45(2), 589–599. 10.1007/s10803-013-2006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M, Harwood R, & Kasari C (2017). The art of camouflage: Gender differences in the social behaviors of girls and boys with autism spectrum disorder. Autism: The International Journal of Research and Practice, 21(6), 678–689. 10.1177/1362361316671845 [DOI] [PubMed] [Google Scholar]
- Di Martino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, & Milham MP (2009). Functional brain correlates of social and nonsocial processes in autism spectrum disorders: An activation likelihood estimation meta-analysis. Biological Psychiatry, 65(1), 63–74. 10.1016/j.biopsych.2008.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, & Bodfish JW (2012). Reward circuitry function in autism spectrum disorders. Social Cognitive and Affective Neuroscience, 7(2), 160–172. 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A, Di Martino A, Hazlett HC, & Gao W (2016). Neural connectivity evidence for a categorical-dimensional hybrid model of autism spectrum disorder. Biological Psychiatry, 80(2), 120–128. 10.1016/j.biopsych.2015.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwin M, Schröder A, Ek L, Wallsten T, & Kjellin L (2017). Sensory clusters of adults with and without autism spectrum conditions. Journal of Autism and Developmental Disorders, 47(3), 579–589. 10.1007/s10803-016-2976-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Georgiades S, Bishop SL, & Hardan AY (2014). Behavioral and cognitive characteristics of females and males with autism in the Simons Simplex Collection. Journal of the American Academy of Child and Adolescent Psychiatry, 53(3), 329-340.e1-3. 10.1016/j.jaac.2013.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, & Ben-Sasson A (2010). Anxiety disorders and sensory over-responsivity in children with autism spectrum disorders: Is there a causal relationship? Journal of Autism and Developmental Disorders, 40(12), 1495–1504. 10.1007/s10803-010-1007-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Bookheimer SY, & Dapretto M (2016). Salience network connectivity in autism is related to brain and behavioral markers of sensory overresponsivity. Journal of the American Academy of Child & Adolescent Psychiatry, 55(7), 618–626.e1. 10.1016/j.jaac.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Lawrence KE, Liu J, Tsang T, Yeargin J, … Bookheimer SY (2019). Distinct patterns of neural habituation and generalization in children and adolescents with autism with low and high sensory overresponsivity. American Journal of Psychiatry, 176(12), 1010–1020. 10.1176/appi.ajp.2019.18121333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez L, Tottenham N, Krasileva K, Bookheimer SY, & Dapretto M (2015). Neurobiology of sensory over responsivity in youth with autism spectrum disorders. Journal of the American Medical Association Psychiatry, 72(8), 778–786. 10.1001/jamapsychiatry.2015.0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Hernandez LM, Bowman HC, Bookheimer SY, & Dapretto M (2018). Sensory overresponsivity and social cognition in ASD: Effects of aversive sensory stimuli and attentional modulation on neural responses to social cues. Developmental Cognitive Neuroscience, 29, 127–139. 10.1016/j.dcn.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green SA, Rudie JD, Colich NL, Wood JJ, Shirinyan D, Hernandez L, … Bookheimer SY (2013). Overreactive brain responses to sensory stimuli in youth with autism spectrum disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 52(11), 1158–1172. 10.1016/j.jaac.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head AM, McGillivray JA, & Stokes MA (2014). Gender differences in emotionality and sociability in children with autism spectrum disorders. Molecular Autism, 5(1), 19. 10.1186/2040-2392-5-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton CL, Harper JD, Kueker RH, Lang AR, Abbacchi AM, Todorov A, & LaVesser PD (2010). Sensory responsiveness as a predictor of social severity in children with high functioning autism spectrum disorders. Journal of Autism and Developmental Disorders, 40(8), 937–945. 10.1007/s10803-010-0944-8 [DOI] [PubMed] [Google Scholar]
- Hofmann SG, & Bitran S (2007). Sensory-processing sensitiveity in social anxiety disorder: Relationship to harm avoidance and diagnostic subtypes. Journal of Anxiety Disorders, 21(7), 944–954. 10.1016/j.janxdis.2006.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmann M, Bölte S, & Poustka F (2007). Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Developmental Medicine and Child Neurology, 49(5), 361–366. 10.1111/j.1469-8749.2007.00361.x [DOI] [PubMed] [Google Scholar]
- Jerome EM, & Liss M (2005). Relationships between sensory processing style, adult attachment, and coping. Personality and Individual Differences, 38(6), 1341–1352. 10.1016/j.paid.2004.08.016 [DOI] [Google Scholar]
- Kientz MA, & Dunn W (1997). A comparison of the performance of children with and without autism on the Sensory Profile. American Journal of Occupational Therapy, 51(7), 530–537. 10.5014/ajot.51.7.530 [DOI] [PubMed] [Google Scholar]
- Klintwall L, Holm A, Eriksson M, Carlsson LH, Olsson MB, Hedvall Å, … Fernell E (2011). Sensory abnormalities in autism: A brief report. Research in Developmental Disabilities, 32(2), 795–800. 10.1016/j.ridd.2010.10.021 [DOI] [PubMed] [Google Scholar]
- Kumazaki H, Muramatsu T, Kosaka H, Fujisawa TX, Iwata K, Tomoda A, … Mimura M (2015). Sex differences in cognitive and symptom profiles in children with high functioning autism spectrum disorders. Research in Autism Spectrum Disorders, 13–14, 1–7. 10.1016/j.rasd.2014.12.011 [DOI] [Google Scholar]
- Lai M-C, Baron-Cohen S, & Buxbaum JD (2015). Under-standing autism in the light of sex/gender. Molecular Autism, 6(1), 24. 10.1186/s13229-015-0021-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lerch JP, Floris DL, Ruigrok ANV, Pohl A, Lombardo MV, & Baron Cohen S (2017). Imaging sex/gender and autism in the brain: Etiological implications. Journal of Neuroscience Research, 95(1–2), 380–397. 10.1002/jnr.23948 [DOI] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Chakrabarti B, Ruigrok AN, Bullmore ET, Suckling J, … Baron-Cohen S (2018). Neural self-representation in autistic women and association with “compensatory camouflaging”. Autism, 23(5), 1210–1223. 10.1177/1362361318807159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M-C, Lombardo MV, Pasco G, Ruigrok ANV, Wheelwright SJ, Sadek SA, … Baron-Cohen S (2011). A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PLoS One, 6(6), e20835. 10.1371/journal.pone.0020835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence KE, Hernandez LM, Bowman HC, Padgaonkar NT, Fuster E, Jack A, … Dapretto M GENDAAR Consortium. (2020). Sex differences in functional connectivity of the salience, default mode, and central executive networks in youth with ASD. Cerebral Cortex, 30(9), 5107–5120. 10.1093/cercor/bhaa105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liss M, Saulnier C, Fein D, & Kinsbourne M (2006). Sensory and attention abnormalities in autistic spectrum disorders. Autism, 10(2), 155–172. 10.1177/1362361306062021 [DOI] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56(6), 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi G, & Bishop S (2012). Autism diagnostic observations schedule (2nd ed.). Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, & Le Couteur A (1994). Autism Diagnostic Interview—Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders, 24(5), 659–685. 10.1007/bf02172145 [DOI] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D (2012). Sex Differences in Autism Spectrum Disorder: Evidence from a Large Sample of Children and Adolescents. Journal of Autism and Developmental Disorders, 42(7), 1304–1313. 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- Mason L, Peters E, Williams SC, & Kumari V (2017). Brain connectivity changes occurring following cognitive behavioural therapy for psychosis predict long-term recovery. Translational Psychiatry, 7(1), e1001–e1001. 10.1038/tp.2016.263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek MO, & Petroski GF (2015). Sleep problems in children with autism spectrum disorder: Examining the contributions of sensory over-responsivity and anxiety. Sleep Medicine, 16(2), 270–279. 10.1016/j.sleep.2014.11.006 [DOI] [PubMed] [Google Scholar]
- McFayden TC, Antezana L, Albright J, Muskett A, & Scarpa A (2019). Sex differences in an autism spectrum disorder diagnosis: Are restricted repetitive behaviors and interests the key? Review Journal of Autism and Developmental Disorders, 7, 119–126. 10.1007/s40489-019-00183-w [DOI] [PubMed] [Google Scholar]
- Menon V, & Uddin LQ (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure & Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen M, Wodka EL, Mostofsky SH, & Puts NAJ (2016). Autism spectrum disorder in the scope of tactile processing. Developmental Cognitive Neuroscience, 29, 140–150. 10.1016/j.dcn.2016.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RL, Hitchiner R, & Kirkby JA (2018). Self-reported sex differences in high functioning adults with autism: A meta-analysis. Molecular Autism, 9, 33. 10.1186/s13229-018-0216-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, & Ronald A (2014). How do girls with low functioning autism compare to boys with autism and typically developing girls with regard to behavior, cognition, and psychopathology?. Scandinavian Journal of Child and Adolescent Psychiatry and Psychology, 2(2), 55–65. [Google Scholar]
- Ochsner KN, Silvers JA, & Buhle JT (2012). Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251, E1–E24. 10.1111/j.1749-6632.2012.06751.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell S, Deitz J, Kartin D, Nalty T, & Dawson G (2012). Sensory processing, problem behavior, adaptive behavior, and cognition in preschool children with autism spectrum disorders. The American Journal of Occupational Therapy, 66(5), 586–594. 10.5014/ajot.2012.004168 [DOI] [PubMed] [Google Scholar]
- Philip RCM, Dauvermann MR, Whalley HC, Baynham K, Lawrie SM, & Stanfield AC (2012). A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neuroscience and Biobehavioral Reviews, 36(2), 901–942. 10.1016/j.neubiorev.2011.10.008 [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, Buitelaar JK, & Beckmann CF (2015). Evaluation of ICA AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage, 112, 278–287. 10.1016/j.neuroimage.2015.02.063 [DOI] [PubMed] [Google Scholar]
- Robinson EB, Lichtenstein P, Anckarsäter H, Happé F, & Ronald A (2013). Examining and interpreting the female protective effect against autistic behavior. Proceedings of the National Academy of Sciences, 110(13), 5258–5262. 10.1073/pnas.1211070110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz A, Schuller B, Marchi E, Piana S, Camurri A, Lassalle A, & Baron-Cohen S (2016). An investigation of the “female camouflage effect” in autism using a computerized ADOS-2 and a test of sex/gender differences. Molecular Autism, 7(1), 10. 10.1186/s13229-016-0073-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D, Grafman J, & Zalla T (2003). The human amygdala: An evolved system for relevance detection. Reviews in the Neurosciences, 14(4), 303–316. 10.1515/revneuro.2003.14.4.303 [DOI] [PubMed] [Google Scholar]
- Schoen SA, Miller LJ, & Green KE (2008). Pilot study of the Sensory Over-Responsivity Scales: Assessment and inventory. The American Journal of Occupational Therapy, 62(4), 393–406. 10.5014/ajot.62.4.393 [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, & Frith U (2008). Levels of emotional awareness and autism: An fMRI study. Social Neuroscience, 3(2), 97–112. 10.1080/17470910701577020 [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, & Menon V (2008). A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences, 105(34), 12569–12574. 10.1073/pnas.0800005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavassoli T, Miller LJ, Schoen SA, Nielsen DM, & Baron-Cohen S (2014). Sensory over-responsivity in adults with autism spectrum conditions. Autism: The International Journal of Research and Practice, 18(4), 428–432. 10.1177/1362361313477246 [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Gustafsson P, Larsson H, Gillberg C, Lundström S, & Lichstenstein P (2018). Examining the association between autistic traits and atypical sensory reactivity: a twin study. Journal of the American Academy of Child & Adolescent Psychiatry, 57(2), 96–102. 10.1016/j.jaac.2017.11.019 [DOI] [PubMed] [Google Scholar]
- Uddin LQ (2015). Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience, 16(1), 55–61. 10.1038/nrn3857 [DOI] [PubMed] [Google Scholar]
- Uddin LQ, & Menon V (2009). The anterior insula in autism: Under-connected and under-examined. Neuroscience and Biobehavioral Reviews, 33(8), 1198–1203. 10.1016/j.neubiorev.2009.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, & Menon V (2013). Reconceptualizing functional brain connectivity in autism from a developmental perspective. Frontiers in Human Neuroscience, 7, 1–11. 10.3389/fnhum.2013.00458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Straten A, Huyser C, Wolters L, Denys D, & van Wingen G (2018). Long-term effects of cognitive behavioral therapy on planning and prefrontal cortex function in pediatric obsessive-compulsive disorder. Biological Psychiatry. Cognitive Neuroscience and Neuroimaging, 3(4), 320–328. 10.1016/j.bpsc.2017.11.009 [DOI] [PubMed] [Google Scholar]
- von dem Hagen EAH, Stoyanova RS, Baron-Cohen S, & Calder AJ (2013). Reduced functional connectivity within and between “social” resting state networks in autism spectrum conditions. Social Cognitive and Affective Neuroscience, 8(6), 694–701. 10.1093/scan/nss053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler Abbreviated Scale of Intelligence. Tex, Harcourt Assessment: San Antonio, CA. [Google Scholar]
- Woo C-W, Roy M, Buhle JT, & Wager TD (2015). Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS Biology, 13(1), e1002036. 10.1371/journal.pbio.1002036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo C-W, Schmidt L, Krishnan A, Jepma M, Roy M, Lindquist MA, … Wager TD (2017). Quantifying cerebral contributions to pain beyond nociception. Nature Communications, 8(1), 1–14. 10.1038/ncomms14211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Suo X, Ding H, Liang M, Yu C, & Qin W (2019). Structural connectivity profile supports laterality of the salience network. Human Brain Mapping, 40(18), 5242–5255. 10.1002/hbm.24769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Anderson KL, Leal SL, Shestyuk A, Gulsen G, Mnatsakanyan L, … Lin JJ (2017). Amygdala-hippocampal dynamics during salient information processing. Nature Communications, 8(1), 1–11. 10.1038/ncomms14413 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1: Supplementary Results
Table S1. Montreal Neurological Institute (MNI) coordinates for salience network maps: autism spectrum disorder group.
Table S2. Descriptive characteristics of sex-matched autism sample
Table S3. Montreal Neurological Institute (MNI) coordinates for brain areas where connectivity with the right anterior insula (rAI) correlated with sensory over-responsivity (SOR): matched sample
Figure S1. Within- and between-group results: sex differences in salience network connectivity in the ASD group
Figure S2. Within- and between-group results: sex differences in salience network connectivity related to SOR in sample-size-matched male and female groups
Figure S3. Within- and between-group results: Sex differences in salience network connectivity related to SOR, not covarying for anxiety


