Abstract
Background
Post-transplant cyclophosphamide (PT-Cy) has become a standard of care in haploidentical hematopoietic stem cell transplantation (HSCT) to reduce the risk of graft-versus-host disease. However, data on cardiac events associated with PT-Cy are scarce.
Objectives
This study sought to assess the incidence and clinical features of cardiac events associated with PT-Cy.
Methods
The study compared clinical outcomes between patients who received PT-Cy (n = 136) and patients who did not (n = 195), with a focus on early cardiac events (ECE) occurring within the first 100 days after HSCT. All patients had the same systematic cardiac monitoring.
Results
The cumulative incidence of ECE was 19% in the PT-Cy group and 6% in the no–PT-Cy group (p < 0.001). The main ECE occurring after PT-Cy were left ventricular systolic dysfunction (13%), acute pulmonary edema (7%), pericarditis (4%), arrhythmia (3%), and acute coronary syndrome (2%). Cardiovascular risk factors were not associated with ECE. In multivariable analysis, the use of PT-Cy was associated with ECE (hazard ratio: 2.7; 95% confidence interval: 1.4 to 4.9; p = 0.002]. Older age, sequential conditioning regimen, and Cy exposure before HSCT were also associated with a higher incidence of ECE. Finally, a history of cardiac events before HSCT and ECE had a detrimental impact on overall survival.
Conclusions
PT-Cy is associated with a higher incidence of ECE occurring within the first 100 days after HSCT. Patients who have a cardiac event after HSCT have lower overall survival. These results may help to improve the selection of patients who are eligible to undergo HSCT with PT-Cy, especially older adult patients and patients with previous exposure to Cy.
Key Words: allogeneic stem cell transplantation, cardiotoxicity, haploidentical transplantation, left ventricular systolic dysfunction, post-transplant cyclophosphamide
Abbreviations and Acronyms: CI, confidence interval; CVD, cardiovascular disease; CVRF, cardiovascular risk factor; Cy, cyclophosphamide; ECE, early cardiac events; GRFS, graft-versus-host disease-free, relapse-free survival; GVHD, graft-versus-host disease; HR, hazard ratio; HSCT, hematopoietic stem cell transplantation; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; PT-Cy, post-transplant cyclophosphamide
Central Illustration
T cell–replete haploidentical (haplo) hematopoietic stem cell transplantation (HSCT) has become feasible with the use of post-transplant cyclophosphamide (PT-Cy) (1). Haplo HSCT allows for the quick identification of an available donor for the majority of patients. With the use of PT-Cy, the incidence of graft-versus-host disease (GVHD) is not increased, and survival outcomes are not inferior in patients with haplo transplants when compared with patients with transplants from a matched-unrelated donor or umbilical cord blood donors (2, 3, 4, 5, 6, 7, 8, 9). For these reasons, PT-Cy is now widely used in the haplo setting (10). PT-Cy has also been applied in other settings, including human leukocyte antigen (HLA)-identical sibling, matched-unrelated, and mismatched unrelated donor HSCT (11, 12, 13, 14). In the last setting, it may provide better outcomes compared with antithymocyte globulin (15). However, PT-Cy is associated with toxicities and organ damage, and the related risk factors, clinical manifestations, and incidence of early cardiac events (ECE) are still poorly understood.
Cardiac events may occur early after HSCT or in the long term, mainly as a result of sepsis or chemotherapy toxicity. Cardiovascular diseases (CVDs) after HSCT include cardiomyopathy, heart failure, valvular dysfunction, arrhythmia, pericarditis, and coronary artery disease (16). The CVD cumulative incidence is 5% to 10% at 10 years after HSCT, thus accounting for 2% to 11% of mortality among long-term survivors. The incidence of CVD and its associated mortality is 1.4- to 3.5-fold higher in transplant recipients compared with the general population (17, 18, 19, 20).
Cy-induced acute cardiotoxicity, in particular, typically develops within 10 days after first administration of the drug. Manifestations may range from endothelial injury, arrhythmias, and heart failure to lethal myopericarditis. The minimum dose to induce cardiotoxicity is not known, although there are a few reports of Cy toxicity at <100 mg/kg. The exact mechanisms of Cy toxicity also remain unclear. Cy may induce endothelial damage followed by extravasation of toxic metabolites, which overproduce reactive oxygen species, cause oxidative stress, and induce myocardial damage and edema (21, 22, 23).
In a large cohort study, including 811 patients undergoing HSCT and receiving a total dose of Cy >100 mg/kg before HSCT as a conditioning regimen, Cy-induced cardiac failure developed in 12 (1.5%) patients (16). Other studies have reported a Cy-induced cardiac toxicity rate of up to 17%, depending on the conditioning regimen and the patients studied (24, 25, 26, 27). However, data on ECE when Cy is used after HSCT are scarce. Thus, we compared clinical outcomes of patients who received PT-Cy with clinical outcomes of patients who did not.
Methods
Patient selection
We report a single-center (Saint Antoine Hospital, AP-HP, Paris, France) retrospective cohort study including all consecutive patients ≥15 years of age who underwent allogeneic HSCT for hematologic malignant disease from January 2013 to June 2018. Patients receiving unrelated cord blood were excluded. All patients provided written informed consent for the use of their data for clinical research, in accordance with the modified Declaration of Helsinki and the local Ethical Committee.
Early cardiac event definition and monitoring
Cardiac events were defined as 1 of the following: left ventricular systolic dysfunction (LVSD), defined as a decrease in the left ventricular ejection fraction (LVEF) of >10 percentage points, to a value <53% (28); acute pulmonary edema; arrhythmia; pericarditis; or acute coronary syndrome. ECE were defined as cardiac events occurring within 3 months after HSCT.
All patients had the same systematic cardiac monitoring: transthoracic echocardiography and an electrocardiogram were performed before the start of the conditioning regimen and at day 90 after HSCT. Echocardiography was also performed in patients with dyspnea or peripheral edema, as clinically indicated. An electrocardiogram was performed after HSCT in all patients with tachycardia or an irregular heartbeat.
Transplantation procedures
PT-Cy was administrated to all patients undergoing haploidentical HSCT. Patients who underwent HSCT after March 2014 with a matched-unrelated or HLA-identical sibling donor also received PT-Cy in case of HLA-mismatch, renal insufficiency, or inclusion in a clinical trial. The dose of PT-Cy was adjusted according to the stem cell source. Bone marrow recipients were scheduled to receive 1 dose of PT-Cy (50 mg/kg/day) at day 3. Peripheral blood stem cell recipients were given 2 doses of PT-Cy at days 3 and 5.
Myeloablative conditioning regimens consisted of fludarabine in combination with busulfan or thiotepa in combination with busulfan and fludarabine with a busulfan dose ≥9.6 mg/kg, except for 2 patients who received busulfan in combination with cyclophosphamide (Cy) in 2013. Reduced-intensity conditioning consisted of fludarabine in combination with busulfan or thiotepa in combination with busulfan and fludarabine with a busulfan dose <9.6 mg/kg, or patients underwent total body irradiation–based regimens. Sequential conditioning regimens were thiotepa based, clofarabine based, or amsacrine based, as previously published (9,29,30).
Supportive care
All patients received 2 to 3 l/day of fluid hyperhydration during the conditioning regimen. PT-Cy administration was associated with 2 to 3 l/day of fluid hyperhydration and mesna to prevent uroepithelial damage. Infections and hepatic veno-occlusive disease were managed as previously described (9,31,32).
Statistical analysis
Continuous variables were recorded as median with 25th and 75th percentiles (Q1, Q3) or ranges and were compared using the Mann-Whitney U test. Categorical qualitative variables were recorded as frequency and percentage and were compared using the chi-square test or the Fisher exact test where appropriate. Overall survival was defined as the time from transplantation to death from any cause; disease-free survival was defined as the time from transplantation to relapse or progression or death from any cause, whichever came first; GVHD-free, relapse-free survival (GRFS) was defined as being alive without grade III to IV acute GVHD, severe chronic GVHD, or disease relapse (33); and nonrelapse mortality was defined as death without evidence of relapse. GVHD was diagnosed and graded according to standard criteria (34,35). The disease risk index (36) and the comorbidity index (37) were defined as previously published. The following cardiovascular risk factors (CVRFs) occurring at the start of the conditioning regimen were considered in the analyses: male sex (vs. female), age >50 years or age of female patient >60 years (vs. less than), obesity (body mass index ≥30 kg/m2 vs. less than), hypertension, diabetes, dyslipidemia, and smoking status (regular smoker, occasional smoker, or former smoker vs. never smoker). History of CVD and previous exposure to anthracyclines and Cy were also considered as covariates.
Cumulative incidence was used to estimate the endpoints of ECE, GVHD, relapse, and nonrelapse mortality to accommodate competing risks. To study ECE and GVHD, we considered relapse and death to be competing events. Probabilities of overall survival, disease-free survival, and GRFS were calculated using the Kaplan-Meier method. Competing risk analyses were performed using Gray’s test for cumulative incidence functions and the log-rank test to compare survival between the 2 groups. All variables differing between the 2 groups and having a significance level of p < 0.10 in the univariable analysis were first entered into a multivariable Cox regression model (38), and then a backward stepwise selection was performed using p < 0.05 and keeping PT-Cy in the model. To analyze the impact of ECE on overall survival, ECE were included in the Cox model as a time-dependent variable, and a landmark analysis was performed on day 100 after allogenic HSCT. Results were expressed as the hazard ratio (HR) with a 95% confidence interval (CI). All tests were 2-sided. The type I error rate was fixed at 0.05 for the determination of factors associated with time-to-event outcomes. Statistical analyses were performed with SPSS software version 24.0 (IBM Corp., Armonk, New York) and R software version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient, disease, and transplant characteristics
Patient and disease characteristics are summarized in Table 1. The median age was 55 years (range 15 to 76 years), and 60% of patients were male. Before transplantation, 276 (83%) patients had at least 1 CVRF, 72 (22%) had a history of CVD, and 31 (9%) had LVSD. The disease risk index was high or very high in 93 (28%) patients. Donors were HLA-identical siblings (n = 89; 27%), unrelated (n = 124; 37%), or haploidentical (n = 118; 36%). A total of 136 patients received PT-Cy, and 195 did not. In the PT-Cy group, 33 patients received PT-Cy at 50 mg/kg/day for 1 day, and 103 patients received PT-Cy at 50 mg/kg/day for 2 days. PT-Cy was also administrated to 13 patients with unrelated donors and 6 patients with HLA-identical sibling donors, according to our center policy. One patient undergoing haplo HSCT did not receive PT-Cy because of severe cardiac failure occurring on day 1 after transplantation.
Table 1.
Patient and Donor Characteristics at Transplantation
| No PT-Cy (n = 195) | PT-Cy (n = 136) | p Value | |
|---|---|---|---|
| Recipient age, yrs | 56 [16–76] | 53 [15–76] | 0.11 |
| Male/female | 114 (58)/81 (42) | 86 (63)/50 (37) | 0.38 |
| Diagnosis | 0.040 | ||
| Acute myeloid leukemia | 83 (43) | 70 (51) | |
| Acute lymphoblastic leukemia | 32 (16) | 17 (13) | |
| Lymphoma | 21 (11) | 23 (17) | |
| Multiple myeloma | 4 (2) | 4 (3) | |
| Myelodysplastic syndrome | 22 (11) | 13 (10) | |
| Myeloproliferative neoplasm | 33 (17) | 9 (7) | |
| Disease status at transplantation | 0.31 | ||
| Complete remission | 95 (49) | 74 (54) | |
| Active or progressive disease or partial response | 100 (51) | 62 (46) | |
| Disease risk index | 0.007 | ||
| Low and intermediate | 151 (77) | 87 (64) | |
| High and very-high | 44 (23) | 49 (36) | |
| Karnofsky index ≤80% | 27 (14) | 25 (18) | 0.26 |
| Comorbidity index ≥3 | 54 (28) | 27 (20) | 0.25 |
| Cardiovascular risk factors | |||
| None | 31 (16) | 24 (18) | 0.67 |
| Male >50 yrs of age or female >60 yrs of age | 110 (56) | 62 (46) | 0.05 |
| Obesity | 32 (16) | 12 (9) | 0.045 |
| Hypertension | 30 (15) | 22 (16) | 0.73 |
| Dyslipidemia | 23 (12) | 7 (5) | 0.038 |
| Smoking∗ | 55 (28) | 37 (27) | 0.84 |
| Diabetes | 17 (9) | 8 (6) | 0.34 |
| Cardiac event before HSCT | 45 (23) | 27 (20) | 0.48 |
| Left ventricular systolic dysfunction | 21 (11) | 10 (7) | 0.30 |
| Exposure to Cy before HSCT | 34 (17) | 32 (24) | 0.17 |
| Cumulative Cy dose before HSCT, mg/kg | |||
| Median [Q1–Q3] | 97.5 [59.5–128] | 134.4 [87.1–--172.5] | 0.030 |
| Missing data | 4 (2) | 4 (2) | |
| Exposure to anthracyclines before HSCT | 113 (68) | 108 (79) | 0.024 |
| Cumulative anthracyclines dose before HSCT, mg/m2 | 0.028 | ||
| Median [Q1–Q3] | 38.7 [0–63.6] | 38.7 [22.5–64.5] | |
| Missing data | 5 (3) | 3 (2) | |
| Previous autologous HSCT | 16 (8) | 16 (12) | 0.28 |
| Previous allogeneic HSCT | 4 (2) | 16 (12) | <0.001 |
| Donor | <0.001 | ||
| HLA-identical sibling | 83 (42.5) | 6 (4) | |
| Matched-unrelated | 111 (57) | 13 (10) | |
| Haploidentical | 1 (0.5) | 117 (86) | |
| Conditioning regimen | <0.001 | ||
| Myeloablative | 99 (51) | 32 (23.5) | |
| Reduced intensity | 39 (20) | 46 (34) | |
| Sequential | 57 (29) | 58 (42.5) | |
| Graft source | <0.001 | ||
| Peripheral blood stem cell | 192 (98) | 111 (82) | |
| Bone marrow | 3 (2) | 25 (18) | |
| ATG | 194 (99.5) | 118 (87) | < 0.001 |
| PT-Cy | |||
| 1 day | 0 (0) | 33 (24) | |
| 2 days | 0 (0) | 103 (76) | |
Values are median [range or Q1–Q3, 25th and 75th percentile] or n (%) unless otherwise indicated.
ATG = antithymocyte globulin; Cy = cyclophosphamide; HLA = human leukocyte antigen; HSCT = hematopoietic stem cell transplantation; PT-Cy = post-transplant cyclophosphamide.
Smoking was defined as ever regular smoker, occasional smoker, or ex-smoker, and it was compared with never smoker.
There was no significant difference between the PT-Cy and the no–PT-Cy groups in terms of age, Karnofsky index, comorbidity index, history of a cardiac event, number of CVRFs, and disease status at transplantation. In the PT-Cy group, the disease risk index was higher (p = 0.007), sequential conditioning was administrated more frequently (p < 0.001), and the incidences of obesity (p = 0.046) and dyslipidemia (p = 0.038) were lower than in the no–PT-Cy group.
Early cardiac events
ECE occurred in 45 patients with a median time of 17 days after transplantation (range 1 to 90 days). The cumulative incidence of ECE was 19% in the PT-Cy group compared with 6% in the no–PT-Cy group (p = 0.001). The incidence of ECE was 14% and 26% in patients receiving a PT-Cy total dose of 50 mg/kg and 100 mg/kg, respectively (a nonsignificant difference). When PT-Cy was administrated to patients with HLA-identical sibling or unrelated donors, the incidence of ECE was 11%. The main complication was LVSD (13% of patients in the PT-Cy group and 2% in the no–PT-Cy group; p < 0.001). Acute pulmonary edema occurred in 7% of patients (n = 9) in the PT-Cy group and 2% (n = 4) in the no–PT-Cy group (p = 0.036). In 7 patients, there was a concomitant decrease in the LVEF >10% (compared with the pre-transplant LVEF) to a value <53%. In 5 patients (3 patients in the PT-Cy group and 2 patients in the no–PT-Cy group) acute pulmonary edema occurred with preserved LVEF. In 1 patient with known underlying cardiac dysfunction, the LVEF was not measured when acute pulmonary edema occurred. Other ECE included arrhythmia (n = 12; 3%), pericarditis (n = 7; 4%), and acute coronary syndrome (n = 3; 2%), with no significant difference between the 2 groups (Table 2).
Table 2.
Cumulative Incidences of Cardiac Events Within 100 Days After Transplantation
| No PT-Cy |
PT-Cy |
p Value | |||
|---|---|---|---|---|---|
| n (%) | % (95% CI) | n (%) | % (95% CI) | ||
| Left ventricular systolic dysfunction | 6 (2.1) | 2.1 (0.7–4.9) | 20 (14.3) | (8.3–19.8) | 0.001 |
| Acute pulmonary edema | 4 (2.1) | 2.1 (0.7–4.9) | 9 (6.7) | (3.3–11.8) | 0.036 |
| Arrhythmia | 7 (3.1) | 3.1 (1.3–6.3) | 5 (3.1) | (1–7.1) | 0.95 |
| Pericarditis | 2 (0.5) | 0.5 (0–2.7) | 5 (3.8) | (1.4–8.1) | 0.09 |
| Acute coronary syndrome | 1 (0.5) | 0.5 (0–2.7) | 2 (1.5) | (0.3–4.8) | 0.36 |
Cumulative incidence was used to estimate all early cardiac events, with relapse and death being the competing events.
CI = confidence interval; PT-Cy = post-transplant cyclophosphamide.
CVRFs were not significantly associated with the incidence of ECE (Table 3). No ECE occurred in patients receiving tyrosine kinase inhibitors before transplantation (n = 31), and the cumulative doses of anthracycline had no significant impact on the incidence of ECE. Patients treated by Cy before HSCT had a higher incidence of ECE (24%) compared with those who were never exposed to Cy (8%; p = 0.004). In multivariable analysis, factors significantly associated with ECE were the use of PT-Cy (HR: 2.7; 95% CI: 1.4 to 4.9; p = 0.002), Cy exposure before HSCT (HR: 2.7; 95% CI: 1.5 to 5.0; p = 0.002), a sequential conditioning regimen (HR: 2.6; 95% CI: 1.5 to 4.8; p = 0.001), and older age (HR: 1.4; 95% CI: 1.1 to 1.7; p = 0.007). These findings were confirmed in sensitivity analyses excluding patients with a history of CVD and LVSD before transplantation or patients with previous HSCT (Table 4). An increased but nonsignificant risk of ECE was observed with respect to pre-transplantation history of cardiac event and comorbidity index ≥ 3.
Table 3.
Univariable Analysis of Factors Associated With Early Cardiac Events
| % (95% CI) | p Value | |
|---|---|---|
| PT-Cy | 0.001 | |
| No | 6 (3.4–10.1) | |
| Yes | 19 (13.0–26.1) | |
| Recipient age | 0.15 | |
| Age < median | 10 (5.8–14.8) | |
| Age > median | 13 (8.6–18.9) | |
| Recipient sex | 0.82 | |
| Male | 12 (7.5–16.4) | |
| Female | 12 (6.7–17.6) | |
| Disease risk index | 0.019 | |
| Low and intermediate | 8 (5.0–11.9) | |
| High and very-high | 20 (12.9–29.2) | |
| Disease status at transplant | 0.020 | |
| No complete remission | 16 (10.9–21.9) | |
| Complete remission | 7 (3.6–11.4) | |
| Cy exposure before HSCT | 0.004 | |
| No Cy before HSCT | 8 (5.4–12.0) | |
| Cy before HSCT | 24 (14.7–35.1) | |
| Cy cumulative dose before HSCT | 0.19 | |
| < median (108.4 mg/kg) | 16 (5.8–31.2) | |
| ≥ median (108.4 mg/kg) | 31 (16.1–47.6) | |
| Anthracycline cumulative dose | 0.08 | |
| < median (38.7 mg/m2) | 14 (8.5–19.8) | |
| > median (38.7 mg/m2) | 10 (6.1–14.7) | |
| Karnofsky index | 0.33 | |
| ≤80 | 15 (7.1–26.5) | |
| >80 | 11 (7.5–14.7) | |
| Comorbidity index | 0.058 | |
| 0 | 10 (5.5–16.3) | |
| 1 or 2 | 8 (4.4–13.9) | |
| ≥3 | 19 (10.9–27.7) | |
| Previous allogeneic HSCT | 0.24 | |
| No | 12 (8.6–15.8) | |
| Yes | 5 (0.3–21.1) | |
| Male >50 or female >60 yrs old | 0.13 | |
| No | 9 (5.5–14.6) | |
| Yes | 13 (8.8–18.9) | |
| Obesity | 0.99 | |
| No | 12 (8.4–15.9) | |
| Yes | 9 (2.9–19.9) | |
| Hypertension | 0.32 | |
| No | 13 (9.0–16.7) | |
| Yes | 6 (1.5–14.5) | |
| Smoking∗ | 0.85 | |
| No | 12 (8.4–16.6) | |
| Yes | 10 (4.8–16.9) | |
| Diabetes | 0.77 | |
| No | 12 (8.5–15.7) | |
| Yes | 8 (1.3–22.9) | |
| Dyslipidemia | 0.61 | |
| No | 12 (8.6–15.9) | |
| Yes | 7 (1.1–19.5) | |
| LVSD before HSCT | 0.27 | |
| No | 11 (7.5–14.5) | |
| Yes | 19 (7.7–34.9) | |
| Cardiac event before HSCT | 0.07 | |
| No | 10 (6.4–13.6) | |
| Yes | 18 (10.2–27.8) | |
| Donor | 0.001 | |
| HLA-identical sibling | 6 (2.1–11.8) | |
| Matched-unrelated | 7 (3.0–11.7) | |
| Haploidentical | 21 (14.3–29) | |
| Conditioning regimen | 0.001 | |
| Reduced intensity | 12 (6.0–19.6) | |
| Myeloablative | 3 (1.0–7.1) | |
| Sequential | 21 (14–28.7) |
Cumulative incidence was used to estimate early cardiac events, relapse and death being the competing events.
Smoking was defined as ever regular smoker, occasional smoker, or ex-smoker, and it was compared with never smoker.
Table 4.
Multivariable Analysis Associated With Early Cardiac Events, Relapse Incidence, and Survival
| HR (95% CI) | p Value | |
|---|---|---|
| Early cardiac event | ||
| PT-Cy (yes vs. no) | 2.65 (1.44–4.90) | 0.002 |
| Age (per 10 yrs) | 1.37 (1.09–1.73) | 0.007 |
| Sequential conditioning (vs. other) | 2.62 (1.45–4.75) | 0.001 |
| Cy exposure before HSCT (yes vs. no) | 2.69 (1.45–5.00) | 0.002 |
| Relapse incidence | ||
| PT-Cy (yes vs. no) | 1.09 (0.68–1.73) | 0.73 |
| DRI (high or very-high vs. low-intermediate) | 2.47 (1.56–3.91) | <0.001 |
| Cardiac event before HSCT (yes vs. no) | 1.85 (1.12–3.07) | 0.017 |
| Disease-free survival | ||
| PT-Cy (yes vs. no) | 1.15 (0.84–1.59) | 0.38 |
| DRI (high or very-high vs. low-intermediate) | 1.97 (1.33–2.90) | 0.001 |
| Sequential conditioning (vs. other) | 1.74 (1.18–2.55) | 0.005 |
| Cardiac event before HSCT (yes vs. no) | 1.94 (1.37–-2.75) | <0.001 |
| Cy exposure before HSCT (yes vs. no) | 1.77 (1.24–2.53) | 0.002 |
| Nonrelapse mortality | ||
| PT-Cy (yes vs. no) | 1.48 (0.94–2.33) | 0.09 |
| DRI (high/very-high vs. low-intermediate) | 2.07 (1.21–3.55) | 0.008 |
| Sequential conditioning (vs. other) | 1.86 (1.10–3.15) | 0.02 |
| Cardiac event before HSCT (yes vs. no) | 1.96 (1.20–3.20) | 0.008 |
| Cy exposure before HSCT (yes vs. no) | 2.22 (1.33–3.70) | 0.002 |
| Anthracycline (cumulative dose > median) | 0.99 (0.98–0.99) | 0.002 |
| Overall survival | ||
| PT-Cy (yes vs. no) | 1.19 (0.79–1.58) | 0.52 |
| DRI (high or very-high vs. low-intermediate) | 2.26 (1.56–3.28) | <0.001 |
| Disease status (CR vs. no CR) | 0.46 (0.31–0.69) | <0.001 |
| Cardiac event before HSCT (yes vs. no) | 1.83 (1.26–2.65) | 0.002 |
| Cy exposure before HSCT (yes vs. no) | 2.04 (1.40–2.98) | <0.001 |
| GRFS | ||
| PT-Cy (yes vs. no) | 1.02 (0.76–1.38) | 0.88 |
| DRI (high or very-high vs. low-intermediate) | 2.34 (1.73–3.17) | <0.001 |
| Cardiac event before HSCT (yes vs. no) | 1.40 (1.00–1.96) | 0.05 |
| Acute grade II–IV GVHD | ||
| PT-Cy (yes vs. no) | 0.61 (0.39–0.97) | 0.037 |
| Age (per 10 years) | 0.90 (0.79–1.05) | 0.18 |
| Chronic GVHD | ||
| PT-Cy (yes vs. no) | 1.01 (0.64–1.58) | 0.98 |
| Myeloablative conditioning (vs. other) | 1.70 (1.10–2.63) | 0.016 |
| Cy exposure before HSCT (yes vs. no) | 0.50 (0.25–0.99) | 0.046 |
All variables differing between the 2 groups and having a significance level of p < 0.10 in the univariable analysis were first entered into a multivariable Cox regression model, considering relapse and death as competing events. Then, a backward stepwise selection was performed using p < 0.05 and keeping PT-Cy in the model.
Graft-versus-host disease
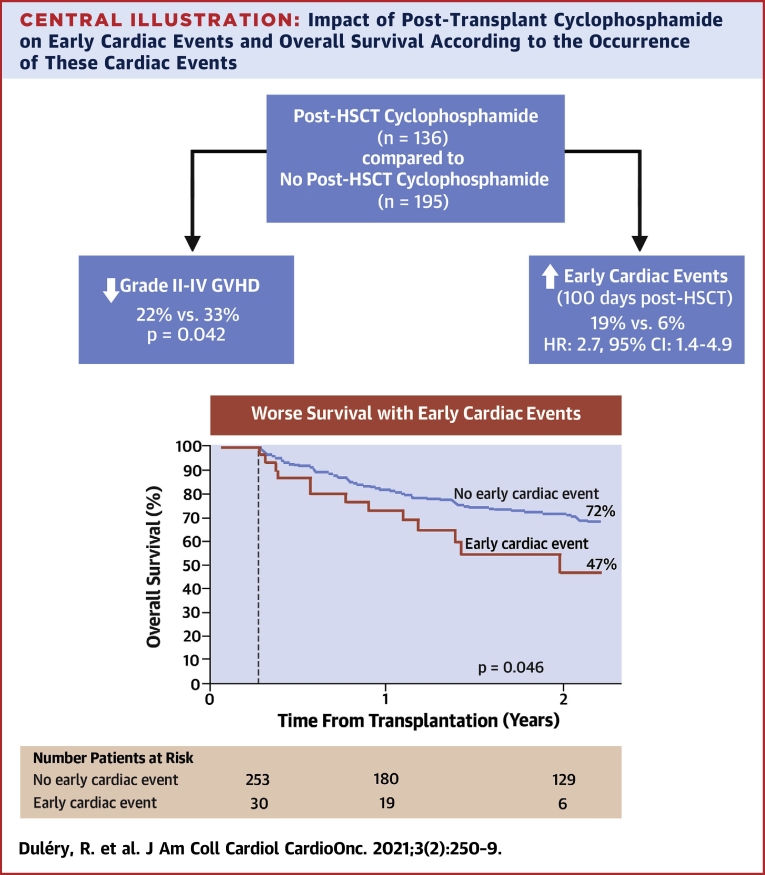
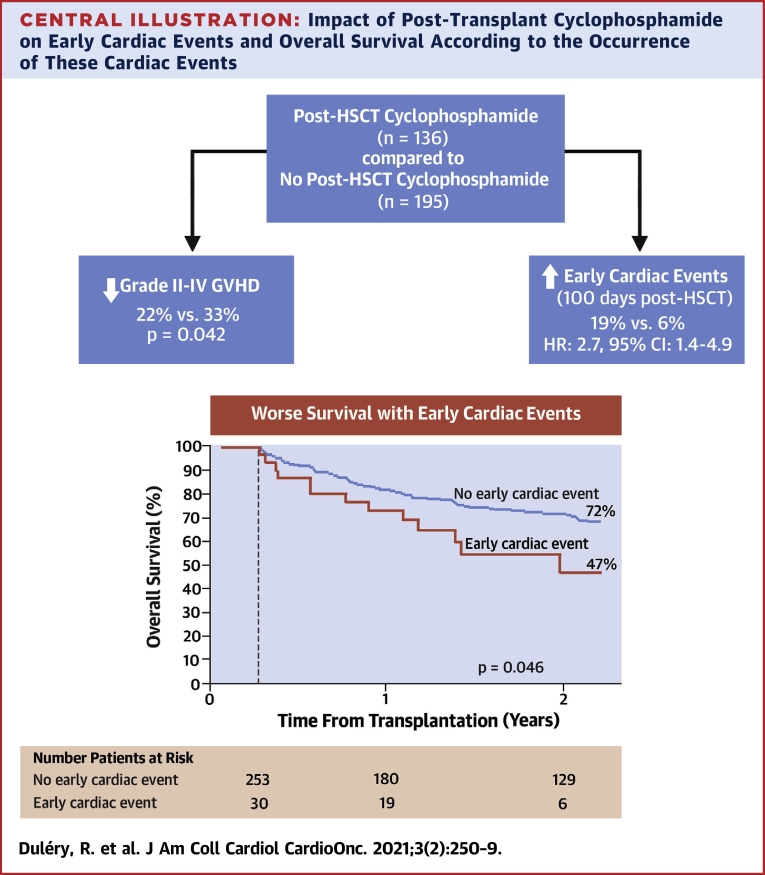
At day 100, the cumulative incidence of grade II to IV and grade III to IV acute GVHD was 22% and 7% in the PT-Cy group compared with 33% and 12% in the no–PT-Cy group, respectively (p = 0.042 for grade II to IV GVHD and p = 0.140 for grade III to IV GVHD) (Table 5). At 2 years, the cumulative incidence of chronic GVHD in the PT-Cy and in the no–PT-Cy group was 25% and 34%, respectively (p = 0.090). Moderate to severe chronic GVHD occurred in 40 (12%) patients with no difference between the 2 groups. In multivariable analysis, the use of PT-Cy was associated with a lower risk of acute grade II to IV GVHD (HR: 0.60; 95% CI: 0.40 to 0.97; p = 0.037). PT-Cy had no significant impact on chronic GVHD (Table 4).
Table 5.
Clinical Outcomes According to Post-Transplant Cyclophosphamide
| No PT-Cy |
PT-Cy |
p Value | |
|---|---|---|---|
| % (95% CI) | % (95% CI) | ||
| Acute GVHD II–IV | 33 (26.1–39.6) | 22 (15.1–30.2) | 0.042 |
| Acute GVHD III–IV | 12 (8.2–17.6) | 7 (3.6–13) | 0.14 |
| 2-yr chronic GVHD | 34 (26.8–40.5) | 25 (17.7–33.4) | 0.09 |
| 2-yr extensive chronic GVHD | 15 (10.4–20.7) | 10 (5.2–15.7) | 0.15 |
| 2-yr relapse incidence | 20 (14.9–26.5) | 23 (16.2–30.9) | 0.67 |
| 2-yr nonrelapse mortality | 21 (15.7–27.4) | 28 (20.6–36) | 0.11 |
| 2-yr disease-free survival | 58 (50.8–65.1) | 49 (39.7–57.1) | 0.06 |
| 2-yr overall survival | 63 (55.3–69.4) | 56 (47–64.5) | 0.15 |
| 2-yr GRFS | 46 (38.7–53.2) | 41 (32.5–50) | 0.45 |
| Median follow-up, months [Q1–Q3] | 41 [27–51] | 25 [15–43] | 0.002 |
Cumulative incidence was used to estimate GVHD, relapse, and nonrelapse mortality to accommodate competing risks. To study GVHD, relapse and death were considered to be competing events. Probabilities of overall survival, disease-free survival, and GRFS were calculated using the Kaplan-Meier method.
Survival outcomes
After a median follow-up of 36.5 months (Q1 to Q3, 32 to 40 months), there were no significant difference in cumulative incidence between the 2 groups with respect to nonrelapse mortality, relapse, overall survival, disease-free survival and GRFS (Table 5). At last-follow-up, 136 patients had died. The main causes of death were disease relapse (n = 60), infection (n = 43), GVHD (n = 20), ECE (n = 7), veno-occlusive disease (n = 2), and miscellaneous causes (n = 4). Univariable analyses are displayed in Table 3 and in Supplemental Table 1. In multivariable analysis, a history of a pre-transplant cardiac event was significantly associated with higher nonrelapse mortality (HR: 2.0; 95% CI: 1.2 to 3.2; p = 0.008) and poorer overall survival (HR: 1.8; 95% CI: 1.3 to 2.7; p = 0.002) and disease-free survival (HR: 1.9; 95% CI: 1.4 to 2.8; p < 0.001). Other significant results in multivariable analysis are presented in Table 4.
ECE resolved in 34 patients (76%). Normalization of LVEF with resolution of signs and symptoms was observed at a median time of 110 days (range 9 to 799 days) in 14 of 23 patients (61%) who experienced LVSD. However, segmental left ventricular motion abnormalities persisted in the majority of patients. At day 90 after HSCT, none of the patients who experienced LVSD had fully normal findings on transthoracic echocardiography. The disease risk index was higher and the Karnofsky index was lower in patients who did not recover from ECE compared with patients who did (p = 0.033 and p = 0.003, respectively). ECE had a significant impact on overall survival (HR: 2.7; 95% CI: 1.8 to 4.2; p < 0.0001). The 11 patients who did not recover from the ECE died. The 2-year overall survival was 31% (95% CI: 17% to 48%) in patients who had ECE compared with 64% (95% CI: 68% to 69%) in the absence of ECE (p = 0.001). The main causes of death in patients with ECE were the following: disease relapse (n = 5), infection (n = 14), GVHD (n = 2), ECE (n = 4), veno-occlusive disease (n = 2), and hemorrhagic shock (n = 1). Fifteen patients with ECE died before day 100, including 3 deaths directly related to a cardiac event. In patients surviving at least 100 days after transplantation, the 2-year overall survival was 47% (95% CI: 25% to 69%) in patients with previous ECE (n = 30) compared with 72% (95% CI: 66% to 78%) in the absence of ECE (n = 253) (p = 0.046) (Central Illustration).
Central Illustration.
Impact of Post-Transplant Cyclophosphamide on Early Cardiac Events and Overall Survival According to the Occurrence of These Cardiac Events
Post-transplant cyclophosphamide reduces the incidence of acute graft-versus-host disease. However, patients who receive post-transplant cyclophosphamide have a higher incidence of cardiac events within the first 100 days after allogeneic stem cell transplantation compared with patients who do not. Kaplan-Meier estimates (with a landmark analysis on day 100) show the detrimental impact of early cardiac events on overall survival. The 2-year overall survival was 47% in patients who had cardiac events compared with 72% in patients who did not (p = 0.046). CI = confidence interval; HR = hazard ratio.
Discussion
This is the first report on cardiac toxicity associated with PT-Cy. The major finding of this study is that the incidence of ECE was significantly higher in patients who received PT-Cy compared with patients who did not. Although the signs of ECE resolved after appropriate treatment in the majority of patients, those with previous ECE had a lower 2-year overall survival compared with patients who did not have any cardiac event.
Our results on patients who did not receive PT-Cy are in line with those of published reports (16,17,24, 25, 26). In the largest cohort study of HSCT recipients receiving Cy in the conditioning regimen, none of those patients who were treated with a total Cy dose of 100 mg/kg had fatal Cy-induced cardiotoxicity (16). In our study, Cy total doses were 100 mg/kg or less, and cardiac toxicity was the direct cause of death in 7 (2%) patients. Thus, the timing of administration of Cy and/or the haplo setting seemed to increase the cardiac toxicity induced by Cy. In terms of physiopathology, the dramatic immunologic changes occurring after the graft infusion may play a causative role. Indeed, PT-Cy prevents GVHD by inducing alloreactive T cell dysfunction and suppression (39). Cytokine release syndrome, noninfectious fevers, and viral or bacterial infections are more frequent when PT-Cy is administrated, especially when peripheral blood stem cells are used (40,41). Those immunologic events may hypothetically induce myocardial damage and contribute to the increased cardiotoxicity associated with PT-Cy.
Pre-transplant CVRFs were not associated with ECE
The impact of CVRFs before HSCT on post-transplantation outcomes is poorly assessed in published reports. In this study, neither the type of CVRF nor the cumulative number of CVRFs was associated with ECE. However, in univariable analysis, hypertension was associated with a lower overall survival, disease-free survival, and GRFS; diabetes was associated with higher nonrelapse mortality and lower overall survival; and dyslipidemia levels were associated with lower overall survival and disease-free survival. The cumulative number of CVRFs had no significant impact on survival. Cancer therapeutics–related cardiac dysfunction was first recognized in the 1960s with the use of anthracyclines. Physicians have since learned to limit the cumulative doses of these agents to avoid cardiac dysfunction. Interestingly, the cumulative doses of anthracyclines administrated before transplantation were not associated with a higher risk ECE or death in our study. However, this is the first study showing that exposure to Cy before HSCT is associated with a higher incidence of ECE and decreased survival. Further studies with a larger sample size and a higher number of cardiac events are warranted to gain additional insight into the impact of CVRFs, anthracyclines, and Cy on ECE and patients’ outcomes.
Identifying patients at higher risk of ECE
This analysis contributes to the identification of patients at higher risk of developing ECE. Older age is, as expected, one of the risk factors of ECE. Although sequential conditioning regimens are associated with a higher incidence of ECE compared with myeloablative and reduced-intensity conditioning, this platform should not be contraindicated in a haplo setting with PT-Cy. We recently published that a thiotepa-based sequential regimen is feasible in a haplo setting, and it allows for a 2-year overall survival of 55% and a 2-year nonrelapse mortality of 15% in patients with refractory hematologic malignant diseases (9). In patients who are in complete remission, a thiotepa-busulfan-fludarabine regimen may be a more appropriate platform in a haplo setting with PT-Cy (31). Finally, a history of a pre-transplant cardiac event had a detrimental impact on outcome, and a trend toward a higher incidence of ECE was observed (18% vs. 10% in the absence of a history of a cardiac event).
To prevent the occurrence of ECE, strategies other than haplo HSCT with PT-Cy should be proposed, when available, to older adult patients and patients with previous exposure to Cy. Cy-induced cardiac toxicity seems to be correlated with the Cy total dose, as previously reported (16). In this study, the incidence of ECE was indeed lower with the reduced PT-Cy dose of 50 mg/kg. Reducing the PT-Cy dose may therefore decrease the risk of ECE. However, firm conclusions cannot be drawn because the difference was not statistically significant, and the stem cell source was adjusted according to the PT-Cy dose. Further studies are needed to confirm whether a reduced PT-Cy dose can decrease the risk of ECE without increasing the incidence of GVHD and compromising outcomes.
Study limitations
One limitation of our study, besides its retrospective nature, is that the 2 groups were unbalanced in terms of disease risk index and use of sequential conditioning. The interaction between those 2 factors is strong because sequential conditioning regimens were actually proposed for patients who were not in complete remission and with a high disease risk index. However, disease risk index was not associated with a higher risk of ECE after HSCT in multivariable analysis. The incidences of obesity and dyslipidemia were also higher in the no–PT-Cy group than in the PT-Cy group. Although those 2 factors had no significant impact on ECE in univariable analysis, they may have only relatively increased the risk of ECE in the no–PT-Cy group.
Another limitation is the presence of patients in the PT-Cy group who did not have a haplo donor. Because PT-Cy was mainly used in haplo transplantation, it was not possible in the multivariable analysis to adjust for donor type and to infer any conclusion on PT-Cy toxicity independently of donor type. Further studies should assess the incidence of ECE in the following: 1) a haplo setting by comparing patients receiving PT-Cy with patients who do not; and 2) patients receiving PT-Cy by comparing patient with a haplo donor to patients with another donor type. The causative role of Cy in the development of the ECE reported in this study should also be discussed. The consensus definition of Cy-induced cardiac toxicity is a cardiac event occurring within 10 days after Cy administration. In this study, some cardiac events occurred later, up to 90 days after transplantation, meaning that these events may not have been directly related to Cy. The risk of ECE development is, regardless of the cause, higher after PT-Cy than without PT-Cy.
Conclusions
Although the use of PT-Cy is an efficient strategy to reduce the incidence of GVHD after HSCT, its administration is associated with a higher incidence of cardiac events within the first 100 days after transplantation. The main cardiac toxicity observed was LVSD, and patients who had a cardiac event after HSCT had lower overall survival. These results may help to improve the selection of patients who are eligible to undergo HSCT with PT-Cy, especially older adult patients and patients with previous exposure to Cy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The incidence of cardiac events within the first 100 days after allogeneic transplantation is 19% with PT-Cy, a percentage that is significantly higher than without PT-Cy. Older patients or patients with exposure to Cy before transplantation are also at higher risk of ECE. The main cardiac toxicity observed is LVSD. Although cardiac events may resolve in the majority of the cases (76%), patients who have a cardiac event with allogeneic transplantation have lower overall survival.
TRANSLATIONAL OUTLOOK: Future studies should assess whether other specific pre-transplant cardiovascular risk factors may be associated with post-transplant cardiac events. In particular, a cumulative maximum Cy dose should be defined. Reducing the PT-Cy dose should also be evaluated.
Funding Support and Author Disclosures
This study was supported by the Association for Training, Education and Research in Hematology, Immunology, and Transplantation (ATERHIT). Drs. Duléry, Mohty, and Malard have received honoraria for lectures from Keocyt and Sanofi, whose drugs were included in this study. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all the clinicians and the nursing staff for providing excellent care for our patients.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Luznik L., O’Donnell P.V., Symons H.J. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashey A., Zhang X., Jackson K. Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant. 2016;22:125–133. doi: 10.1016/j.bbmt.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Raiola A.M., Dominietto A., di Grazia C. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014;20:1573–1579. doi: 10.1016/j.bbmt.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 4.Ringdén O., Labopin M., Ciceri F. Is there a stronger graft-versus-leukemia effect using HLA-haploidentical donors compared with HLA-identical siblings? Leukemia. 2016;30:447–455. doi: 10.1038/leu.2015.232. [DOI] [PubMed] [Google Scholar]
- 5.Ruggeri A., Labopin M., Sanz G. Comparison of outcomes after unrelated cord blood and unmanipulated haploidentical stem cell transplantation in adults with acute leukemia. Leukemia. 2015;29:1891–1900. doi: 10.1038/leu.2015.98. [DOI] [PubMed] [Google Scholar]
- 6.Martínez C., Gayoso J., Canals C. Post-transplantation cyclophosphamide-based haploidentical transplantation as alternative to matched sibling or unrelated donor transplantation for Hodgkin lymphoma: a registry study of the Lymphoma Working Party of the European Society for Blood and Marrow Transplantation. J Clin Oncol. 2017;35:3425–3432. doi: 10.1200/JCO.2017.72.6869. [DOI] [PubMed] [Google Scholar]
- 7.Gauthier J., Poiré X., Gac A.-C. Better outcome with haploidentical over HLA-matched related donors in patients with Hodgkin’s lymphoma undergoing allogeneic haematopoietic cell transplantation-a study by the Francophone Society of Bone Marrow Transplantation and Cellular Therapy. Bone Marrow Transplant. 2018;53:400–409. doi: 10.1038/s41409-017-0018-z. [DOI] [PubMed] [Google Scholar]
- 8.Ciurea S.O., Zhang M.-J., Bacigalupo A.A. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–1040. doi: 10.1182/blood-2015-04-639831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duléry R., Ménard A.-L., Chantepie S. Sequential conditioning with thiotepa in T cell-replete hematopoietic stem cell transplantation for the treatment of refractory hematologic malignancies: comparison with matched related, haplo-mismatched, and unrelated donors. Biol Blood Marrow Transplant. 2018;24:1013–1021. doi: 10.1016/j.bbmt.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Passweg J.R., Baldomero H., Bader P. Use of haploidentical stem cell transplantation continues to increase: the 2015 European Society for Blood and Marrow Transplant activity survey report. Bone Marrow Transplant. 2017;52:811–817. doi: 10.1038/bmt.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mielcarek M., Furlong T., O’Donnell P.V. Posttransplantation cyclophosphamide for prevention of graft-versus-host disease after HLA-matched mobilized blood cell transplantation. Blood. 2016;127:1502–1508. doi: 10.1182/blood-2015-10-672071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jorge A.S., Suárez-Lledó M., Pereira A. Single antigen-mismatched unrelated hematopoietic stem cell transplantation using high-dose post-transplantation cyclophosphamide is a suitable alternative for patients lacking HLA-matched donors. Biol Blood Marrow Transplant. 2018;24:1196–1202. doi: 10.1016/j.bbmt.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Ruggeri A., Labopin M., Bacigalupo A. Post-transplant cyclophosphamide for graft-versus-host disease prophylaxis in HLA matched sibling or matched unrelated donor transplant for patients with acute leukemia, on behalf of ALWP-EBMT. J Hematol Oncol. 2018;11:40. doi: 10.1186/s13045-018-0586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorentino F., Labopin M., Ciceri F. Post-transplantation cyclophosphamide GvHD prophylaxis after hematopoietic stem cell transplantation from 9/10 or 10/10 HLA-matched unrelated donors for acute leukemia. Leukemia. 2021;35:585–594. doi: 10.1038/s41375-020-0863-4. [DOI] [PubMed] [Google Scholar]
- 15.Battipaglia G., Labopin M., Kröger N. Posttransplant cyclophosphamide versus antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood. 2019;134:892–899. doi: 10.1182/blood.2019000487. [DOI] [PubMed] [Google Scholar]
- 16.Ishida S., Doki N., Shingai N. The clinical features of fatal cyclophosphamide-induced cardiotoxicity in a conditioning regimen for allogeneic hematopoietic stem cell transplantation (allo-HSCT) Ann Hematol. 2016;95:1145–1150. doi: 10.1007/s00277-016-2654-6. [DOI] [PubMed] [Google Scholar]
- 17.Tichelli A., Bucher C., Rovó A. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 18.Tichelli A., Passweg J., Wójcik D. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 19.Chow E.J., Mueller B.A., Baker K.S. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 20.Bhatia S., Francisco L., Carter A. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viswanatha Swamy A.H.M., Patel U.M., Koti B.C., Gadad P.C., Patel N.L., Thippeswamy A.H.M. Cardioprotective effect of Saraca indica against cyclophosphamide induced cardiotoxicity in rats: a biochemical, electrocardiographic and histopathological study. Indian J Pharmacol. 2013;45:44–48. doi: 10.4103/0253-7613.106434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeJarnett N., Conklin D.J., Riggs D.W. Acrolein exposure is associated with increased cardiovascular disease risk. J Am Heart Assoc. 2014;3 doi: 10.1161/JAHA.114.000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurauchi K., Nishikawa T., Miyahara E., Okamoto Y., Kawano Y. Role of metabolites of cyclophosphamide in cardiotoxicity. BMC Res Notes. 2017;10:406. doi: 10.1186/s13104-017-2726-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cazin B., Gorin N.C., Laporte J.P. Cardiac complications after bone marrow transplantation. A report on a series of 63 consecutive transplantations. Cancer. 1986;57:2061–2069. doi: 10.1002/1097-0142(19860515)57:10<2061::aid-cncr2820571031>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Gottdiener J.S., Appelbaum F.R., Ferrans V.J., Deisseroth A., Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141:758–763. [PubMed] [Google Scholar]
- 26.Braverman A.C., Antin J.H., Plappert M.T., Cook E.F., Lee R.T. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 27.Morandi P., Ruffini P.A., Benvenuto G.M., Raimondi R., Fosser V. Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant. 2005;35:323–334. doi: 10.1038/sj.bmt.1704763. [DOI] [PubMed] [Google Scholar]
- 28.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmid C., Schleuning M., Schwerdtfeger R. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood. 2006;108:1092–1099. doi: 10.1182/blood-2005-10-4165. [DOI] [PubMed] [Google Scholar]
- 30.Mohty M., Malard F., Blaise D. Sequential regimen of clofarabine, cytosine arabinoside and reduced intensity transplantation for primary refractory acute myeloid leukemia. Haematologica. 2017;102:184–191. doi: 10.3324/haematol.2016.150326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duléry R., Bastos J., Paviglianiti A. Thiotepa, busulfan, and fludarabine conditioning regimen in T cell-replete HLA-haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019;25:1407–1415. doi: 10.1016/j.bbmt.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Picod A., Bonnin A., Battipaglia G. Defibrotide for sinusoidal obstruction syndrome/veno-occlusive disease prophylaxis in high-risk adult patients: a single-center experience study. Biol Blood Marrow Transplant. 2018;24:1471–1475. doi: 10.1016/j.bbmt.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Ruggeri A., Labopin M., Ciceri F., Mohty M., Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016;51:610–611. doi: 10.1038/bmt.2015.305. [DOI] [PubMed] [Google Scholar]
- 34.Przepiorka D., Weisdorf D., Martin P. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 35.Jagasia M.H., Greinix H.T., Arora M. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. the 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401. doi: 10.1016/j.bbmt.2014.12.001. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armand P., Gibson C.J., Cutler C. A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood. 2012;120:905–913. doi: 10.1182/blood-2012-03-418202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorror M.L., Maris M.B., Storb R. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fine J., Gray R.J. A Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 39.Wachsmuth L.P., Patterson M.T., Eckhaus M.A., Venzon D.J., Gress R.E., Kanakry C.G. Posttransplantation cyclophosphamide prevents graft-versus-host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest. 2019;129:2357–2373. doi: 10.1172/JCI124218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimoto M., Hirose A., Koh H. Clinical impacts of using serum IL-6 level as an indicator of cytokine release syndrome after HLA-haploidentical transplantation with post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. 2019;25:2061–2069. doi: 10.1016/j.bbmt.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Abid M.B., Hamadani M., Szabo A. Severity of cytokine release syndrome and its association with infections after T cell-replete haploidentical related donor transplantation. Biol Blood Marrow Transplant. 2020;26:1670–1678. doi: 10.1016/j.bbmt.2020.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.