Abstract
Background
Cancer treatment can lead to left ventricular (LV) dysfunction in female cancer survivors of reproductive age, and pregnancy-related hemodynamic stress may result in LV dysfunction or heart failure (HF).
Objectives
We performed a systematic review and meta-analysis to determine the incidence of LV systolic dysfunction or HF during or soon after pregnancy in cancer survivors and evaluated the impact of history of cancer therapeutics-related cardiac dysfunction (CTRCD).
Methods
We systematically searched electronic databases (MEDLINE and EMBASE) from inception to January 2020 to identify cohort studies that examined cardiac disease in pregnant cancer survivors. Meta-analysis was performed using the inverse-variance fixed effects method. Potential sources of heterogeneity were explored using subgroup analyses and meta-regression.
Results
Of 13,782 identified articles, 6 studies consisting of 2,016 pregnancies, predominantly in childhood cancer survivors, were included. Overall, there were 33 cardiac events. The total weighted incidence of LV dysfunction or HF with pregnancy was 1.7% (95% confidence interval [CI]: 0.9% to 2.7%) overall; 28.4% (95% CI: 14.6% to 43.9%) in those with a history of CTRCD and 0.24% (95% CI: 0% to 0.81%) in those without, translating into an odds ratio of 47.4 (95% CI: 17.9 to 125.8). Interstudy heterogeneity was low (I2 = 17.5%). Metaregression did not reveal significant sources of heterogeneity.
Conclusions
The incidence of LV dysfunction or HF during pregnancy in cancer survivors was low. Although risk estimates are limited by the small number of events, women with a history of CTRCD compared to those without had a 47.4-fold higher odds of experiencing pregnancy-related LV dysfunction or HF.
Key Words: cancer survivors, cancer therapeutics-related cardiac dysfunction, cardiotoxicity, heart failure, pregnancy
Abbreviations and Acronyms: CTRCD, cancer therapeutics-related cardiac dysfunction; HF, heart failure; LV, left ventricle; LVEF, left ventricular ejection fraction
Central Illustration
Advances in cancer treatment in the last several decades have led to an increasing number of young adult cancer survivors. In the United States alone, 1 in 640 young adults between the ages of 20 and 39 years is a cancer survivor (1). Cardiac toxicity from cancer treatment, including chemotherapy and radiation, is the leading nonmalignant cause of death among adult cancer survivors (2). Manifestations of adverse cardiovascular outcomes for both chemotherapy and radiation treatment include cancer therapeutics-related cardiac dysfunction (CTRCD) (ie, reduction in left ventricular ejection fraction [LVEF] or heart failure [HF]), premature coronary artery disease, valvular disorders, pericardial injury, and arrhythmia (3, 4, 5). Onset of cardiovascular complications can be delayed by up to 30 years after cancer treatment (6) and may be accelerated by either conventional cardiovascular risk factors or hemodynamic stress (7).
The increasing number of female cancer survivors with reproductive potential raises concern about cardiac complications associated with pregnancy. Pregnancy results in plasma volume expansion, increased heart rate, and higher cardiac output resulting in increased myocardial wall stress. Furthermore, there is a rise in oxidative stress during normal pregnancy, culminating in the last trimester (8). These changes may be tolerated poorly in patients with a history of CTRCD who have a vulnerable myocardium (9,10). Furthermore, up to one-third of patients with prior cancer treatment, regardless of a CTRCD history, can have subclinical LV systolic dysfunction which can be unmasked by the hemodynamic stress of pregnancy (11). Prior studies examining maternal adverse cardiac events in cancer survivors are individually limited by small sample size and single-center recruitment, and some were dependent on self-reported events (12, 13, 14, 15, 16, 17). Drawing definitive conclusions from each study in isolation to guide management may be challenging.
The present objective was to perform a systematic review and meta-analysis of prior published studies to quantify the impact of a pre-pregnancy history of CTRCD on the risk of maternal LV systolic dysfunction or HF during pregnancy or within 12 months of delivery. The present authors hypothesized that the incidence of maternal LV systolic dysfunction or HF would be low and that a history of CTRCD would be associated with a higher risk.
Methods
Systematic review
A systematic review was performed, adhering to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (18) (Supplemental Table 1) and registered prospectively in the PROSPERO database (Cardiac Outcomes in Pregnant Women with Prior Treated Cancer: a Systematic Review and Meta-Analysis; CRD42019123849). Electronic searches of MEDLINE and EMBASE (OvidSP) were performed from inception to January 2020. Due to multiple potential sources of variability, including different cardiomyopathy definitions and sources of CTRCD, a liberal search strategy was used to improve sensitivity (Supplemental Appendix). In addition, references of relevant publications were searched, and experts in the field were contacted to improve the sensitivity of the search criteria. Inclusion criteria consisted of publications in peer-reviewed, English-language journals reporting data for: 1) study type: either prospective or retrospective cohort studies with ≥10 patients who underwent ≥1 pregnancy during study duration; 2) study population: females of reproductive age with prior treatment of cancer using potentially cardiotoxic therapeutics, including any chemotherapy or radiotherapy with fields including the thorax and reported cardiac function or HF status prior to pregnancy; 3) follow-up: from cancer diagnosis until at least first pregnancy; and 4) outcome: LV systolic dysfunction (reduction in LVEF or fractional shortening) or HF during pregnancy or within 12 months of delivery. Exclusion criteria were: 1) nonhuman studies; and 2) abstracts or conference proceedings. All papers were reviewed for inclusion and exclusion factors by 2 authors (M.N. and E.K.O.), who also extracted data from the primary publications. Any discrepancies were resolved by consensus. Information for year of publication, sample size, follow-up duration, type and dose of cancer therapy drugs, number of patients with CTRCD prior to pregnancy, and number of patients with pregnancy-related LV dysfunction and HF were extracted independently from each study. When required data were missing, the corresponding authors were contacted. Quality of individual studies was assessed using the Newcastle-Ottawa Scale (19).
Statistical analysis
The combined weighted incidence of LV dysfunction or HF from all studies was calculated using Freeman-Tukey double-arcsine transformation to weigh by inverse variance and a fixed-effects model. Between-study heterogeneity was assessed by calculation of the Cochran Q and I2 statistics. Meta-analysis for the odds ratio (OR) for LV dysfunction or HF in pregnant women with or without CTRCD was performed by first calculating the log odds ratio (OR) and 95% confidence interval (CI) for each study identified by the systematic review. The log (OR) was used as the summary statistic, as this estimate has a better normal approximation than that of the OR. A continuity correction, adding 0.05 to each cell, was done in the presence of any 0 outcome cell counts. The combined log (OR) was calculated using the inverse-variance fixed-effect meta-analysis method. The overall OR with 95% CI was obtained by exponentiating the log (OR) results. A sensitivity analysis was performed using a random effects meta-analysis method (DerSimonian and Laird method).
The impact of baseline characteristics on the association between CTRCD and pregnancy-related cardiac events was explored using meta-regression techniques. Meta-regression was performed using a fixed-effects model with OR for CTRCD as the dependent variable and using previously defined study level characteristics including year of publication, number of patients enrolled, and cumulative anthracycline dose as the independent variables.
Publication bias was assessed visually by funnel plots of effect estimates and by the Egger regression statistical test. Because the number of studies assessed was small, further assessment of possible publication bias was undertaken by using the Duval and Tweedie Trim and Fill test. Statistical analysis was performed using the “metafor” application in R statistical software (Vienna, Austria) (20). Statistical tests were 2-sided, and significance was defined as a p value of <0.05.
Results
Study summary
Systematic review identified 13,782 potential studies (Supplemental Figure 1). After review, 6 studies met eligibility criteria (Table 1). All studies were of good quality according to the Newcastle-Ottawa Scale (Supplemental Table 2). A total of 5 of 6 studies were retrospective, and most focused on pediatric, adolescent, and young adult cancer survivors. Three studies used cardiac dysfunction defined by echocardiography as the outcome, 2 studies used symptomatic cardiac disease as the preferred outcome, and 1 study used both. A standard previous definition of CTRCD was not used in all studies, and most studies did not provide a formal definition (Table 1).
Table 1.
Summary of Prior Studies Describing Pregnancy-Related Outcomes in Cancer Survivors
| First Author, Year (Ref. #) | Participants | Recruited Population | Study Design | Median Age at Cancer Diagnosis (yrs) | Median Age at First Pregnancy (yrs) | Median Follow-Up Duration (yrs) | Distribution of Cancers | Cancer Therapy Details | Anthracycline Dose (Median or Mean) |
Prior Cardiomyopathy/Abnormal LV Function Pre-Pregnancy | CTRCD Definition |
Pregnancy-Related Cardiac Outcome Definition | Pregnancy-Related Cardiac Outcomes | Predictors of Pregnancy-Related Cardiac Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bar et al. 2003, (12) |
72 pregnancies (37 women) | Schneider Children’s Medical Centre (Israel). Inclusion: childhood cancer, doxorubicin treatment; exclusions: none |
Prospective cohort study | 12 (range, 3-18) | 24 (range, 18-32) | 17 (range, 6-29) | Leukemia, 35%; lymphoma, 27%; sarcoma, 32%; Wilms’ tumor 6% |
All received anthracyclines, no information on RT | 400 mg/m2 (range, 150-500 mg/m2) | 8 of 37 (22%)/8 of 37 (22%)∗ | FS <30% on TTEs or RNV-EF <50% on 2 sequential tests 1 month apart | ICU admission for HF during or after delivery | 2 of 37 (5%) | Pre-pregnancy LV function |
| van Dalen et al. 2006, (13) |
100 pregnancies (53 women) | Emma Children’s’ Hospital (the Netherlands). Inclusion: age ≥17 yrs; childhood cancer survival ≥5 yrs post-cancer; anthracycline treated; exclusions: none |
Retrospective cohort study | 11.2 (range, 1.5–17.8) | Not stated | Mean 20.3 (range, 5.8-28) | Leukemia 26%; lymphoma 30%; osteosarcoma, 10%; Ewing’s sarcoma, 19%; Wilms’ tumor, 2%; others, 13% |
All received anthracyclines; 10 patients received RT | 267 mg/m2 (range, 60-552 mg/m2) | 2 of 53 (4%)/NA | Clinical HF (signs + symptoms treated with diuretics during or after chemotherapy | Clinical HF (signs + symptoms treated with diuretics, during pregnancy or <5 months after delivery) | No clinical HF events; no routine cardiac imaging performed | No events |
| Hines et al. 2016, (14) |
1,554 pregnancies (847 women) | St. Jude Children’s Hospital (U.S.). Inclusions: childhood cancer, survival ≥5 yrs after cancer, >13 yrs of age at follow-up; had at least 1 delivery; exclusions: none |
Retrospective cohort study | 10.3 (range, 0.02-22.6) | 22.4 (range, 13.8-40.1) | 26.5 (range, 6.0-48.4) | Leukemia, 38%; lymphoma, 23%; sarcoma, 14%; embryonic tumors, 10% others 15% |
484 patients received anthracyclines (248 also received RT); 363 patients received nonanthracycline therapy (140 received RT) | 200 mg/m2 (39-721 mg/m2) | 26 of 847 (3%)/8 of 847 (1%) | EF <50% or FS <28% by TTE or treatment for HF | LVEF <50% or FS <28% by TTE or treatment for HF within 5 months of delivery (outcomes were self-reported) | 8 of 26 (31%) inpatients with previous CTRCD; 3 of 821 (4%) had new diagnoses during pregnancy (2 asymptomatic LV dysfunction, 1 HF) |
Higher anthracycline dose |
| Thompson et al., 2017 (15)† |
86 pregnancies (58 women) | MD Anderson Cancer Center (U.S.). Inclusions: age 16-55 yrs; cancer diagnosis before age 20 yrs; treated with anthracyclines or chest irradiation. Exclusions: history of abortion or miscarriage; Down syndrome; death without adequate follow-up |
Retrospective cohort study | 11.8 (range, 0.5-19.5)† | 23.0 (range, 16-37)† | 20.2 (range, 5.2-48.2)† | Childhood cancer survivors (no details) | All received anthracycline and/or XRT (numbers in each group not provided) | 292.5 mg/m2 (0-480 mg/m2)† | 3 of 58 (5%)/NA | EF <50% on 2 TTEs or CAD | LVEF <50% on 2 TTEs or CAD within 12 months of delivery | 11 of 58 (19%; all asymptomatic LV dysfunction; 2 of 3 in patients with previous CTRCD; 9 of 55 had new diagnoses during pregnancy.† | High anthracycline dose; younger age at cancer diagnosis; longer time from cancer therapy to first pregnancy |
| Liu, et al., 2018 (16) | 94 pregnancies (78 women) | Mt. Sinai Hospital (Canada). Inclusions: Female; potentially cardiotoxic treatment; exclusions: unknown cancer or treatment; familial cardiomyopathies |
Retrospective cohort study | 28 (range, 2-41) | 34 (range, 22-43) | During pregnancy and peripartum period | Lymphoma, 33%; leukemia, 10%; breast cancer, 32%; Wilms' tumor, 8%; osteosarcoma, 7%; others, 10% |
55 patients received anthracyclines; 16 received nonanthracycline (33 among this 71 received RT); 7 received RT only | 290 mg/m2 (90-500) mg/m2‡ | 13 of 78 (17%); 7 of 78; (10%) |
LVEF to <50% with or without HF symptoms | Composite of cardiac death, clinical HF (signs + symptoms + diuresis escalation or admission), ACS, arrhythmia up to 16 weeks after delivery | 5 HF events in 4 patients; all in patients with previous CTRCD | History of CTRCD; LVEF <53% at the start of pregnancy; cardiac medications |
| Chait-Rubinek, et al., 2019 (17) | 110 pregnancies (64 women) | Peter MacCallum Cancer Centre (Australia). Inclusions: age <30 yrs at cancer diagnosis; ≥5 yrs since cancer treatment; or ≥2 yrs after allogeneic stem cell transplantation. Exclusions: pregnancies prior to 5-yr timepoint since cancer treatment |
Retrospective cohort study | 18 (range, 2-29) | 31 (range, 19-42) | NA | Leukemia, 13%; lymphoma, 66%; Ewing sarcoma, 8%; Wilms’ tumor, 6%; osteosarcoma (1.5%); hepatoblastoma (1.5%); others, 5% |
55 patients received anthracyclines (28 received RT); 9 received nonanthracycline (4 received RT); 5 had RT only | 270 mg/m2 (150–600 mg/m2) | 1/64 (2%); 1/64 (2%) |
Treatment-induced cardiotoxicity (as diagnosed by a cardiologist) prior to pregnancy | Symptomatic cardiac dysfunction defined (clinical signs of HF requiring diuresis therapy with LVEF <50% or FS <28%). Subclinical dysfunction was defined as the absence of clinical features with LVEF <50% or FS <28% during pregnancy or <5 months after delivery |
3 symptomatic cardiac dysfunction events (0 in patients with prior CTRCD) 2 subclinical cardiac dysfunction events (1 in a patient with prior CTRCD) |
Younger age at time of cancer diagnosis; higher cumulative anthracycline dose; diagnosis of solid tumor |
CTRCD = cancer therapy-related cardiac dysfunction; ACS = acute coronary syndrome; CAD = coronary artery disease; FS = fractional shortening; HF = heart failure; ICU = intensive care unit; LVEF = left ventricular ejection fraction; RNV-EF = Radionuclide Ventriculography; TTE = transthoracic echocardiogram; XRT/RT = radiation involving the chest.
Based on a fractional shortening of <30%.
Data were obtained through personal communication with the author. All outcomes were EF <50%; none of the patients experienced coronary artery disease.
Data were available only in 23 of 55 patients who received anthracyclines.
The 6 studies included a cumulative total of 1,137 women with 2,016 pregnancies. Median age at cancer diagnosis was 12.1 years (range: 0.02 to 41 years). Median age at first pregnancy was 21.8 years, and median follow-up since the cancer diagnosis was 24.8 years for the 5 studies that provided data. Cancer treatment spanned from 1963 to 2015. Among the patients, 66.5% received anthracyclines with a mean cumulative anthracycline dose of 234.0 mg/m2. For the 5 studies that provided data for cancer subtypes, 60.6% were hematological malignancies.
Incidence of LV dysfunction or HF
Using data from all studies, 33 cancer survivors (2.9%) had LV dysfunction or HF during pregnancy or in the 12 months after delivery. Seventeen of the 33 survivors (51.5%) had CTRCD previously, 16 (48.5%) did not; 32 (97.0%) had prior anthracycline treatment, and 1 (3.0%) did not. The total weighted incidence of LV dysfunction or HF during pregnancy was 1.7% (95% CI: 0.9% to 2.7%). In patients with a history of CTRCD, the weighted incidence was 28.4% (95% CI: 14.6% to 43.9%), whereas in patients without a history of CTRCD, the weighted incidence was 0.24% (95% CI: 0% to 0.81%). This translated to a number-needed-to-harm of 4. There were no maternal or fetal pregnancy-associated mortalities reported in the studies, although 3 studies reported incidence rates of spontaneous abortion of 15.1%, 10.8%, and 6.0% (12,13,17).
Predictors of LV dysfunction or HF
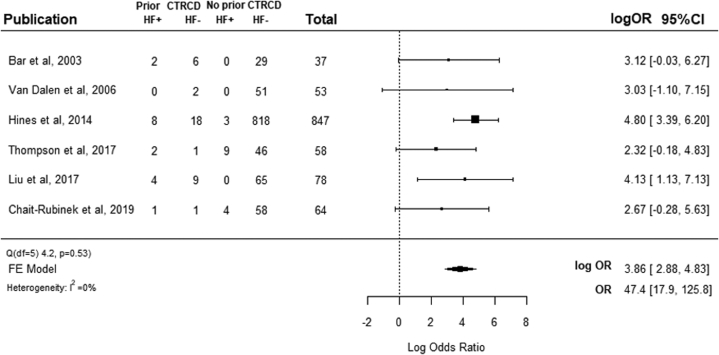
The association between pre-pregnancy CTRCD and development of LV dysfunction or HF during pregnancy or within 12 months of delivery based on the meta-analysis is shown in Figure 1. Low and nonsignificant interstudy heterogeneity were observed (Cochran Q: 4.2; p = 0.53, and I2 = 17.5%). Patients with a history of pre-pregnancy CTRCD had significantly higher odds of LV dysfunction or HF during pregnancy or within 12 months of delivery (OR: 47.4; 95% CI: 17.9 to 125.8; p < 0.001). Analysis repeated with a random effects model demonstrated a similar OR (OR: 40.4; 95% CI: 12.8 to 126.5). A sensitivity analysis that excluded the largest single study, which accounted for 74.5% of all patients, still found that pre-pregnancy CTRCD was associated with higher odds of LV dysfunction or HF (OR: 19.9; 95% CI: 5.1 to 77.0; p < 0.001).
Figure 1.
Forest Plot for LV Dysfunction or HF Related to Pregnancy in Women With and Without Prior CTRCD
Forest plot shows the odds ratio for LV systolic dysfunction or HF during pregnancy in women with and without a history of CTRCD prior to pregnancy. CTRCD = cancer therapeutics-related cardiac dysfunction; FE = fixed effect; HF = heart failure; HF+ = LV dysfunction or heart failure related to pregnancy; HF- = No LV dysfunction or heart failure related to pregnancy; LV = left ventricular; OR = odds ratio.
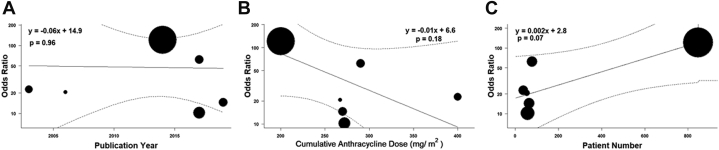
Meta-regression did not demonstrate any significant contribution toward interstudy heterogeneity using study level variables of publication year (r = −0.06; p = 0.96), cumulative anthracycline dose (r = −0.01; p = 0.18), or number of patients enrolled (r = 0.002; p = 0.07) (Figure 2).
Figure 2.
Metaregression of Study-Level Variables
Metaregression of study-level variables of (A) publication year, (B) cumulative anthracycline dose, and (C) number of patients enrolled. Each filled circle represents a study, and the size of the circle is directly proportional to the study’s weight in the analysis.
Assessing publication bias
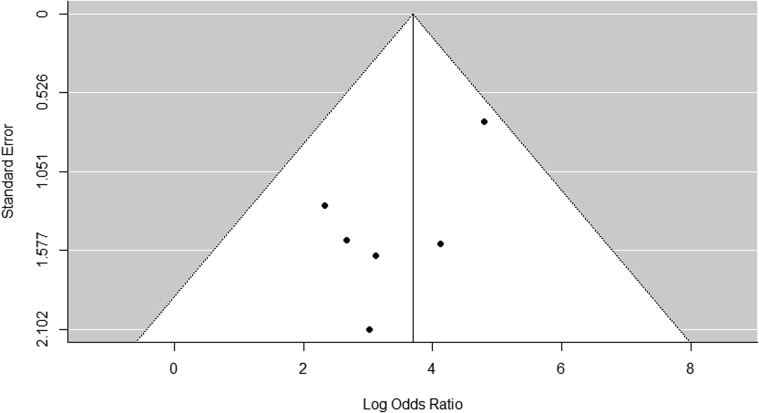
Risk of publication bias as assessed by visual inspection of funnel plot did not reveal any asymmetry (Figure 3). The Egger regression test did not demonstrate evidence of bias (p = 0.12), although it was underpowered due to the small number of studies. The Duval and Tweedie Trim and Fill test results did not significantly predict missing studies, nor did they alter the effect size.
Figure 3.
Funnel Plot for Assessing Publication Bias
Data points represent individual studies. The y-axis represents the measurement of study precision (plotted as SE of effect size), and the x-axis represents point estimates for each study. Dashed triangular lines represent the region in which 95% of studies are expected to lie in the absence of bias and heterogeneity.
Discussion
In this systematic review and meta-analysis of 1,137 women previously treated for cancer who underwent at least 2,106 pregnancies, the incidence of LV dysfunction or HF with pregnancy was 1.7% in the cancer survivor population. There were significant differences in the incidence of LV dysfunction or HF in patients with a history of CTRCD compared to patients without CTRCD (28.4% vs. 0.24%, respectively). The number needed to harm was 4, meaning that among every 4 women with a history of CTRCD, one would develop LV dysfunction or HF during pregnancy or within 12 months after delivery. These findings add to existing reviews of this subject (21) by providing data that can be used for patient counseling, including a weighted risk estimate of LV dysfunction or HF in cancer survivors going through pregnancy, an OR for risk based on a history of CTRCD, and a number-needed-to-harm estimate.
Incidence of LV dysfunction or HF associated with pregnancy in cancer survivors
The overall reported incidence of LV dysfunction or HF associated with pregnancy in survivors of adult and pediatric cancer survivors has varied between 0.0% and 7.8% (Table 1). The studies that reported the lowest incidence either did not have pre- and post-pregnancy cardiac assessment (13) or relied on self-reporting of peripartum HF, which can be associated with recall and survival bias (14). In the present meta-analysis of 6 published studies, the weighted risk of pregnancy-associated LV systolic dysfunction or HF in cancer survivors was 1.7% with no reported maternal cardiac deaths. It is possible, but we believe less likely, that the cases of HF in the published studies represent superimposed conditions such as peripartum cardiomyopathy or viral myocarditis, as opposed to decompensation of subclinical dysfunction in patients with a history of CTRCD. However, the reported incidence of peripartum cardiomyopathy is approximately 0.03% in the general population (22), which is much lower than the incidence seen in our meta-analysis.
Predictors of LV dysfunction or HF during pregnancy in cancer survivors
Potential predictors of LV dysfunction or HF during pregnancy or in the post-partum period based on results presented in the individual studies but not from our primary analysis are summarized in Table 1. These predictors include lower pre-pregnancy LV systolic function (12,16), younger age at cancer diagnosis (15,17), longer time from cancer treatment to first pregnancy (15), and cumulative anthracycline dose (14,15,17). The younger age at cancer diagnosis and longer time from cancer treatment to pregnancy may reflect patients who received older treatment regimens with higher doses of anthracycline, subclinical cardiomyopathy, or the development of additional cardiovascular risk factors. In the present meta-analysis, the weighted incidence of pregnancy associated HF or worsening LV function in cancer survivors restricted to those with a history of CTRCD was 28.4%. A history of CTRCD was associated with 47.4-fold higher odds of LV dysfunction (95% CI: 17.9 to 125.8) or HF with pregnancy. This incidence of cardiac events in those with a history of CTRCD is similar to the reported incidence of new onset HF in women with a history of peripartum cardiomyopathy (29%) going through a second pregnancy (23). These scenarios demonstrate that persistent myocardial injury can be unmasked by the stress of pregnancy. Interestingly, the majority of the reported LV dysfunctions or HF events (32 of 33) occurred in those who had previously undergone anthracycline therapy. However, the number of events in the nonanthracycline subgroup was inadequate to accurately and precisely perform subgroup analysis to examine the impact of cancer treatment regimen on LV dysfunction or HF related to pregnancy.
Study limitations
The present analysis has some limitations worth discussing. Although the results are based on 6 single-center studies, there were a limited number of events. Still, this is the only meta-analysis of this subject, and it provides summary statistics that help guide clinical care. However, the overall small number of outcomes led to wide confidence intervals for the present estimates. Hence these estimates should be interpreted in the context of this limitation. Patients in the present study received cancer treatment spanning 52 years, and the treatment regimens have evolved. However, the absence of interstudy heterogeneity of studies published over a 16-year span and the lack of relationship between publication year and outcome in the present meta-regression suggest that the present findings are likely still relevant to current treatments. Furthermore, the main risk factor studied, CTRCD, remains an important consideration with current treatment regimens (24). Inconsistent definitions of CTRCD were used among studies, and cardiac imaging was not consistently performed before and after pregnancy in all patients. Despite this, there remained concordance of findings between studies with minimal interstudy heterogeneity, suggesting that the results of this study are relevant. Most studies focused on pediatric, adolescent, and young adult cancer survivors leading to under-representation of older adult cancer survivors. We were also unable to adjust the odds ratio for high-risk baseline demographics, such as extremes of patient age at pregnancy, due to lack of patient-level data. We were also unable to perform subgroup analysis examining the effects of radiotherapy or type of cancer therapy on outcomes due to incomplete reporting of these baseline characteristics and/or a small number of events. Future development of registries and prospective studies are needed to provide more accurate identification of risk factors and ideal diagnostic and therapeutic strategies for cancer survivors going through pregnancy. Several other risk factors beyond CTRCD have been outlined for LV dysfunction and HF during pregnancy or post-delivery in Discussion and in Table 1, based on prior publications. However, data from individual studies were not adequate to determine their independent and incremental predictive value over that of CTRCD history. Furthermore, important information regarding cardiac medications in patients with a history of CTRCD or their cessation during pregnancy was not consistently available in our studies (Supplemental Table 3). Therefore, whether cessation of cardiac medications during pregnancy contributed to the LV dysfunction or HF cannot be determined.
Clinical recommendations
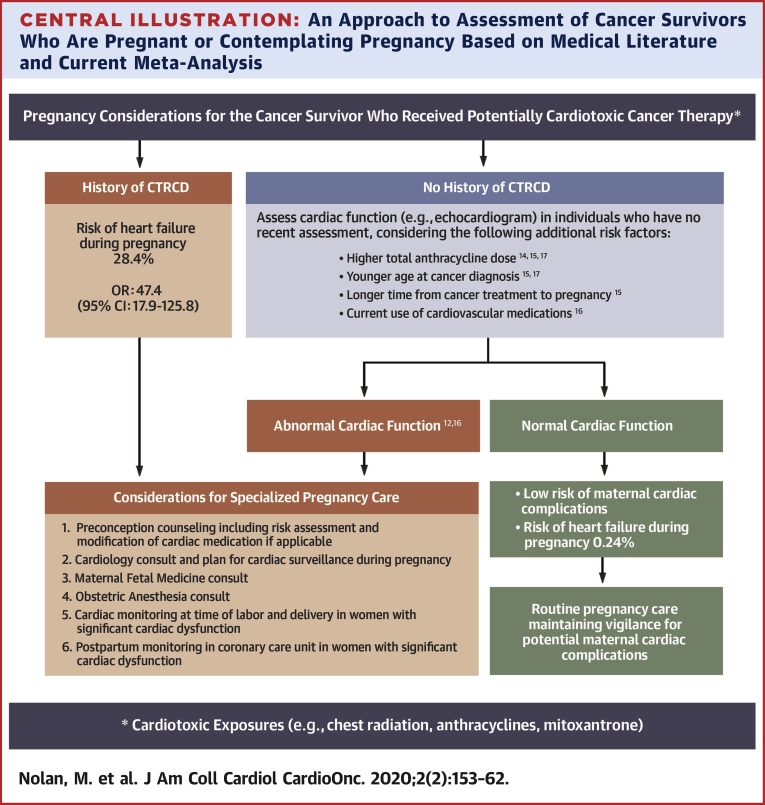
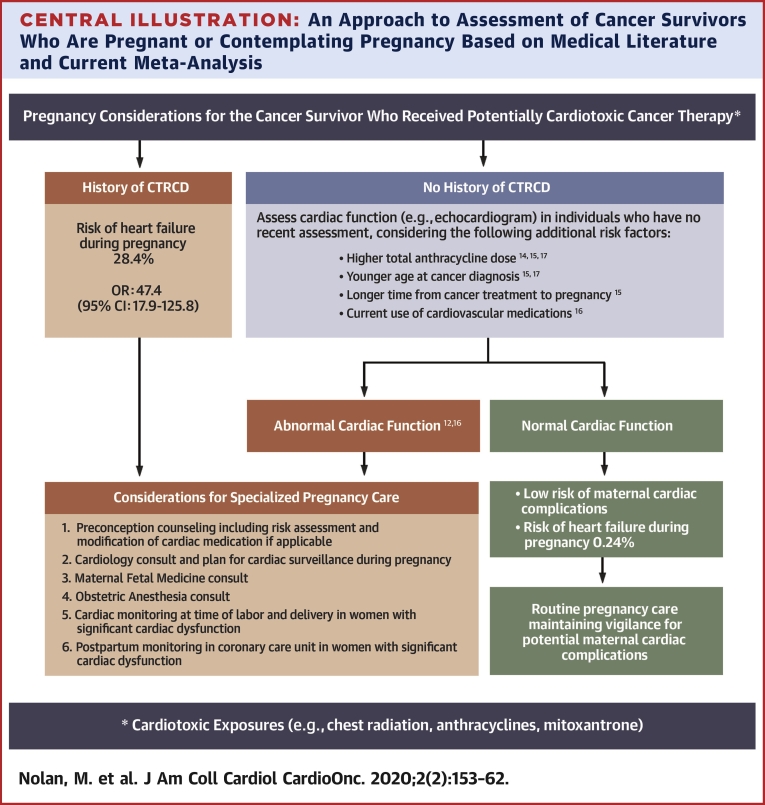
Current guidelines for cancer survivorship provide different recommendations, including consideration of cardiomyopathy surveillance before pregnancy or in the first trimester in those treated with anthracyclines or chest radiation without specific recommendations for ongoing surveillance if the LVEF is normal (25); cardiologist referral prior to pregnancy (26); or performing cardiac imaging in the third trimester in all cancer survivors (27). There appears to be a lack of consensus regarding patient selection, modality, and timeline of cardiac monitoring and use of cardioprotective strategies. The present meta-analysis provides important information for pre-conception counseling in female cancer survivors of reproductive age. Overall, the risk of developing LV dysfunction or HF is rare with pregnancy in women with prior exposure to cancer therapy but without a history of CTRCD. However, in women with a history of CTRCD, the risk of developing HF during or within 1 year of pregnancy is 28.4%. Therefore, although all women with prior potentially cardiotoxic cancer therapy could benefit from a baseline evaluation of cardiac function (25) and counseling pre-conception or early during pregnancy, the present authors believe those with a history of CTRCD should receive close cardiac surveillance during pregnancy at a center with expertise in cardiac disease in pregnancy (Central Illustration). Women without prior CTRCD may benefit from a baseline assessment of LV function, and in the absence of LV systolic dysfunction, these women can be reassured that they are at a low risk of developing LV dysfunction or HF during pregnancy with follow-up determined on an individual basis. Cardioprotection with β-blockers for patients with peri-partum cardiomyopathy going through subsequent pregnancies has demonstrated benefit in small observational studies (28) and could plausibly be used to manage high-risk cancer survivors during pregnancy. This will, however, have to be confirmed in prospective studies.
Central Illustration.
An Approach to Assessment of Cancer Survivors Who Are Pregnant or Contemplating Pregnancy Based on Medical Literature and Current Meta-Analysis
A threshold anthracycline dose to define high risk for pregnancy-related LV dysfunction or HF is unavailable, however, pediatric survivorship guidelines suggest that general screening for cardiomyopathy is reasonable beyond doxorubicin equivalent doses ≥100 mg/m2 (25). Thresholds to define younger age and longer time to pregnancy were not available, but the median value for younger age in those who developed LV dysfunction or HF in the studies that reported this as a risk factor were 8.1 years (15) and 14.5 years (17). Similarly, the median value for longer time to pregnancy in the study that reported this variable as a risk factor were 16.9 years (15). CTRCD = cancer-therapeutics related cardiac dysfunction; heart failure = asymptomatic left ventricular systolic dysfunction or clinical heart failure; OR = odds ratio.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The present systematic review and meta-analysis demonstrated that the pooled incidence of pregnancy-related cardiac dysfunction in cancer survivors was 1.7%. There was a 47.4-fold higher odds (95% CI: 17.9 to 125.8) of LV dysfunction or HF if there was a history of CTRCD. These findings indicate that patients going through pregnancy with a history of CTRCD represent a high-risk cohort that requires cardiac surveillance.
TRANSLATIONAL OUTLOOK: There is a need for large prospective studies to quantify the long-term risk of pregnancy-related cardiac dysfunction attributable to differences in cancer type, cancer treatment modalities, and clinical factors to facilitate individualized risk prediction. Furthermore, prospective studies to understand the role of cardiac medications (e.g., beta-blockers) to prevent LV dysfunction or HF during pregnancy in high risk cancer survivors are needed.
Acknowledgments
The authors acknowledge Michelle A.T. Hilderbrandt, PhD, and Lori Chait-Rubinek, MBBS, for providing additional data related to their publications.
Footnotes
Dr. Thavendiranathan is supported by the Canadian Institutes of Health Research New Investigator Award. Dr. Nolan was compensated as a speaker for Novartis. Dr. Hines had a relationship with Incyte. Dr. Amir presented expert testimony for Genentech and Roche; and is a consultant for Sandoz and Apobiologix. Dr. Maxwell is a consultant for Guidepoint Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Armstrong G.T., Liu Q., Yasui Y. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2328–2338. doi: 10.1200/JCO.2008.21.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M.H., Colan S.D., Diller L. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res. 2011;108:619–628. doi: 10.1161/CIRCRESAHA.110.224519. [DOI] [PubMed] [Google Scholar]
- 3.Lipshultz S.E., Adams M.J., Colan S.D. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927–1995. doi: 10.1161/CIR.0b013e3182a88099. [DOI] [PubMed] [Google Scholar]
- 4.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khanna A., Pequeno P., Gupta S. Increased risk of all cardiovascular disease subtypes among childhood cancer survivors: population-based matched cohort study. Circulation. 2019;140:1041–1043. doi: 10.1161/CIRCULATIONAHA.119.041403. [DOI] [PubMed] [Google Scholar]
- 6.Oeffinger K.C., Mertens A.C., Sklar C.A. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 7.Nolan M.T., Marwick T.H., Plana J.C. Effect of traditional heart failure risk factors on myocardial dysfunction in adult survivors of childhood cancer. J Am Coll Cardiol Img. 2018;11:1202–1203. doi: 10.1016/j.jcmg.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton G.J., Jauniaux E. Oxidative stress. Best Pract Res Clin Obstet Gynaecol. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edgar A.B., Wallace W.H. Pregnancy in women who had cancer in childhood. Eur J Cancer. 2007;43:1890–1894. doi: 10.1016/j.ejca.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Siu S.C., Colman J.M., Sorensen S. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation. 2002;105:2179–2184. doi: 10.1161/01.cir.0000015699.48605.08. [DOI] [PubMed] [Google Scholar]
- 11.Armstrong G.T., Joshi V.M., Ness K.K. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar J., Davidi O., Goshen Y., Hod M., Yaniv I., Hirsch R. Pregnancy outcome in women treated with doxorubicin for childhood cancer. Am J Obstet Gynecol. 2003;189:853–857. doi: 10.1067/s0002-9378(03)00837-8. [DOI] [PubMed] [Google Scholar]
- 13.van Dalen E.C., van der Pal H.J.H., van den Bos C., Kok W.E.M., Caron H.N., Kremer L.C.M. Clinical heart failure during pregnancy and delivery in a cohort of female childhood cancer survivors treated with anthracyclines. Eur J Cancer. 2006;42:2549–2553. doi: 10.1016/j.ejca.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Hines M.R., Mulrooney D.A., Hudson M.M. Pregnancy-associated cardiomyopathy in survivors of childhood cancer. J Cancer Surviv. 2016;10:113–121. doi: 10.1007/s11764-015-0457-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson K.A., Hildebrandt M.A.T., Ater J.L. Cardiac outcomes with pregnancy after cardiotoxic therapy for childhood cancer. J Am Coll Cardiol. 2017;69:594–595. doi: 10.1016/j.jacc.2016.11.040. [DOI] [PubMed] [Google Scholar]
- 16.Liu S., Aghel N., Belford L. Cardiac outcomes in pregnant women with treated cancer. J Am Coll Cardiol. 2018;72:2087–2089. doi: 10.1016/j.jacc.2018.07.085. [DOI] [PubMed] [Google Scholar]
- 17.Chait-Rubinek L., Mariani J.A., Goroncy N. A retrospective evaluation of risk of peripartum cardiac dysfunction in survivors of childhood, adolescent and young adult malignancies. Cancers. 2019;11:E1046. doi: 10.3390/cancers11081046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liberati A., Altman D.G., Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G.A., Shea B., O'Connell D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at:
- 20.Viechtbauer Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 21.Thompson K.A. Pregnancy and cardiomyopathy after anthracyclines in childhood. Front Cardiovasc Med. 2018;5:1–5. doi: 10.3389/fcvm.2018.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearson G.D., Veille J.C., Rahimtoola S. Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA. 2000;283:1183–1188. doi: 10.1001/jama.283.9.1183. [DOI] [PubMed] [Google Scholar]
- 23.Elkayam U., Tummala P.P., Rao K. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. N Engl J Med. 2001;344:1567–1571. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 24.Thavendiranathan P., Abdel-Qadir H., Fischer H.D. Risk-imaging mismatch in cardiac imaging practices for women receiving systemic therapy for early-stage breast cancer: a population-based cohort study. J Clin Oncol. 2018;36:2980–2987. doi: 10.1200/JCO.2018.77.9736. [DOI] [PubMed] [Google Scholar]
- 25.Armenian S.H., Hudson M.M., Mulder R.L. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carver J.R., Szalda D., Ky B. Asymptomatic cardiac toxicity in long–term cancer survivors: defining the population and recommendations for surveillance. Semin Oncol. 2013;40:229–238. doi: 10.1053/j.seminoncol.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sieswerda E., Postma A., van Dalen E.C. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23:2191–2198. doi: 10.1093/annonc/mdr595. [DOI] [PubMed] [Google Scholar]
- 28.Codsi E., Rose C.H., Blauwet L.A. Subsequent pregnancy outcomes in patients with peripartum cardiomyopathy. Obstet Gynecol. 2018;131:322–327. doi: 10.1097/AOG.0000000000002439. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.