Abstract
Objectives
This study will compare the incidence of major adverse cardiovascular events (MACEs) with androgen deprivation therapy (ADT) among men with advanced prostate cancer who are being treated with a gonadotropin-releasing hormone (GnRH) antagonist versus a GnRH agonist.
Background
Treatment of advanced prostate cancer with ADT might increase the risk of subsequent cardiovascular events among men with known atherosclerotic cardiovascular disease (ASCVD), but a recent meta-analysis suggested that this risk might be lower with ADT using a GnRH antagonist versus a GnRH agonist.
Methods
PRONOUNCE is a multicenter, prospective, randomized, open, blinded endpoint trial that will enroll approximately 900 patients with advanced prostate cancer and pre-existing ASCVD who will be treated with ADT. Participants will be randomized to receive the GnRH antagonist degarelix or the GnRH agonist leuprolide as ADT for 12 months. The primary endpoint is time from randomization to first confirmed, adjudicated occurrence of a MACE, which is defined as a composite of all-cause death, nonfatal myocardial infarction, or nonfatal stroke through 12 months of ADT treatment. Baseline cardiovascular biomarkers (high-sensitivity C-reactive protein, high-sensitivity troponin T, and N-terminal pro-brain natriuretic peptide), as well as serial inflammatory and immune biomarkers, will be evaluated in exploratory analyses.
Results
As of October 1, 2019, a total of 364 patients have been enrolled. The mean age is 74 years, 90% are white, 80% have hypertension or dyslipidemia, 30% diabetes mellitus, 40% have had a previous myocardial infarction, and 65% have had previous revascularization. Regarding prostate cancer features at randomization, 48% of the patients had localized disease, 23% had locally advanced disease, and 18% had metastatic disease.
Conclusions
PRONOUNCE is the first prospective cardiovascular outcomes trial in advanced prostate cancer that will delineate whether the risk of subsequent cardiovascular events associated with ADT is lower with a GnRH antagonist versus a GnRH agonist for men with pre-existing ASCVD. (A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease [PRONOUNCE]; NCT02663908)
Key Words: cardiovascular safety, outcomes, prostate cancer
Abbreviations and Acronyms: ASCVD, atherosclerotic cardiovascular disease; ADT, androgen deprivation therapy; CI, confidence interval; DSMB, Data Safety Monitoring Board; GnRH, gonadotropin-releasing hormone; MACE, major adverse cardiovascular event
Central Illustration
The known concurrence of cancer and atherosclerotic cardiovascular disease (ASCVD) in certain patient populations has been informed by improved methods of early cancer detection and integrated treatment approaches that have resulted in significant cancer-related survival gains over the past few decades (1, 2, 3). With increasing survival trends associated with cancer, the competing risks of downstream morbidity and mortality for patients with cancer may be more influenced by concomitant ASCVD (when present) than from the incident type of cancer (4, 5, 6). As a result, ASCVD has emerged as the predominant cause of mortality, particularly among older cancer survivors (4,5,7, 8, 9). More specifically, prostate cancer is the most common form of cancer among older men and has an increasing occurrence with older age; similar age-related trends have been found in the prevalence and consequences of ASCVD (10,11). As a result, ASCVD has become the second most common cause of death among men with prostate cancer (12,13).
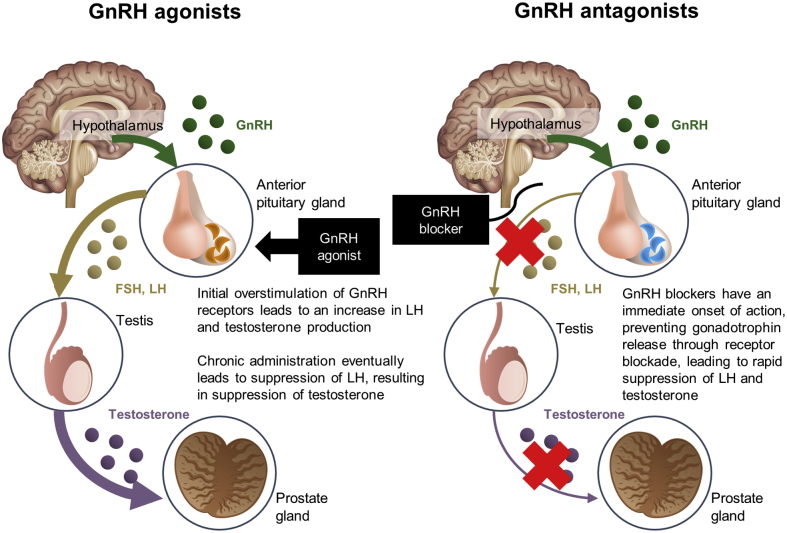
For locally advanced, relapsed, or metastatic prostate cancer, androgen deprivation therapy (ADT) is the backbone of treatment, alone or in combination with radiation therapy or other agents because of the role of testosterone in the development and progression of prostate cancer (14). Medical castration with ADT is accomplished with either a gonadotropin-releasing hormone (GnRH) agonist or a GnRH antagonist because both methods effectively achieve a castration level of testicular testosterone suppression. Whether the different mechanisms of action between these 2 agents might have differing effects on off-target tissues (e.g., atherosclerotic plaque) remains unknown (15). Initial administration of a GnRH agonist causes luteinizing hormone and follicle-stimulating hormone release, which results in an increase in serum testosterone, or “testosterone flare.” Long-term exposure to a GnRH agonist eventually shuts down luteinizing hormone and follicle-stimulating hormone; consequently, testicular production of testosterone is stopped. In contrast, GnRH antagonists inhibit pituitary GnRH receptors, which immediately shuts down luteinizing hormone secretion, which leads to subsequent suppression of testosterone production without an associated testosterone flare (Figure 1).
Figure 1.
Mechanism of Action
Mechanism of action of GnRH agonist and antagonist. FSH = follicle-stimulating hormone; GnRH = gonadotropin-releasing hormone; LH = luteinizing hormone.
Over the past 2 decades, several observational studies have demonstrated an association between the use of ADT and an increased risk of thrombotic cardiovascular events, including pulmonary embolism, myocardial infarction, and cardiovascular-related mortality (16, 17, 18). Findings from the 2 initial studies that demonstrated this association analyzed data from the Surveillance Epidemiology and End Results-Medicare linked database and identified an increased risk of incident coronary heart disease, myocardial infarction, and cardiovascular death among men with prostate cancer treated with a GnRH agonist (19,20). Although this signal was not uniformly observed in other studies published at that time, these new data led to the 2010 publication of a joint scientific statement from the American Heart Association, American Cancer Society, and American Urological Association that suggested a possible association between ADT and risk of cardiovascular events (16,21). Shortly after this publication, the U.S. Food and Drug Administration and Health Canada asked manufacturers of GnRH agonists to add extra safety information to drug labels with a warning about the possible increased risks for cardiovascular events. Although the Food and Drug Administration did not request a label change for GnRH antagonists (22), the European Medical Agency requested similar label warnings for both GnRH agonists and GnRH antagonists. Thereafter, further insights were provided from a meta-analysis of pooled data from 6 prospective randomized clinical trials that compared short-term (up to 12 months) treatment of advanced prostate cancer with a GnRH antagonist (degarelix) versus a GnRH agonist (leuprolide). In the overall population, the risk of cardiovascular events was similar between the treatment arms. However, in those with pre-existing ASCVD before the start of ADT, the frequency of cardiovascular events was substantially lower with the GnRH antagonist versus the GnRH agonist (6.5% vs. 14.7%, respectively) with a separation of event curves that occurred approximately 3 to 6 months after ADT initiation that lasted throughout the first year of treatment (23).
Prompted by this additional evidence that demonstrated a potential differential risk of cardiovascular events by type of ADT when treatment was administered for up to 12 months, the PRONOUNCE (A Trial Comparing Cardiovascular Safety of Degarelix Versus Leuprolide in Patients With Advanced Prostate Cancer and Cardiovascular Disease) trial was designed to rigorously and prospectively evaluate the cardiovascular safety of a GnRH receptor antagonist (degarelix) versus a GnRH receptor agonist (leuprolide) among men with prostate cancer who had pre-existing ASCVD. To our knowledge, the PRONOUNCE trial is the first prospective cardiovascular outcomes trial in advanced prostate cancer to compare 2 different types of cancer treatment, thereby representing an important development in the field of cardio-oncology (24).
Methods
PRONOUNCE is a phase IIIb, multicenter, prospective, randomized, open, blinded endpoint trial designed to compare the occurrence of major adverse cardiovascular events (MACEs) in patients with advanced prostate cancer and pre-existing ASCVD who will receive either a GnRH antagonist (degarelix) or a GnRH agonist (leuprolide) as ADT for 12 months (25). The trial plans to enroll approximately 900 patients at approximately 100 sites in North America and Europe. The first patient was randomized in April 2016.
This trial is being conducted in compliance with the study protocol, the Declaration of Helsinki, and Good Clinical Practice, as defined by the International Conference on Harmonization. Before patient enrollment, written informed consent is obtained from each patient, and approval is obtained from appropriate institutional review boards and ethics committees for participating sites. The steering and operations committees, which include academic members and sponsor representatives, oversee the medical, scientific, and operational conduct of the study. The PRONOUNCE trial is supported by the manufacturer of degarelix, Ferring Pharmaceuticals (Parsippany, New Jersey).
Study population
Eligible patients must have a pathological diagnosis of adenocarcinoma of the prostate with newly diagnosed localized disease, biochemical recurrence after definitive therapy, or hormone-sensitive metastatic disease. If a participant received previous ADT for neoadjuvant or adjuvant therapy, then the last dose of therapy must have been at least 12 months before randomization. Serum testosterone level must be in the non-castration range, and the duration of planned ADT must be at least 12 months. In addition, eligible patients must have pre-existing ASCVD defined as: a history of myocardial infarction; previous percutaneous or surgical revascularization of the carotid, coronary, iliac, femoral, or popliteal arteries; previous documentation of a stenosis of >50% in these vessels by angiography or carotid ultrasound; or peripheral arterial disease (PAD) confirmed with a diminished ankle-brachial pressure index (Tables 1 and 2, Supplemental Appendix).
Table 1.
Inclusion Criteria
| Main inclusion criteria for prostate cancer |
| Histologically confirmed adenocarcinoma of the prostate |
| Tumor, node, metastasis staging available before treatment start (bone scan and/or CT scan and/or MRI) <12 weeks before study start. |
| If no radiographic image is available at the time of screening, a bone scan should be performed |
| Investigator judgement to initiate continued ADT therapy with intended duration of 12 months or longer. |
| Patients with metastatic prostate cancer at time of diagnosis |
| Patients with prostate cancer who develop metastases after local therapy |
| Patients with prostate cancer with very high-risk, high-risk, or intermediate risk disease with feature of unfavorable prognosis who will be treated with definitive radiation therapy in combination with at least 12 months of neoadjuvant/adjuvant ADT |
| Patients must be treatment-naive (ADT) |
| If patients received previous ADT for neoadjuvant or adjuvant therapy, then the last dose of therapy must be at least 12 months before randomization |
| Any additional hormonal therapy upfront (i.e., abiraterone) is prohibited in the study; however, anti-androgen use for initial flare protection is allowed for a maximum period of up to 28 days after randomization |
| Main cardiovascular inclusion criteria |
| Pre-existing ASCVD (confirmed diagnosis, documented) according to at least 1 of the following criteria |
| Previous myocardial infarction ≥30 days before randomization |
| Previous revascularization procedure ≥30 days before randomization |
| Coronary artery: stent placement/balloon angioplasty or coronary artery bypass graft surgery |
| Carotid artery: stent placement/balloon angioplasty or endarterectomy surgery |
| Iliac, femoral, popliteal arteries: stent placement/balloon angioplasty or vascular bypass surgery |
| At least 1 vascular stenosis ≥50% at any time point before randomization by angiography or CT angiography |
| Coronary artery |
| Carotid artery |
| Iliac, femoral, or popliteal arteries |
| Carotid ultrasound results that documented a vascular stenosis ≥50% at any time point before randomization |
| Ankle−brachial pressure index <0.9 at any time point before randomization |
ADT = androgen deprivation therapy; ASCVD = atherosclerotic cardiovascular disease; CT = computed tomography; MRI = magnetic resonance imaging.
Table 2.
Main Exclusion Criteria
| Main prostate cancer exclusion criteria |
| Previous or current hormonal management of prostate cancer |
| Surgical castration |
| Any hormonal manipulation |
| Any previous neoadjuvant/adjuvant hormonal therapy, unless treatment terminated >12 months before study start |
| Main cardiovascular exclusion criteria |
| Uncontrolled type 1 or type 2 diabetes mellitus (defined as HbA1c >10%) at time of randomization |
| Uncontrolled hypertension (SBP >180 mm Hg or DBP >110 mm Hg) at time of randomization |
| A history of congenital long QT syndrome or risk factors for Torsade de pointes ventricular arrhythmias (e.g., heart failure, hypokalemia, concomitant medication known to cause QT prolongation) |
| Within 30 days before randomization: |
| Myocardial infarction |
| Stroke (hemorrhagic/ischemic) |
| Coronary, carotid, or peripheral artery revascularization |
| Planned or scheduled cardiac surgery or PCI procedure that is known at the time of randomization |
DBP = diastolic blood pressure; HbA1c = glycosylated hemoglobin A1c; PCI = percutaneous coronary intervention; SBP = systolic blood pressure.
During screening, potentially eligible patients are evaluated by a local cardiovascular specialist to ensure that baseline secondary prevention medications for ASCVD are optimized according to guideline recommendations and to provide verification of the ASCVD inclusion criteria for the trial (26,27). Furthermore, to support sites in properly confirming the ASCVD inclusion criteria, cardiovascular disease information and source medical documents for the first series of patients screened by each site are reviewed centrally by a cardiologist at the Duke Clinical Research Institute (Durham, North Carolina), which is the academic coordinating center for the trial. The investigators are also required to ensure that a cardiovascular specialist is treating the patients during their trial participation to ensure optimization of secondary prevention medications for ASCVD for the duration of the trial.
Randomization and treatment
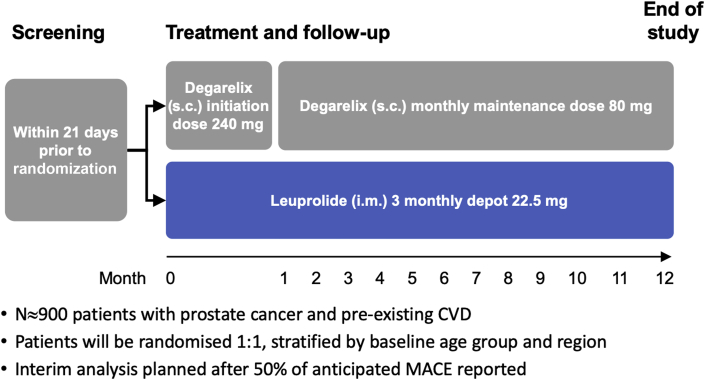
Eligible patients are randomized 1:1 (with a fixed block size of 4) to degarelix or leuprolide acetate in an open-label fashion. Randomization lists are prepared by an independent statistician not involved with the trial and sent to an external electronic Case Report Form vendor for upload to an eCRF/online randomization. Each patient receives a unique randomization number. Randomization is stratified by baseline age group (younger than 75 years vs. 75 years or older) and region (North America vs. other geographic regions). Patients randomized to degarelix receive a starting dose of 240 mg degarelix (2 subcutaneous injections, each of 120 mg) followed by 11 subcutaneous injections of 80 mg degarelix given at 28-day intervals. Patients randomized to leuprolide receive 22.5 mg administered intramuscularly every 84 days, for 4 doses. Each patient is treated with 12 months of ADT (Figure 2). Because of the different frequency (once a month vs. once every 3 months), modality of dosing (subcutaneous vs. intramuscular), and the known difference in injection site reactions of the 2 randomized treatment regimens, a double-blind, placebo-controlled treatment design was determined to be too challenging to successfully implement, as well as too difficult and uncomfortable for patients because of the need for multiple sham injections. Nonetheless, several mechanisms and approaches are used to minimize bias in the ascertainment, classification, adjudication, and confirmation of suspected cardiovascular events as detailed in subsequent sections (Central Illustration). Any additional hormonal therapy upfront (i.e., abiraterone) is prohibited in the study; however, anti-androgen use for initial flare protection is allowed for a maximum period of ≤28 days after randomization. If a patient progresses and requires additional hormonal therapy, these subjects are subsequently excluded from the per protocol analysis.
Figure 2.
Trial Design
The trial design, from the initial screening period through treatment and/or follow-up, and the end of the trial. CVD = cardiovascular disease; i.m. = intramuscularly; MACE = major adverse cardiovascular event; s.c. = subcutaneously.
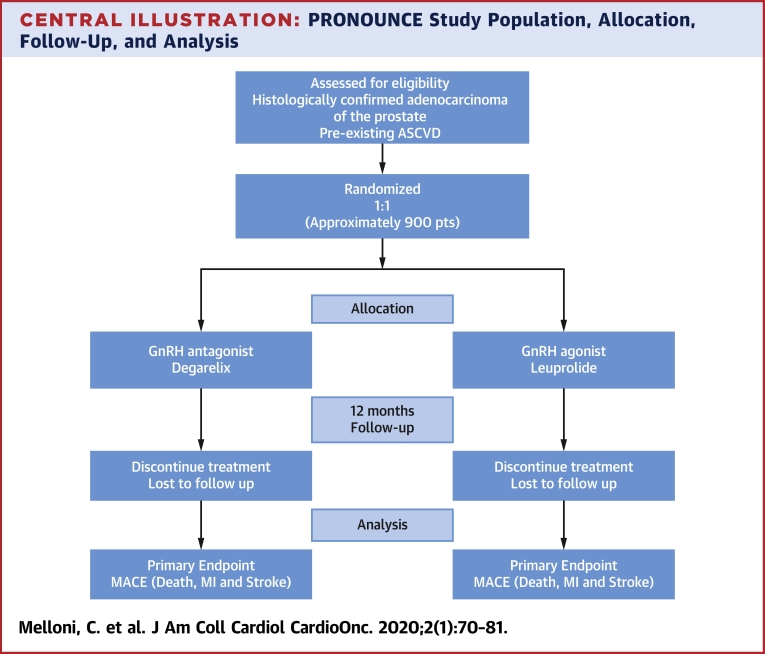
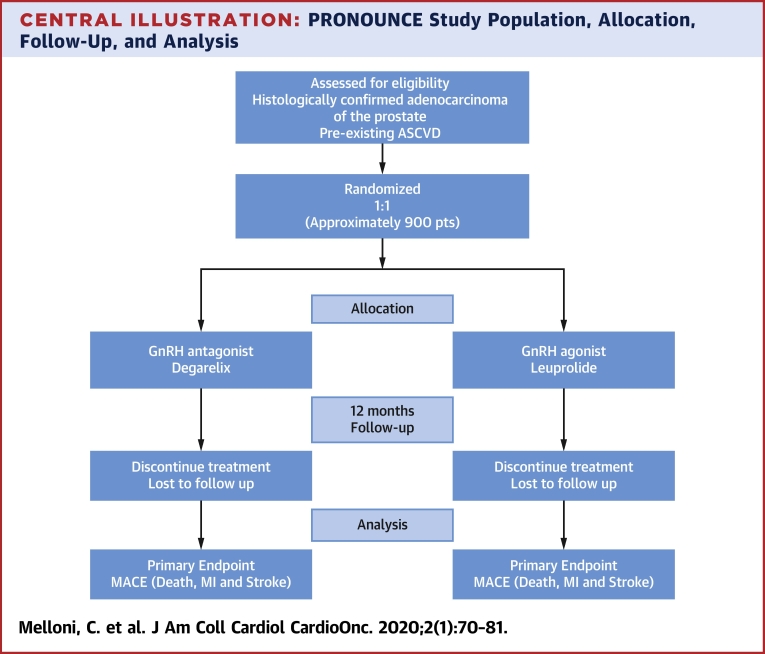
Central Illustration.
PRONOUNCE Study Population, Allocation, Follow-Up, and Analysis
The study population for the PRONOUNCE trial for initial inclusion, allocation, follow-up, and analysis. ASCVD = atherosclerotic cardiovascular disease; GnRH = gonadotropin-releasing hormone; MACE = major adverse cardiovascular event.
As of October 1, 2019, a total of 364 patients have been enrolled with 60% from North America and 40% from Europe. The mean age is 74 years, 90% are white, approximately 80% have hypertension or dyslipidemia, 30% have diabetes mellitus, 40% have had a previous myocardial infarction, and 65% have had previous revascularization. Regarding prostate cancer features at randomization, 48% of the patients had localized disease, 23% had locally advanced disease, and 18% had metastatic disease.
Schedule of assessments
A detailed schedule of study assessments is delineated in Supplemental Tables 1 and 2. Suspected cardiovascular events, adverse events, and use of concomitant medications are assessed at the baseline visit and during pre-specified monthly visits. In addition, at the baseline visit, blood samples are obtained to evaluate cardiovascular biomarkers (high-sensitivity C-reactive protein, high-sensitivity troponin T, and N-terminal pro-brain natriuretic peptide). At each monthly trial-related visit, each participant is administered a detailed questionnaire that captures information on potential cardiovascular events, such as hospitalizations for cardiovascular causes, occurrence of angiographic and revascularization procedures, use of brain imaging procedures, and so forth. Patient responses to this monthly questionnaire prompt sites to report potential cardiovascular events and submit requisite source documents through a standard cardiovascular endpoint reporting process. Each patient has a final clinic visit 1 month after the last dose of their assigned treatment regimen.
Primary endpoints
The primary objective of this trial is to evaluate the impact of degarelix versus leuprolide on the first occurrence of a MACE (all-cause death, nonfatal myocardial infarction, or nonfatal stroke) through 12 months of ADT.
Secondary endpoints
The main secondary cardiovascular objectives are to assess the frequency of the individual components of the composite MACE primary endpoint (myocardial infarction, stroke, and all-cause death); cardiovascular-related death; and a composite of cardiovascular-related death, myocardial infarction, or stroke; and unstable angina. Definitions of cardiovascular endpoints are standardized based upon published regulatory recommendations (28). The main prostate cancer-related objectives are to monitor testosterone levels on days 28, 168, and 336, to evaluate the progression-free survival failure rates (defined as either death, radiographic disease progression, introduction of additional prostate cancer therapies for progression, or prostate-specific antigen failure, whichever is first), and to compare urinary and prostate cancer-related symptoms with the International Prostate Symptom Score questionnaire. Health economics and patient-reported outcome objectives include comparing healthcare resource use, health status through the EuroQol Group 5 Dimensions 5 Levels Questionnaire, functional capacity and quality of life through the Duke Activity Status Index, and heart-focused anxiety through the Cardiac Anxiety Questionnaire. Adverse events are collected on a monthly basis and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events. These events will be reported as part of the secondary safety assessment.
Exploratory endpoints
Exploratory endpoints for the comparison of degarelix with leuprolide include: 1) time from first adjudicated nonfatal MACE to a second confirmed (adjudicated) occurrence of the composite MACE endpoint in the subgroup of patients that survived the first cardiovascular event, including an analysis of all (total) MACE events by treatment arm; 2) the regression coefficient associated with the interaction between treatment and baseline cardiovascular biomarker status with respect to a Cox regression model of the time from initial dosing to the first confirmed occurrence of the composite MACE endpoint; 3) the area under the receiver-operating characteristics curve for cardiovascular biomarkers based on the prediction of event-free survival; and 4) the difference in the area under the receiver-operating characteristics curves based on Cox regression models, including the traditional baseline cardiovascular risk factors, and the traditional cardiovascular risk factors plus cardiovascular biomarkers (high-sensitivity C-reactive protein, high-sensitivity troponin T, and N-terminal pro-brain natriuretic peptide) as covariates.
Statistical considerations: sample size calculation and assumptions for the primary mace endpoint
Based on the aforementioned published data from the meta-analysis of pooled randomized clinical trial data (23), the 1-year MACE event rates for sample size calculations were set to 5.1% and 10.2% for degarelix versus leuprolide, respectively (23). With a hypothesized hazard ratio of 0.49 based upon previous observations, 66 MACE events will be required at final analysis, corresponding to a sample size of 876 patients, to reject the null hypothesis of equal hazards at the 2-sided 5% type I error level with 80% power. One interim analysis is planned after 50% of the expected number of adjudicated MACE endpoints have been collected. The objective at the interim analysis is to test whether there is any reason to stop the trial early for futility purposes. In the event that the stopping boundaries are not crossed for futility, the required sample size will be reassessed based upon MACE event rates observed at the time of the interim analysis, to achieve a conditional power of 80% at trial conclusion. All randomized patients will be included in the intention-to-treat analysis set at trial conclusion. All analyses will be performed based on the planned (randomized) treatment. The data of all patients who received at least 1 dose of study drug will be included in the safety analysis set according to the actual treatment received.
The analysis of the primary MACE endpoint will be performed for the intention-to-treat and the per-protocol analysis sets. The time from randomization to the first confirmed occurrence of the composite MACE endpoint in the 2 treatment groups will be analyzed based on the Kaplan-Meier estimate of the survival function and the log-rank test stratified by age group and geographic region. The null hypothesis of equal hazard functions between the 2 treatment groups will be rejected if the inverse normal test statistics exceed the critical level for a 2-sided hypothesis test with a type I error level of 5%. Unless otherwise specified, time-to-event endpoints will be censored at the time when a patient initiates new and/or different ADT, is lost to follow-up and/or withdraws from the study or day 336, whichever occurs first. In addition, all hypothesis tests will be 2-sided at a significance level of 5%, and missing data will not be imputed.
Safety monitoring
The independent, external Data and Safety Monitoring Board (DSMB) is composed of 1 chairperson (cardiologist), 1 independent statistician, 1 cardiologist, and 1 urologist (Supplemental Appendix). The major roles of the DSMB are to periodically evaluate safety data and to perform the pre-planned, unblinded interim analysis after approximately 33 positively adjudicated MACEs have been observed. Based on pre-specified criteria, the DSMB will make recommendations to the Steering Committee on whether to continue the trial as is, modify (increase the sample size), or stop the trial due to futility according to the pre-determined criteria. The interim analysis will be based on data from the intention-to-treat analysis set.
Steering committee
The Steering Committee consists of external clinical and scientific experts, including cardiologists, oncologists, and urologists, as well as a Sponsor representative (Supplemental Appendix). The Steering Committee will be responsible for overseeing trial integrity and making decisions related to the trial conduct, such as potential protocol amendments and decisions based on interim recommendations from the DSMB, as previously described. The Duke Clinical Research Institute is the academic coordinating center for the trial and supports and organizes the Steering Committee.
Clinical event classification committee
An independent, firewalled, blinded clinical events adjudication committee from the Duke Clinical Research Institute will adjudicate all potential cardiovascular endpoints with the endpoint definitions listed in the Supplemental Appendix. Members of the adjudication committee have no other role in the trial conduct. Potential MACEs will be evaluated by applying a specific clinical events classification process to ensure blinding of the adjudicators, which includes extensive redaction of treatment-related details from source documents used during the adjudication processes. Each potential event will be adjudicated independently by 2 physician adjudicators, and disagreements will be reviewed by a committee of the adjudicators. Cardiologists will adjudicate death, myocardial infarction, and unstable angina events; neurologists will review stroke events; and oncologists will participate in the cause of death adjudication activities together with cardiologists.
Discussion
The primary goal of the PRONOUNCE study is to prospectively evaluate the occurrence of MACE events in patients with advanced prostate cancer and concomitant ASCVD who are treated with a GnRH antagonist degarelix compared with the GnRH agonist leuprolide over the initial 12 months of ADT. In addition, several non-cardiovascular−related secondary endpoints will be studied to further investigate the potential differences in the risk−benefit profile of degarelix versus leuprolide. Our main hypothesis is that patients treated with degarelix will have a lower risk of cardiovascular events than those treated with leuprolide. The PRONOUNCE trial is the first prospective cardiovascular outcomes trial to evaluate different cancer treatments.
Although ADT is the mainstay treatment of advanced prostate cancer, post hoc analyses have demonstrated an association between treatment with a GnRH receptor agonist and an increased risk of downstream cardiovascular events (20,29,30). For the most part, cardiovascular events occurred early after ADT initiation (typically after 1 to 4 months of exposure), which could suggest a short-term, treatment-related risk for aggravation or destabilization of existing atherosclerotic plaques (16,29, 30, 31). At the same time, other analyses have shown that although the risk seems to peak in the first 6 months of treatment, the event curve continues to diverge over longer follow-up (20,32). In line with these observations, studies have consistently shown that a history of ASCVD is strongly associated with subsequent cardiovascular complications during ADT therapy (33, 34, 35). A recent meta-analysis has also demonstrated that new hormonal agents (e.g., enzalutamide, darolutamide, and others) are associated with improvement in terms of metastases-free survival, but these agents come with a higher grade risk of cardiovascular events (36). Because new hormonal agents (e.g., abiraterone, docetaxel, and enzalutamide) all have their own added toxicities, including cardiovascular toxicity, we found that co-administration of these agents is not always in the best interest of patients with pre-existing cardiovascular disease or diabetes. In this study, we want to minimize any outside toxicities that can be contributed by these drugs; therefore, new hormonal agents are prohibited in the PROUNOUNCE trial.
Recently, results from a small, investigator-initiated, open-label trial that randomized 80 men with prostate cancer and pre-existing ASCVD to receive degarelix versus leuprolide for 12 months demonstrated that the number of cardiovascular events was lower with degarelix versus leuprolide (a pre-specified secondary endpoint) (37). Although the results from this study are hypothesis-generating and not definitive, these findings further support the rationale for an adequately powered, prospective trial such as PRONOUNCE to definitively ascertain the relative cardiovascular safety of ADT with a GnRH antagonist versus a GnRH agonist.
Mechanisms of increased cardiovascular risk with ADT appear to be multifactorial and are believed to be related to both metabolic and immunomodulatory changes that may destabilize pre-existing atherosclerotic plaques and potentially accelerate the progression of atherosclerosis.
Pre-clinical studies of androgen-receptor knockout and orchiectomized low-density lipoprotein−receptor knockout models demonstrated that androgens could exert both favorable direct and indirect effects on the development and progression of atherosclerotic lesions (38). The administration of testosterone to animal and cell models of atherosclerosis showed a decrease in the expression of vascular cell adhesion molecules (e.g., VCAM-1) and pro-inflammatory cytokines (tumor necrosis factor-alpha, interleukin-1) (39,40).
After post hoc analyses indicated that GnRH receptor agonists and GnRH receptor antagonists might have different levels of cardiovascular risk, the qualitative difference in the mechanism of action between GnRH receptor antagonists and GnRH receptor agonists, including the effect on the follicle-stimulating hormone, as well as potentially functional GnRH receptors identified in peripheral tissues, was investigated. These studies raised the possibility that GnRH receptor agonists and GnRH receptor antagonists might have different profiles with respect to short-term cardiovascular safety in patients with established ASCVD (23,41). For example, T cells present in atherosclerotic plaque may express GnRH receptors, and, consequently, may be stimulated by a GnRH agonist, thereby potentially promoting fibrotic cap disruption and plaque destabilization (23).
Although the pathophysiological mechanisms of potential differential cardiovascular risk with a GnRH antagonist versus GnRH agonist remain to be fully elucidated, putative differences in mechanisms of action of these agents may underlie the observed findings previously mentioned.
There are several distinctive features that make the PRONOUNCE trial unique. First, a multispecialty group of cardiologists, urologists, and oncologists work together on the Steering Committee and DSMB (42). Second, numerous strategies have been implemented to support investigators (mainly urology/oncology investigators) and to help in the assessment of cardiovascular inclusion criteria. Several training sessions covering cardiovascular disease definitions and cardiovascular inclusion criteria have been conducted during the course of the study and are offered on an as-needed basis to all sites. At the site level, local urology/oncology site investigators are supported by their cardiovascular specialists to confirm cardiovascular inclusion criteria and optimal background cardiovascular medication treatment. Furthermore, a cardiologist at Duke Clinical Research Institute (C.M.) reviews and confirms cardiovascular eligibility criteria for at least the first 3 patients screened at each site and is available thereafter based on a site’s needs. Clinical trial educators who are trained nurses with previous cardiovascular trial experience visit sites on a regular basis to support investigators in the screening and enrollment process and to assist sites with performing well in the trial. Third, structured questions to ensure full and complete cardiovascular endpoint ascertainment have been created and are administered by urology/oncology site research personnel to patients every month to inquire about potential cardiovascular events in a process that likely has not been used by these sites in previous clinical trials. Finally, several exploratory biomarkers (cardiovascular and immune) will be collected and assessed to determine how these biomarkers may relate to putative differences in cardiovascular events between the GnRH antagonist (degarelix) and the GnRH agonist (leuprolide); these analyses will also explore several potential biological mechanisms that may underlie the increased cardiovascular risk observed with ADT.
Conclusions
Cardio-oncology involves caring for patients with cancer who have concomitant cardiovascular disease at the time of cancer diagnosis or who develop cardiovascular disease during increasingly more complex and efficacious, but also potentially more cardio-toxic, cancer treatment. Due to the frequent co-existence of cardiovascular disease and cancer, the field of cardio-oncology is rapidly expanding. Effective communication and collaboration between different specialty providers (oncologists, urologists, and cardiologists) as essential partners in a care team is critical to balance cancer care and cardiovascular outcomes toward optimal survival. Cooperation among specialties also has regulatory repercussions, where different divisions at the Food and Drug Administration and European Medical Agency must collaborate to oversee and approve these types of trials. In this context, the PRONOUNCE trial is the first randomized trial that is designed to prospectively capture cardiovascular outcomes as a primary study endpoint comparing different treatments for prostate cancer.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Contemporary management of advanced prostate cancer involves the use of ADT, which is associated with several immunomodulatory and metabolic changes that may increase the risk of subsequent cardiovascular events among men with known ASCVD.
TRANSLATIONAL OUTLOOK: The PRONOUNCE trial will delineate the relative cardiovascular safety of degarelix (GnRH antagonist) versus leuprolide (GnRH agonist) and is the first prospective cardiovascular outcomes trial comparing treatments for patients with prostate cancer.
Acknowledgment
The authors acknowledge Erin Campbell, MS (Duke Clinical Research Institute), for her editorial contributions.
Footnotes
The PRONOUNCE trial is supported by Ferring Pharmaceuticals Inc. Dr. Melloni has received research grant funding through Duke University for conduct of the PRONOUNCE trial; and has received research grant or contract from Amgen, AstraZeneca, Bristol-Myers Squibb, Ferring Pharmaceuticals, GlaxoSmithKline, IntraCellular Therapies, Luitpold Pharmaceuticals, Merck, Roche, Sanofi, St. Jude Medical, and Pfizer. Dr. Slovin has been on the advisory boards for Amgen, Bayer, OncLive, and PER. Dr. Goodman has received research grant support (e.g., steering committee or data monitoring committee) and/or speaker/consulting honoraria (e.g., advisory boards) from Ferring Pharmaceuticals, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CSL Behring, Daiichi-Sankyo, Eli Lilly, Esperion, Fenix Group International, GlaxoSmithKline, HLS Therapeutics, Janssen/Johnson & Johnson, Luitpold Pharmaceuticals, Matrizyme, Merck, Novartis, Novo Nordisk A/C, Pfizer, Regeneron, Sanofi, Servier, Tenax Therapeutics, Heart and Stroke Foundation of Ontario/University of Toronto, Canadian Heart Research Centre and MD Primer, Canadian VIGOUR Centre, Duke Clinical Research Institute, and PERFUSE. Dr. Evans has received research support from Astellas/Pfizer, Ferring, Sanofi, Janssen, National Institutes of Health, SU2C/AACR (Stand Up for cancer/American Association for Cancer Research), Department of Defense, and Prostate Cancer Foundation; has been a consultant for Astellas/Pfizer and Janssen; has been a member of the Speakers Bureau for Astellas/Pfizer, Janssen; has received honoraria from Astellas/Pfizer, Janssen, and MDxHealth; and has been a member of the Scientific Advisory Board for Astellas, Janssen, and MDxHealth. Dr. Nilsson has received unrestricted research support from Ferring Pharmaceuticals, Novo Nordisk A/S, Follicum AB, and Medimmune. Dr. Bhatt has been a member of the Advisory Board for Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, and Regado Biosciences; has been a member of the Board of Directors for Boston VA Research Institute, Society of Cardiovascular Patient Care, and TobeSoft; has been the chair of the American Heart Association Quality Oversight Committee; has been a member of the Data Monitoring Committees for Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), and Population Health Research Institute; has received honoraria from the American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim, AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); and has received other funding from Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); has received research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; has received royalties from Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); has been the site co-investigator for Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; has been a trustee for the American College of Cardiology; and has received unfunded research from FlowCo, Fractyl, Merck, Novo Nordisk, PLx Pharma, and Takeda. Dr. Higano has been a member of the Advisory Boards for Aptevo, Asana, Astellas, Bayer, Blue Earth Diagnostics, Churchill Pharma, Clovis Oncology, Dendreon, Endocyte, Ferring, Hinova, Janssen, Myriad, Orion Corporation, and Pfizer; has been a consultant for Astellas, Bayer, Blue Earth Diagnostics, Carrick Therapeutics, Clovis, Ferring Pharmaceuticals, Janssen, Hinova, Merck, Novartis, Pfizer, Asana, Aptevo, Churchill Pharma, Dendreon, Myriaad, and Orion; and has done sponsored research for Aptevo, Aragon, Astellas, AstraZeneca, Bayer, Clovis, Dendreon, eFFECTOR Therapeutics, Emergent, Ferring, Genentech, Hoffman-Laroche, Medivation, Sanofi, and Pfizer. Dr. Roe has received research grant funding from Sanofi-Aventis, AstraZeneca, Patient Centered Outcomes Research Institute, Ferring Pharmaceuticals, Myokardia, Familial Hypercholesterolemia Foundation, Bayer; and has been a consultant and received honoraria from AstraZeneca, Amgen, Cytokinetics, Eli Lilly, Roche-Genentech, Janssen Pharmaceuticals, Regeneron, Novo Nordisk, Pfizer, Sanofi-Aventis, Signal Path, and Elsevier Publishers. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For a list of the members of the Data and Safety Monitoring Board and the Steering Committee, an expanded Methods section, and supplemental tables, please see the online supplement.
Appendix
References
- 1.Jemal A., Ward E., Hao Y., Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A., Ward E., Thun M. Declining death rates reflect progress against cancer. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N., Ries L.A., Mariotto A.B., Reichman M.E., Ruhl J., Cronin K.A. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102:1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman J.A., Meng D., Shepherd L. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J Natl Cancer Inst. 2008;100:252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanrahan E.O., Gonzalez-Angulo A.M., Giordano S.H. Overall survival and cause-specific mortality of patients with stage T1a,bN0M0 breast carcinoma. J Clin Oncol. 2007;25:4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D.M., Leip E.P., Larson M.G. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113:791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 7.Schairer C., Mink P.J., Carroll L., Devesa S.S. Probabilities of death from breast cancer and other causes among female breast cancer patients. J Natl Cancer Inst. 2004;96:1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 8.Patnaik J.L., Byers T., Diguiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colzani E., Liljegren A., Johansson A.L. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J Clin Oncol. 2011;29:4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 10.Epstein M.M., Edgren G., Rider J.R., Mucci L.A., Adami H.O. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104:1335–1342. doi: 10.1093/jnci/djs299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driver J.A., Djoussé L., Logroscino G., Gaziano J.M., Kurth T. Incidence of cardiovascular disease and cancer in advanced age: prospective cohort study. BMJ. 2008;337:a2467. doi: 10.1136/bmj.a2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Studer U.E., Whelan P., Albrecht W. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24:1868–1876. doi: 10.1200/JCO.2005.04.7423. [DOI] [PubMed] [Google Scholar]
- 13.Calais da Silva F.E., Bono A.V., Whelan P. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: results from a randomised phase 3 study of the South European Uroncological Group. Eur Urol. 2009;55:1269–1277. doi: 10.1016/j.eururo.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Scherr D., Swindle P.W., Scardino P.T., National Comprehensive Cancer Network National Comprehensive Cancer Network guidelines for the management of prostate cancer. Urology. 2003;61:14–24. doi: 10.1016/s0090-4295(02)02395-6. [DOI] [PubMed] [Google Scholar]
- 15.Sharifi N., Gulley J.L., Dahut W.L. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–244. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 16.Tsai H.K., D’Amico A.V., Sadetsky N., Chen M.H., Carroll P.R. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 17.Hu J.C., Williams S.B., O'Malley A.J., Smith M.R., Nguyen P.L., Keating N.L. Androgen-deprivation therapy for nonmetastatic prostate cancer is associated with an increased risk of peripheral arterial disease and venous thromboembolism. Eur Urol. 2012;61:1119–1128. doi: 10.1016/j.eururo.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ehdaie B., Atoria C.L., Gupta A. Androgen deprivation and thromboembolic events in men with prostate cancer. Cancer. 2012;118:3397–3406. doi: 10.1002/cncr.26623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keating N.L., O’Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 20.Saigal C.S., Gore J.L., Krupski T.L., Hanley J., Schonlau M., Litwin M.S. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 21.Bolla M., de Reijke T.M., Van Tienhoven G. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 22.United States Food and Drug Administration (FDA) FDA drug safety communication: ongoing safety review of GnRH antagonists and possible increased risk of diabetes and certain cardiovascular diseases. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-ongoing-safety-review-gnrh-agonists-and-possible-increased-risk Updated March 8, 2016. Available at:
- 23.Albertsen P.C., Klotz L., Tombal B., Grady J., Olesen T.K., Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. Eur Urol. 2014;65:565–573. doi: 10.1016/j.eururo.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 24.Bhatt D.L. Birth and maturation of cardio-oncology. J Am Coll Cardiol CardioOnc. 2019;1:114–116. doi: 10.1016/j.jaccao.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hansson L., Hedner T., Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Prospective Randomized Open Blinded End-Point. Blood Press. 1992;1:113–119. doi: 10.3109/08037059209077502. [DOI] [PubMed] [Google Scholar]
- 26.Fihn S.D., Blankenship J.C., Alexander K.P. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64:1929–1949. doi: 10.1016/j.jacc.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 27.Task Force Members. Montalescot G., Sechtem U. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 28.Hicks K.A., Mahaffey K.W., Mehran R. 2017 cardiovascular and stroke endpoint definitions for clinical trials. J Am Coll Cardiol. 2018;71:1021–1034. doi: 10.1016/j.jacc.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 29.Smith M.R., Lee H., Fallon M.A., Nathan D.M. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–322. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Basaria S. Androgen deprivation therapy, insulin resistance, and cardiovascular mortality: an inconvenient truth. J Androl. 2008;29:534–539. doi: 10.2164/jandrol.108.005454. [DOI] [PubMed] [Google Scholar]
- 31.Lester-Coll N.H., Goldhaber S.Z., Sher D.J., D’Amico A.V. Death from high-risk prostate cancer versus cardiovascular mortality with hormonal therapy: a decision analysis. Cancer. 2013;119:1808–1815. doi: 10.1002/cncr.27980. [DOI] [PubMed] [Google Scholar]
- 32.O’Farrell S., Garmo H., Holmberg L., Adolfsson J., Stattin P., Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–1251. doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 33.Nanda A., Chen M.H., Braccioforte M.H., Moran B.J., D’Amico A.V. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 34.D'Amico A.V., Denham J.W., Crook J. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 35.Ly-Yao G., Nikita N., Keith S.W. Mortality and hospitalization risk following oral androgen signaling inhibitors among men with advanced prostate cancer by pre-existing cardiovascular comorbidities. Eur Urol. 2019;77:158–166. doi: 10.1016/j.eururo.2019.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Nunno V., Mollica V., Santoni M. New hormonal agents in patients with nonmetastatic castration-resistant prostate cancer: meta-analysis of efficacy and safety outcomes. Clin Genitourin Cancer. 2019;17:e871–e877. doi: 10.1016/j.clgc.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Margel D., Peer A., Ber Y. Cardiovascular morbidity in a randomized trial comparing GnRH-agonist and GnRH-antagonist among patients with advanced prostate-cancer and pre-existing cardiovascular disease. J Urol. 2019;202:1199–1208. doi: 10.1097/JU.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 38.Bourghardt J., Wilhelmson A.S., Alexanderson C. Androgen receptor-dependent and independent atheroprotection by testosterone in male mice. Endocrinology. 2010;151:5428–5437. doi: 10.1210/en.2010-0663. [DOI] [PubMed] [Google Scholar]
- 39.Hatakeyama H., Nishizawa M., Nakagawa A., Nakano S., Kigoshi T., Uchida K. Testosterone inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human aortic endothelial cells. FEBS Lett. 2002;530:129–132. doi: 10.1016/s0014-5793(02)03440-3. [DOI] [PubMed] [Google Scholar]
- 40.Corcoran M., Meydani M., Lichtenstein A., Schaefer E., Dillard A., Lamon-Fava S. Sex hormone modulation of proinflammatory cytokine and C-reactive protein expression in macrophages from older men and postmenopausal women. J Endocrinol. 2010;206:217–224. doi: 10.1677/JOE-10-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith M.R., Klotz L., van der Meulen E., Colli E., Tankó L.B. Gonadotropin-releasing hormone blockers and cardiovascular disease risk: analysis of prospective clinical trials of degarelix. J Urol. 2011;186:1835–1842. doi: 10.1016/j.juro.2011.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campia U., Moslehi J.J., Amiri-Kordestani L. Cardio-oncology: vascular and metabolic perspectives: a scientific statement from the American Heart Association. Circulation. 2019;139:e579–e602. doi: 10.1161/CIR.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.