Abstract
Echocardiographic imaging is crucial for patient management during cardiotoxic cancer therapy. Left ventricular ejection fraction is the most commonly used parameter for identifying left ventricular dysfunction. However, it lacks sensitivity to detect subclinical changes in cardiac function due to cardiotoxic treatment. Global longitudinal strain (GLS) is the best studied strain parameter with established diagnostic and prognostic value. Multiple studies have demonstrated changes in GLS as an early marker of cardiotoxicity. This document serves as a primer to help clinicians in the acquisition and interpretation of strain in cardio-oncology. Cases with embedded videos illustrate a step-by-step approach to obtaining GLS measurements and common pitfalls to avoid. The document includes a concise summary of the indications of GLS in cardio-oncology and its role in guiding oncological therapy. Practical approaches on how to implement strain in the echo laboratory with guidance on training and quality assurance are also discussed.
Key Words: cancer, cardiotoxicity, echocardiography, global longitudinal strain, left ventricular function
Abbreviations and Acronyms: 2D, 2-dimensional; 3D, 3-dimensional; ACC, American College of Cardiology; AL, amyloid light chains; ASE, American Society of Echocardiography; CMRI, cardiac magnetic resonance imaging; CTRCD, cancer treatment–related cardiac dysfunction; DICOM, Digital Imaging and Communications in Medicine; EACVI, European Association of Cardiovascular Imaging; GLS, global longitudinal strain; LV, left ventricle; LVEF, left ventricular ejection fraction; ROI, region of interest; STE, speckle tracking echocardiography; VEGF, vascular endothelium growth factor
Central Illustration
Highlights
-
•
Compared with LVEF, GLS is a more sensitive and reproducible measure of LV systolic function and has emerged as an early marker of cardiotoxicity.
-
•
Despite evidence supporting the clinical utility of GLS imaging, familiarity with the practical process of strain imaging among clinicians is lacking.
-
•
Although each vendor has its own proprietary software for strain imaging, the basic steps of measuring GLS are similar.
-
•
Education is needed to enhance the performance, analysis, and interpretation of GLS for the management of cardio-oncology patients.
Echocardiographic imaging has been crucial for the management of patients treated with cardiotoxic cancer agents. Although the spectrum of cardiovascular diseases in cancer patients is broad, there is a specific interest in the early detection of cardiomyopathy due to its implication for ongoing cancer treatment and the association with poor prognosis (1). Left ventricular ejection fraction (LVEF) is the most commonly used parameter for identifying left ventricular (LV) dysfunction before, during, and after cancer therapy. Although LVEF is a robust diagnostic and prognostic marker in various cardiovascular diseases, it lacks sensitivity to detect subclinical changes in cardiac function caused by early myocyte damage due to cardiotoxic treatment.
LV deformation, or strain, which is now feasible using speckle-tracking echocardiography (STE), provides a quantitative measure of cardiac contractile function. Strain imaging has been shown to have clinical utility in a variety of settings. Global longitudinal strain (GLS) is the best studied strain parameter with the largest body of literature supporting its diagnostic and prognostic value (2). It is a more sensitive and reproducible measure of LV systolic function than LVEF. GLS is considered the optimal deformation parameter for the detection of subclinical LV dysfunction. When reduction in LVEF during chemotherapy is established, it may be too late for treatment to allow complete recovery (1). GLS has emerged as an early marker of cardiotoxicity (3). But despite evidence supporting the clinical utility of this technique, familiarity with the practical process of strain imaging is lacking among clinicians. The objective of this document is to serve as a primer for echocardiography laboratories in the training of sonographers, cardiology fellows, and physicians on the use of strain imaging in cardio-oncology. This document will provide a basic understanding of LV strain measured using STE and illustrate with embedded videos a step-by-step approach to the performance, analysis, and interpretation of GLS for the management of cardio-oncology patients.
2-Dimensional STE
What is speckle tracking imaging?
Strain measures LV deformation in longitudinal, radial, and circumferential directions and represents the percent change in LV fiber length from relaxed to contractile state. LV strain is commonly measured with STE, which capitalizes on constructive and destructive interference of ultrasound backscatter from structures within the heart that are smaller than the wavelength of the ultrasound beams. Once random noise is filtered out, unique features (“speckles”) within the heart can be identified (4). Groups of these speckles can then be tracked from frame to frame over multiple segments simultaneously allowing the calculation of strain and strain rate. Optimal speckle tracking is typically achieved at frame rates between 40 and 90 frames/s at normal heart rates. Low frame rates (<40 frames/s) are associated with the loss of speckles and accuracy, whereas high frame rates can lead to noisy signals that are difficult to smooth. Long axis images are obtained to measure longitudinal strain, whereas short axis images are obtained to measure circumferential and radial strain as well as other aspects of cardiac function including rotation, torsion, and twist. GLS has shown to be more reproducible and accurate than other strain parameters for the detection of cancer therapy–related cardiotoxicity, hence it is most commonly used in clinical practice. A recent study compared cardiac magnetic resonance imaging (CMRI) and two-dimensional (2D) echocardiographic LVEF and global strain measurements for detection of cardiotoxicity and found that CMRI-derived LVEF and echocardiography-derived GLS had the optimal temporal and observer variability for detection of cancer therapy cardiotoxicity (5). Thus, in the absence of CMRI LVEF, echo 2D GLS could be considered the method with the least variability for monitoring cardiac function changes in patients receiving cancer therapy. Current advantages and limitations of 2D STE are listed in Table 1.
Table 1.
Advantages and Limitations of 2D STE
| Advantages |
| Uses standard grey-scale images obtained on routine transthoracic echocardiogram |
| Good signal to noise ratio; minimal angle dependence |
| Ability to measure strain in multiple LV segments from a single acquisition |
| High reproducibility with GLS |
| Availability of automated post-processing software to streamline GLS measurements requiring minimal user input |
| Limitations |
| Accuracy of STE measurements depends on 2D image quality |
| Inability to track speckles moving out of scan plane of the 2D image |
| Reproducibility or reliability of segmental strain values not established |
| Vendor-specific STE techniques: |
| Intervendor variability in strain measurements |
| Data stored in proprietary scan line format not analyzable using another vendor software |
2D = 2-dimensional; GLS = global longitudinal strain; LV = left ventricle; STE = speckle tracking echocardiography.
Three-dimensional (3D) strain measurements can be obtained using 3D STE, which is inherently better for analyzing complex myocardial fiber architecture and 3D mechanics, overcoming the limitations of 2D STE. An important advantage of 3D STE is that speckles can be followed in any direction including out of plane motion, enabling the simultaneous calculation of all strain parameters from a single volumetric dataset of the LV. Video 1 demonstrates an example of 3D GLS measurement using a vendor-neutral system (4D LV Analysis; Tom Tec Imaging System, Munich, Germany) capable of processing images in the DICOM (Digital Imaging and Communications in Medicine) format. Besides saving time, it avoids errors caused by heart rate variability with multiple image acquisitions needed for 2D STE. Although studies have shown incremental value of 3D STE over 2D STE for the detection of cardiotoxicity (6), at the present time 3D STE is predominantly a research tool due to a number of critical limitations preventing its widespread use in real-world everyday practice. The ability to obtain good image quality with sufficient frame rate requires dedicated training and skill, and the need for an excellent acoustic window limits 3D STE applicability in a significant portion of patients. Furthermore, the lack of standardization of algorithms and definitions used among vendors, as well as lack of data on normative values, represent major barriers to implementation in clinical practice. In summary, despite significant technological advances in 3D STE, further progress is needed to establish its feasibility and incremental clinical value over conventional 2D STE.
Calculation of GLS
Global strain is calculated by computing the deformation using the entire LV layer specific line length or by averaging the values computed from various segments. Selection of the region of interest (ROI) for GLS may include endocardial, midwall, epicardial, or full-thickness strain. Although measurement of endocardial GLS was specifically chosen by the American Society of Echocardiography (ASE)/European Association of Cardiovascular Imaging (EACVI) task force when comparing intervendor global strain differences because this was the only parameter that could be provided by all vendors, data thus far have not favored one method over another (7). Strain values may differ across layers of the LV such that longitudinal strain is highest at the endocardium and lowest at the epicardium (8). Hence, the location where strain is measured should be noted, particularly when comparisons between measurements are made. The fact that different vendors’ software packages provide different strain measurements as a default partially contributes to the intervendor variability of strain measurements. Calculation of GLS requires a reference timepoint to report displacement or deformation. End-diastole is commonly defined as the beginning of the QRS complex on electrocardiography or at the largest LV diameter or volume on 2D echo. End-systole is often defined by the end of spectral tracing of the Doppler of the aortic valve flow or by visualization of aortic valve closure in the apical long-axis view. Alternatively, the nadir of a volume curve has also been used as a surrogate of end-systole. Options for strain measurements include peak systolic strain (highest value in systole), end-systolic strain (strain measurements at the pre-defined end-systolic point), and peak strain (highest strain through the entire cardiac cycle). The EACVI/ASE/Industry Task Force recommends reporting end-systolic strain as the default parameter with additional parameters reported as needed (9).
When To Use Strain: Which Patients? Which Oncology Therapies?
Some of the earliest studies investigating clinical applications of 2D echocardiography-measured strain were conducted in oncology populations. Among patients with breast cancer receiving anthracyclines (e.g., doxorubicin or epirubicin) and/or trastuzumab, LV deformation imaging was shown to detect preclinical myocardial injury and predict development of subsequent LVEF decrease (10). In a systematic review (2), an early decrease in peak systolic GLS was identified as the best predictor of a subsequent decrease in LVEF or heart failure across 8 studies including 452 patients with cancer treated with anthracyclines and/or trastuzumab. These findings, coupled with the growth and maturation of STE, have accelerated the interest in incorporating strain into cardio-oncology decision making. Indeed, the ASE/EACVI expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy recommended the use of GLS in the evaluation of patients before, during, and after cancer therapy (11). Although the predictive value of echocardiographic strain continues to be demonstrated (3), several challenges have limited its widespread use and are discussed in this article.
Current indications for echocardiographic assessment in oncology patients
Most guideline and consensus documents recommend routine LVEF assessment, preferably using echocardiography, before initiation of anthracyclines or HER2 (human epidermal growth factor receptor 2)-molecular targeted therapies (e.g., trastuzumab) (11,12). Similarly, echocardiographic imaging is recommended in oncology patients presenting with clinical signs or symptoms concerning for cardiac dysfunction. In contrast, the frequency and need for surveillance imaging during and after the completion of oncology treatment in asymptomatic patients have been the subject of active debate (13,14). Routine imaging assessment every 3 months during treatment and every 6 months for 2 years after treatment with any HER2-targed therapies (trastuzumab, pertuzumab, and ado-trastuzumab emtasine [T-DM1]) has been recommended by the Food and Drug Administration, although with varying rates of adoption (15). The American Society of Clinical Oncology guideline recommends that the frequency of surveillance imaging during active cancer treatment be determined by the provider based on the patient’s risk, while a single follow-up echocardiogram may be considered 6 to 12 months after treatment among patients at increased risk (12).
Although cardiac dysfunction and reduced strain have been reported in the setting of other cancer therapeutics, in particular, agents inhibiting the vascular endothelium growth factor (VEGF) pathway (such as sunitinib, sorafenib, and bevacizumab) (16) and proteasome inhibitors (17), the American Society of Clinical Oncology guideline on surveillance for cardiac dysfunction notes an inability to make a standard recommendation regarding LVEF assessment prior to the initiation of treatment with non-anthracycline– and non-HER2–containing regimens given the insufficient evidence (12). In contrast, the ASE/EACVI consensus document in 2014 recommended LVEF assessment with other targeted agents, in particular VEGF inhibitors at baseline and during treatment, largely extrapolating from trastuzumab data (8). Among the many reasons for discrepancies in recommendations are the lack of inclusion of cardiac imaging in most oncology clinical trials and the absence of evidence that cardiac function monitoring leads to improved patient outcomes (11). With rapid evolution of oncology treatments and increasing awareness of complex cardiovascular effects, it is likely that recommendations for cardiac imaging with specific oncological therapies will evolve. For example, the recently published European Society of Medical Oncologists document on management of cardiac disease in patients with cancer recommends baseline LVEF evaluation in patients treated with VEGF inhibitors and certain proteasome inhibitors, such as carfilzomib (18).
Value of strain before, during, and after completion of oncological therapy
In patients scheduled to receive anthracyclines and/or HER2 targeted therapy, measurement of strain is recommended as part of a comprehensive cardiovascular and echocardiographic assessment (11,12). GLS before anthracycline treatment has demonstrated superiority compared with LVEF in the prediction of heart failure (19). In a recent study that developed a risk score model for predicting heart failure in patients with leukemia after anthracycline treatment, GLS alone was independently associated with all-cause mortality after adjusting for age and leukemia type (20). A population in whom strain may be especially useful are patients with LVEF in the lower limits of normal (21). Abnormally low strain among patients with normal LV systolic function requires further investigation to identify potential causes such as cardiac amyloidosis, infiltrative processes, or hypertensive cardiomyopathy. The decision to initiate cancer therapy in patients with reduced baseline strain depends on the underlying etiology which in turn will drive personalized multidisciplinary discussion about potential treatment options and optimal cancer therapy (22).
Decrease in LV strain during cancer therapy has been well described among patients receiving trastuzumab and/or anthracyclines. In the ASE/EACVI consensus document, a 15% worsening in GLS is defined as clinically significant, likely indicative of subclinical LV dysfunction (11). In a recent meta-analysis of 21 studies comprising 1,782 patients with various cancer diagnoses, Oikonomou et al. (3) found that the absolute GLS values and a relative GLS decrease from baseline to during treatment were each predictive of subsequent cancer treatment–related cardiac dysfunction (CTRCD). The range of identified GLS cut-off values was wide, reflecting heterogeneity in sample size and CTRCD definitions as well as publication bias. Together, these studies support GLS as an independent prognostic marker of subsequent cardiac dysfunction in patients receiving anthracyclines and/or trastuzumab, however, larger prospective multicenter studies are needed to define and validate optimal cut-off ranges.
Another subgroup of oncology patients in whom assessment of LV strain carries clinical relevance is light-chain cardiac amyloidosis (AL). It is important to emphasize that GLS is an important echocardiographic tool for the diagnosis of cardiac amyloidosis, regardless of etiology, and recent growth of available therapeutic options for patients with transthyretin amyloidosis is likely to increase the use of GLS in screening at risk populations. Among patients with AL cardiac amyloidosis, reduced GLS has been shown to predict survival and has been proposed as a prognostic marker in risk stratification of patients undergoing hematopoietic stem cell transplantation (23,24).
After cancer therapy, the St. Jude Lifetime Cohort Study showed that the prevalence of cardiac dysfunction, defined by decreased GLS, was 31.8% at a median interval of 23 years from diagnosis in long-term adult survivors of childhood cancers treated with anthracyclines, chest radiation, or both. Abnormal GLS was associated with treatment exposure (25). A more recent report from the same cohort identified an association between reduced strain and exercise intolerance, which represents an important predictor of long-term outcomes in this population (26). Similar findings of the high prevalence of abnormal GLS among long-term cancer survivors exposed to anthracyclines and/or chest radiotherapy have been reported in other studies (27,28). Furthermore, an association between reduced GLS and impaired cardiopulmonary fitness many years after cancer therapy, as measured by peak oxygen consumption, has recently been reported among survivors of breast cancer that developed cardiotoxicity during trastuzumab treatment (29).
Value of strain in guiding oncological therapy
Although decrease in LV strain may indicate an increased risk of CTRCD, there are no data to support routine holding or stopping of oncological therapy. Rather, the findings should initiate a conversation between oncology and cardiology, or cardio-oncology teams where available, for investigation of possible contributing causes and consideration of preventive strategies (22). As an example, presence of hypertension has been associated with reduced strain as well as increased incidence of heart failure among cancer survivors who received anthracycline-based therapy (30). Other disease states that may affect strain measurements such as concurrent coronary artery disease should also be considered. The prospective SUCCOUR (Strain Surveillance of Chemotherapy for Improving Cardiovascular Outcomes) trial (31) aims to identify whether reduced strain can guide a therapeutic intervention (initiation of neurohormonal blockade) (32) that will limit the development of reduced LVEF. The 1-year interim results, presented at the European Society of Cardiology Congress 2020, demonstrated a decreased incidence of cardiotoxicity in the GLS-guided arm compared with the standard LVEF-guided arm, suggesting that GLS was an effective marker for identifying subclinical cardiotoxicity that responded to beta blocker and angiotensin-converting enzyme inhibitor therapy. These initial findings are encouraging for the use of GLS in cardiotoxicity surveillance and support the use of cardioprotective medications on detection of subclinical dysfunction using GLS. However, the primary endpoint, change in 3D LVEF from baseline, was not different between the 2 arms at 1-year follow-up. Final analysis of the primary and secondary endpoints on completion of 3-year follow-up will hopefully provide more evidence to understand the potential role of GLS in the detection and management of CTRCD.
In summary, the main use of strain in contemporary oncology care is to improve risk prediction and stratification with the potential to guide cardioprotective therapy in patients receiving potentially cardiotoxic therapies (Central Illustration). Its impact on clinical decision making is likely to grow and will be determined by the prospective incorporation of this imaging modality in oncology clinical trials and the establishment of its association with cardiovascular and oncology outcomes. In addition to changes in LVEF, we feel that changes in GLS should be included in oncology clinical trial endpoints. Future investigations should focus on incorporating cardiac imaging with targeted and other cancer therapies.
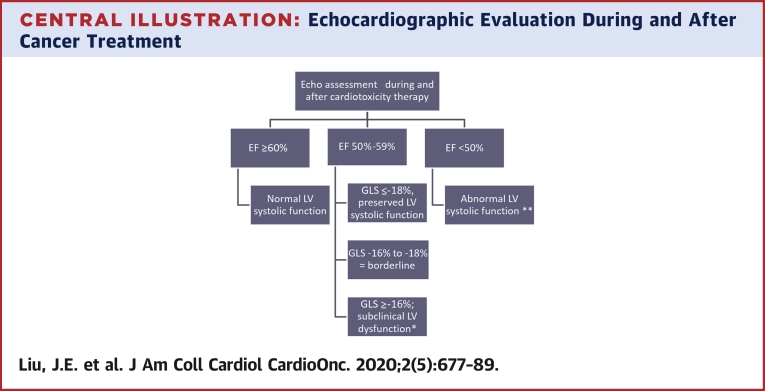
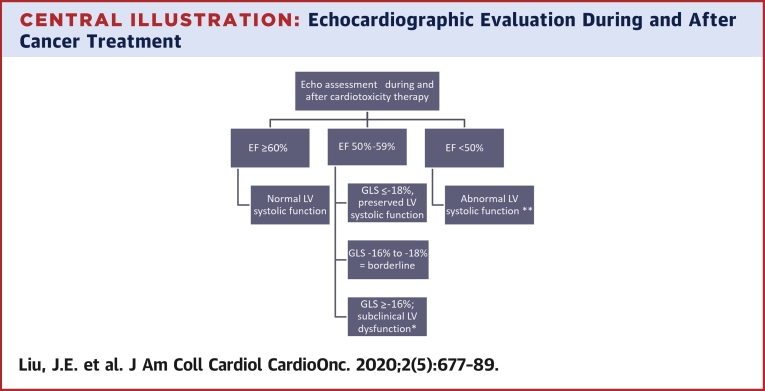
Central Illustration.
Echocardiographic Evaluation During and After Cancer Treatment
Proposed algorithm on the use of left ventricular ejection fraction (LVEF) and global longitudinal strain (GLS) to guide clinical decision making pertaining to asymptomatic patients at risk for left ventricular (LV) dysfunction. Patients with cardiac symptoms warrant additional evaluation. ∗Marker of increased risk of cancer therapeutic related cardiac dysfunction. Optimize existing cardiovascular risk factors, consider cardioprotective medications. ∗∗Initiate cardioprotective medications as per American College of Cardiology/American Heath Association guideline for the management of Stage B heart failure.
How To Obtain 2D Images And Measure GLS
Many ultrasound manufacturers now offer the capability to perform STE with user-friendly postprocessing software that streamlines GLS measurement. The necessary images are acquired on the echocardiography machine and the STE analysis can be performed online on the machine itself or offline on a workstation.
Step-by-step instructions on image acquisition and analysis
Strain imaging using STE is a gray scale–based technique that relies on proper image acquisition for accurate measurement and high reproducibility of the strain data (Table 2, Video 2). Although each system uses its own proprietary software for LV deformation imaging, the basic steps of measuring GLS are very similar (Table 3). Step-by-step instructions on how to measure GLS with commonly used software platforms are illustrated in clinical scenarios (case 1 to 3) during the cancer treatment continuum.
Table 2.
Image Acquisition
|
|
|
|
|
|
|
|
|
|
See Video 2.
ECG = electrocardiogram; LV = left ventricle; RV = right ventricle.
Table 3.
Steps for GLS Measurement Common to Various Software
| Step 1 | Image choice: select the best image acquired for each view for analysis. |
| Step 2 | Event timing: define timing of end-systole (see Calculation of GLS to define) |
| Step 3 | Software border detection: automated vs. semi-automated tracing with fiducial marking of the mitral annulus and apex. Ensure that the contour borders do not extend pass the mitral annulus into the left atrium. |
| Step 4 | Tracking quality: visually inspect the moving images to determine the adequacy of tracking; check that the software tracing actually moves with the underlying myocardium. Manually adjust ROI or endocardial contour to optimize tracking if necessary. Exclude segments that do not adequately track after 3 attempts to optimize. |
| Step 5 | Analysis: after speckle tracking is performed in the 3 apical views, the strain values for all the segments are integrated to obtain GLS. Visually inspect the curves from each view for any segment that is a clear outlier. |
| Step 6 | Verification: if there are clear outliers in the examination of the strain curves, return to the tracking to ensure that the image quality in that segment is appropriate and that tracking is accurate. |
GLS = global longitudinal strain; ROI = region of interest.
Case 1
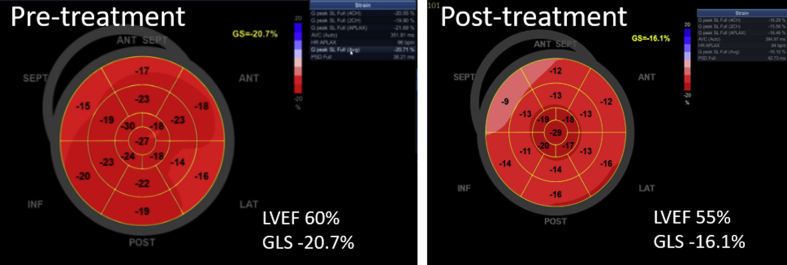
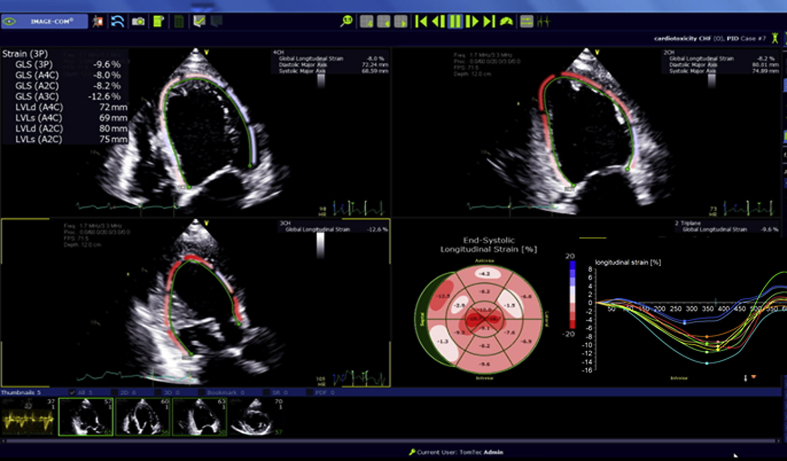
A 42-year-old woman with invasive HER2+ breast cancer planned to receive doxorubicin-based chemotherapy followed by trastuzumab and pertuzumab. Pretreatment LVEF and GLS were 60% and -20.7%, respectively. Step-by-step approach to measuring GLS using automated function imaging (GE Medical Systems, Waukesha, Wisconsin) is shown in Videos 3 to 5 (Figure 1). Follow-up echocardiogram post–doxorubicin treatment showed a modest LVEF decrease to 55% but a significant GLS decrease to -16.0% (Figure 2).
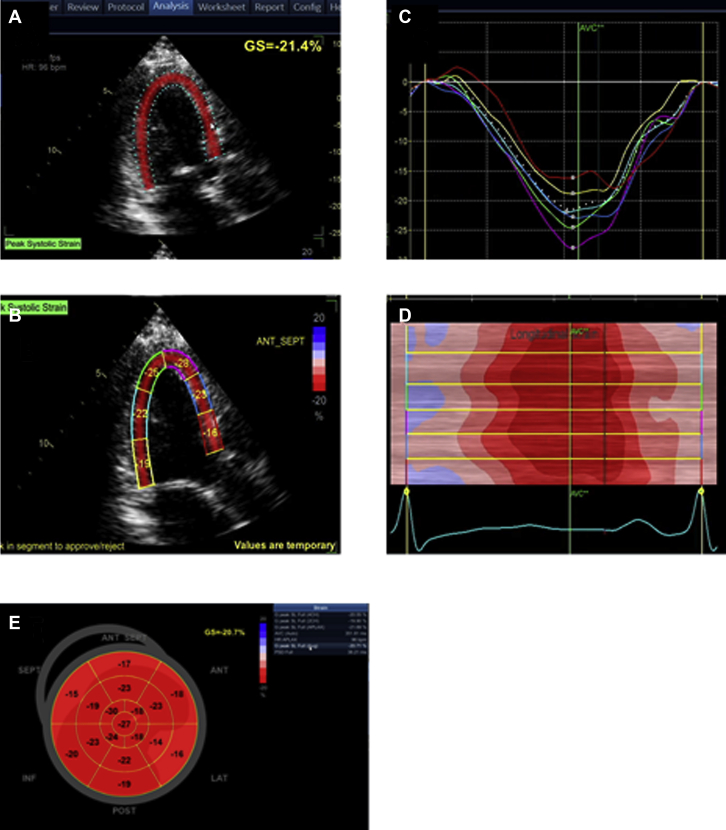
Figure 1.
Display of Segmental and GLS
(A) Parametric image provides a quick, visual impression of the timing and the extent of segmental LV deformation with tracking of the full LV (left ventricular) thickness. In this software, calculated GLS (global longitudinal strain) represents the average of all 3 layers of the LV. The cardiac motion is color coded with shortening displayed as a shade of red. The higher the shortening, the darker the shade. (B and C) A quantitative segmental peak systolic strain can be assigned for each segment. A color-coded strain curve is generated for each of the 6 LV segments per apical view. The strain curves are displayed as a negative wave below the baseline because the myocardium generally shortens in the longitudinal direction during systole and should peak around aortic valve closure. (D) The time-strain plot displays cardiac deformation of all the segments simultaneously at a specific time point in the cardiac cycle. The x-axis represents time during the cardiac cycle and the y-axis represents the 6 color-coded LV segments. (E) After finishing the measurements in the 3 planes, a bullseye (BE) display of the peak or systolic segmental and global strain values is generated using a 17 or 18 LV segment model. The BE plot is color coded such that shortening is displayed as a shade of red and lengthening is displayed as blue. The higher the percentage of shortening, the darker the shade. When multiple segments are color-coded blue (i.e., lengthening is systole), it is worthwhile rechecking those segments to ensure good image quality and appropriate tracking.
Figure 2.
Change in GLS Precedes LVEF Decrease
The bullseye plot demonstrates a significant change global longitudinal strain (GLS) (-20.7% to -16.1%) whereas only a mild decrease in left ventricular (LV) ejection fraction (EF) (60% to 55%) from pre- to post-anthracycline treatment.
Case 2
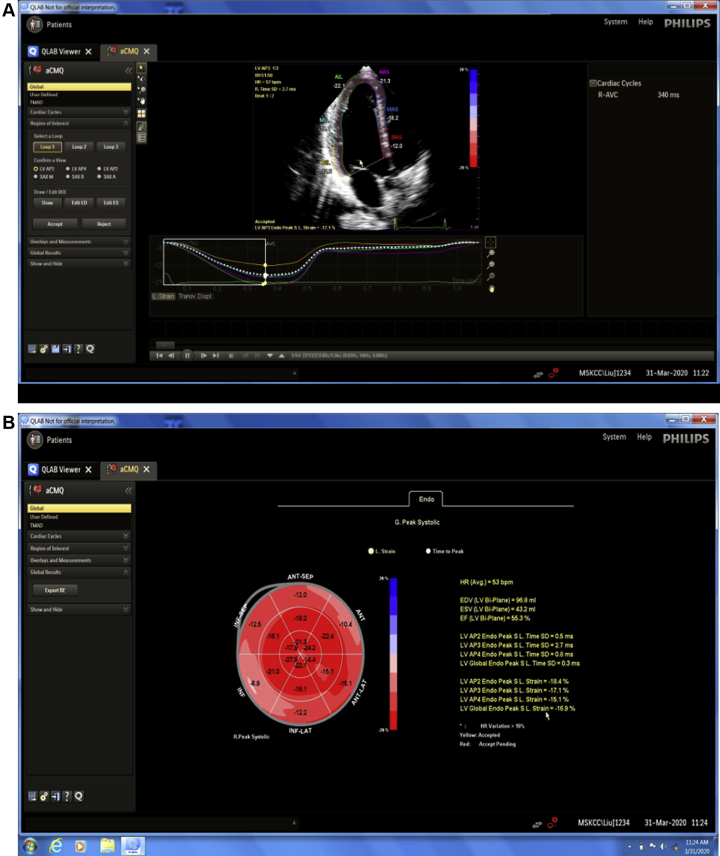
A 65-year-old woman with hypertension, diabetes, and invasive ER/PR (estrogen receptor/progesterone receptor) (+), HER2+ breast cancer. Pretreatment echo showed LVEF of 57% with low normal GLS of -17%. The GLS was measured with Automated Cardiac Motion (aCMQ, Philips, Amsterdam, the Netherlands), which is demonstrated in Videos 6 and 7 (Figure 3).
Figure 3.
Display of Longitudinal Strain
This software provides automated tracing of the endocardial border. It allows the user to select where strain should be calculated, either along the endocardium, along the mid-wall, or along the epicardium. Endocardial longitudinal strain is most commonly used in clinical practice and, hence, set as the default option. The user can also select the timing of the strain measurement at peak strain, peak systolic strain, or end systolic strain although peak systolic strain is most widely used. (A) The software displays segmental and global strain for each of the apical views and strain curves for each of the 6 segments per view. (B) A bullseye plot is generated after completion of strain analysis in all 3 apical views, then integrated to derive global longitudinal strain. The same principles of border adjustment, visualization of tracking, and assessment of strain curves should be performed as described.
Case 3
A 72-year-old woman with hypertension and history of breast cancer with no evidence of disease, status post treatment with doxorubicin-based chemotherapy followed by trastuzumab and radiation therapy, presented with acute heart failure 5 years after treatment. Echo showed EF of 30% and GLS of -10.2%. The GLS was measured with AutoStrain (Image Arena, Tom Tec Imaging System, Germany), a vendor-neutral system capable of processing images in the DICOM format, as demonstrated in Video 8 (Figure 4). A PVC (premature ventricular contraction) beat is noted in the image clip, which should be avoided when measuring GLS.
Figure 4.
Display of Segmental and GLS
Tracking of the endocardial layer with calculation of segmental and global strain for each apical view was then integrated to derive a global strain value. This software measures endocardial longitudinal strain and it is important to place the strain tracing along the endocardial border. A bullseye plot demonstrating segmental strain values and waveforms for each of the 16 segments are generated. The same principles of border adjustment, visualization of tracking, and assessment of strain curves should be performed as described.
Tips and tricks for measuring strain
To obtain accurate and reproducible data, there are common pitfalls to avoid when measuring strain that can lead to erroneous GLS calculation.
-
1.
Image acquisition
| Pitfalls | Impact on GLS |
| Poor endocardial definition | Suboptimal speckle tracking causing error in GLS measurement |
| Foreshortened apex | Can cause overestimation of apical strain |
| Not including all LV segments in the sector | Missing strain for the LV segments not included or inadequate tracking of partially included segments |
| Images captured at different heart rates | Precludes integration of the 3 apical views to derive GLS |
| Low frame rate (<40 frames/s) | Decreases the quality of the tracking: beware when using compressed DICOM cine loops |
| Use of contrast | Strain images should be obtained before use of contrast. Use of contrast decreases reliability of the tracking algorithms. There is currently no commercially available software that reliably tracks speckles in the presence of echo contrast, although software is currently under development. Until approved software is available, strain values should not be reported in the presence of echo contrast. |
-
2.
Tracking
| Suboptimal tracking | Leads to strain values discordant with the visual wall motion assessment and nonphysiological waveform tracing (Video 9) |
| Tracking mimicking structures (papillary muscle/trabeculation) | Underestimates GLS due to lower strain derived from nonmyocardial segments (Video 10) |
| Tracking the pericardium | Results in underestimation of LV strain |
-
3.
Tracing contour/ROI
-
4.
Timing
| Incorrect timing of end-systole | Varying approaches to defining end systole depending on the software. Some systems use beginning of QRS complex and end of T wave to define event timing and, thus, it is important to have a good quality electrocardiography tracing. Setting end systole too early or too late can affect peak strain in some segments and lead to inaccurate GLS measurement (Video 13). It is important to know timing of the strain measurement: peak strain from the entire cardiac cycle, peak systole, or end systole |
Interpreting and reporting GLS values
There is significant heterogeneity in the normal ranges of GLS in published reports (33). Multiple factors can influence STE-based measurement of GLS. These include patient-specific factors such as age, gender, and loading conditions (blood pressure) as well as technical factors related to differences in software packages and algorithms between vendors. The negative sign of systolic GLS can lead to confusion when describing increases or decreases in strain as an increase in contraction leads to a decrease in arithmetic value of strain. Thus, there are some that advocate to express the absolute value of strain to avoid confusion when communicating changes in strain values (34). As a general guide in adults, GLS >-16% is considered abnormal, GLS <-18% normal, and GLS -16% to -18% borderline (7). It is important to consider the use of the same vendor for longitudinal follow-up due to potential intervendor variability in strain measurements. Baseline GLS should include the value as well as the vendor software used. When reporting comparison with prior measurements, GLS and a relative change from the prior measurement should be included given its value for predicting cardiotoxicity (3). Furthermore, there needs to be direct communication between the readers and oncology care providers to facilitate communication of a strategy for managing the results.
How to Implement
Practical approaches
Strain has been recognized by the ASE as a full component of the echocardiographic examination (35). Laboratories should develop protocols to acquire apical views for measurement of global longitudinal strain. When setting up strain imaging in the echo lab, we recommend that one start by training a small group of sonographers to gain expertise, then spreading this expertise to the rest of the laboratory. Currently, there is no societal recommendation to acquire dedicated parasternal short axis views for measurement of circumferential strain or torsion. Strain rate has not been used routinely in clinical practice. Analysis can be performed directly on the machine or on a separate station. The latest strain measurement software program is fully automated, however, in the high-throughput workflow of most echocardiographic laboratories, strain is most often measured after image acquisition has ended. Serial strain imaging studies should be performed on the same ultrasound system to reduce variability caused by different equipment and software (35).
For successful implementation of strain in clinical practice, there needs to be institutional recognition of the diagnostic and therapeutic value of strain imaging to improve patient care along with a commitment to invest in the latest software with artificial intelligence for adequate tracking of strain images and incorporation into hospital image visualization and analysis systems to efficiently allow readers to review and retrace strain images as appropriate. The Centers for Medicare and Medicaid Services established the myocardial strain imaging CPT (current procedural terminology) code +93356 in January 2020, acknowledging GLS imaging as a clinically useful diagnostic service.
Training and quality
The 2019 American College of Cardiology (ACC)/American Heart Association/ASE Advanced Training Statement on Echocardiography recommends that strain be incorporated into training in echocardiography (36). In particular, level III training should include understanding of the principles of LV mechanics, of the acquisition, analysis, and interpretation of strain/strain rate, of the applications of these parameters, and of the limitations and pitfalls of these measurements. For level III echocardiography training, the ACC Competency Management Committee recommends the interpretation of at least 50 studies that involve strain and strain rate assessment. A recent study demonstrated that expert competency in the tracing and interpretation of GLS (based on the intraclass correlation coefficient >0.90 of a previously novice reader with an expert reader) could be achieved with a minimum of 50 studies (37). The recently published opinion paper of the ACC Cardio-oncology Council on the preparation of the workforce to practice cardio-oncology emphasizes the need to acquire knowledge in echocardiography and imaging (38). Assessment of the laboratory intraobserver and interobserver variability of strain tracing is recommended. Quality improvement sessions both for sonographers and echocardiography readers should include strain measurements. Accreditation agencies for echo laboratories (IAC-Echo) should require laboratories to submit high-quality strain cases as a requirement for accreditation.
Conclusions
Although there have been great advances in the research applications of strain and strain rate, widespread acceptance and routine use of the technique have been slow. There have been significant gains in the speed of strain analysis, theoretically allowing its efficient implementation in a busy laboratory. One important obstacle has been the variability in the acquisition and analysis of strain. Laboratories that desire optimal implementation of strain into clinical practice should develop and follow careful acquisition protocols, standardization of the positioning of the ROI, and detailed feedback and quality improvement sessions among the team. Such approaches will help the standardization of strain and allow its use in cardiovascular diseases, particularly for cardio-oncology.
Author Disclosures
Dr. Liu has received consultant fees for Bay Labs, Pfizer, and Philips; and is supported by the National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support Grant (P30 CA008748). Dr. Barac has received advisory fees for Bristol Myers Squibb and Takeda Inc. Dr. Thavendiranathan is supported by the Canadian Institutes of Health Research New Investigator Award; and has been on the Speakers Bureau of Amgen, Takeda, and Blood Institute. Dr. Scherrer-Crosbie is supported by NIH-NHLBI R01 130539.
Footnotes
Judy Mangion, MD, served as Guest Associate Editor for this paper. Anju Nohria, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
3D GLS of the LV. Using 4D LV Analysis (Tom Tec Imaging System, Germany), 3D speckle tracking strain measurement of GLS from a 3D full volume of the LV. Measurements are performed along the endocardial border. A 16-segment bullseye plot is generated. 3D = three-dimensional; 4D = four-dimensional; GLS = global longitudinal strain; LV = left ventricle.
2D Image Acquisition. Acquire 3 apical views sequentially to ensure similar heart rates and frame rates. Optimize image quality and avoid foreshortening. See Table 2 for further details. 2D = two-dimensional.
GLS Measurement With Automated Function Imaging (GE Medical Systems). Start with apical 3 chamber view. Tracing is automated. Region of interest (ROI) should cover the full LV thickness from the endocardial to the epicardial border. Important to assess tracking quality to confirm that the bullets are moving with the underlying myocardium. See Table 3 for further details. Abbreviations as in Video 1.
GLS Measurement With Automated Function Imaging (GE Medical Systems). Proceed with the next 2 views (AP4, AP2). Repeat the same steps as above. The ROI should be at the annulus and not in the left atrium. Abbreviations as in Video 1 and 3.
Event Timing. Timing of end systole can also be identified using the timing of the aortic valve closure measured off the spectral Doppler of the aortic valve.
GLS Measurement With Automated Cardiac Motion (Philips Healthcare). Tracing is automated. Important to assess that the automated software detection is correctly detecting the endocardial border and tracking the underlying myocardium. Manually adjust the endocardial contour to optimize tracking if necessary. Abbreviation as in Video 1.
GLS Measurement With Automated Cardiac Motion (Philips Healthcare). Proceed with the next 2 views (AP4, AP2). After the 3 views are completed, a bullseye plot is generated. Abbreviation as in Video 1.
GLS Measurement With AutoStrain (Image Area, Tom Tec Imaging System). GLS measurement using a vendor-neutral system capable of processing images in the DICOM format. Abbreviation as in Video 1.
Suboptimal Tracking. Suboptimal tracking as shown in the video leads to abnormal or suspicious strain values with nonphysiological waveform tracing that are discordant with the visual wall motion.
Tracking Mimicking Structures. The automated software detection tracking papillary muscle instead of the LV endocardial border. Abbreviation as in Video 1.
Marking of the Annulus. ROI should be placed at the insertion of the mitral leaflets. ROI in the left atrium as shown in the video leads to abnormal strain of the basal segments. Abbreviation as in Video 3.
ROI Placement of the LV Walls. ROI including the pericardium can lead to underestimation of GLS. Abbreviations as in Videos 1 and 3.
Incorrect Timing of End-Systole. Incorrect timing of end systole due to poor electrocardiogram (ECG) tracing can affect peak strain in some segments and lead to inaccurate GLS measurement. Abbreviation as in Video 1.
References
- 1.Cardinale D., Colombo A., Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 2.Thavendiranathan P., Poulin F., Lim K.D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 3.Oikonomou E.K., Kokkinidis D.G., Kampaktsis P.N. Assessment of prognostic value of left ventricular global longitudinal strain for early prediction of chemotherapy-induced cardiotoxicity: a systematic review and meta-analysis. JAMA Cardiol. 2019;4:1007–1018. doi: 10.1001/jamacardio.2019.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pirat B., Khoury D.S., Hartley C.J. A novel feature-tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia-reperfusion. J Am Coll Cardiol. 2008;51:651–659. doi: 10.1016/j.jacc.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lambert J., Lamacie M., Thampinathan B. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart. 2020;106:817–823. doi: 10.1136/heartjnl-2019-316297. [DOI] [PubMed] [Google Scholar]
- 6.Zhang K.W., Finkelman B.S., Gulati G. Abnormalities in 3-dimensional left ventricular mechanics with anthracycline chemotherapy are associated with systolic and diastolic dysfunction. J Am Coll Cardiol Img. 2018;11:1059–1068. doi: 10.1016/j.jcmg.2018.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farsalinos K.E., Daraban A.M., Unlu S., Thomas J.D., Badano L.P., Voigt J.U. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: The EACVI/ASE Inter-Vendor Comparison Study. J Am Soc Echocardiogr. 2015;28:1171–1181,e2. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Nagata Y., Wu V.C., Otsuji Y., Takeuchi M. Normal range of myocardial layer-specific strain using two-dimensional speckle tracking echocardiography. PloS One. 2017;12 doi: 10.1371/journal.pone.0180584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voigt J.U., Pedrizzetti G., Lysyansky P. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. 2015;16:1–11. doi: 10.1093/ehjci/jeu184. [DOI] [PubMed] [Google Scholar]
- 10.Sawaya H., Sebag I.A., Plana J.C. Assessment of echocardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circulation Cardiovasc Imaging. 2012;5:596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 13.Kenigsberg B., Wellstein A., Barac A. Left ventricular dysfunction in cancer treatment: is it relevant? J Am Coll Cardiol HF. 2018;6:87–95. doi: 10.1016/j.jchf.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 14.Dang C.T., Yu A.F., Jones L.W. Cardiac Surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. 2016;34:1030–1033. doi: 10.1200/JCO.2015.64.5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chavez-MacGregor M., Niu J., Zhang N. Cardiac monitoring during adjuvant trastuzumab-based chemotherapy among older patients with breast cancer. J Clin Oncol. 2015;33:2176–2183. doi: 10.1200/JCO.2014.58.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nhola L.F., Abdelmoneim S.S., Villarraga H.R. Echocardiographic assessment for the detection of cardiotoxicity due to vascular endothelial growth factor inhibitor therapy in metastatic renal cell and colorectal cancers. J Am Soc Echocardiogr. 2019;32:267–276. doi: 10.1016/j.echo.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Cole D.C., Frishman W.H. Cardiovascular complications of proteasome inhibitors used in multiple myeloma. Cardiol Rev. 2018;26:122–129. doi: 10.1097/CRD.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 18.Curigliano G., Lenihan D., Fradley M. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. 2020;31:171–190. doi: 10.1016/j.annonc.2019.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali M.T., Yucel E., Bouras S. Myocardial strain is associated with adverse clinical cardiac events in patients treated with anthracyclines. J Am Soc Echocardiogr. 2016;29:522–527e3. doi: 10.1016/j.echo.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y., Assuncao B.L., Denduluri S. Symptomatic heart failure in acute leukemia patients treated with anthracyclines. J Am Coll Cardiol CardioOnc. 2019;1:208–217. doi: 10.1016/j.jaccao.2019.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mousavi N., Tan T.C., Ali M., Halpern E.F., Wang L., Scherrer-Crosbie M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50-59% treated with anthracyclines. Eur Heart J Cardiovasc Imaging. 2015;16:977–984. doi: 10.1093/ehjci/jev113. [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Banchs J., Mousavi N. Contemporary role of echocardiography for clinical decision making in patients during and after cancer therapy. J Am Coll Cardiol Img. 2018;11:1122–1131. doi: 10.1016/j.jcmg.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 23.Pun S.C., Landau H.J., Riedel E.R. Prognostic and added value of two-dimensional global longitudinal strain for prediction of survival in patients with light chain amyloidosis undergoing autologous hematopoietic cell transplantation. J Am Soc Echocardiogr. 2018;31:64–70. doi: 10.1016/j.echo.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buss S.J., Emami M., Mereles D. Longitudinal left ventricular function for prediction of survival in systemic light-chain amyloidosis: incremental value compared with clinical and biochemical markers. J Am Coll Cardiol. 2012;60:1067–1076. doi: 10.1016/j.jacc.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong G.T., Joshi V.M., Ness K.K. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ness K.K., Plana J.C., Joshi V.M. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J Clin Oncol. 2020;38:29–42. doi: 10.1200/JCO.19.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu A.F., Raikhelkar J., Zabor E.C. Two-dimensional speckle tracking echocardiography detects subclinical left ventricular systolic dysfunction among adult survivors of childhood, adolescent, and young adult cancer. Biomed Res Int. 2016;2016:9363951. doi: 10.1155/2016/9363951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai H.R., Gjesdal O., Wethal T. Left ventricular function assessed by two-dimensional speckle tracking echocardiography in long-term survivors of Hodgkin's lymphoma treated by mediastinal radiotherapy with or without anthracycline therapy. Am J Cardiol. 2011;107:472–477. doi: 10.1016/j.amjcard.2010.09.048. [DOI] [PubMed] [Google Scholar]
- 29.Yu A.F., Flynn J.R., Moskowitz C.S. Long-term cardiopulmonary consequences of treatment-induced cardiotoxicity in survivors of ERBB2-positive breast cancer. JAMA Cardiol. 2020;5:309–317. doi: 10.1001/jamacardio.2019.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marwick T.H., Gillebert T.C., Aurigemma G. Recommendations on the use of echocardiography in adult hypertension: a report from the European Association of Cardiovascular Imaging (EACVI) and the American Society of Echocardiography (ASE) Eur Heart J Cardiovasc Imaging. 2015;16:577–605. doi: 10.1093/ehjci/jev076. [DOI] [PubMed] [Google Scholar]
- 31.Negishi T., Thavendiranathan P., Negishi K., Marwick T.H. SUCCOUR investigators. Rationale and design of the strain surveillance of chemotherapy for improving cardiovascular outcomes: The SUCCOUR Trial. J Am Coll Cardiol Img. 2018;11:1098–1105. doi: 10.1016/j.jcmg.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Barac A. Optimal treatment of Stage B heart failure in cardio-oncology?: the promise of strain. J Am Coll Cardiol Img. 2018;11:1106–1108. doi: 10.1016/j.jcmg.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Yingchoncharoen T., Agarwal S., Popovic Z.B., Marwick T.H. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell C., Rahko P.S., Blauwet L.A. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr. 2019;32:1–64. doi: 10.1016/j.echo.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Wiegers S.E., Ryan T., Arrighi J.A. 2019 ACC/AHA/ASE Advanced Training Statement on Echocardiography (Revision of the 2003 ACC/AHA Clinical Competence Statement on Echocardiography): A Report of the ACC Competency Management Committee. J Am Coll Cardiol. 2019;74:377–402. doi: 10.1016/j.jacc.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Chan J., Shiino K., Obonyo N.G. Left ventricular global strain analysis by two-dimensional speckle-tracking echocardiography: the learning curve. J Am Soc Echocardiogr. 2017;30:1081–1090. doi: 10.1016/j.echo.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Hayek S.S., Ganatra S., Lenneman C. Preparing the cardiovascular workforce to care for oncology patients: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:2226–2235. doi: 10.1016/j.jacc.2019.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3D GLS of the LV. Using 4D LV Analysis (Tom Tec Imaging System, Germany), 3D speckle tracking strain measurement of GLS from a 3D full volume of the LV. Measurements are performed along the endocardial border. A 16-segment bullseye plot is generated. 3D = three-dimensional; 4D = four-dimensional; GLS = global longitudinal strain; LV = left ventricle.
2D Image Acquisition. Acquire 3 apical views sequentially to ensure similar heart rates and frame rates. Optimize image quality and avoid foreshortening. See Table 2 for further details. 2D = two-dimensional.
GLS Measurement With Automated Function Imaging (GE Medical Systems). Start with apical 3 chamber view. Tracing is automated. Region of interest (ROI) should cover the full LV thickness from the endocardial to the epicardial border. Important to assess tracking quality to confirm that the bullets are moving with the underlying myocardium. See Table 3 for further details. Abbreviations as in Video 1.
GLS Measurement With Automated Function Imaging (GE Medical Systems). Proceed with the next 2 views (AP4, AP2). Repeat the same steps as above. The ROI should be at the annulus and not in the left atrium. Abbreviations as in Video 1 and 3.
Event Timing. Timing of end systole can also be identified using the timing of the aortic valve closure measured off the spectral Doppler of the aortic valve.
GLS Measurement With Automated Cardiac Motion (Philips Healthcare). Tracing is automated. Important to assess that the automated software detection is correctly detecting the endocardial border and tracking the underlying myocardium. Manually adjust the endocardial contour to optimize tracking if necessary. Abbreviation as in Video 1.
GLS Measurement With Automated Cardiac Motion (Philips Healthcare). Proceed with the next 2 views (AP4, AP2). After the 3 views are completed, a bullseye plot is generated. Abbreviation as in Video 1.
GLS Measurement With AutoStrain (Image Area, Tom Tec Imaging System). GLS measurement using a vendor-neutral system capable of processing images in the DICOM format. Abbreviation as in Video 1.
Suboptimal Tracking. Suboptimal tracking as shown in the video leads to abnormal or suspicious strain values with nonphysiological waveform tracing that are discordant with the visual wall motion.
Tracking Mimicking Structures. The automated software detection tracking papillary muscle instead of the LV endocardial border. Abbreviation as in Video 1.
Marking of the Annulus. ROI should be placed at the insertion of the mitral leaflets. ROI in the left atrium as shown in the video leads to abnormal strain of the basal segments. Abbreviation as in Video 3.
ROI Placement of the LV Walls. ROI including the pericardium can lead to underestimation of GLS. Abbreviations as in Videos 1 and 3.
Incorrect Timing of End-Systole. Incorrect timing of end systole due to poor electrocardiogram (ECG) tracing can affect peak strain in some segments and lead to inaccurate GLS measurement. Abbreviation as in Video 1.