Abstract
Recent advances in microalgae biotechnology have proven that these microorganisms contain a number of bioactive molecules, that can be used as food additives that help prevent disease. The green microalga Chlorella vulgaris presents several biomolecules, such as lutein and astaxanthin, with antioxidant capacity, which can play a protective role in tissues. In this study, we produced and analyzed a C. vulgaris functional alcoholic beverage (produced using a traditional Brazilian alcoholic beverage, cachaça, and C. vulgaris biomass). Assays were conducted in vitro by radical scavenging tests, and in vivo, by modeling cortical spreading depression in rat brains. Scavenging radical assays showed that consumption of the C. vulgaris alcoholic beverage had a DPPH inhibition of 77.2%. This functional alcoholic beverage at a concentration of 12.5 g L-1 significantly improved cortical spreading depression velocity in the rat brains (2.89 mm min-1), when compared with cachaça alone (3.68 mm min-1) and control (distilled water; 3.25 mm min-1). Moreover, animals that consumed the functional beverage gained less weight than those that consumed just alcohol and the control groups. These findings suggest that the C. vulgaris functional alcoholic beverage plays a protective physiologic role in protecting brain cells from the effects of drinking ethanol.
Introduction
Ethyl alcohol consumption is one of the most common causes of general health disabilities, lower life expectancy, and also is associated to many chronic diseases [1, 2]. Due to the neurotoxic effect of alcohols, excessive ethyl alcohol consumption has been linked to an increased risk of some diseases, such as dementia, [3]. Controversially, epidemiological studies have indicated that moderate consumption of red wine may have a positive impact on human health [4, 5]. Phytochemicals, chemical compounds produced by plants, may be found in various beverages, such as wine. People who are moderate wine drinkers (three to four glasses a day) have a lower incidence of dementia compared to nondrinkers [6–9]. The possible mechanisms for these protective effects include antioxidant and anti-inflammatory properties as well as higher plasma apolipoprotein E levels [8, 9]. Therefore, moderate wine drinking is seen as a possible preventive measure against senile dementia, even though a direct demonstration of the protective effects of wine or a delineation of its mechanisms have not yet been fully elucidated [7].
The interest in biocompounds with antioxidant and antibacterial properties, such as polyphenols and carotenoids, has been increasing over the years. Due to the diversity of these compounds, they can exhibit a broad range of biological activities. For example, some plants and algae are particularly rich in polyphenols identified as having potential antioxidant activity [10], while other phenolic compounds and carotenoids have been most commonly related to free radical scavenging activity [11]. Therefore, the study of such biological compounds has been identified as a promising research field.
A growing attention to functional food has driven research into the physiological effects of high biological value components [12]. Microalgae are among the most promising sources of bioactive compounds for new food products, which can be used to enhance the nutritional value of foods due to their well-balanced chemical composition and their potential anticancer, anti-diabetes, anti-inflammatory and antioxidant properties [13, 14]. The addition of microalgae biomass to food products has become an interesting method for providing nutritional supplementation with biologically active compounds, like fatty acids, essential amino acids, polysaccharides, vitamins, carotenoids, and others [15–17]. In this context, Chlorella and Arthrospira spp. (Spirulina) are already being commercialized in various forms, mainly as powders, tablets and capsules, that can be added to drinks or food. Chlorella spp., particularly C. vulgaris, have a wide variety of compounds including polysaccharides [18, 19], flavonoids [20] and polyphenols [21], with reported biological activities.
The concern over the nutritional value and beneficial effects of food and beverages, aiming a longer and healthier life expectancy, and the growing of vegan food market have a major impact on consumer acceptance of using algae as food source [22, 23]. The market of microalgae-based functional foods is very promising and grows with the demand of the population that seeks balanced foods that can improve physical and mental well-being of the consumers. In recent years, microalgae are being considered a potential functional food source to prevent, treat or ameliorate of various diseases such as cardiovascular diseases, cancer, inflammations, neurological disorders etc. [23–25].
The cortical spreading depression (CSD) phenomenon is an interesting electrophysiological model that is valuable for understanding the relationship between oxidative stress, nutrition, and neural development and function [26]. CSD has been studied rigorously in rodents and humans because of its clinical significance in neurologic disorders, which has contributed to better knowledge and clinical improvement in neural diseases. To gain a better understanding of the action of compounds from microalgae-based alcoholic extracts and their application to human health, particularly on the nervous system, we studied the effects of consumption of a Chlorella functional alcoholic beverage on changes in propagation of CSD in the cerebral cortex of young-adult rats treated chronically.
Material and methods
Microalgae production
The green microalga Chlorella vulgaris used in this study were obtained from the Laboratório de Produção de Alimento Vivo at the Universidade Federal Rural de Pernambuco, Brazil. The microalga inoculum was cultivated in semi-continuous mode, in both laboratory and pilot-scale. Cultures of C. vulgaris were conducted using autoclaved freshwater enriched with Provasoli medium in 2–L Erlenmeyer type glass flasks, under 150 μmol photons m−2 s−1, homogenized continuously with atmospheric air at 24±0.5°C. After increasing the cultivation to 100–L cultures, a 500-L pilot-scale fiberglass tank was used as the final stage of cultivation, using N:P:K (2:1:2) fertilizer as the culture medium. The pilot-scale tank was under ~1.850 μmol photons m−2 s−1 in a natural photoperiod (12:12h light/dark cycle). The microalgae cells were harvested at exponential growth phase by flocculation, using NaOH (1.0 M), and then freeze-dried (-50±1°C and 150×10−3 mbar) [15].
Ultra-Performance Liquid Chromatography with coupled Mass Spectroscopy (UPLC–MS)
Chromatographic runs were performed in an ultra-performance liquid chromatography (UPLC, Acquity H–Class system, Waters Inc, Milford, MA, USA), with a C18 HSS 2.1 × 100 mm column and particle size of 1.8 μm (Waters Inc, Milford, MA, USA). The mobile phase was composed of 0.1% formic acid in acetonitrile (eluent A), 0.1% formic acid in ethyl acetate (eluent B) and 0.1% formic acid in methanol (eluent C), at an elution rate of 0.37 mL min-1 and an isocratic run, with a proportion of 10% A/40% B/50% C for 5 min, and sample injection volume of 10 μL. Column temperature was kept at 30°C, while the auto injector was at 10°C. The UPLC detection system was composed of a coupled mass spectrometer single quadrupole SQ Detector 2. Capilar voltage was 5.5 kV and cone voltage 50 V, the desolvation temperature was 350°C, and gas flow was 650 L h-1. Data acquisition was carried out in full scan mode, for masses between 100 and 1000 Da in positive ionization. Acquisition of chromatograms and mass spectra was performed with the software MassLynx™ (Waters Inc, Milford, MA, USA).
Preparation of the C. vulgaris functional alcoholic beverage
Samples of dried microalgae biomass were extracted in a commercial Brazilian alcoholic beverage (Pitu Ltda., Recife, PE, Brazil) that contains 40% ethanol from sugar cane (cachaça), resulting in a hydroalcoholic microalgae extract (C. vulgaris functional alcoholic beverage, CFAB) at a final concentration of 12.5 g L-1. The intracellular compounds were extracted under 30 min of ultrasound disruption (40 kHz) in an ultrasonic batch (model Ultra Cleaner 1400, Ultrasonic Unique, Brazil) followed by chamber shaking for 2 h and centrifugation (2,486 g, Herolab UniCen MR Centrifuge, Wiesloch, BW, Germany) for 10 min to obtain the supernatant liquid. The CFAB was stored in a freezer (-20±1°C) and analyzed daily to evaluate the quality of the samples for antioxidant activity during the experiment. We established three days as the CFAB expiration date, after which a new microalgal hydroalcoholic extract was prepared.
Antioxidant activity
The antioxidant activity of the C. vulgaris extracts was evaluated by 1, 1–diphenyl–2–picryl hydrazyl (DPPH) free-radical scavenging activity as reported by Dantas et al. [12]. The extracts were analyzed to evaluate the DPPH percentage of inhibition and a possible change over the days of the experiment.
Experimental design
Wistar rats were reared in polypropylene cages (51 × 35.5 × 18.5 cm) in a room maintained at 23 ± 1°C with a photoperiod of 12 h (lights on 7:00 AM). A total of 27 rats were divided into three groups (at age of 55–73 days). For 18 days each group received by gavage (a method for administering substances directly into the stomach through a cannula inserted in the mouth) 4.5 mL kg-1 d-1 doses of Chlorella functional alcoholic beverage, CFAB (CFAB-group); cachaça (cachaça-group) and distilled water (control-group). All groups had free access to water and a diet of commercial laboratory feed (23% crude protein, Purina do Brazil Ltd, Paulínia, SP, Brazil).
Electrophysiological recordings
Immediately after the gavage period, the CSD recording session was carried out, as previously described [27]. Briefly, under anesthesia with a mixture of 1 g kg-1 urethane plus 40 mg kg-1 chloralose (Sigma; injected intraperitoneally), a tracheal cannula was inserted and 3 trephine holes were made on the right side of the skull, aligned along the anteroposterior direction and parallel to the midline.
One hole was placed in the frontal bone (2 mm of diameter) and was used to apply the stimulus to elicit CSD. The other 2 holes were drilled in the parietal bone (3 to 4 mm in diameter) and were used to record the propagating CSD wave. The distance between the centers of contiguous holes was about 3 to 5 mm. Rectal temperature was continuously monitored and maintained at 37 ± 1° C with a heating blanket. At 20-min intervals, CSD was elicited by application, for 1 min, of a cotton ball (1 to 2 mm in diameter) soaked with 2% KCl solution (270 mM) placed on the frontal cortical surface through the hole drilled on that region. At the 2 parietal holes, both the slow change in direct current (DC) voltage and the reduction in the spontaneous cortical electrical activity accompanying CSD were continuously recorded for 4 h using a pair of Ag–AgCl Agar-Ringer electrodes (1 in each hole), as previously described [28]. A common reference electrode, of the same type, was placed on the nasal bones. The changes in DC voltage were recorded by connecting the electrodes to GRASS DC-amplifiers (Astro-Med Industrial Park, West Warwick, RI, USA), and the ECoG was recorded with AC-amplification (band pass filters set at 1 to 35 Hz range). Both DC-recording and ECoG were performed with a model 7–D GRASS chart recorder (Astro-Med Industrial Park, West Warwick, RI, USA). In some experiments, DC-recording was also computer-digitalized and recorded. The CSD propagation velocity was calculated from the time required for a CSD wave to cover the distance between the 2 cortical electrodes. In the measurement of CSD velocities, the initial point of each DC negative rising phase was used.
Statistical analysis
A one-way analysis of variance (ANOVA), followed by the Tukey’s test, when necessary, was applied using a significance level of 0.05. Normality and homoscedasticity were evaluated by the Shapiro-Wilk and Levene tests, respectively [29].
Ethics statement
The animals (male Wistar rats) were handled in accordance with the norms of the Ethics Committee for Animal Research of the Universidade Federal de Pernambuco, Brazil (where the experiments were conducted), which comply with the “Principles of Laboratory Animal Care” (National Institutes of Health, Bethesda, USA). The study was conducted after approval by the Ethics Committee on Animal Experimentation at UFPE (process number 23076.006249-2004-82). All electrophysiological recordings were performed under anesthesia, and subsequently, the rats were euthanized by an overdose of anesthetics. All efforts were made to minimize suffering.
Results and discussion
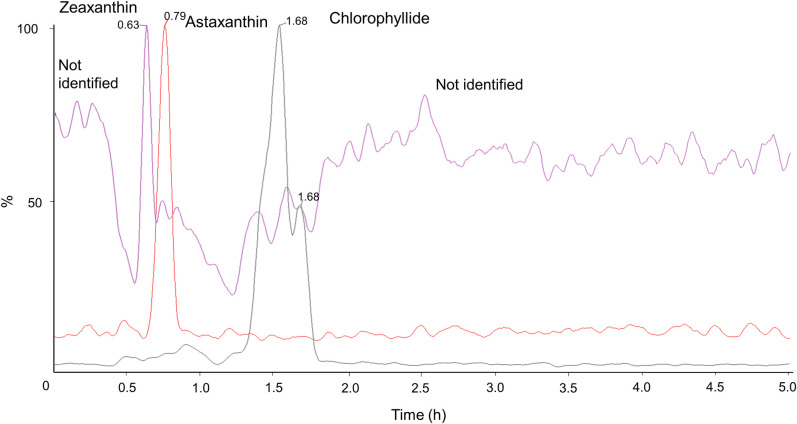
This study evaluated the production of an alcoholic beverage with the microalga C. vulgaris and both the antioxidant activity (in vitro) and the CSD model in brains of rats that consumed this functional beverage. Zeaxanthin, astaxanthin and chlorophyllide were the most abundant antioxidant pigments found in the CFAB (Fig 1). These four compounds contributed to almost 50% of the total antioxidant potential in the CFAB, with their isomers and derivatives comprising the rest. This information indicates that hydrophilic compounds, pigments and pheophytin were compounds with strong antioxidant activity and that can attach positive properties in CFAB [30]. Astaxanthin is the major carotenoid marketed in the world, and is already widely used for aquaculture purposes and also for human nutrition and supplementation [31], and a number of studies have demonstrated bioactive properties of this carotenoid [32, 33]. Furthermore, other carotenoids, such as lutein and violaxanthin, are commonly found in C. vulgaris biomass, but were not found in the present study. According to Yusof et al. [34], C. vulgaris has a higher concentration of carotenoids when grown under continuous lighting (i.e., 24:0 light/dark cycle). Thus, once cultures of C. vulgaris in the present study were conducted in natural photoperiod (i.e., 12:12 light/dark cycle) is likely that photoperiod had a negative influence on the carotenoid profile. In addition, other growing conditions can influence on the carotenoid profile in microalgae, such as nutritional metabolism, growth curve phase and salt stress [35, 36].
Fig 1. Chromatogram of the Chlorella vulgaris functional alcoholic beverage in ethyl acetate.
Growth performance of young-adult rats that received alcoholic beverages (CFAB or cachaça) and water are shown in Table 1. All animals survived and were in good health throughout the assays, however, the longevity of rats that did not consume alcoholic beverages was 9 days longer. In addition, rats that consumed alcoholic beverages had lower body weight and weight gain compared to the control group during the assays.
Table 1. Growth performance of young-adult rats (79–88 days of life) treated per gavage with C. vulgaris functional alcoholic beverage (CFAB), cachaça and water.
| Control | CFAB | Cachaça | |
|---|---|---|---|
| Final weight (g) | 361.8 ± 5.34a | 292.2 ± 9.66b | 297.8 ± 5.29b |
| Weight gain (g) | 62.15 ± 18.74a | 32.63 ± 9.84b | 46.5 ± 14.02ab |
| Age (days) | 88 | 79 | 79 |
| Radical scavenging (%) | 0 | 77.2 | 36.7 |
Data represent mean ± standard deviation of nine individuals for each treatment. Different letters on the same line indicate a significant difference by the Tukey’s post-hoc test (p <0.05).
These responses in weight gain are congruent with findings reported by Nuño et al. [37] who observed similar results when evaluating the effect of the diets containing microalgae Isochrysis galbana and Nannochloropsis oculata in nutritional treatment of diabetic rats. These authors reported that both healthy and diabetic rats that received a microalgae-based diet had a lower final weight than the control group (no microalgae), and suggested that consumption of the microalga I. galbana could be beneficial to rats with diabetes mellitus because it promoted body weight loss in healthy animals and helped to maintain weight in diabetic rats, while lowering glucose and cholesterol values. In addition, studies conducted with rats that received Chlamydomonas reinhardtii biomass showed that consuming lyophilized microalgae biomass improved body-weight loss and mitigated the extent of colonic damage [38, 39]. Thus, body-weight loss may be attributed to the anti-obesity effects of microalgae biomass intake, which facilitate a reduction in body fat and body-weight loss.
In a previous study, we observed the presence of bioactive compounds (phenols, flavonoids and tannins) in C. vulgaris extract. Furthermore, the extracts with solvents of ethanol and water showed DPPH percentage of inhibition of 37.2% and 68.5%, respectively, higher than the standardly used Gallic acid (28.7%), showing that they are potential inhibitors of cellular oxidation by free radicals [20]. Herein, CFAB and cachaça showed a percentage of inhibition of 77.2% and 36.7%, respectively. The level of DPPH inhibition of the extracts prepared did not change during the experiment, showing that the beverage’s functionalities were preserved during the gavage period. Moreover, the three-day shelf life was also suitable for conducting the assays, but we did not evaluate a maximum shelf life for the CFAB.
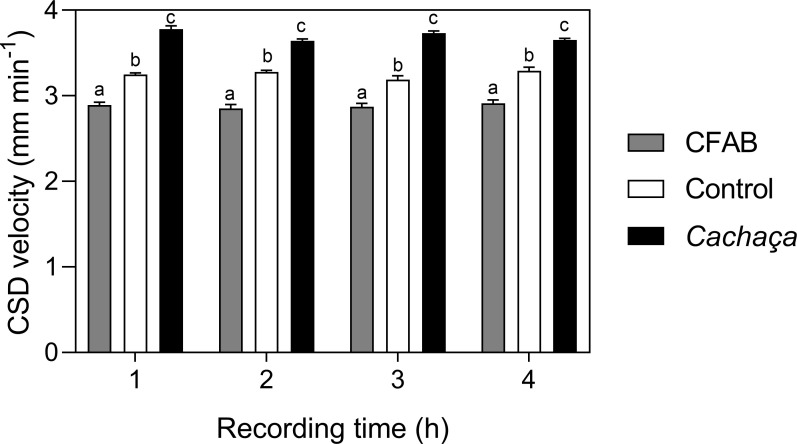
We observed a reduction of CSD-velocity in young-adult rats (at 79–88 days of life) that consumed CFAB, lower than the control group (water). The CSD–velocity in adult rats was significantly different in all treatments (p <0.05), and no significant difference was observed over the four-hour section for the same treatment (Fig 2).
Fig 2. Electrophysiological recordings during cortical spreading depression effect of the alcohol consumption (CFAB, cachaça and only water) on cortical spreading depression propagation velocities in young-adult rats.
Several studies have demonstrated the ability of substances that facilitate the spread of CSD, such as monosodium glutamate [40] and tianeptine [41], and other compounds that prevent the spread of this electrophysiological phenomenon, such as lectins [42]. In particular, ethanol has different effects depending on the concentration or exposure frequency, that can facilitate the CSD, and this justifies the higher CSD velocity in cachaça-group rats treated. Abadie-Guedes et al. [28] demonstrated that the antioxidant compounds exert an antagonistic action against the effect of chronic EtOH on CSD in rats. These authors found that a dose of astaxanthin alone was able to counteract EtOH action on CSD, suggesting that this effect is related to the antioxidant activities of this molecule and its protective effect on the brain. CSD is a wave of neuronal and glial depolarization, which is slowly propagated in the cortex (3–5 mm min-1), and followed by a long-lasting suppression of neuronal activity and excitability, also observed by Bogdanov et al. [43]. Interestingly, in the present study, CFAB-group had a lower CSD velocity than even the water-group treated, in short, this information suggests that consumption of CFAB exert an interesting effect in protecting the brain. Furthermore, for both CFAB and water the CSD-velocity remained close to the order of 3 mm min-1 –velocity reported for comfortable situations.
The electrophysiological recordings (electrocorticogram and slow cortical depression-potential changes) during CSD are show in Fig 3. Application of KCl for 1 min on a cortical point in the frontal region has been an effective technique in eliciting a single spreading depression-wave [26]. In the present study, waves have propagated without interruption and was recorded in the parietal region of the same hemisphere, as documented by the electrophysiological recordings.
Fig 3. Recordings of spontaneous cortical electrical activity (ECoG; two upper traces in each panel) and slow potential change (P; two lower traces) during spreading depression in rats.
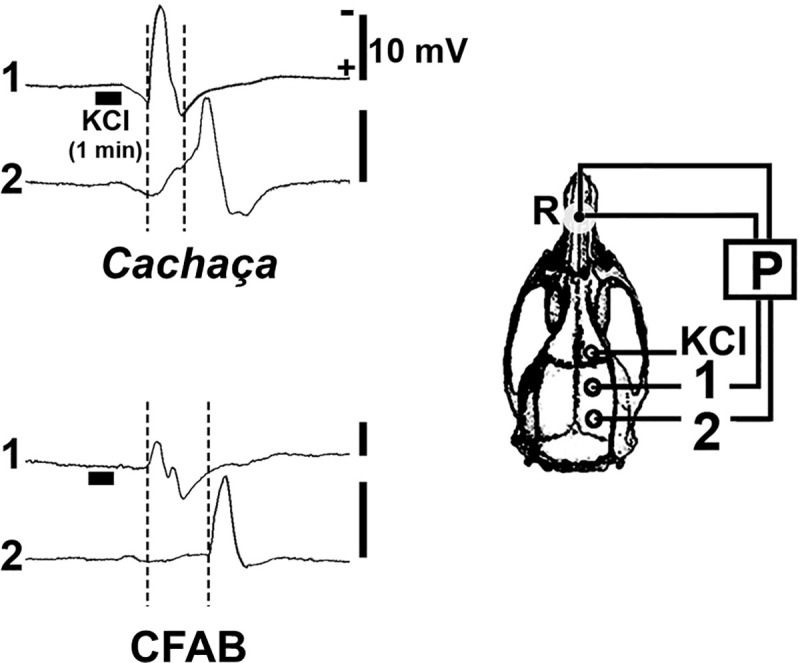
Spreading depression was elicited by applying a cotton ball (1–2 mm diameter) soaked in 2% KCl solution for 1 min, on the frontal cortex. Vertical calibration bars equal 1 mV for the ECoG- and 10 mV for the P-recordings.
In recent decades, considerable scientific efforts have been made to determine whether antioxidant supplementation can help prevent various neurological dysfunctions. The implication is that understanding the specific molecular effects of reactive oxygen species (ROS) on brain functioning is of great importance in determining the role of antioxidants on these diseases and may help to shed light on epidemiological findings, which sometimes point to contrasting neuropsychological effects of antioxidants [44]. Chronic alcoholism represents a well reported scenario of increased ROS production and, under such a condition, carotenoids can protect tissues from alcohol-induced injuries by capturing free radicals and improving the redox balance [26, 45]. In addition, other studies have demonstrated the brain tissues protection in rats by antioxidant compounds in CDS assays [27, 28, 40]. Our findings contribute to establishing a correlation between the antioxidant activities of microalgae and their neuroprotective effects in rats.
Despite this, it is necessary to assess the antioxidant power of a single molecule (or a combination of molecules) from microalgae biomass. Some “minor carotenoids” (such as neoxanthin, violaxanthin and β-carotene) also have functional properties. For example, β–carotene may play an important role in preventing degenerative diseases due to its associated antioxidant and pro-vitamin A activity [46, 47].
The data obtained showed that the rats that consumed the CFAB had a lower CSD propagation velocity. This may be associated to a protective action from C. vulgaris antioxidants compounds. ROS can be formed in the metabolism of ethanol and the presence of antioxidants may help protect tissues from the deleterious effects of ethanol. Lutein, chlorophyll-a and -b, and pheophytin a, were the most abundant antioxidants in the CFAB. These four compounds contributed to almost 50% of the total antioxidant potential in the CFAB, with their isomers and derivatives comprising the rest. This result indicates that hydrophilic compounds, lutein, chlorophylls a and b, and pheophytin a were major contributors to brain protection in rats [30]. Experimental increasing or decreasing of the brain’s ability to counteract CSD may help to understand the phenomenon and the diseases related to it.
According to Zuccalà et al. [6], studies on the potential benefits of alcohol use should not be banned from current research. In fact, the results of this study, together with those focusing on the favorable cardiovascular effects of alcohol consumption, indicate that moderate drinking may confer health benefits that should not be disregarded, from the perspectives of both the patients and society as a whole. Several research groups have been formed to investigate the neural protective effects of antioxidant molecules, mainly those from nutraceutical- and dietary sources, both in laboratory animals and in humans, using in vivo and in vitro models. Therefore, it is suggested that C. vulgaris biocompounds extracted with cachaça can be used as a functional alcoholic beverage that provides protection against the deleterious effects of ethanol on the brain, when moderately consumed. Nevertheless, it is worth noting that our objective was not to encourage or discourage the consumption of alcoholic beverages, but to highlight the advantages and potential for the use of microalgae biomass by the alcohol industry, contributing to the supply of functional alcoholic beverages.
Conclusions
The findings presented in this study provide evidence that an alcoholic beverage containing the microalga C. vulgaris contains compounds with antioxidant activity and exert antioxidant effect in vivo and in vitro. This was demonstrated by resistance to CDS in the cerebral cortex of young-adult rats that consumed the beverage, which indicates a neuroprotective action of the functional beverage. The action from this functional alcoholic beverage, in this context, is greater than what might have been expected from the cachaça content alone. The antioxidant compounds in C. vulgaris may play a role in preventing oxidative damage to the brain by free radicals.
Informed consent, human/animal rights
The rats utilized in this study were handled in accordance with the "Principles of Laboratory Animal Care" (National Institutes of Health, USA) and with the norms of the Ethics Committee for Animal Research of the Universidade Federal de Pernambuco.
Supporting information
(TIF)
Acknowledgments
The authors are thankful to the Department of Antibiotics, Federal University of Pernambuco for valuable assistance.
Abbreviations
- CFAB
Chlorella vulgaris functional alcoholic beverage
- CSD
cortical spreading depression
- DC
direct current
- DPPH– 1
1-diphenyl-2-picryl hydrazyl
- ROS
reactive oxygen species
Data Availability
1. Dantas DMM, Santos SD, Abadie-Guedes R, Guedes R, Gálvez AO, Bezerra RS. "Microalgae functional alcoholic beverage: cultivation and production process" [Text in Portuguese], registration number: BR1020120329301 - National Institute of Industrial Property - Brazil (INPI). Deposit in 21 December 2012 2. Dantas DMM. Biological activities from extract of Chlorella vulgaris and Scenedesmus subspicatus and their potential biotechnology applications [Text in Portuguese]. Doctoral thesis. Federal University of Pernambuco - Brazil. Available in: < https://repositorio.ufpe.br/bitstream/123456789/13360/1/Tese%20Danielli%20Dantas.pdf>.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 and Fundação de Amparo à Ciência e Tecnológica de Pernambuco (FACEPE). Alfredo O. Gálvez (PQ 308063/2019-8), Rubem C. A. Guedes (PQ 303636/2014-9), and Ranilson S. Bezerra (PQ 307107/2019-1) are thankful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the research productivity fellowships.
References
- 1.Manthey J, Shield KD, Rylett M et al. Global alcohol exposure between 1990 and 2017 and forecasts until 2030: a modelling study. The Lancet 2019;393:2493–2502. doi: 10.1016/S0140-6736(18)32744-2 [DOI] [PubMed] [Google Scholar]
- 2.WHO—World Health Organization. Global status report on alcohol and health 2018. Geneva; 2019. [Google Scholar]
- 3.Henriques JF, Portugal CC, Canedo T, et al. Microglia and alcohol meet at the crossroads: Microglia as critical modulators of alcohol neurotoxicity. Toxicol Let 2018;283:21–31. doi: 10.1016/j.toxlet.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Dunbar RIM, Launay J, Wlodarski R, et al. Functional benefits of (modest) alcohol consumption. Adapt Hum Behav Physiol 2017;3(2):118–133. doi: 10.1007/s40750-016-0058-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiol Aging 2002; 23: 891–897. doi: 10.1016/s0197-4580(02)00024-6 [DOI] [PubMed] [Google Scholar]
- 6.Zuccalà G, Onder G, Pedone C, et al. Dose-related impact of alcohol consumption on cognitive function in advanced age: Results of a multicenter survey. Alcohol Clin Exp Res 2001;25:12. doi: 10.1111/j.1530-0277.2001.tb02185.x [DOI] [PubMed] [Google Scholar]
- 7.Letenneur L. Moderate alcohol consumption and risk of developing dementia in the elderly: The contribution of prospective studies. Ann Epidemiol 2007;17:S43–S45. doi: 10.1016/j.annepidem.2007.01.010 [DOI] [Google Scholar]
- 8.Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol 1997;153:185–192. doi: 10.1016/j.annepidem.2007.01.010 [DOI] [PubMed] [Google Scholar]
- 9.Shukitt-Hale B, Youdim KA, Joseph JA. Neurological Aging and Nutritional Intervention. In: Cutler RG, Rodriguez H, editors. Critical Reviews of Oxidative Stress and Aging: Advances in Basic Science, Diagnostics and Intervention. New Jersey: World Scientific Publishing; 2003. pp. 526–542 [Google Scholar]
- 10.Kris-Etherton PM, Keen CL. Evidence that the antioxidant flavonoids in tea and cocoa are beneficial for cardiovascular health. Curr Opin Lipidol 2002;13:41–49. doi: 10.1097/00041433-200202000-00007 [DOI] [PubMed] [Google Scholar]
- 11.Goya L, Mateos R, Bravo L. Effect of the olive oil phenol hydroxytyrosol on human hepatoma HepG2 cells: Protection against oxidative stress induced by tert–butylhydroperoxide. Eur J Nutr 2007;46:70–78. doi: 10.1007/s00394-006-0633-8 [DOI] [PubMed] [Google Scholar]
- 12.Dantas DMM, Oliveira CYB, Costa RMPB, et al. Evaluation of antioxidant and antibacterial capacity of green microalgae Scenedesmus subspicatus. Food Sci Technol Int 2019;25:318–326. doi: 10.1177/1082013218825024 [DOI] [PubMed] [Google Scholar]
- 13.Lauritano C, Andersen JH, Hansen E, et al. Bioactivity screening of microalgae for antioxidant, anti-inflammatory, anticancer, anti-diabetes, and antibacterial activities. Front Mar Sci 2016;3:68. doi: 10.3389/fmars.2016.00068 [DOI] [Google Scholar]
- 14.Oliveira CYB, Oliveira CDL, Prassad R, et al. A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquacult 2021;13(3):1594–1618. doi: 10.1111/RAQ.12536 [DOI] [Google Scholar]
- 15.Oliveira CYB, Viegas TL, Silva MFO, et al. Effect of trace metals on growth performance and accumulation of lipids, proteins, and carbohydrates on the green microalga Scenedesmus obliquus. Aquac Int 2020;28:1435–1444. doi: 10.1007/s10499-020-00533-0 [DOI] [Google Scholar]
- 16.Sathasivam R, Ki JS. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar Drugs 2018;16(1):26. doi: 10.3390/md16010026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matos J, Cardoso C, Bandarra NM, Afonso C. Microalgae as healthy ingredients for functional food: a review. Food Funct 2017;8(8):2672–2685. doi: 10.1039/c7fo00409e [DOI] [PubMed] [Google Scholar]
- 18.Yu M, Chen M, Gui J, et al. Preparation of Chlorella vulgaris polysaccharides and their antioxidant activity in vitro and in vivo. Int J Biol Macromol 2019;137:139–150. doi: 10.1016/j.ijbiomac.2019.06.222 [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Liu L, Chen F. Production and characterization of exopolysaccharides from Chlorella zofingiensis and Chlorella vulgaris with anti-colorectal cancer activity. Int J Biol Macromol 2019;134:976–983. doi: 10.1016/j.ijbiomac.2019.05.117 [DOI] [PubMed] [Google Scholar]
- 20.Dantas DMM, Costa RMPB, Carneiro-Da-Cunha MG et al. Bioproduction, antimicrobial and antioxidant activities of compounds from Chlorella vulgaris. J Bot Sci 2015;4:12–18. [Google Scholar]
- 21.Zielinski D, Fraczyk J, Debowski M, et al. Biological activity of hydrophilic extract of Chlorella vulgaris grown on post-fermentation leachate from a biogas plant supplied with stillage and maize silage. Molecules 2020;25:1790. doi: 10.3390/molecules25081790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koyande AK, Chew KW, Rambabu K, Tao Y, Chu DT, Show PL. Microalgae: A potential alternative to health supplementation for humans. Food Sci Hum Well 2019;8(1):16–24. doi: 10.1016/j.fshw.2019.03.001 [DOI] [Google Scholar]
- 23.Boukid F, Castellari M. Food and beverages containing algae and derived ingredients launched in the market from 2015 to 2019: a front-of-pack labeling perspective with a special focus on Spain. Foods 2021;10(1):173. doi: 10.3390/foods10010173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siro I, Kápolna E, Kápolna B, Lugasi A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 2008;51(3):456–467. doi: 10.1016/j.appet.2008.05.060 [DOI] [PubMed] [Google Scholar]
- 25.Villarruel-López A, Ascencio F, Nuño K. Microalgae, a potential natural functional food source–a review. Pol J Food Nutr Sci 2017;67(4):251–263. doi: 10.1515/pjfns-2017-0017 [DOI] [Google Scholar]
- 26.Guedes RCA, Abadie-Guedes R, Bezerra RDS. The use of cortical spreading depression for studying the brain actions of antioxidants. Nutr Neurosci 2012;15(3):111–119. doi: 10.1179/1476830511Y.0000000024 [DOI] [PubMed] [Google Scholar]
- 27.de Souza Bezerra R, Abadie-Guedes R, Melo FRM et al. Shrimp carotenoids protect the developing rat cerebral cortex against the effects of ethanol on cortical spreading depression. Neurosci Let 2005;391:51–55. doi: 10.1016/j.neulet.2005.08.040 [DOI] [PubMed] [Google Scholar]
- 28.Abadie‐Guedes R, Santos SD, Cahú TB, et al. Dose‐dependent effects of astaxanthin on cortical spreading depression in chronically ethanol‐treated adult rats. Alcohol Clin Exp Res 2008;32:1417–1421. doi: 10.1111/j.1530-0277.2008.00710.x [DOI] [PubMed] [Google Scholar]
- 29.Zar JH. Biostatistical analysis: Pearson new international edition. 5th edition. Harlow: Pearson Education, 2014. [Google Scholar]
- 30.Cha KH, Kang SW, Kim CY, et al. Effect of pressurized liquids on extraction of antioxidants from Chlorella vulgaris. J Agric Food Chem 2010;58:4756–4761. doi: 10.1021/jf100062m [DOI] [PubMed] [Google Scholar]
- 31.Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM. Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 2006;46:185–196. doi: 10.1080/10408690590957188 [DOI] [PubMed] [Google Scholar]
- 32.Farruggia C, Kim MB, Bae M, et al. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. J Nutr Biochem 2018;62:202–209. doi: 10.1016/j.jnutbio.2018.09.005 [DOI] [PubMed] [Google Scholar]
- 33.Sztretye M, Dienes B, Gönczi M, et al. Astaxanthin: A Potential Mitochondrial-Targeted Antioxidant Treatment in Diseases and with Aging. Oxid Med Cell Longev 2019;2019:3849692. doi: 10.1155/2019/3849692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yusof NS, Yeong YS, Zakeri HA, Wahid MEA, Ab N, Ghafar NY. Photoperiod influenced the growth and antioxidative responses of Chlorella vulgaris, Isochrysis galbana, and Tetraselmis chuii. J Appl Pharm Sci 2021;11(4):125–134. doi: 10.7324/JAPS.2021.110415 [DOI] [Google Scholar]
- 35.Oliveira CYB D ’Alessandro EB, Antoniosi Filho NR, Lopes RG, Derner RB. Synergistic effect of growth conditions and organic carbon sources for improving biomass production and biodiesel quality by the microalga Choricystis minor var. minor. Sci Total Environ 2021;759:143476. doi: 10.1016/j.scitotenv.2020.143476 [DOI] [PubMed] [Google Scholar]
- 36.Ali HEA, El-fayoumy EA, Rasmy WE, et al. Two-stage cultivation of Chlorella vulgaris using light and salt stress conditions for simultaneous production of lipid, carotenoids, and antioxidants. J Appl Phycol 2020;33:227–239. doi: 10.1007/s10811-020-02308-9 [DOI] [Google Scholar]
- 37.Nuño K, Villarruel-López A, Puebla-Pérez AM, et al. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J Funct Foods 2013;5:106–115. doi: 10.1016/j.jff.2012.08.011 [DOI] [Google Scholar]
- 38.Avila-Román J, Talero E, Alcaide A, et al. Preventive effect of the microalga Chlamydomonas debaryana on the acute phase of experimental colitis in rats. Brit J Nutr 2014;112:1055–1064. doi: 10.1017/S0007114514001895 [DOI] [PubMed] [Google Scholar]
- 39.Fields FJ, Lejzerowicz F, Schroeder D, et al. Effects of the microalgae Chlamydomonas on gastrointestinal health. J Funct Foods 2020;65:103738. doi: 10.1016/j.jff.2019.103738 [DOI] [Google Scholar]
- 40.Vitor-de-Lima SM, Medeiros LDB, Benevides RSL, Dos Santos CN, Lima da Silva NO, Guedes RCA. Monosodium glutamate and treadmill exercise: anxiety-like behavior and spreading depression features in young adult rats. Nutr Neurosci 2019;22(6):435–443. doi: 10.1080/1028415X.2017.1398301 [DOI] [PubMed] [Google Scholar]
- 41.de Seixas Maia LMS, Amancio-dos-Santos A, da Silva Germano PCP, Falcão ACSM, Duda-de-Oliveira D, Guedes RCA. Do the accelerating actions of tianeptine and l-arginine on cortical spreading depression interact? An electrophysiological analysis in young and adult rats. Neurosci Lett 2017;650:134–138. doi: 10.1016/j.neulet.2017.04.044 [DOI] [PubMed] [Google Scholar]
- 42.Soares GSF, Lima CB, Cavalcanti LC, Villacampa N, Castellano B, Guedes RCA. Brain effects of the lectin from Canavalia ensiformis in adult rats previously suckled in favorable and unfavorable conditions: A spreading depression and microglia immunolabeling study. Nutr Neurosci 2015;18(7):307–315. doi: 10.1179/1476830514Y.0000000128 [DOI] [PubMed] [Google Scholar]
- 43.Bogdanov VB, Bogdanova OV, Koulchitsky SV, et al. Behavior in the open field predicts the number of KCl-induced cortical spreading depressions in rats. Behav Brain Res 2013;236:90–93. doi: 10.1016/j.bbr.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abadie‐Guedes R, Guedes RC, Bezerra RS. The impairing effect of acute ethanol on spreading depression is antagonized by astaxanthin in rats of 2 young‐adult ages. Alcohol Clin Exp Res 2012;36:1563–1567. doi: 10.1111/j.1530-0277.2012.01766.x [DOI] [PubMed] [Google Scholar]
- 45.Reddy VD, Padmavathi P, Bulle S, Hebbani AV, Marthadu SB, Venugopalacharyulu NC, et al. Association between alcohol-induced oxidative stress and membrane properties in synaptosomes: a protective role of vitamin E. Neurotoxicol Teratol 2017;63:60–65. doi: 10.1016/j.ntt.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 46.Plaza L, Colina C, de Ancos B, et al. Influence of ripening and astringency on carotenoid content of high-pressure treated persimmon fruit (Diospyros kaki L.). Food Chem 2012;130:591–597. doi: 10.1016/j.foodchem.2011.07.080 [DOI] [Google Scholar]
- 47.Sansone C, Brunet C. Promises and challenges of microalgal antioxidant production. Antioxidants 2019;8:199. doi: 10.3390/antiox8070199 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Data Availability Statement
1. Dantas DMM, Santos SD, Abadie-Guedes R, Guedes R, Gálvez AO, Bezerra RS. "Microalgae functional alcoholic beverage: cultivation and production process" [Text in Portuguese], registration number: BR1020120329301 - National Institute of Industrial Property - Brazil (INPI). Deposit in 21 December 2012 2. Dantas DMM. Biological activities from extract of Chlorella vulgaris and Scenedesmus subspicatus and their potential biotechnology applications [Text in Portuguese]. Doctoral thesis. Federal University of Pernambuco - Brazil. Available in: < https://repositorio.ufpe.br/bitstream/123456789/13360/1/Tese%20Danielli%20Dantas.pdf>.