Case 1
In 2015, a 31-year-old woman presented with cough and lymphadenopathy. On positron emission tomography-computed tomography (PET-CT), she was found to have cervical and mediastinal lymph node involvement and superior vena cava encasement. Open left neck lymph node biopsy confirmed the diagnosis of nodular sclerosing Hodgkin’s lymphoma, with immunohistochemistry positive for CD30 and CD15. She underwent transthoracic echocardiography (TTE), which revealed a large pericardial effusion with evidence of tamponade. She was admitted and underwent pericardiocentesis, with drainage of 500 ml of serosanginous fluid. Repeat TTE revealed a small pericardial effusion. The patient developed sinus pauses of up to 9 s in duration, in addition to episodes of Mobitz I second-degree atrioventricular block.
Cardiac magnetic resonance (CMR) imaging revealed a normal left ventricular ejection fraction (EF) of 67% and an irregularly contoured tissue prominence on the posterior aspect of left and right atria that measured 1.4 × 0.9 cm with perivascular thickening adjacent to the aorta and superior vena cava. Tissue characterization with contrast enhancement was consistent with neoplastic invasion. The patient continued to have frequent sinus pauses consistent with direct sinoatrial node or carotid sinus involvement. A temporary transvenous pacemaker (TVP) was placed. Cycle 1 of adriamycin, bleomycin, vinblastine, dicarbazine (ABVD) chemotherapy was initiated in two 14-day infusions. The TVP was removed due to reduced sinus pauses. However, a repeat CMR showed an increase in the size of the right atrial mass (1.8 × 1.0 cm), with a septal thickness of 1.1 cm (Figure 1). She also developed nonocclusive thrombi in both the proximal left internal jugular and right subclavian veins.
Figure 1.
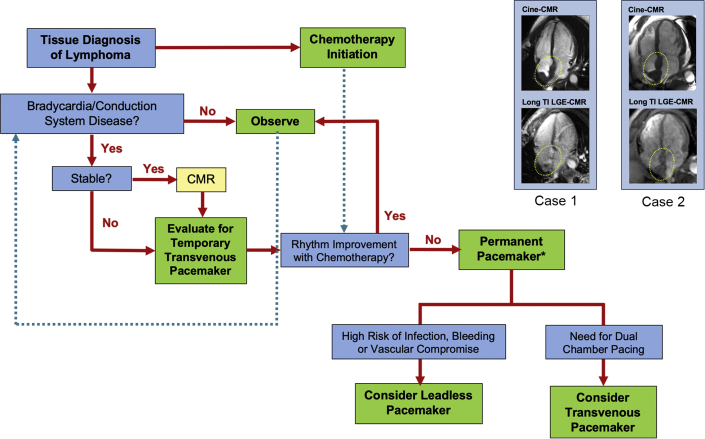
Summary of Managing Patients With Lymphoma
Summary of approach to managing patients with lymphoma at risk for bradycardia and cardiac conduction disease. Timing of permanent pacemaker implantation (asterisk) requires consideration of timing of chemotherapy and its impact on tumor burden and on blood cell counts. (Top) Representative cine cardiac magnetic resonance (CMR) imaging and (Bottom) late gadolinium enhancement (LGE) CMR images of Case 1 and Case 2 patients are also shown to the upper right. Cine-CMR was acquired at different phases of the cardiac cycle. LGE CMR was acquired using long inversion recovery pulse sequence for which inversion times (TI) were tailored to null thrombus (TI 600), whereas contrast enhancement was observed in tissue with vascularity. Neoplastic invasion of the interatrial septum (yellow circles) was present in both patients.
Despite receiving chemotherapy, the patient continued to have prolonged sinus pauses. After a discussion between the cardiology and oncology services, the decision was made to implant a permanent pacemaker. A compassionate use exemption for a leadless pacemaker being studied at our institution as part of a clinical trial was denied. Therefore, the patient underwent placement of a single-chamber right ventricular lead pacemaker (Biotronik Eluna, Portland, Oregon) due to presence of continued sinus node dysfunction and atrioventricular block. The patient completed 6 cycles of ABVD and has remained in full remission. She has had minimal pacing requirements and has been planned for device removal and lead extraction.
Case 2
In 2019, a 57-year-old man presented with abdominal pain and jaundice and was found to have biliary obstruction. He underwent CT imaging, which was notable for lung and mediastinal masses. Following an endoscopic retrograde cholangio-pancreatography to relieve the biliary obstruction, a duodenal biopsy was performed. Pathology revealed diffuse large B-cell lymphoma. At subsequent follow-up, he complained of dizziness, and an electrocardiogram (ECG) was notable for complete heart block with a junctional escape rhythm at a ventricular rate of 40 beats/min.
The patient was admitted, and he remained in a stable junctional rhythm for several days. CMR revealed an irregularly contoured intrapericardial mass involving the interatrial septum, pulmonary veins, aortic root, and membranous interventricular septum, measuring up to 1.9 cm in diameter (Figure 1). Post-contrast enhancement demonstrated tissue characterization consistent with neoplasm, with a preserved EF of 62%, and no evidence of myocardial scar or infarction. PET-CT showed fluorodeoxyglucose (FDG)−avid areas in the mediastinum that extended into the pericardium and right lung, and encased the left mainstem bronchus, left main pulmonary artery, proximal biliary duct, and proximal gastric wall. Before biopsy, a TVP was placed in anticipation of worsening conduction disease. Right lung core biopsy was performed and confirmed the diagnosis of germinal center-like diffuse large B-cell lymphoma. Immunohistochemistry was positive for BCL-2 and BCL-6 and negative for MYC, and FISH did not demonstrate translocations of BCL-2 and MYC.
Cycle 1 of ritixubimab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin (R-EPOCH) was completed. He remained in complete heart block. Because of the extent of the patient’s tumor burden, full recovery of conduction disease was believed to be unlikely to occur within a short time frame. The decision was made to implant a permanent pacemaker and the options of a dual-chamber pacemaker and leadless pacemaker were considered. To minimize the risk of infection and hematoma with anticipated pancytopenia following completion of chemotherapy, a leadless pacemaker (Medtronic Micra, Minneapolis, Minnesota) was implanted after cycle 1 of R-EPOCH. The patient has been stable and is continuing chemotherapy. Pacemaker interrogation 1-month post-implantation revealed 1% ventricular pacing burden.
Discussion
Although primary cardiac lymphoma is rare, lymphomas are one of the most common cancers with cardiac metastases (1,2). In an autopsy study of 150 patients with lymphoma, 13 (8.7%) had histological evidence of cardiac involvement, one-half of whom had abnormal ECGs but did not have specific conduction abnormalities (3). Despite the histological presence of cardiac involvement in a substantial percentage of patients with lymphoma, its presentation is often subclinical, and its prevalence is poorly understood. Other infiltrative masses can cause similar conduction disturbances, including neck masses such as thymoma, catecholamine-secreting tumors, cardiac metastasis, and amyloidosis (4). The overall prevalence of the association of cardiac conduction disease with these malignancies is unknown.
Generally, T-cell lymphomas invade the myocardium more frequently than B-cell lymphomas (3,5). There are 3 mechanisms for cardiac involvement by lymphoma: 1) extension of tumor from the mediastinum; 2) hematogeneous spread; and 3) retrograde flow via cardiac lymphatics. Direct extension is the most destructive, with the highest frequency of cardiac dysfunction (5).
Who is at risk?
It is important to maintain a high index of suspicion for the development of cardiac conduction disease if lymphoma rapidly spreads to different anatomic structures, especially in patients with previous conduction disease. It is essential to understand the anatomic locations of tumor involvement and its proximity to the primary cardiac conduction system or structures that mediate cardiac autonomic input.
How to diagnose?
The optimal tools for visualizing cardiac involvement in lymphoma include echocardiography, CMR, and PET-CT. Tissue characterization by CMR or PET provides added usefulness for identification of neoplastic involvement in the heart. Long inversion time late gadolinium enhancement CMR (LGE-CMR) identifies neoplasms based on tissue vascularity, whereas PET identifies neoplasms based on metabolic activity. Studies have shown that CMR tissue characterization can stratify prognosis among patients with cardiac neoplasms (6,7). PET imaging with FDG-avid uptake can allow early detection of cardiac involvement by neoplasm (8). However, tumors with low metabolic activity may be challenging to discern on PET.
The diagnostic sensitivity of FDG-PET for detection of cardiac neoplasms may be dependent upon the extent of tissue necrosis. In a study of 121 patients with cardiac neoplasms who underwent CMR and PET within a 3-month interval, PET sensitivity for tumor detection varied in relation to the extent of CMR-evidenced avascularity (9). PET detection of diffusely enhanced or mixed lesions was higher than for predominantly avascular neoplasms (87% vs. 63%). PET-FDG uptake decreased stepwise when neoplasms were stratified based on the extent of avascularity on LGE-CMR (p ≤ 0.001). Therefore, in cases with a high suspicion of cardiac neoplasm, CMR with tissue mass characterization will have a higher yield (10).
What is the treatment strategy?
The management of lymphoma-associated bradycardia and cardiac conduction disease requires close coordination between cardiology and oncology teams because decisions on both cardiac and cancer treatment should be made in parallel. On the basis of our clinical experience and expert consensus, we propose a flowchart for management of this clinical problem in Figure 1. In the acute presentation, if advanced atrioventricular block or sinus node dysfunction with symptoms or signs of hemodynamic instability are encountered, then temporary pacing is indicated. If the patient is stable, CMR imaging should be performed before placement of a TVP to determine the extent of cardiac involvement by the tumor. After placement of a TVP, there are 2 decision points: 1) timing of transition from a temporary to permanent pacemaker if necessary; and 2) implantation of a TVP versus a leadless pacemaker. Transition to a permanent pacemaker is recommended if there is a persistent need for pacing and if the extent of lymphoma burden and its impact on cardiac conduction are not expected to be resolved within several weeks. The decision to implant a TVP versus a leadless pacemaker should be based on weighing the need for dual-chamber pacing against the risks associated with device system infection, pocket hematoma, and lead-related vascular issues. Currently, there are no published reports comparing outcomes of infection, bleeding, and complication rates associated with TVPs versus leadless pacemakers in patients with cancer.
Proper timing of device implantation with respect to chemotherapy is critical because chemotherapy-induced pancytopenia can significantly increase the risks of infection and device-related hematoma formation. In addition, tumor involvement or thrombotic occlusion of venous structures may preclude the use of transvenous pacing. Overall, we recommend the timing and type of implantable pacemaker be decided collectively and within clinical context (9).
Is there a chance of recovery?
The extent of cardiac involvement by tumor invasion, the chance of complete cancer remission with treatment, and the presence of pre-existing conduction disease will determine the chances of cardiac recovery. Patients with extensive tumor burden or significant baseline conduction disease before cancer diagnosis are more likely to require long-term permanent pacing.
Conclusions
These cases highlight the need for recognition of bradycardia and cardiac conduction abnormalities caused by lymphoma infiltration, the usefulness of imaging (e.g., CMR and PET-CT), and the importance of a multidisciplinary and patient-centered shared decision-making approach to management.
Footnotes
Dr. Mahmood is supported by the New York Academy of Medicine’s Glorney-Raisbeck Fellowship Award; and has received consulting fees from Medicure, OMR Globus, Alpha Detail, and Opinion Research Team. Dr. Pastore has received consulting fees from Sanofi Genzyme, Dova, and Rigel; has been a member of the Medical Advisory Boards of Rigel, Dova, and BioVerativ; and has been a speaker for Sanofi Genzyme. Dr. Martine has been a consultant for Bayer, Celgene, Cellectar, Janssen, Kit, Morphosys, Regeneron, and Teneobio. Dr. Cheung has received consulting fees from Abbott and Biotronik; and has received fellowship grant support from Abbott, Biotronik, Boston Scientific, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Allen D.C., JA, Morton P., Mollan P.A., Morris T.C. Pathology of the heart and conduction system in lymphoma and leukemia. J Clin Pathol. 1987;40:746–750. doi: 10.1136/jcp.40.7.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palaskas N.T.K., Gladish G., Agha A.M. Evaluation and management of cardiac tumors. Curr Treat Options Cardiovasc Med. 2018;20:29. doi: 10.1007/s11936-018-0625-z. [DOI] [PubMed] [Google Scholar]
- 3.McDonnell P.J.M.R., Bulkley B.H. Involvement of the heart by malignant lymphoma: a clinicopathologic study. Cancer. 1982;49:944–951. doi: 10.1002/1097-0142(19820301)49:5<944::aid-cncr2820490519>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 4.Chang H.M., Okwuosa T.M., Scarabelli T., Moudgil R., Yeh E.T.H. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: part 2. J Am Coll Cardiol. 2017;70:2552–2565. doi: 10.1016/j.jacc.2017.09.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinen K., Izumo T. Cardiac involvement by malignant lymphoma: a clinicopathologic study of 25 autopsy cases based on the WHO classification. Ann Hematol. 2005;84:498–505. doi: 10.1007/s00277-005-1009-5. [DOI] [PubMed] [Google Scholar]
- 6.Chan A.T., Plodkowski A.J., Pun S.C. Prognostic utility of differential tissue characterization of cardiac neoplasm and thrombus via late gadolinium enhancement cardiovascular magnetic resonance among patients with advanced systemic cancer. J Cardiovasc Magn Reson. 2017;19:76. doi: 10.1186/s12968-017-0390-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pun S.C., Plodkowski A., Matasar M.J. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cui J., Ren Z., Wang X. Evaluation of 18F-FDG PET/CT in the diagnosis of cardiac lymphoma. J Nucl Med. 2018;59:S1574. [Google Scholar]
- 9.Nagano M., Uike N., Suzumiya J. Successful treatment of a patient with cardiac lymphoma who presented with a complete atrioventricular block. Am J Hematol. 1998;59:171–174. doi: 10.1002/(sici)1096-8652(199810)59:2<171::aid-ajh12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 10.Chan A.T., Fox J., Perez Johnston R. Late gadolinium enhancement cardiac magnetic resonance tissue characterization for cancer-associated cardiac masses: metabolic and prognostic manifestations in relation to whole-body positron emission tomography. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.011709. [DOI] [PMC free article] [PubMed] [Google Scholar]