Abstract
Background
The relation between cancer and arterial thromboembolism (ATE) remains unclear.
Objectives
The purpose of this study was to evaluate ATE risk in cancer patients.
Methods
Danish registries were used to identify all cancer patients between 1997 and 2017, each matched to three cancer-free comparator individuals. ATE was defined as the composite of myocardial infarction, ischemic/unspecified stroke, and peripheral arterial occlusion. A competing risk approach was used to compute cumulative incidences and subdistribution hazard ratios (SHRs). Cause-specific hazard ratios (HRs) were calculated using Cox regression. Among cancer patients, mortality risk was estimated in Cox regression analysis by treating ATE as a time-varying exposure. Patients were followed for 12 months.
Results
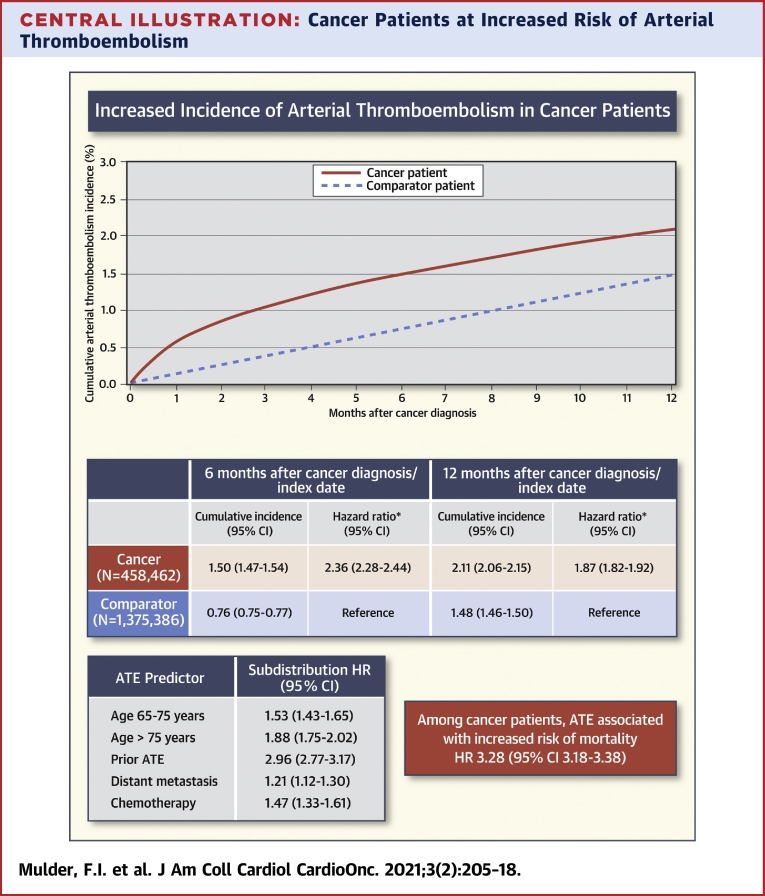
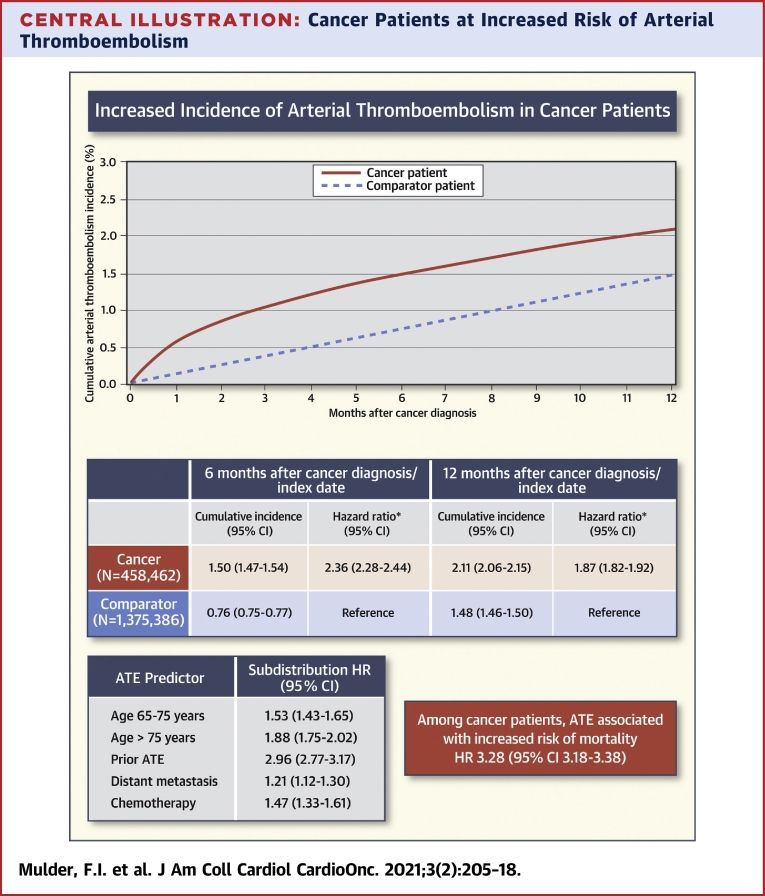
The study included 458,462 cancer patients and 1,375,386 comparator individuals. In the 6-month period following cancer diagnosis/index date, the cumulative incidence for ATE was 1.50% (95% confidence interval [CI]: 1.47% to 1.54%) in cancer patients and 0.76% (95% CI: 0.75% to 0.77%) in comparator individuals (HR: 2.36; 95% CI: 2.28 to 2.44). Among cancer patients age <65 years, 65 to 75 years, and >75 years, this was 0.79% (95% CI: 0.74% to 0.83%), 1.61% (95% CI: 1.55% to 1.67%), and 2.30% (95% CI: 2.22% to 2.38%), respectively. Other predictors for ATE among cancer patients were prior ATE (SHR: 2.96; 95% CI: 2.77 to 3.17), distant metastasis (adjusted SHR: 1.21; 95% CI: 1.12 to 1.30), and chemotherapy (SHR: 1.47; 95% CI: 1.33 to 1.61). Among cancer patients, ATE was associated with an increased risk of mortality (HR: 3.28; 95% CI: 3.18 to 3.38).
Conclusions
Cancer patients are at increased risk of ATE. Clinicians should be aware of this risk, which is associated with mortality.
Key Words: arterial occlusion, arterial thromboembolism, cancer, cohort study, ischemic stroke, myocardial infarction, neoplasm
Abbreviations and Acronyms: CI, confidence interval; HR, hazard ratio; SHR, subdistribution hazard ratio
Central Illustration
The relation between cancer and venous thromboembolism, due to the prothrombotic state induced by cancer and systemic cancer therapies, is well established (1). However, it is less clear whether cancer also increases the risk of arterial thromboembolism. Emerging data based on cohort studies suggest that risks of myocardial infarction, ischemic stroke, and peripheral arterial occlusion are higher in cancer patients than in the general population (2, 3, 4, 5, 6, 7, 8, 9). However, these data were often restricted due to specific cancer types, not reporting on all types of arterial thromboembolism, providing only relative risks, or having a limited sample size.
Large population-based databases permit evaluation of the relation between cancer and arterial thromboembolism with high precision in subgroups and for various subtypes of this outcome. For example, a study using Medicare health care data demonstrated that the risk of cancer-associated arterial thromboembolism was 4.7% in the 6 months following cancer diagnosis (10). However, Medicare data only include insured patients older than 65 years, and may therefore not be generalizable to all cancer patients (10).
A better understanding of the risk of arterial thromboembolism in cancer patients is needed to increase awareness among clinicians and to advance the development of prediction models and preventive measures. Danish population-based national health registries are well known for their completeness and for the validity of clinical outcomes, including cardiovascular diseases. The positive predictive value of the diagnosis codes for myocardial infarction, ischemic stroke, and peripheral arterial occlusion are 97%, 88%, and 91%, respectively (11,12). Therefore, they represent a unique resource for evaluating the association between cancer and arterial thromboembolism. We aimed to examine the absolute and relative risks of arterial thromboembolism in cancer patients compared to the general population. Additionally, we evaluated several predictors for arterial thromboembolism in cancer patients.
Methods
Study design and use of Danish registries
We studied a cohort of cancer patients and matched comparator individuals from the general population using data from Danish population-based health registries, which contain high-quality health care data that can be linked using a unique identifier assigned to each Danish resident (13). Clinical data for this study were obtained from the DNPR (Danish National Patient Registry) (14), cancer-specific data from the DCR (Danish Cancer Registry) (15), and data on medication use from the Danish National Prescription Registry (16). All codes for disease diagnoses and medication use that were used in this study are provided in Supplemental Table 1. This study was approved by the Danish Data Protection Agency (record number 2016-051-000001), which does not require informed consent from subjects when they are not contacted or assigned to an intervention.
Cancer and general population comparator cohorts
Danish residents age 18 years or older with a first-time diagnosis of cancer between 1997 and 2017 were included in the cancer cohort. Patients with all cancer types were eligible, except for those with skin cancer. Information on cancer stage and treatment during the first 4 months following cancer diagnosis was obtained from the DCR, which defines cancer stage as localized, regional, or distant (15). For each cancer patient, three comparator individuals who were alive and free of cancer at the time of the matched person’s cancer diagnosis (defined as the index date) were randomly selected with replacement (17) from the general population by means of the Civil Registration System, which tracks the vital status of all Danish residents (13). Comparator individuals were matched to cancer patients on year of birth, sex, and date of the cancer diagnosis/index date.
Follow-up
Cancer patients and members of the comparator cohort were followed from the date of cancer diagnosis/index date until a first diagnosis of arterial thromboembolism, death, emigration, loss to follow-up, or end of study follow-up (December 2017), whichever occurred first. Follow-up of members of the comparator cohort was stopped in the event of a cancer diagnosis, after which the affected individual was censored in the comparator cohort and shifted to the cancer cohort. The maximum follow-up duration was 12 months.
Study outcomes
The primary outcome was a primary or secondary inpatient diagnosis of arterial thromboembolism, defined as the composite of myocardial infarction, ischemic stroke, unspecified stroke, and peripheral arterial occlusion. Unspecified stroke was included in the primary outcome, because the majority of strokes in the DNPR are classified as unspecified and more than two-thirds of these events are ischemic in nature (18). Secondary study outcomes were myocardial infarction, the combination of ischemic and unspecified stroke, and additionally, all-cause mortality in the cancer cohort.
Outcomes were evaluated at 6 months after cancer diagnosis/index date in the main analysis because the risk was assumed to be highest during this period. In a secondary analysis, outcomes were also evaluated at 12 months. To evaluate the period prevalence of arterial thromboembolism prior to the diagnosis of cancer, an additional analysis was performed in which patients were followed from 6 months preceding the cancer diagnosis/index date up until the cancer diagnosis/index date. Fatal arterial thromboembolic events in this period were thus not included in this analysis by definition. The association between arterial thromboembolism and all-cause mortality was evaluated during the period between cancer diagnosis and 12-month follow-up.
Confounding factors
The following variables were identified from the DNPR from 1977 onward because they were considered potential confounders: prior arterial thromboembolism, atrial fibrillation or flutter, venous thromboembolism, heart failure, atherosclerosis and peripheral vascular disease, human immune deficiency virus, inflammatory bowel disease, chronic obstructive pulmonary disease, liver disease, renal disease, diabetes, alcoholism and alcoholism-related conditions, obesity, rheumatoid arthritis, and hypertension.
Statistical analyses
Cumulative incidences among cancer patients and members of the comparator cohort were calculated with 95% confidence intervals (CIs) in a competing risk analysis, in which death was regarded as a competing event for arterial thromboembolism (19). Cause-specific hazard ratios (HRs) for arterial thromboembolism were calculated in a Cox proportional hazards regression analysis to compare cancer patients with comparator individuals. These HRs were adjusted for matching variables by study design and were adjusted for the potential confounders listed in the previous text. Visual inspection of log-log plots indicated no violation of the assumption of proportionality. Additionally, incidence rates were calculated per 1,000 person-years of follow-up. The robust Poisson regression model was used to estimate the period prevalence of arterial thromboembolism in the 6 months prior cancer/index date, and the adjusted prevalence ratios between the cohorts (20).
Study results were reported separately for individuals age younger than 65 years, 65 to 75 years, and older than 75 years. Because myocardial infarction and ischemic stroke are almost never treated in the outpatient setting, and to reduce misclassification, only in-hospital diagnoses were used in these analyses. An additional analysis was performed in which both inpatient and outpatient clinic diagnoses were included. Another subgroup analysis was performed for both cohorts excluding cancer patients and comparator individuals receiving anticoagulant or antiplatelet therapy at cancer diagnosis/index date.
Within the cancer cohort, predictors for arterial thromboembolism were evaluated by calculating crude and adjusted subdistribution hazard ratios (SHRs) using the Fine & Gray subdistribution hazard model (19), which focuses on prediction rather than causation, and takes the competing risk of death into account.
In the cancer cohort, the association between arterial thromboembolism and subsequent mortality was evaluated by calculating the HR using Cox proportional hazards regression analysis, in which arterial thromboembolism was treated as a time-varying exposure. The analysis was adjusted for age, sex, calendar year, cancer type, and the potentially confounding factors listed in the previous text. Statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
The cancer cohort comprised 458,462 patients with a first-time cancer diagnosis between 1997 and 2017. The median age was 69 years (25th to 75th percentiles: 60 to 77 years) at cancer diagnosis, and 51% were women. Among cancer patients with solid cancer, 143,282 (34%) had localized cancer, 112,837 (27%) had regional cancer, and 91,202 (22%) had distant metastasis; for 68,675 (17%), the cancer stage was unknown. The comparator cohort comprised 1,375,386 persons from the general population with similar baseline characteristics, except for a slightly lower prevalence of comorbid conditions (Table 1).
Table 1.
Baseline Characteristics of the Cancer and Comparator Cohorts
| Cancer Cohort (n = 458,462) | Comparator Cohort (n = 1,375,386) | |
|---|---|---|
| Female | 234,915 (51.2) | 704,745 (51.2) |
| Age, yrs | 69 (60–77) | 69 (60–77) |
| Age group, yrs | ||
| <65 | 168,807 (36.8) | 506,421 (36.8) |
| 65–75 | 159,538 (34.8) | 478,614 (34.8) |
| >75 | 130,117 (28.4) | 390,351 (28.4) |
| Cancer stage at diagnosis∗ | ||
| Localized | 143,282 (34.4) | — |
| Regional | 112,837 (27.1) | — |
| Distant | 91,202 (21.9) | — |
| Missing | 68,675 (16.5) | — |
| Comorbidities | ||
| Atrial fibrillation or flutter | 31,311 (6.8) | 81,237(5.9) |
| Heart failure | 20,132 (4.4) | 51,689 (3.8) |
| Atherosclerosis and peripheral vascular disease | 11,243 (2.5) | 23,525 (1.7) |
| COPD | 43,608 (9.5) | 94,765 (6.9) |
| IBD | 9,595 (2.1) | 24,066 (1.7) |
| Liver disease | 8,887 (1.9) | 15,914 (1.2) |
| Chronic kidney disease | 8,548 (1.9) | 19,567 (1.4) |
| Diabetes | 31,821 (6.9) | 79,112 (5.8) |
| Obesity | 16,776 (3.7) | 42,145 (3.1) |
| Alcoholism and alcoholism-related conditions | 20,307 (4.4) | 44,525 (3.2) |
| Hypertension | 75,204 (16.4) | 197,871 (14.4) |
| Rheumatoid arthritis | 6,816 (1.5) | 18,356 (1.3) |
| HIV | 327 (0.1) | 606 (0.0) |
| Previous ATE | 47,712 (10.4) | 128,893 (9.4) |
| Previous VTE | 13,227 (2.9) | 30,971 (2.3) |
| Antiplatelet therapy | 96,759 (21.1) | 270,468 (19.7) |
| Anticoagulant therapy | 24,679 (5.4) | 60,692 (4.4) |
| Lipid-lowering therapy | 85,940 (18.7) | 249,009 (18.1) |
Values are n (%) or median (25th to 75th percentiles).
ATE = arterial thromboembolism; COPD = chronic obstructive pulmonary disease; HIV = human immune deficiency virus; IBD = inflammatory bowel disease; VTE = venous thromboembolism.
For solid cancers and lymphoma.
Arterial thromboembolism
In the 6 months prior to the cancer diagnosis/index date, the period prevalence of arterial thromboembolism was 1.52% (95% CI: 1.48% to 1.55%) in the cancer cohort and 0.62% (95% CI: 0.61% to 0.63%) in the comparator cohort (prevalence ratio 2.40; 95% CI: 2.32 to 2.48) (Table 2).
Table 2.
Incidence of Arterial Thromboembolism During Follow-Up Intervals, Overall and by Cancer Type
| n | 6 Months Prior Cancer Diagnosis/Index Date |
6 Months After Cancer Diagnosis/Index Date |
12 Months After Cancer Diagnosis/Index Date |
||||
|---|---|---|---|---|---|---|---|
| Period Prevalence (95% CI) | Prevalence Ratio∗ (95% CI) | Cumulative Incidence (95% CI) | Hazard Ratio∗ (95% CI) | Cumulative Incidence (95% CI) | Hazard Ratio∗ (95% CI) | ||
| Matched comparator cohort | 1,375,386 | 0.62 (0.61–0.63) | Reference | 0.76 (0.75–0.77) | Reference | 1.48 (1.46–1.50) | Reference |
| Cancer cohort | 458,462 | 1.52 (1.48–1.55) | 2.40 (2.32–2.48) | 1.50 (1.47–1.54) | 2.36 (2.28–2.44) | 2.11 (2.06–2.15) | 1.87 (1.82–1.92) |
| Cancer types | |||||||
| Bladder | 16,051 | 1.60 (1.41–1.81) | 1.68 (1.44–1.97) | 2.49 (2.25–2.74) | 2.61 (2.27–3.02) | 3.49 (3.21–3.79) | 2.14 (1.90–2.40) |
| Lung cancer | 75,084 | 2.33 (2.22–2.44) | 3.39 (3.15–3.65) | 2.08 (1.98–2.18) | 3.76 (3.46–4.08) | 2.70 (2.59–2.82) | 3.10 (2.89–3.32) |
| Colon | 51,436 | 1.95 (1.84–2.08) | 2.62 (2.40–2.85) | 2.08 (1.96–2.21) | 2.64 (2.41–2.90) | 2.66 (2.52–2.80) | 1.97 (1.83–2.12) |
| Rectal | 26,191 | 1.40 (1.26–1.55) | 2.13 (1.86–2.44) | 2.07 (1.90–2.25) | 3.15 (2.76–3.58) | 2.81 (2.61–3.02) | 2.31 (2.08–2.56) |
| Pancreatic | 16,044 | 1.99 (1.78–2.22) | 2.65 (2.27–3.09) | 1.94 (1.74–2.17) | 4.78 (3.93–5.83) | 2.46 (2.23–2.71) | 4.14 (3.51–4.89) |
| Esophageal | 7,956 | 1.32 (1.09–1.59) | 1.83 (1.41–2.36) | 1.92 (1.63–2.24) | 2.83 (2.21–3.62) | 2.34 (2.02–2.69) | 2.18 (1.77–2.68) |
| Stomach | 10,296 | 1.97 (1.71–2.26) | 2.64 (2.18–3.20) | 1.79 (1.55–2.06) | 2.35 (1.90–2.91) | 2.35 (2.07–2.66) | 2.08 (1.74–2.49) |
| Brain | 8,500 | 5.97 (5.48–6.50) | 13.31 (10.89–16.27) | 1.74 (1.48–2.04) | 4.56 (3.44–6.05) | 2.18 (1.88–2.51) | 3.87 (3.05–4.89) |
| Hematological† | 42,466 | 1.51 (1.40–1.64) | 2.48 (2.23–2.75) | 1.42 (1.31–1.53) | 2.32 (2.07–2.60) | 2.02 (1.89–2.16) | 1.79 (1.63–1.96) |
| Renal | 12,333 | 1.99 (1.75–2.25) | 3.28 (2.70–3.98) | 1.35 (1.16–1.57) | 2.29 (1.82–2.88) | 2.09 (1.85–2.36) | 1.93 (1.62–2.31) |
| Liver | 6,103 | 1.80 (1.49–2.16) | 2.81 (2.11–3.75) | 1.31 (1.05–1.62) | 2.64 (1.75–3.98) | 1.59 (1.30–1.93) | 2.41 (1.71–3.39) |
| Prostate | 68,334 | 1.09 (1.01–1.17) | 1.32 (1.21–1.43) | 1.26 (1.18–1.35) | 1.30 (1.19–1.41) | 2.14 (2.03–2.25) | 1.16 (1.09–1.23) |
| Gynecological‡ | 31,922 | 0.96 (0.85–1.07) | 2.50 (2.14–2.91) | 0.97 (0.86–1.08) | 2.44 (2.06–2.89) | 1.42 (1.29–1.55) | 1.93 (1.69–2.20) |
| Breast | 85,746 | 0.47 (0.43–0.52) | 1.38 (1.22–1.55) | 0.58 (0.54–0.64) | 1.37 (1.22–1.54) | 0.97 (0.90–1.03) | 1.14 (1.05–1.24) |
CI = confidence interval.
Matching factors controlled for by study design and adjusted for prior arterial thromboembolism, venous thromboembolism, atrial fibrillation or flutter, heart failure, atherosclerosis and peripheral vascular disease, chronic obstructive pulmonary disease, inflammatory bowel disease, liver disease, renal disease, diabetes, obesity, alcoholism and alcoholism-related conditions, rheumatoid arthritis, human immune deficiency virus, and hypertension.
Hematological malignancies included multiple myeloma, Hodgkin lymphoma, non-Hodgkin lymphoma, and leukemia.
Gynecological cancers included ovarian, uterine, and endometrial cancer.
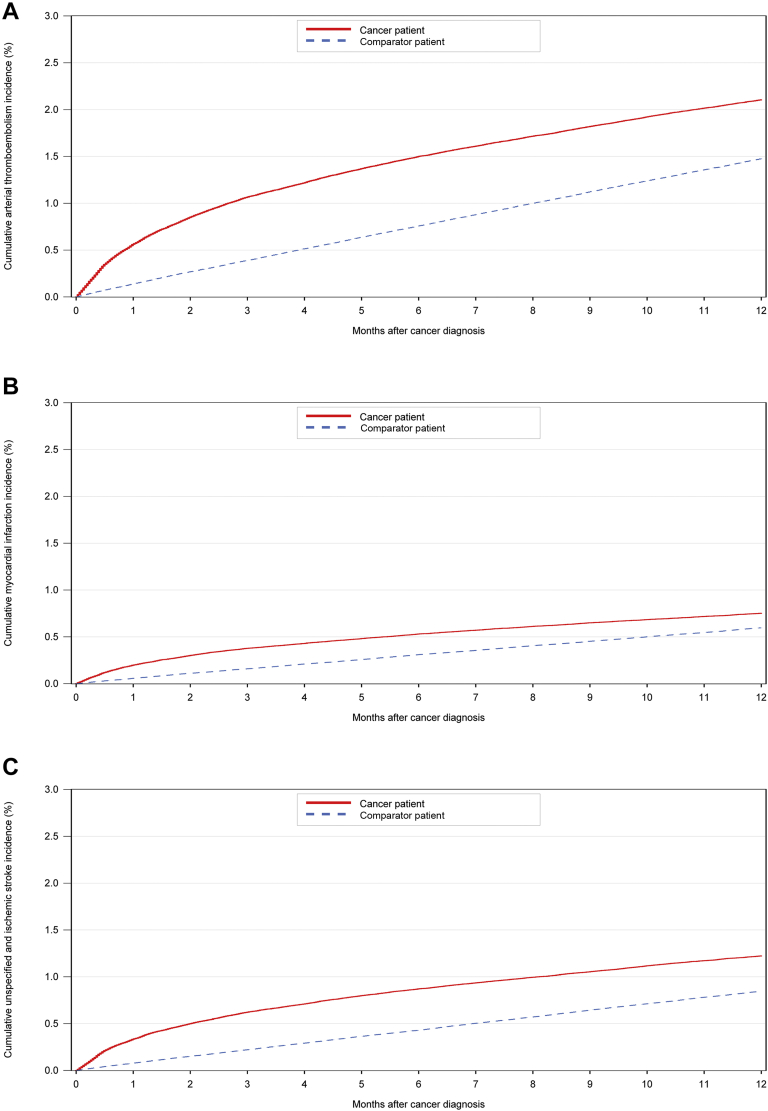
During the 6-month period after the cancer diagnosis/index date, the cumulative incidence of arterial thromboembolism was 1.50% (95% CI: 1.47% to 1.54%) in the cancer cohort and 0.76% (95% CI: 0.75% to 0.77%) in the comparator cohort (HR: 2.36; 95% CI: 2.28 to 2.44). During the 12-month study period after the cancer diagnosis/index date, the cumulative incidence was 2.11% (95% CI: 2.06% to 2.15%) in the cancer cohort and 1.48% (95% CI: 1.46% to 1.50%) in the matched comparator cohort (HR: 1.87; 95% CI: 1.82 to 1.92) (Figure 1). The risk of arterial thromboembolism in the cancer cohort was slightly lower when patients receiving anticoagulant/antiplatelet therapy at cancer diagnosis were excluded (6-month cumulative incidence 1.06% [95% CI: 1.03% to 1.10%]) (Supplemental Table 2). In contrast, the analysis in which both inpatient and outpatient diagnoses of the primary outcome were used yielded a somewhat higher incidence among the cancer patients (6-month cumulative incidence 1.68% [95% CI: 1.65% to 1.72%]) (Supplemental Table 3). The increased risk of arterial thromboembolism in the cancer cohort diminished during 24 months. The cumulative incidence of arterial thromboembolism in the 24 months after cancer diagnosis/index date is shown in Supplemental Figure 1.
Figure 1.
12-Month Cumulative Incidence Curves for ATE, Myocardial Infarction, and Ischemic Stroke
Cumulative arterial thromboembolism (ATE) (A), myocardial infarction (B), and ischemic stroke (C) incidence for patients with cancer and for the matched comparator cohort in the 12 months after cancer diagnosis/index date. The cumulative incidence was calculated with a competing risk approach.
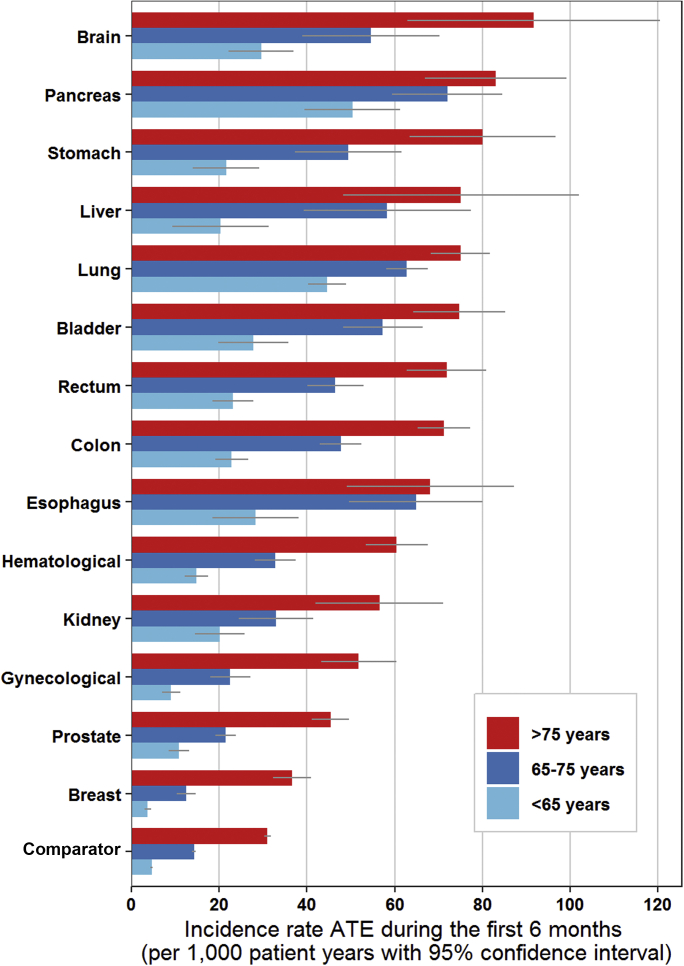
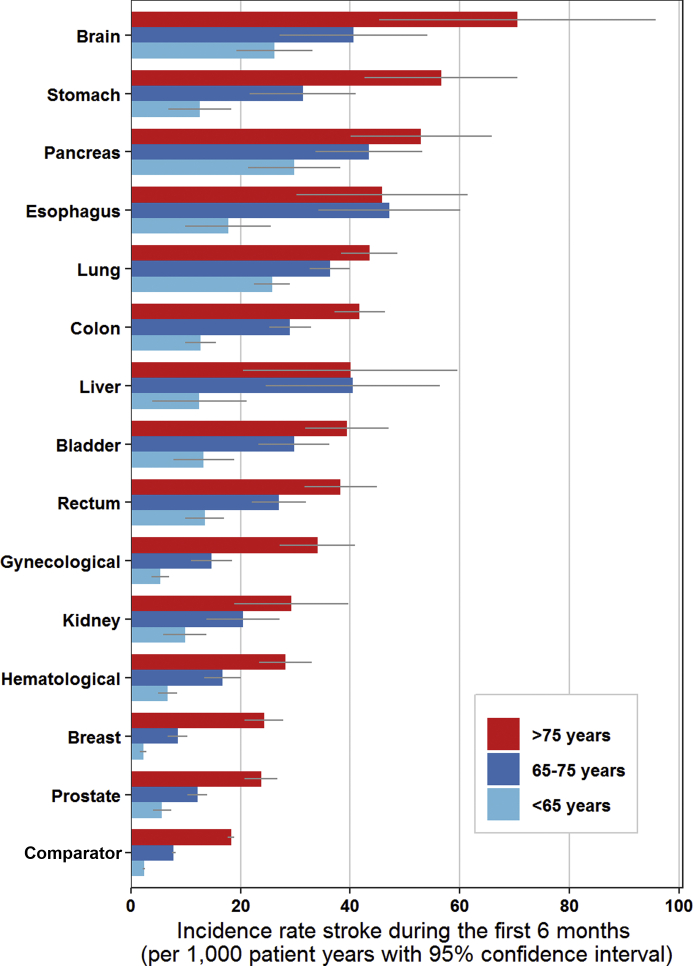
The risk varied substantially across cancer types and age groups. The cumulative incidence of arterial thromboembolism in the 6 months following a cancer diagnosis was highest in patients with bladder cancer (2.49%; 95% CI: 2.25% to 2.74%), lung cancer (2.08%; 95% CI: 1.98% to 2.18%), and colon cancer (2.08%; 95% CI: 1.96% to 2.21%), and was lowest in those with breast cancer (0.58%; 95% CI: 0.54% to 0.64%). In the group younger than age 65 years, the 6-month cumulative incidence of arterial thromboembolism was 0.79% (95% CI: 0.74% to 0.83%) in the cancer cohort and 0.23% (95% CI: 0.22% to 0.24%) in the comparator cohort (HR: 3.61; 95% CI: 3.31 to 3.95). In the group age 65 to 75 years, this was 1.61% (95% CI: 1.55% to 1.67%) in the cancer cohort and 0.71% (95% CI: 0.68% to 0.73%) in the comparator cohort (HR: 2.56; 95% CI: 2.42 to 2.71). In the group older than age 75 years, the 6-month cumulative incidence of arterial thromboembolism was 2.30% (95% CI: 2.22% to 2.38%) in the cancer cohort and 1.52% (95% CI: 1.48% to 1.55%) in the comparator cohort (HR: 1.93; 95% CI: 1.84 to 2.03). Study outcomes for the three age groups are reported separately in Supplemental Tables 4, 5, and 6. Figure 2 graphically depicts the incidence rate of arterial thromboembolism per 1,000 person-years in the three age groups during the first six months after cancer diagnosis, for each cancer type separately.
Figure 2.
Incidence Rate of ATE for Each Cancer Type During the First 6 Months After Cancer Diagnosis for Patients <65, 65 to 75, and >75 Years
The incidence rate was calculated as number of events per 1,000 person years. The gray bar depicts the 95% confidence interval. ATE = arterial thromboembolism.
The risk of arterial thromboembolism decreased over calendar time in the comparator cohort, but not in the cancer cohort. The 12-month cumulative incidence of arterial thromboembolism was 1.70 (95% CI: 1.59 to 1.82) in 1997 and 0.96 (95% CI: 0.84 to 1.09) in 2017 in the comparator cohort. In the cancer cohort, it was 1.72 (95% CI: 1.54 to 1.93) in 1997 and 1.76 (95% CI: 1.44 to 2.14) in 2017 (Supplemental Figure 2).
In the analysis among cancer patients, in which arterial thromboembolism was treated as a time-varying exposure, there was a significant association with mortality (time-varying HR: 3.28; 95% CI: 3.18 to 3.38).
Myocardial infarction
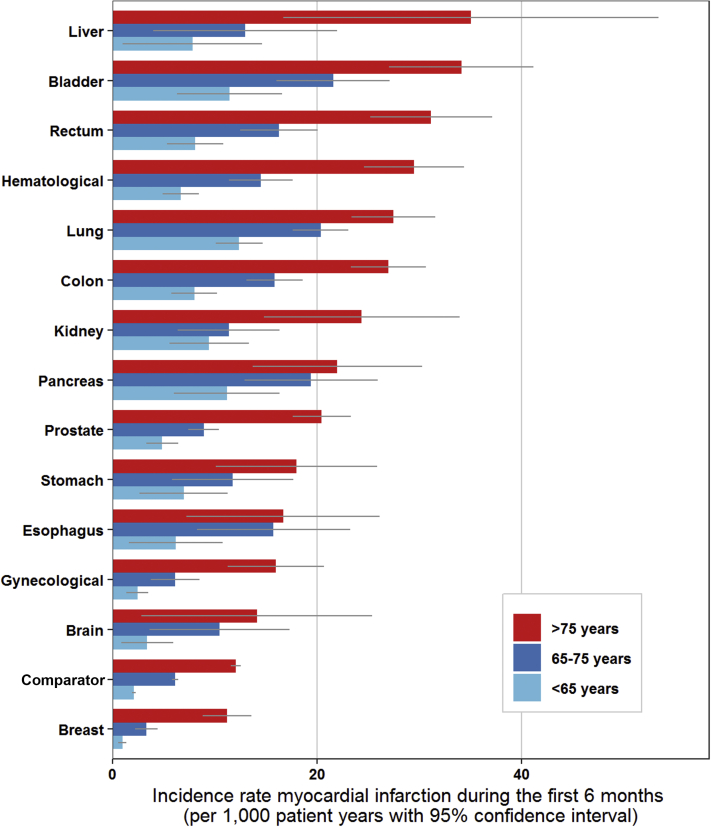
The period prevalence of myocardial infarction in the 6 months preceding the cancer diagnosis/index date was 0.53% (95% CI: 0.51% to 0.55%) in the cancer cohort and 0.24% (95% CI: 0.23% to 0.25%) in the comparator cohort (prevalence ratio 2.13; 95% CI: 2.02 to 2.25). Similarly, the cumulative incidence of myocardial infarction in the 6-month period after the cancer diagnosis/index date was 0.53% (95% CI: 0.51% to 0.55%) in the cancer cohort and 0.31% (95% CI: 0.30% to 0.32%) in the comparator cohort (HR: 2.08; 95% CI: 1.96 to 2.19). The 12-month cumulative incidence was 0.75% (95% CI: 0.73% to 0.78%) in the cancer cohort and 0.60% (95% CI: 0.58% to 0.61%) in the comparator cohort (HR: 1.66; 95% CI: 1.59 to 1.73) (Figure 1, Supplemental Table 7). Figure 3 shows the incidence rate of myocardial infarction in the first 6 months after cancer diagnosis, stratified by age group.
Figure 3.
Incidence Rate of Myocardial Infarction for Each Cancer Type During the First 6 Months After Cancer Diagnosis for Patients <65, 65 to 75, and >75 Years
The incidence rate was calculated as number of events per 1,000 person years. The gray bar depicts the 95% confidence interval.
Stroke
The period prevalence of the combination of ischemic and unspecified stroke was 0.93% (95% CI: 0.91% to 0.96%) for members of the cancer cohort in the 6 months preceding cancer diagnosis and 0.36% (95% CI: 0.35% to 0.37%) for members of the comparator cohort in the 6 months preceding the index date (prevalence ratio 2.51; 95% CI: 2.41 to 2.62). In the 6 months after the cancer diagnosis/index date, the cumulative incidence was 0.87% (95% CI: 0.85% to 0.90%) in the cancer cohort and 0.43% (95% CI: 0.42% to 0.44%) in the comparator cohort (HR: 2.39; 95% CI: 2.28 to 2.50). The 12-month cumulative incidence was 1.22% (95% CI: 1.19% to 1.26%) in the cancer cohort and 0.85% (95% CI: 0.83% to 0.86%) in the comparator cohort (HR: 1.89; 95% CI: 1.82 to 1.96) (Figure 1, Supplemental Table 8). Figure 4 shows the incidence rate of stroke in the first 6 months after cancer diagnosis, stratified by age group.
Figure 4.
Incidence Rate of Stroke for Each Cancer Type During the First 6 Months After Cancer Diagnosis for Patients <65, 65 to 75, and >75 Years
The incidence rate was calculated as number of events per 1,000 person years. The gray bar depicts the 95% confidence interval.
Just as for stroke and myocardial infarction, the risk of peripheral arterial occlusions was increased in cancer patients. The risk of peripheral arterial occlusions in the cancer and comparator cohorts are shown in Supplemental Table 9.
Predictors for arterial thromboembolism
In the cancer cohort, age was a predictor for arterial thromboembolism during the first 6 months after cancer diagnosis. Compared with patients younger than 65 years, the risk was higher in patients age 65 to 75 years (adjusted SHR: 1.53; 95% CI: 1.43 to 1.65) and in those older than 75 years (adjusted SHR: 1.88; 95% CI: 1.75 to 2.02). Other predictors were male sex (adjusted SHR: 1.15; 95% CI: 1.08 to 1.22), prior arterial thromboembolism (adjusted SHR: 2.96; 95% CI: 2.77 to 3.17), hypertension (adjusted SHR: 1.29; 95% CI: 1.21 to 1.37), and diabetes mellitus (adjusted SHR: 1.20; 95% CI: 1.10 to 1.29). Compared with patients with localized cancer, the risk was higher in patients with regional cancer (adjusted SHR: 1.16; 95% CI: 1.08 to 1.25) and in those with distant cancer (adjusted SHR: 1.21; 95% CI: 1.12 to 1.30). Patients who received chemotherapy (adjusted SHR: 1.47; 95% CI: 1.33 to 1.61) and surgery (adjusted SHR: 1.16; 95% CI: 1.09 to 1.24) during the first 4 months of follow-up had a higher risk compared with patients receiving no treatment. All predictors are presented in Table 3. Predictors for myocardial infarction and ischemic and unspecified stroke separately are shown in Supplemental Tables 10 and 11.
Table 3.
Analysis of Predictors for Arterial Thromboembolism During the 6-Month Period Following Cancer Diagnosis
| Cumulative Incidence (95% CI) | Unadjusted Subdistribution Hazard Ratio (95% CI) | Adjusted Subdistribution Hazard Ratio∗ (95% CI) | |
|---|---|---|---|
| Female | 1.18 (1.14–1.23) | Reference | Reference |
| Male | 1.84 (1.78–1.90) | 1.56 (1.49–1.64) | 1.15 (1.08–1.22) |
| Age groups, yrs | |||
| <65 | 0.79 (0.74–0.83) | Reference | Reference |
| 65–75 | 1.61 (1.55–1.67) | 2.06 (1.92–2.20) | 1.53 (1.43–1.65) |
| >75 | 2.30 (2.22–2.38) | 2.96 (2.77–3.15) | 1.88 (1.75–2.02) |
| Prior arterial thromboembolism | |||
| No | 1.10 (1.07–1.14) | Reference | Reference |
| Yes | 4.94 (4.74–5.13) | 4.59 (4.36–4.82) | 2.96 (2.77–3.17) |
| Cancer stage at diagnosis† | |||
| Localized | 1.15 (1.09–1.20) | Reference | Reference |
| Regional | 1.54 (1.47–1.61) | 1.34 (1.25–1.44) | 1.16 (1.08–1.25) |
| Distant | 1.87 (1.78–1.96) | 1.64 (1.53–1.75) | 1.21 (1.12–1.30) |
| Unknown | 1.75 (1.65–1.85) | 1.53 (1.42–1.65) | 1.07 (0.98–1.16) |
| Cancer treatment during first 4 months after cancer diagnosis‡ | |||
| No treatment | 1.67 (1.60–1.74) | Reference | Reference |
| Included chemotherapy | 1.30 (1.23–1.37) | 0.77 (0.72–0.83) | 1.47 (1.33–1.61) |
| Included radiotherapy | 1.23 (1.14–1.32) | 0.73 (0.67–0.79) | 1.20 (1.08–1.33) |
| Included surgery | 1.36 (1.32–1.41) | 0.81 (0.77–0.86) | 1.16 (1.09–1.24) |
| Included hormonal therapy | 1.00 (0.90–1.11) | 0.59 (0.53–0.66) | 1.05 (0.91–1.22) |
| Atrial fibrillation or flutter | |||
| No | 1.41 (1.37–1.44) | Reference | Reference |
| Yes | 2.83 (2.65–3.02) | 2.03 (1.89–2.18) | 1.06 (0.96–1.16) |
| Hypertension | |||
| No | 1.29 (1.25–1.33) | Reference | Reference |
| Yes | 2.60 (2.49–2.72) | 2.03 (1.93–2.14) | 1.29 (1.21–1.37) |
| Lipid-lowering therapy | |||
| No | 1.33 (1.30–1.37) | Reference | Reference |
| Yes | 2.24 (2.14–2.34) | 1.69 (1.60–1.78) | 0.96 (0.89–1.02) |
| Diabetes mellitus | |||
| No | 1.42 (1.38–1.45) | Reference | Reference |
| Yes | 2.64 (2.47–2.82) | 1.88 (1.74–2.02) | 1.20 (1.10–1.29) |
| Antiplatelet therapy | |||
| No | 1.14 (1.10–1.17) | Reference | Reference |
| Yes | 2.88 (2.77–2.98) | 2.56 (2.44–2.69) | 1.23 (1.16–1.31) |
| Anticoagulant therapy | |||
| No | 1.43 (1.40–1.47) | Reference | Reference |
| Yes | 2.77 (2.57–2.98) | 1.95 (1.80–2.11) | 1.23 (1.11–1.36) |
CI = confidence interval.
Adjusted for age, sex, calendar year, prior arterial thromboembolism, prior venous thromboembolism, atrial fibrillation or flutter, heart failure, atherosclerosis and peripheral vascular disease, chronic obstructive pulmonary disease, inflammatory bowel disease, liver disease, renal disease, diabetes, obesity, alcoholism and alcoholism-related conditions, rheumatoid arthritis, HIV, hypertension, cancer type, lipid-lowering therapy, antiplatelet use, and anticoagulant use.
For solid cancers only.
Only estimated for patients that were still alive at 4 months to avoid immortal time bias.
Discussion
In this Danish population-based cohort study, the risk of arterial thromboembolism in cancer patients was evaluated using data from almost one-half million cancer patients (Central Illustration). We found that risk of myocardial infarction, ischemic stroke, and peripheral arterial embolism was approximately two-fold higher in cancer patients than in matched members of the general population. This risk was increased before the cancer diagnosis and during the 12 months after the cancer diagnosis. Elderly patients and those with bladder, lung, and colon cancer were at highest risk. Arterial thromboembolic events were strongly associated with increased mortality in cancer patients. These findings underscore the relevance of this disease complication in cancer patients, for which preventive efforts might be considered in high-risk patients. International guidelines propose several possible preventive measures, including a thorough cardiovascular risk assessment, early identification and treatment of comorbidities, minimization of cardiac irradiation, the limitation of the cumulative dose of several systemic cancer treatments, and promoting exercise and positive lifestyle behavior (21,22).
Central Illustration.
Cancer Patients at Increased Risk of Arterial Thromboembolism
The 12-month cumulative incidence of arterial thromboembolism (ATE) is higher for cancer patients than for comparator individuals. In cancer patients, ATE is associated with an increased risk of mortality. Age, prior arterial thromboembolism, distant metastasis, and chemotherapy were important predictors for ATE. ATE was defined as the composite of myocardial infarction, ischemic and unspecified stroke, and peripheral arterial occlusion. CI = confidence interval; HR = hazard ratio.
It is well known that cancer patients are at increased risk of venous thromboembolism, which develops in approximately 3% of the total cancer population (23). This study identified a somewhat lower 1.5% 6-month risk of arterial thromboembolism. However, it is important to note that the case fatality rate of arterial events is substantially higher than that of venous thromboembolic events (24, 25, 26). This is reflected by the strong association of arterial events with mortality in our study. Several studies showed that venous thromboembolism is associated with systemic anticancer therapies, such as cisplatin and angiogenesis inhibitors (23,27,28). Large-scale studies are needed to evaluate whether a similar association exists between cancer and arterial thromboembolism.
Although the risk of arterial thromboembolism in the overall cancer population was modest, the risk appeared to be substantial in certain patient groups. For instance, the 6-month incidence of arterial thromboembolism among men older than age 75 years with diabetes mellitus and a diagnosis of bladder cancer was 4.09% (95% CI: 2.48% to 6.32%) compared with only 0.12% (95% CI: 0.09% to 0.16%) among women younger than age 65 years with no comorbidities and a diagnosis of breast cancer.
Risk stratification scores are available to select cancer patients at high risk of venous thromboembolism for thromboprophylaxis. Such tools allow clinicians to identify cancer patients with a 6-month incidence of venous thromboembolism of roughly 9% (29,30). Consequently, international guidelines now suggest 6 months of outpatient primary thromboprophylaxis to prevent venous thromboembolism in high-risk cancer patients (31, 32, 33). To the best of our knowledge, no such validated risk scores exist to identify cancer patients at high risk for arterial thromboembolism. In the present study, we identified several predictors (Table 3), which can aid identification of patients for whom intensive preventive measures, such as antiplatelet or lipid-lowering therapy, might be beneficial.
Navi et al. (10) evaluated the risk of arterial thromboembolism in a cohort of 279,719 cancer patients and a matched comparator cohort using Medicare data, which include health care data for Americans older than age 65 years. In their study, the 6-month incidence of arterial thromboembolism was 4.7% (95% CI: 4.6% to 4.8%) in the cancer cohort, and 2.2% (95% CI: 2.1% to 2.2%) in the comparator cohort (HR: 2.2; 95% CI: 2.1 to 2.3). Although the 2-fold increased relative risk for cancer patients is consistent with the present study, we observed a substantially lower absolute risk in the cancer cohort overall (1.50%; 95% CI: 1.47% to 1.54%), but also in the groups of patients age 65 to 75 years (1.61%; 95% CI: 1.55% to 1.67%) and >75 years (2.30%; 95% CI: 2.22% to 2.38%). A potential explanation, pointed out by Navi et al., is that Medicare data might be prone to overcoding in some situations due to diagnostic reclassifications (34). The observation could also be explained by differences between the study populations in unmeasured risk factors, such as smoking and body weight. In contrast to Navi et al. (10), we did not include outpatient diagnosis in our primary analysis, because this might result in delayed entry of previous arterial thromboembolic events. Nonetheless, our additional analysis, in which we used both inpatient and outpatient diagnoses, suggests that this explanation does not fully explain the difference. An Austrian prospective cohort study that included 1,880 cancer patients yielded a 6-month cumulative incidence of 1.1% (95% CI: 0.7% to 1.7%), which was somewhat lower than our finding (35). As in the present study, male sex, older age, and hypertension were associated with arterial thromboembolism.
Study strengths and limitations
Strengths of the current study include its use of comprehensive routine clinical care data available for all Danish residents regardless of age, insurance status, sociodemographic factors, or ethnic background. The use of competing risk analysis, which recently was shown to be appropriate in this setting (36), mitigated overestimation of risks. However, some limitations also need to be acknowledged. First, the higher risk of arterial thromboembolism could, at least in part, be due to differences between the patient and comparator cohorts in unmeasured confounders, such as smoking. Unfortunately, information on smoking was not available in our dataset. However, we reduced the effect of smoking by adjusting for chronic lung disease, and our findings were consistent for cancer types that are not strongly associated with smoking, such as breast cancer and hematological malignancies. Other unmeasured potential confounders include obesity and diet. Second, although transient ischemic attacks also may be considered a type of arterial thromboembolism, we excluded this diagnosis from the primary outcome given its low positive predictive value in the DNPR (37). This approach may potentially have resulted in conservative estimates. Third, cancer patients likely receive closer clinical surveillance after a cancer diagnosis than persons in the comparator cohort, leading to earlier detection of study outcomes. However, our analysis of outcomes before cancer diagnosis yielded similar results. Fourth, the analysis focusing on the 6 months prior to the cancer diagnosis introduced immortal time bias for both cohorts, because fatal events were excluded by definition. This resulted in underestimated risks in this period, especially for the cancer cohort. Fifth, cancer treatment was not limited to a single modality and was recorded only during the first 4 months following cancer diagnosis. Sixth, data on comorbidities were available from 1977 onwards, meaning that comorbidities occurring before that year were not available. Finally, our results do not permit any inferences about the disease mechanism underlying arterial thromboembolism in cancer patients. Cancer types with the highest risk of arterial thromboembolism were bladder, lung, and colon cancer, which are all associated with smoking. This observation suggests that smoking (and potentially other lifestyle factors) may contribute to the increased risk in cancer patients. However, the higher risk in patients with metastasized cancer and in those receiving chemotherapy suggests that hypercoagulability may play a role as well, as observed for cancer-associated venous thromboembolism.
Conclusions
Our study showed that cancer patients are at increased risk of arterial thromboembolic events, including myocardial infarction and stroke. As these events are associated with mortality, clinicians should be aware of this disease complication, in particular in risk groups including the elderly and those with certain cancers such as bladder and lung cancer.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Cancer patients are at increased risk of arterial thromboembolism, including myocardial infarction, ischemic stroke, and peripheral arterial occlusion. Cancer patients should be informed about their increased risk of arterial thromboembolism and preventive measures should be considered by clinicians.
TRANSLATIONAL OUTLOOK: Clinical prediction models should be developed to identify cancer patients at highest risk of arterial thromboembolism. This could aid clinicians in selecting patients for preventive measures. A better understanding of the association between arterial thromboembolism and specific anticancer systemic agents is needed. Future population-based cohort studies should address this need.
Funding Support and Author Disclosures
The Department of Clinical Epidemiology at Aarhus University receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies has any relation to the present study. This study was supported by a research grant of the Karen Elise Jensen’s Foundation. Dr. van Es is supported by an Amsterdam Cardiovascular Sciences MD/Postdoc grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank Hanne Kjeldahl Schlosser for her important role in this collaboration, and L.F. Reeskamp for his help on optimizing the visual layout of the figures.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Trousseau A. New Sydenham Society; Paris. London, England: 1865. Plegmasia alba dolens. Lectures on clinical medicine, delivered at the Hotel-Dieu; pp. 281–332. [Google Scholar]
- 2.Navi B.B., Singer S., Merkler A.E. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. doi: 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graus F., Rogers L.R., Posner J.B. Cerebrovascular complications in patients with cancer. Medicine (Baltimore) 1985;64:16–35. doi: 10.1097/00005792-198501000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Aleman B.M.P., Van Den Belt-Dusebout A.W., De Bruin M.L. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 5.De Bruin M.L., Dorresteijn L.D.A., Van’t Veer M.B. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 6.Tsai S.-J., Huang Y.-S., Tung C.-H. Increased risk of ischemic stroke in cervical cancer patients: a nationwide population-based study. Radiat Oncol. 2013;8:41. doi: 10.1186/1748-717X-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nilsson G., Holmberg L., Garmo H., Terent A., Blomqvist C. Increased incidence of stroke in women with breast cancer. Eur J Cancer. 2005;41:423–429. doi: 10.1016/j.ejca.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Chen P.-C., Muo C.-H., Lee Y.-T., Yu Y.-H., Sung F.-C. Lung cancer and incidence of stroke: a population-based cohort study. Stroke. 2011;42:3034–3039. doi: 10.1161/STROKEAHA.111.615534. [DOI] [PubMed] [Google Scholar]
- 9.Navi B.B., Reiner A.S., Kamel H. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. doi: 10.1002/ana.24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navi B.B., Reiner A.S., Kamel H. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundbøll J., Adelborg K., Munch T. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt M., Schmidt S.A.J., Adelborg K. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidt M., Pedersen L., Sørensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt M., Schmidt S.A.J., Sandegaard J.L., Ehrenstein V., Pedersen L., Sørensen H.T. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gjerstorff M.L. The Danish Cancer Registry. Scand J Public Health. 2011;39:42–45. doi: 10.1177/1403494810393562. [DOI] [PubMed] [Google Scholar]
- 16.Pottegård A., Schmidt S.A.J., Wallach-Kildemoes H., Sørensen H.T., Hallas J., Schmidt M. Data resource profile: the Danish National Prescription Registry. Int J Epidemiol. 2017;46:798f. doi: 10.1093/ije/dyw213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heide-Jørgensen U., Adelborg K., Kahlert J., Sørensen H.T., Pedersen L. Sampling strategies for selecting general population comparison cohorts. Clin Epidemiol. 2018;10:1325–1337. doi: 10.2147/CLEP.S164456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krarup L.-H., Boysen G., Janjua H., Prescott E., Truelsen T. Validity of stroke diagnoses in a national register of patients. Neuroepidemiology. 2007;28:150–154. doi: 10.1159/000102143. [DOI] [PubMed] [Google Scholar]
- 19.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 20.Petersen M.R., Deddens J.A. A comparison of two methods for estimating prevalence ratios. BMC Med Res Methodol. 2008;8:1–9. doi: 10.1186/1471-2288-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines. Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 22.Alexandre J., Cautela J., Ederhy S. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European Cardio-Oncology Guidelines. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mulder F.I., Horvàth-Puhó E., van Es N. Venous thromboembolism in cancer patients: a population-based cohort study. Blood. 2021;137:1959–1969. doi: 10.1182/blood.2020007338. [DOI] [PubMed] [Google Scholar]
- 24.Wiener R.S., Schwartz L.M., Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171:831–837. doi: 10.1001/archinternmed.2011.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdulla A., Davis W.M., Ratnaweera N., Szefer E., Ballantyne Scott B., Lee A.Y.Y. A meta-analysis of case fatality rates of recurrent venous thromboembolism and major bleeding in patients with cancer. Thromb Haemost. 2020;120:702–713. doi: 10.1055/s-0040-1708481. [DOI] [PubMed] [Google Scholar]
- 26.Smolina K., Wright F.L., Rayner M., Goldacre M.J. Incidence and 30-day case fatality for acute myocardial infarction in England in 2010: national-linked database study. Eur J Public Health. 2012;22:848–853. doi: 10.1093/eurpub/ckr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verso M., Agnelli G., Barni S., Gasparini G., LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: The Protecht score. Intern Emerg Med. 2012;7:291–292. doi: 10.1007/s11739-012-0784-y. [DOI] [PubMed] [Google Scholar]
- 28.Moore R.A., Adel N., Riedel E. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khorana A.A., Kuderer N.M., Culakova E., Lyman G.H., Francis C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mulder F.I., Candeloro M., Kamphuisen P.W. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica. 2019;104:1277–1287. doi: 10.3324/haematol.2018.209114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Key N.S., Khorana A.A., Kuderer N.M. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 32.Farge D., Frere C., Connors J.M. 2019 International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;2045:1–16. doi: 10.1016/S1470-2045(19)30336-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang T., Zwicker J.I., Ay C. The use of direct oral anticoagulants for primary thromboprophylaxis in ambulatory cancer patients: Guidance from the SSC of the ISTH. J. Thromb Haemost. 2019;17:1772–1778. doi: 10.1111/jth.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke J.F., Skolarus L.E. Are more young people having strokes? A simple question with an uncertain answer. JAMA Neurol. 2017;74:639. doi: 10.1001/jamaneurol.2017.0161. [DOI] [PubMed] [Google Scholar]
- 35.Grilz E., Königsbrügge O., Posch F. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103:1549–1556. doi: 10.3324/haematol.2018.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blix K., Gran O.V., Severinsen M.T. Impact of time since diagnosis and mortality rate on cancer-associated venous thromboembolism: the Scandinavian Thrombosis and Cancer (STAC) cohort. J Thromb Haemost. 2018;16:1327–1335. doi: 10.1111/jth.14130. [DOI] [PubMed] [Google Scholar]
- 37.Johnsen S.P., Overvad K., Sorensen H.T., Tjonneland A., Husted S.E. Predictive value of stroke and transient ischemic attack discharge diagnoses in The Danish National Registry of Patients. J Clin Epidemiol. 2002;55:602–607. doi: 10.1016/s0895-4356(02)00391-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.