Abstract
The majority of endocervical adenocarcinomas (EAs) are caused by human papillomavirus (HPV). Gastric-type EA, the second most common EA and unrelated to HPV, is biologically different with a more aggressive clinical course. Our knowledge of the molecular fingerprint of these important EA types and its role in diagnosis, prognosis and management is still evolving. Thus, we sought to evaluate the genomic profile of HPV-related and gastric EA. Clinical information including patient outcome was gathered for fifty-six tumors (45 HPV-associated and 11 gastric-type) which were evaluated by a targeted massively parallel sequencing assay (OncoPanel platform) which surveys exonic DNA sequences of 447 cancer genes and 191 regions across 60 genes for rearrangement detection. KRAS, TP53, and PIK3CA were the most commonly mutated genes (10, 10, and 9 cases, respectively). Alterations in TP53, STK11, CDKN2A, ATM, and NTRK3 were significantly more common in gastric-type EA (p < 0.05, Fisher’s exact test). Disease recurrence and/or death occurred in 14/49 (29%) cases with clinical information available (7 HPV-related (18% of HPV-related cases with clinical information available) and 7 gastric-type (64% of gastric-type cases with clinical information available). Tumors associated with adverse outcome, regardless of histotype, more commonly had alterations in KRAS (2 HPV-related, 4 gastric-type), GNAS (3 HPV-related, 1 gastric-type), and CDKN2A (0 HPV-related, 3 gastric type) compared to indolent-behaving cases (p < 0.05, Fisher’s exact test). A total of 8/56 (14%) tumors harbored at least one actionable mutation; of these, 6 (75%) were associated with recurrence and/or cancer-related death. Copy number variations were detected in 45/56 cases (80%). The most frequent were chromosome 20 gain and 16q loss, identified in 7 cases each (all HPV-associated EA). The mutational profile of EA is diverse and correlates with clinical behavior and EA subtype. Thus, targeted sequencing assays can potentially serve as a diagnostic and prognostic tool. It can also identify targetable alterations, which may benefit patients with recurrent/metastatic disease.
Keywords: Cervix, endocervical adenocarcinoma, histotype, next generation sequencing, outcome
INTRODUCTION
Endocervical adenocarcinoma (EA) is the 2nd most common malignancy of the uterine cervix1. Like its squamous counterpart, a large proportion of EAs are associated with oncogenic human papilloma virus (HPV) infection. However, it is well recognized that a significant proportion of EAs are not associated with HPV infection2–8. Because of this dichotomy in pathogenesis, an international group of pathologists recently proposed an updated classification scheme entitled the International Endocervical Criteria and Classification (IECC) which categorizes EAs based on the presence or absence of HPV infection-related features9 (Figure 1). The IECC is further supported by the original descriptions of gastric-type EA (the most common HPV-unrelated EA) which described aggressive clinical behavior in this tumor type (Figure 2)10–12. Recent clinicopathologic studies based on the IECC system have confirmed the differences in presentation and prognosis between gastric-type EA and HPV-associated EA13,14.
Figure 1.
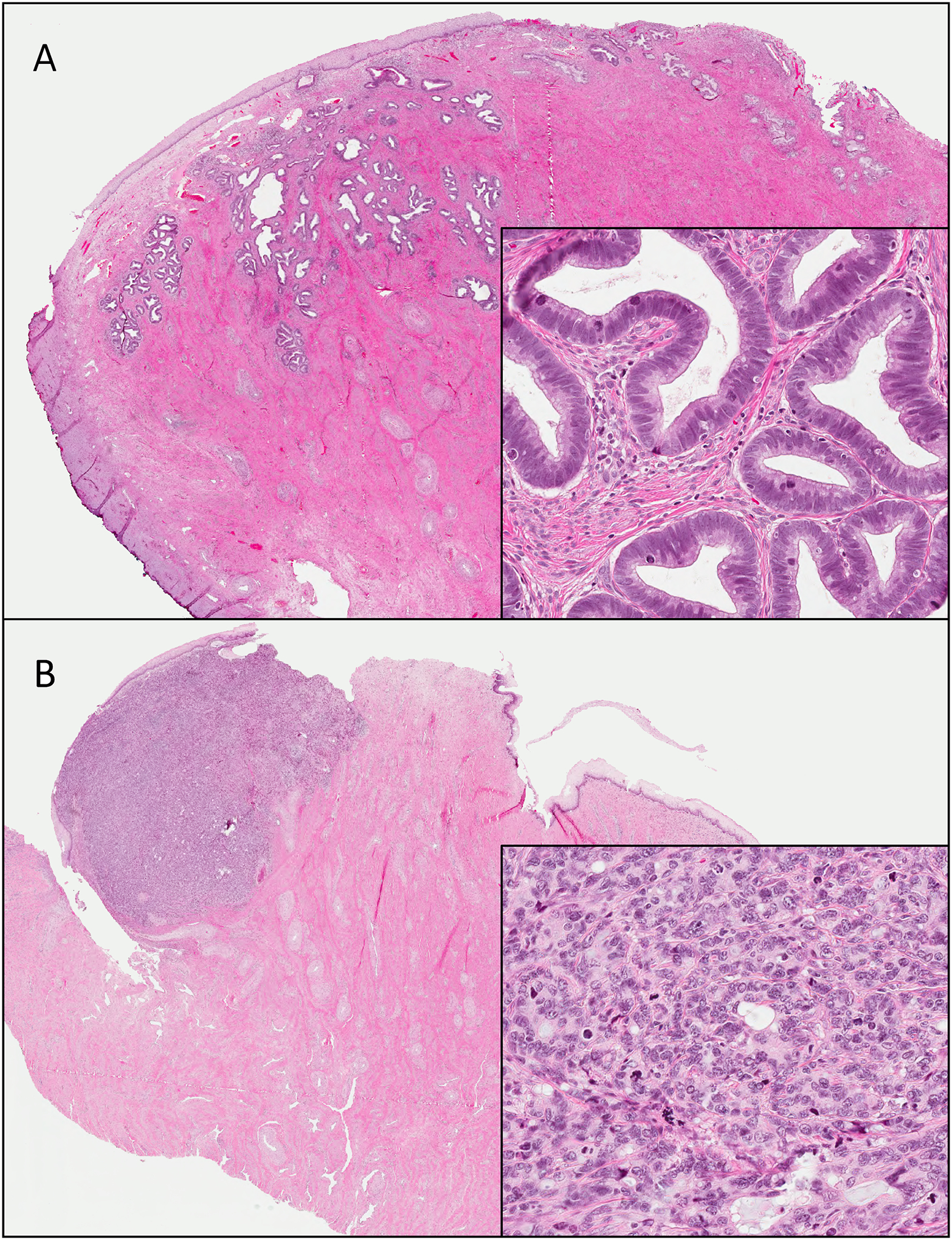
Human papillomavirus-associated endocervical adenocarcinoma. These lesions are centered in the squamous-columnar junction. Tumors ranged from gland-forming / well-differentiated (A) to solid and poorly differentiated (B). Note in both the presence of conspicuous mitoses and apoptoses.
Figure 2.
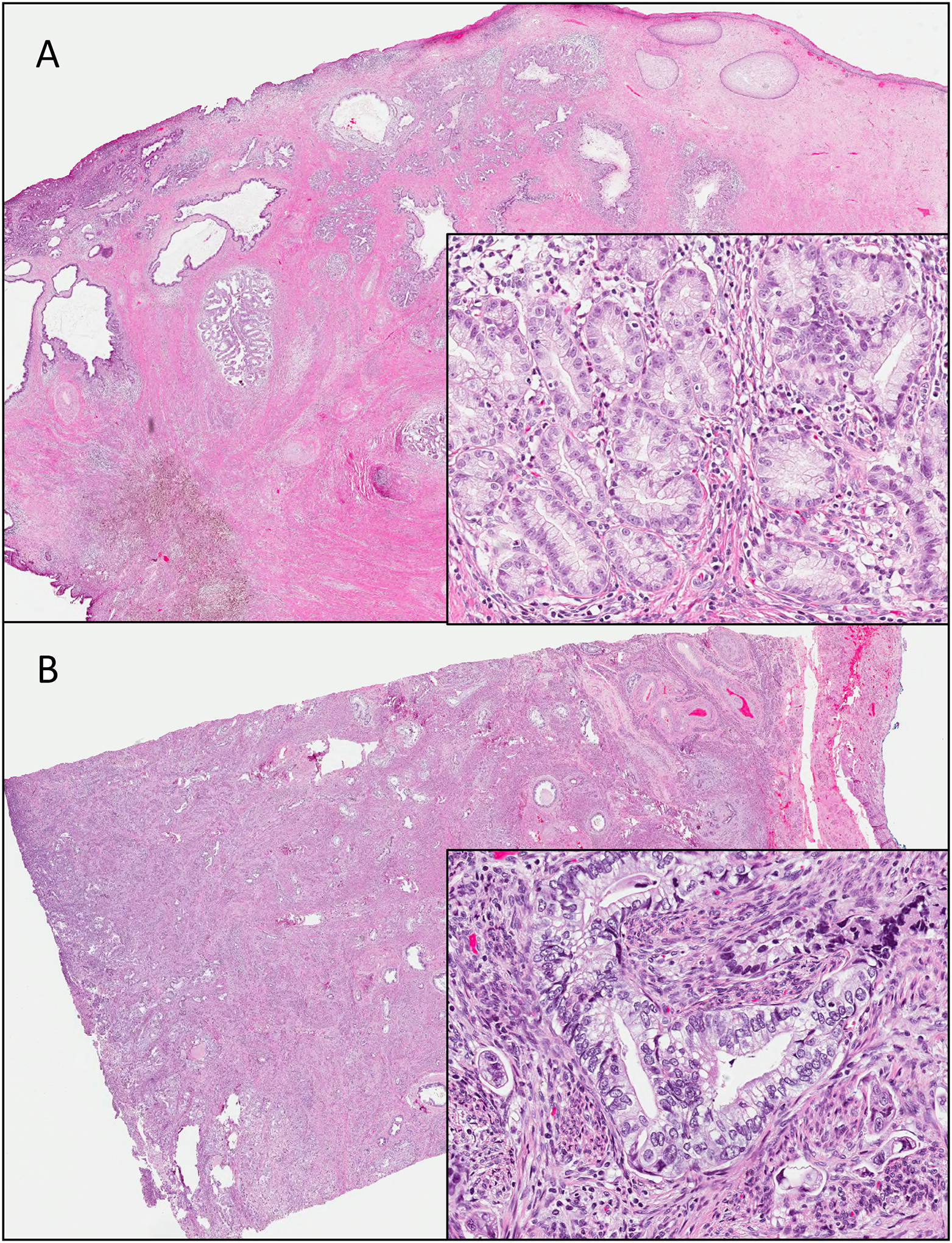
Gastric-type endocervical adenocarcinoma. The tumor is composed of mucinous cells with clear to foamy cytoplasm, distinct cell borders and atypical round nuclei. Tumors range from well-differentiated (A) to poorly differentiated (B).
Genomic evaluation of EA thus far has shown diverse results. Recurrently altered genes include PIK3CA, KRAS and PTEN (PI3K-Akt-mTOR pathway), mostly found in HPV-related adenocarcinomas15–21. Interestingly, mutations in both PIK3CA and KRAS have been shown to be prognostically significant22,23 and additionally, targeted therapies exist against alterations in these genes24,25. In contrast to HPV-associated EAs, gastric-type tumors tend to be enriched for TP53, STK11, GNAS, SMAD4, and CDKN2A mutations26,27; in addition, there appears to be some molecular overlap between this subtype and HPV-associated EAs as KRAS and PIK3CA alterations have also been described. Importantly, previous studies have not separated cases into subtypes as per the IECC and were based mostly on targeted sequencing panels.
With this information in mind, we sought to evaluate the genomic profile of an institutional cohort of HPV-associated EAs and gastric-type EAs using a comprehensive massively parallel sequencing platform, with the aim of identifying prevalent and specific alterations for IECC subtypes and correlating these molecular alterations with patient outcome.
MATERIALS AND METHODS
Case selection and review
Consecutive cases of invasive EA from Sunnybrook Health Sciences Centre (Toronto, Ontario, Canada) diagnosed between 2002 and 2018 were retrieved for initial review (see Figure 3). The search included resection (cone biopsy, loop electrosurgical excision procedure, trachelectomy, or hysterectomy) and biopsy specimens. Those with histologic material available for examination were further reviewed and complete slide sets for each case were examined by one gynecologic pathologist (CPH) in order to confirm the diagnosis of EA. Tumors of uncertain origin, adenosquamous carcinomas and metastases to the cervix were excluded. After the diagnosis of EA was confirmed, subtyping as per the IECC9 was completed by three gynecologic pathologists (BH, MN, CPH) who reviewed full slide sets (hematoxylin and eosin stained slides only) for each case. Fully concordant diagnoses (agreement by all three pathologists) required no further review. Discrepant cases were assigned a majority diagnosis (when two reviewers concurred). The reviewers were blinded to all clinicopathologic characteristics as well as immunohistochemistry and HPV in situ hybridization and/or polymerase chain reaction results (if available).
Figure 3.
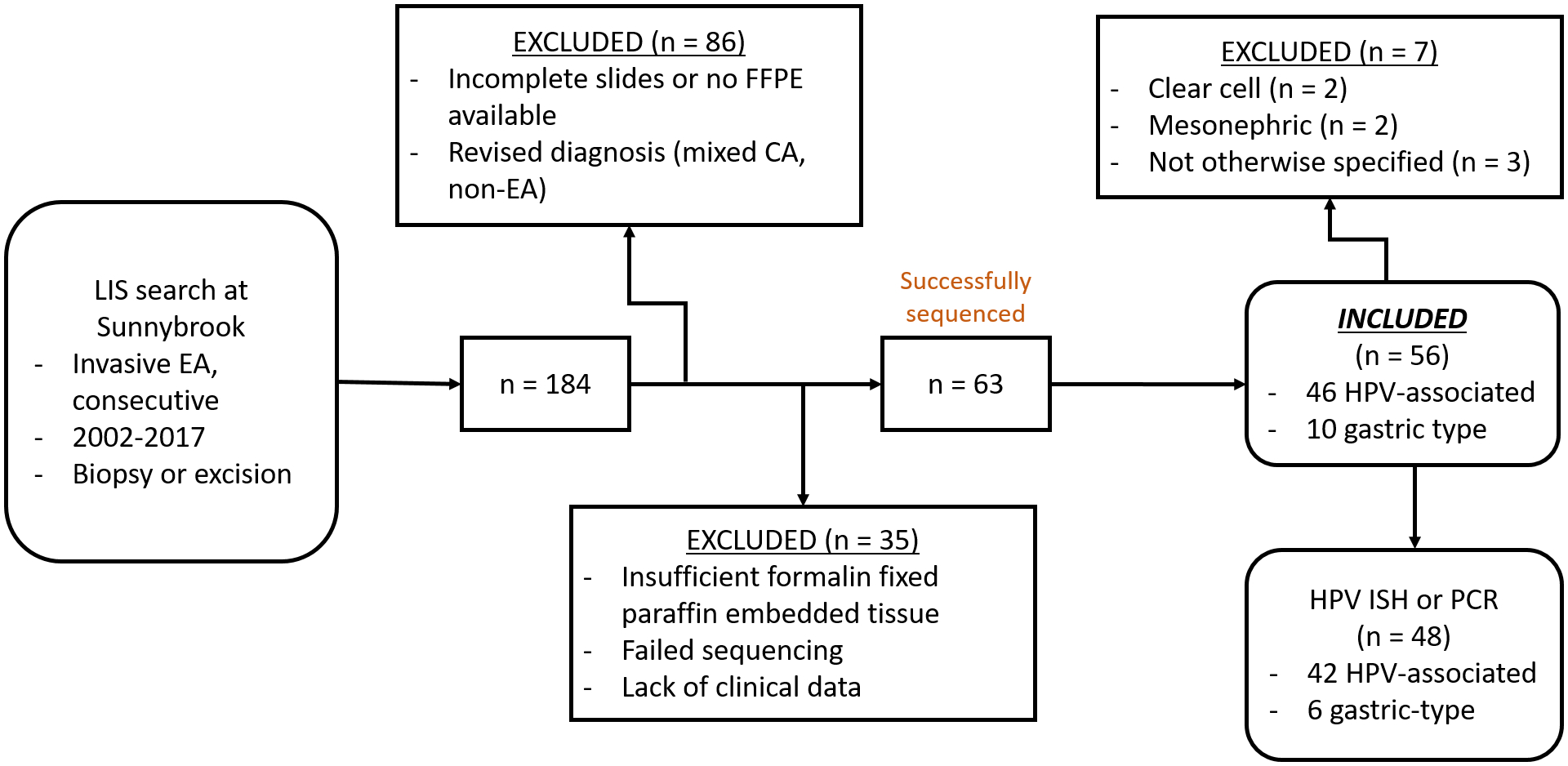
Flowchart depicting case selection and inclusion. LIS, laboratory information system; EA, endocervical adenocarcinoma; FFPE, formalin fixed paraffin embedded; HPV, human papillomavirus; PCR, polymerase chain reaction; ISH, in situ hybridization.
For each case, age at diagnosis, stage at diagnosis and follow up information was collected (if available) including documentation of tumor recurrence, time to recurrence, time to last follow up (in months) and clinical status at last follow up (alive with no evident disease, alive with ongoing disease, died of disease, or died of other causes). Detailed clinical and pathologic information of the cohort, including patient outcome, was previously documented by our group13.
HPV in situ hybridization and polymerase chain reaction
In situ hybridization with chromogen for HPV types was performed on tissue microarrays, which were constructed using two 2-mm cores of representative paraffin-embedded tissue blocks per case. Normal tissue controls were included in each TMA block. In situ hybridization was completed using the Advanced Cell Diagnostics (Hayward, CA) RNAscope® system (catalogue no. 312598). The RNAscope® Probe “HPV HR18” contains probes targeting E6 and E7 mRNA for the following high risk types: HPV16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73 and 82. Historic tumors known to contain high risk-HPV were used as positive controls, while those known to be high risk HPV-negative were used as negative controls during assay optimization. Subsequently, a negative control slide lacking application of the probes was prepared and examined, particularly when adjudicating tumors with rare or equivocal signals. A full range of cytoplasmic and nuclear signals were encountered, as has been previously described28.
Tumor tissue for polymerase chain reaction testing was attempted in tumors not represented in tissue microarrays. The Roche cobas 4800 system (Pleasanton, California) was utilized for human papillomavirus detection which evaluates for the presence of 14 types of the following HPV DNA: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68.
Genomic DNA extraction and sequencing
Tumor samples from selected paraffin-embedded blocks were processed at the Center for Advanced Molecular Diagnostics at Brigham and Women’s Hospital (Boston, MA, USA) for all stages of the next generation sequencing assay. Tumor cellularity was estimated by one pathologist (CPH) as a percentage of the overall cellularity in each selected tumor block (all cases > 20% cellularity). Unstained 4-micron formalin-fixed paraffin-embedded tissue sections of tumor were manually scraped for all cases and DNA was isolated using a commercially available kit (Qiagen, Valencia, California) following the manufacturer’s instructions. Of note, paired normal tissue samples were not analyzed. DNA was quantified (PicoGreen) and samples with at least 50 ng/ul of DNA proceeded to library preparation. Hybrid capture libraries were prepared following previously published protocols29,30. Sheared DNA was hybridized to a set of custom-designed capture probes (Agilent SureSelect) targeting the complete exonic regions of 447 cancer genes and 191 intronic regions across 60 genes for the evaluation of structural rearrangements (OncoPanel). Sequencing was performed using Illumina HiSeq 2500.
Sequencing data analysis
Data were analyzed by an internally developed bioinformatics pipeline composed of reconfigured publicly available tools and internally developed algorithms (VisCap Cancer, Phaser, BreaKmer)29. Pooled sample reads were demultiplexed using Picard (http://picard.sourceforge.net/command-line-overview.shtml), aligned to Human Genome Reference Consortium reference sequence GRCh37p1331 and duplicate reads were removed. GATK32 was used to refine alignments around insertion/deletion (indel) sites. Single nucleotide variants were called using MuTect33 and indels using Indelocator (http://www.broadinstitute.org/cancer/cga/indelocator). Annotation was performed using Oncotator34. Because tumor tissues were tested without a paired normal from individual patients, additional informatics steps were taken to identify common single nucleotide polymorphisms: any single nucleotide polymorphism present at >0.1% in Exome Variant Server, NHLBI GO Exome Sequencing Project, Seattle, WA (URL: http://evs.gs.washington.edu.myaccess.library.utoronto.ca/EVS/) was filtered, however, variants also present in the Catalogue of Somatic Mutations in Cancer (COSMIC; cancer.sanger.ac.uk) were rescued for manual review. VisCap Cancer calls copy number changes based on log2 ratios that are calculated using a normalized depth of coverage against a median from a panel of normal (non-cancer) samples. Circular binary segmentation was used to segment the data; segments were called via strict thresholding. Unique, aligned (hg19) sequence reads with PHRED>30 were reviewed, annotated, and interpreted using Integrated Genome Viewer (Broad Institute, Cambridge, MA) and a suite of internally developed Web-based tools. Samples with a mean target coverage of <50 × were failed and excluded from further analysis. Individual variants present at <10% allele fraction or in regions with <50 × coverage were flagged for manual review and interpreted by the reviewing laboratory scientists and molecular pathologists (NL, BH, CPH) based on overall tumor percentage, read depth, complexity of alteration, and evidence for associated copy number alterations. Manual inspection also included cross-reference with the Catalogue of Somatic Mutations in Cancer (COSMIC) database (https://cancer.sanger.ac.uk/cosmic). After filtering and manual inspection, the number of single nucleotide variations per case was obtained (mutational burden).
Prevalent mutations (seen in two cases or more) and copy number variations were correlated with clinicopathologic variables including tumor subtype and patient outcome (in terms of disease-free survival, disease-specific survival and overall survival). “Actionable” pathogenic mutations were defined as an alteration which applies to at least one of the following contexts: predicts response to treatment with an FDA-approved therapy or an investigational therapy in clinical trials for the same cancer type, proven association of response to treatment with an FDA-approved therapy in a different type of cancer, or is a similar alteration with a proven association with response with an FDA-approved therapy in this cancer type.
Statistical analysis
Inferential analysis was performed to compare clinicopathologic variables and genomic data using GraphPad QuickCalcs (GraphPad Software Inc, La Jolla, CA, United States). Categorical variables were compared using the Fisher’s exact test, and numerical variables with the unpaired t-test. A p value of less than 0.05 was considered statistically significant.
RESULTS
Case selection and final inclusion is depicted in Figure 3. A total of 184 cases of EA were identified in our database. Of these, 86 cases were excluded for a number of reasons: external cases seen in consultation, incomplete slide set or no blocks available, or revised diagnosis (non-EA or mixed carcinoma). Of the remaining 98 tumors, 35 were further excluded due to insufficient tissue for tissue coring or after failed sequencing and 63 cases were successfully sequenced. After exclusion of other tumor histotypes (with very low frequency for further analysis), the final cohort consisted of 56 EAs: 45 (80%) HPV-associated EAs and 11 (20%) gastric-type EAs. The 45 HPV-associated tumors were further subclassified into subtypes according to the IECC as follows: 31 usual type, 8 mucinous not otherwise specified, 1 mucinous intestinal type, and 5 invasive stratified mucin producing carcinoma. The distribution amongst tumor categories was the result of full concordance (3/3 reviewers) in 36 (64%) cases and majority consensus (2/3 reviewers) in 20 (36%) cases. Instances of complete disagreement among the three reviewers were not encountered. Classification of the included cases is shown in Table 1.
Table 1.
Case distribution and HPV status for 56 endocervical adenocarcinomas morphologically classified according to the International Endocervical Adenocarcinoma Criteria and Classification (IECC)
| Number of cases | Number of cases tested for HPV by ISH or PCR | Number of cases positive for HPV by PCR or ISH | |
|---|---|---|---|
| All endocervical adenocarcinoma cases | 56 | 49 | 42 |
| HPV-associated endocervical adenocarcinoma subtypes | 45 | 42 | 42 |
| Usual | 31 | 29 | 29 |
| Mucinous, not otherwise specified | 8 | 7 | 7 |
| Mucinous, intestinal type | 1 | 1 | 1 |
| Invasive stratified mucin producing carcinoma | 5 | 5 | 5 |
| Gastric-type endocervical adenocarcinoma | 11 | 7 | 0 |
HPV: human papillomavirus; ISH: in situ hybridization; PCR: polymerase chain reaction.
Clinical information
The mean and median age at diagnosis for HPV-associated EAs was 43 and 40 years, respectively. Gastric-type EA patients were older, with mean and median age of 56 and 58 years, respectively (p = 0.005, unpaired t test). Staging information was available for 53/56 cases (95%), of which 42 were HPV-associated EAs and 11 were gastric-type EAs. 37/42 (88%) HPV-associated EAs were stage 1 while the remaining 5 (12%) were stage 2; there were no stage 3 or stage 4 HPV-associated EAs. In contrast, 3/11 (27%) of gastric-type EAs were stage 1, 1 (9%) was stage 2, 2 (18%) were stage 3, and the remaining 5 (46%) were stage 4. The difference in stage distribution between HPV-associated EAs and gastric type EAs was statistically significant (p = 0.0002, Fisher’s exact test).
A total of 49/56 cases (88%) had clinical outcome information available. The mean time of follow up was 33 months (range 1–133 months). Tumor recurrence and/or cancer-related death was documented in 14/49 (29%) patients. Of these, 7 were HPV-associated (18% of total HPV-associated EAs with outcome information available) and 7 were gastric-type (64% of total gastric-type tumors with outcome information available). Importantly, of the HPV-associated EAs that recurred, 4 were invasive stratified mucin producing carcinomas, 2 were usual type and 1 was an intestinal type mucinous carcinoma.
In order to mitigate bias, we compared the clinico-pathologic profile of the 56 included cases with the 36 excluded due to insufficient tissue or failed sequencing. No statistically significant clinicopathologic differences were observed between these groups in terms of patient age, histologic type distribution, stage at presentation or clinical outcome (Supplemental File 1).
HPV detection analysis
49/56 tumors (88%) were successfully tested for the presence of HPV (42/45 HPV-associated tumors and 7/11 gastric-type tumors). Of these, 45 were tested by in-situ hybridization (cases represented in tissue microarrays) and 4 by PCR. The remaining 7 lesions had no remaining material for HPV testing. All 42 cases (100%) classified as HPV-associated EA per methodology were positive for HPV. Similarly, all 7 (100%) of the tumors classified as gastric-type EA that were tested for HPV resulted negative (Table 1).
Mutation profile of HPV-associated and gastric-type endocervical adenocarcinomas
Prevalent altered genes across the entire cohort are shown in Table 2 and Figure 4. Specific mutations are listed in Supplemental File 2. All single nucleotide variants identified after filtration and manual inspection are listed as pathogenic or likely pathogenic in the COSMIC database. Overall, KRAS and TP53 were most commonly altered (10 cases each), followed by PIK3CA (9 cases), and GNAS (7 cases). TP53, CDKN2A, STK11, ATM and NTRK3 alterations were more frequently seen in gastric-type compared to HPV-associated tumors (p < 0.05 for all, Fisher’s exact test). Of note, we found 2 HPV-associated EA which had STK11 alterations; interestingly, both of these cases were classified as invasive stratified mucin producing carcinomas, a recently described entity with specific architectural and cytomorphology35. A higher mutation burden was noted in gastric-type EAs (average 3.2 per case) compared to HPV-associated EAs (average 2.0 per case; p = 0.04, unpaired t test).
Table 2.
Altered genes seen in at least 2 cases of endocervical adenocarcinoma cohort
| Gene | Number of total affected cases | Human papillomavirus-associated endocervical adenocarcinoma (n = 45) |
Gastric-type endocervical adenocarcinoma (n = 11) |
p value (Fisher’s exact test) |
|---|---|---|---|---|
| n (%) | ||||
| KRAS | 10 (18%) | 6 (13%) | 4 (36%) | 0.0934 |
| TP53 | 10 (18%) | 5 (11%) | 5 (46%) | 0.0179 |
| PIK3CA | 9 (16%) | 7 (16%) | 2 (18%) | 1.00 |
| GNAS | 7 (12%) | 6 (13%) | 1 (9%) | 1.00 |
| ERBB2 | 6 (11%) | 5 (11%) | 1 (9%) | 1.00 |
| STK11 | 5 (9%) | 2 (4%) | 3 (27%) | 0.0468 |
| CDKN2A | 4 (7%) | 1 (2%) | 3 (27%) | 0.0211 |
| SMAD4 | 4 (7%) | 3 (7%) | 1 (9%) | 1.00 |
| ATRX | 3 (5%) | 3 (7%) | 0 | 1.00 |
| TET2 | 3 (5%) | 3 (7%) | 0 | 1.00 |
| ATM | 2 (4%) | 0 | 2 (18%) | 0.0357 |
| NTRK3 | 2 (4%) | 0 | 2 (18%) | 0.0357 |
| ERBB3 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| SF3B1 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| NTRK1 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| CARD11 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| CREBBP | 2 (4%) | 2 (4%) | 0 | 1.00 |
| MED12 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| HNF1A | 2 (4%) | 2 (4%) | 0 | 1.00 |
| MAP2K2 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| TSC2 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| CHEK2 | 2 (4%) | 2 (4%) | 0 | 1.00 |
| ETV6 | 2 (4%) | 2 (4%) | 0 | 1.00 |
Figure 4.
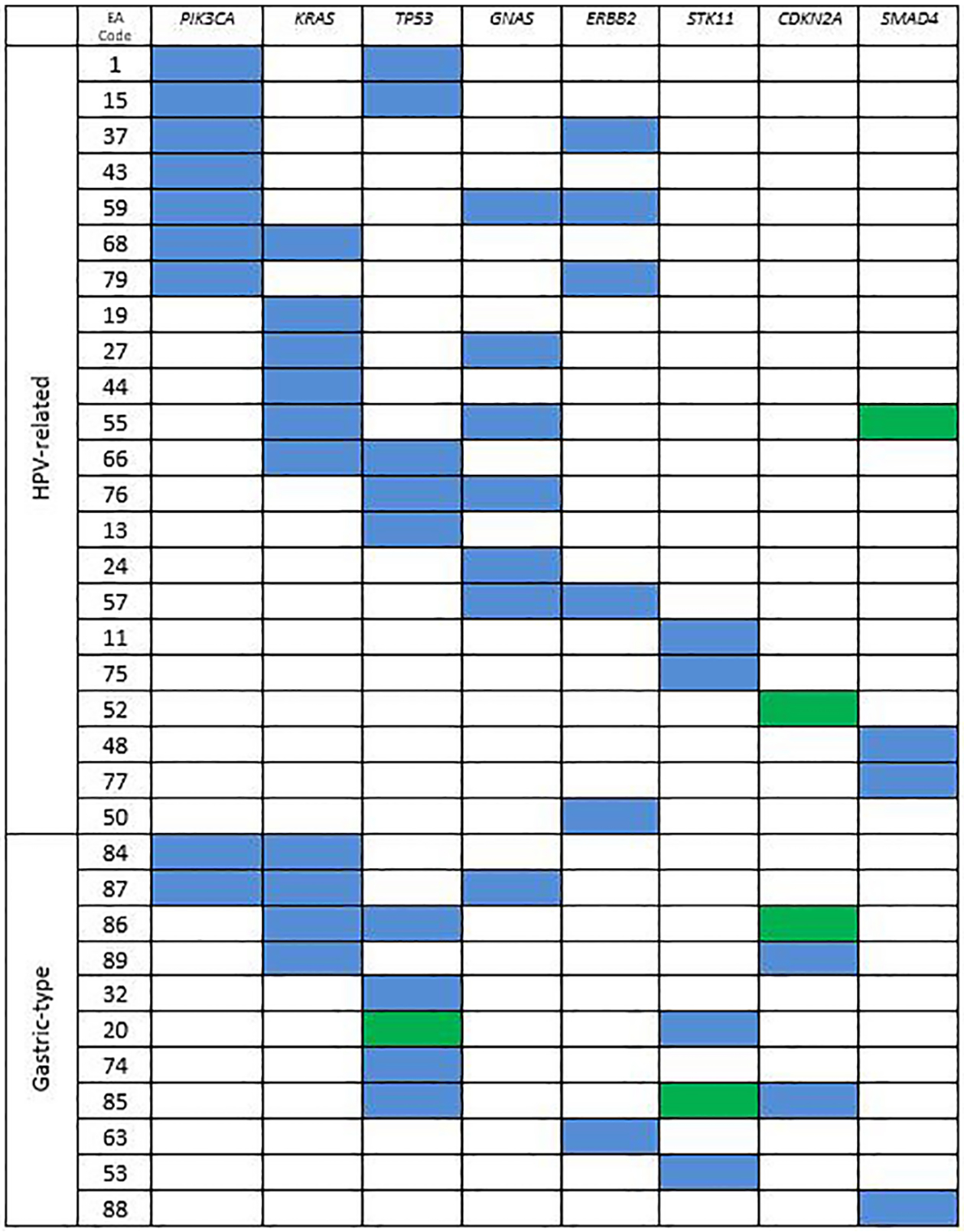
Most commonly altered genes in endocervical adenocarcinoma. Blue = missense mutation, green = truncating/frameshift mutation.
Table 3 depicts the number of cases in the cohort with actionable mutations. Overall, 8 cases were found to have actionable mutations, of which 4 (9%) were HPV-associated EAs and 4 (40%) were gastric-type EAs (p = 0.027, Fisher’s exact test).
Table 3.
Genes with actionable alterations in endocervical adenocarcinoma
| Gene | Human papillomavirus-associated endocervical adenocarcinoma (n = 45) |
Gastric-type endocervical adenocarcinoma (n = 11) |
|---|---|---|
| KRAS | 1 | 2 |
| PIK3CA | 2 | 0 |
| KRAS and PIK3CA | 0 | 2 |
| ERBB2 | 1 | 0 |
| Total number of cases with actionable alterations | 4 (9%) | 4 (36%) |
Correlation of mutation profile and clinical outcome
Overall, EAs with adverse outcome had a higher mutational burden compared to those cases which did not have an adverse outcome (3.2 vs. 1.7 gene alterations / case, p = 0.004, unpaired t test) and more commonly had mutations in KRAS, GNAS, and CDKN2A (p < 0.05 for all, Fisher’s exact test), compared to cases without an adverse outcome. Worse outcome in STK11-altered cases approached significance. Of note, 6/8 (75%) of the patients who had tumors with actionable mutations suffered a recurrence and/or died of their disease; 4 of those 6 were gastric-type tumors. Indeed, 100% of gastric type tumors which had actionable alterations suffered some form of adverse outcome. To the best of our knowledge, no tumors in the cohort were treated with targeted therapy.
Copy number variations in HPV-associated and gastric-type endocervical adenocarcinomas
Copy number variations were detected in 45/56 cases (80%) of which 36 were HPV-associated EAs and 9 were gastric-type EAs. Gastric-type EAs did not show any significant recurring copy number variations, although whole chromosome 4 and arm level chromosome 13q losses were each seen in 2 cases. The most common recurring copy number alterations were identified exclusively in HPV-associated EAs: arm level (20p) or whole chromosome 20 gain, arm level chromosome 16q loss (7 cases each), arm level chromosome 5p gain (5 cases each), and arm level chromosome 3p and 1q losses (3 cases each). Some copy number alterations were identified in both HPV-associated and gastric-type EA: arm level chromosome 18q loss in 5 cases (4 HPV-associated EA, 1 gastric-type EA) and arm level 17p loss in 4 cases (3 HPV-associated EA, 1 gastric-type EA). Gene specific amplifications were identified in a minority of cases. MYC amplifications (chromosome 8q) were identified in two HPV-associated EAs (4%) and ERBB2 (HER2) amplification was noted in one gastric-type EA (9%). None of the identified copy number variations correlated significantly with patient outcome.
DISCUSSION
Previous studies have evaluated the genomic landscape of EAs although only a fraction have separated lesions based on subtype (i.e. HPV-related versus unrelated). In HPV-associated tumors, PIK3CA and KRAS alterations have been reported in several studies15–21, and we again show that these genes are prevalently altered in our cohort of EAs. We also identified copy number variations (ex. involving chromosome 18 and 20) in our study which have also been previously reported16 and we confirm that the vast majority of these are found in HPV-associated EAs.
Gastric-type EA, including minimal deviation adenocarcinoma, is associated with Peutz-Jehger’s syndrome, a hereditary tumor disorder characterized by alterations in STK11. The STK11 protein is a serine/threonine kinase which functions, in part, to regulate the AMP-activated protein kinase pathway and therefore, has an important role in the regulation of cell metabolism. Previous studies have demonstrated alterations in STK11 in gastric type EA27,36,37, a finding also observed in our series. Nonetheless, the specificity of STK11 mutations for gastric-type EA does not appear to be as good as predicted in earlier studies as we identified STK11 alterations in two HPV-associated EAs, both of which were positive for HPV by in situ hybridization and were classified as invasive stratified mucin producing carcinomas. It is important to note that the mutations in those cases were different than those in the gastric-type EA group and that no case from either group shared an identical STK11 mutation. All of the detected STK11 mutations appear to involve the protein kinase domain of the STK11 protein and although the exact biochemical significance of all of the mutations is not entirely clear, the majority have been reported to negatively impact protein function38–41. Overall, 2/5 (40%) invasive stratified mucin producing carcinomas in the cohort had STK11 mutations. Recently, our group studied the clinicopathologic characteristics of EAs including invasive stratified mucin producing carcinoma: these neoplasms behave more aggressively than other HPV-associated EAs13. Of note, none of the patients with STK11-mutated tumors (either HPV-related or gastric-type) had clinical findings compatible with Peutz-Jehgers Syndrome.
Murali et al26 noted the morphologic and genomic similarities between gastric-type EA and pancreatic ductal adenocarcinoma. Modern molecular analysis of pancreatic ductal adenocarcinomas has shown a number of recurrently altered genes including KRAS, TP53, GNAS, CDKN2A, and SMAD442 and we show, like Murali et al, that gastric-type EA tends to harbor alterations in the these genes26. Interestingly, alterations in all of these genes can be also seen in HPV-associated tumors, albeit in different proportions.
Of interest, we identified both NTRK1 and NTRK3 mutations in our cohort. NTRK rearrangements have been described in cervical sarcomas as well as some carcinomas and Larotrectinib, a tropomyosin receptor kinase (TRK) inhibitor, has been approved for use in the treatment of solid tumors which harbor an NTRK gene fusion. However, little data exists regarding the use of Larotrectinib in the setting of NTRK mutations. As such, the therapeutic significance of these alterations in not only EAs, but other tumor types with NTRK mutations, remains to be established.
Importantly, KRAS, GNAS, and CDKN2A-altered tumors, irrespective of histotype, behaved more aggressively than tumors without alterations in these genes. This finding suggests that information from next generation sequencing may be used in a prognostic sense. Potentially actionable mutations were seen in 14% of the total cohort (in addition to one additional case with ERBB2 amplification). This is a relatively small but clinically important finding: a) all patients with gastric-type EA and adverse outcome (4/10, 40%) harbored an actionable mutation, and b) 2 of the 4 patients with HPV-associated tumors with actionable mutations also recurred and/or died of their disease. This observation further underscores the potential utility of next generation sequencing in the assessment of EAs, particularly of those tumors from patients who are suffering from recurrent and/or refractory disease. Of note, efforts to successfully target KRAS mutations pharmacologically are ongoing43 while PI3K inhibitors such as Alpelisib and Idelalisib are FDA-approved and are already being used in the clinic in the treatment of solid tumors such as carcinomas arising from the breast44 and different hematological malignancies45.
Our study is limited by the relative paucity of gastric-type EAs compared to HPV-associated EAs. However, the distribution of cases in our cohort generally reflects the expected proportion of EAs9. We also purposely excluded other HPV-unrelated variants of EA (clear cell, mesonephric etc.) as we had too few patients in these categories. Larger studies with proper representation of these variants are certainly warranted. In addition, a number of cases were excluded which, theoretically, could introduce bias into the analysis. However, we found no clinicopathologic differences between these cases and those which were included and thus, we can be reassured that our results are applicable to a larger and general population.
In summary, in this comprehensive analysis of EA we demonstrate a correlation between the mutational profile and the etiologic subtype, namely HPV-related versus gastric-type EA. Nonetheless, no single gene alteration is entirely sensitive or specific for either type. Furthermore, we show an association between certain genomic alterations and clinical behavior. Lastly, next generation sequencing serves to identify actionable mutations. Thus, sequencing of EAs can serve as a prognostic and predictive clinical tool, particularly in patients with recurrent or refractory disease.
Supplementary Material
Conflicts of Interest and sources of funding:
The authors have no conflicts of interest to disclose.
This research was funded in part by the International Society of Gynecologic Pathologists through their Young Investigator Grant (C Parra-Herran) as well as the NIH/NCI Support Grant P30 CA008748 (KJ Park).
REFERENCES
- 1.Smith HO, Tiffany MF, Qualls CR, et al. The Rising Incidence of Adenocarcinoma Relative to Squamous Cell Carcinoma of the Uterine Cervix in the United States—A 24-Year Population-Based Study. Gynecol Oncol. 2000;78:97–105. [DOI] [PubMed] [Google Scholar]
- 2.Pirog EC, Kleter B, Olgac S, et al. Prevalence of Human Papillomavirus DNA in Different Histological Subtypes of Cervical Adenocarcinoma. Am J Pathol. 2000;157:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holl K, Nowakowski AM, Powell N, et al. Human papillomavirus prevalence and type‐distribution in cervical glandular neoplasias: Results from a European multinational epidemiological study. Int J Cancer 2015;137:2858–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molijn A, Jenkins D, Chen W, et al. The complex relationship between human papillomavirus and cervical adenocarcinoma. Int J Cancer 2016;138:409–416. [DOI] [PubMed] [Google Scholar]
- 5.Pirog EC, Lloveras B, Molijn A, et al. HPV prevalence and genotypes in different histological subtypes of cervical adenocarcinoma, a worldwide analysis of 760 cases. Mod Pathol. 2014;27:1559–1567. [DOI] [PubMed] [Google Scholar]
- 6.An HJ, Kim KR, Kim IS, et al. Prevalence of human papillomavirus DNA in various histological subtypes of cervical adenocarcinoma: a population-based study. Mod Pathol. 2005;18:528–534. [DOI] [PubMed] [Google Scholar]
- 7.Kusanagi Y, Kojima A, Mikami Y, et al. Absence of high-risk human papillomavirus (HPV) detection in endocervical adenocarcinoma with gastric morphology and phenotype. Am J Pathol. 2010;177:2169–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park KJ, Kiyokawa T, Soslow RA, et al. Unusual endocervical adenocarcinomas: an immunohistochemical analysis with molecular detection of human papillomavirus. Am J Surg Pathol. 2011;35:633–646. [DOI] [PubMed] [Google Scholar]
- 9.Stolnicu S, Barsan I, Hoang L, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): A New Pathogenetic Classification for Invasive Adenocarcinomas of the Endocervix. Am J Surg Pathol. 2018;42:214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kojima A, Mikami Y, Sudo T, et al. Gastric morphology and immunophenotype predict poor outcome in mucinous adenocarcinoma of the uterine cervix. Am J Surg Pathol. 2007;31:664–672. [DOI] [PubMed] [Google Scholar]
- 11.Rodríguez-Carunchio L, Soveral I, Steenbergen RDM, et al. HPV-negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG 2015;122:119–127. [DOI] [PubMed] [Google Scholar]
- 12.Karamurzin YS, Kiyokawa T, Parkash V, et al. Gastric-type Endocervical Adenocarcinoma: An Aggressive Tumor With Unusual Metastatic Patterns and Poor Prognosis. Am J Surg Pathol. 2015;39:1449–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodgson A, Olkhov-Mitsel E, Howitt BE, et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): correlation with adverse clinicopathological features and patient outcome. J Clin Pathol. 2019;72:347–353. [DOI] [PubMed] [Google Scholar]
- 14.Stolnicu S, Hoang L, Chiu D, et al. Clinical Outcomes of HPV-associated and Unassociated Endocervical Adenocarcinomas Categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am J Surg Pathol. 2019;43:466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network, Albert Einstein College of Medicine, Analytical Biological Services, et al. Integrated genomic and molecular characterization of cervical cancer. Nature 2017;543:378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojesina AI, Lichtenstein L, Freeman SS, et al. Landscape of genomic alterations in cervical carcinomas. Nature 2014;506:371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wright AA, Howitt BE, Myers AP, et al. Oncogenic mutations in cervical cancer: genomic differences between adenocarcinomas and squamous cell carcinomas of the cervix. Cancer 2013;119:3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lou H, Villagran G, Boland JF, et al. Genome Analysis of Latin American Cervical Cancer: Frequent Activation of the PIK3CA Pathway. Clin Cancer Res. 2015;21:5360–5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tornesello ML, Annunziata C, Buonaguro L, et al. TP53 and PIK3CA gene mutations in adenocarcinoma, squamous cell carcinoma and high-grade intraepithelial neoplasia of the cervix. J Transl Med. 2014;12:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung TKH, Van Hummelen P, Chan PKS, et al. Genomic aberrations in cervical adenocarcinomas in Hong Kong Chinese women. Int J Cancer 2015;137:776–783. [DOI] [PubMed] [Google Scholar]
- 21.Hodgson A, Amemiya Y, Seth A, et al. Genomic abnormalities in invasive endocervical adenocarcinoma correlate with pattern of invasion: biologic and clinical implications. Mod Pathol. 2017;30:1633–1641. [DOI] [PubMed] [Google Scholar]
- 22.Xiang L, Li J, Jiang W, et al. Comprehensive analysis of targetable oncogenic mutations in chinese cervical cancers. Oncotarget 2014;6:4968–4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang L, Jiang W, Li J, et al. PIK3CA mutation analysis in Chinese patients with surgically resected cervical cancer. Sci Rep. 2015;5:14035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fruman DA, Rommel C. PI3K and cancer: lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chappell WH, Steelman LS, Long JM, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR Inhibitors: Rationale and Importance to Inhibiting These Pathways in Human Health. Oncotarget 2011;2:135–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murali R, De Filippo M, Weigelt B, et al. Genomic characterization of gastric type endocervical adenocarcinomas. Mod Pathol. 2016;29:279A–279A. [Google Scholar]
- 27.Garg S, Nagaria TS, Clarke B, et al. Molecular characterization of gastric-type endocervical adenocarcinoma using next-generation sequencing. Mod Pathol. 2019. doi: 10.1038/s41379-019-0305-x; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 28.Evans MF, Peng Z, Clark KM, et al. HPV E6/E7 RNA in situ hybridization signal patterns as biomarkers of three-tier cervical intraepithelial neoplasia grade. PloS One 2014;9:e91142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abo RP, Ducar M, Garcia EP, et al. BreaKmer: detection of structural variation in targeted massively parallel sequencing data using kmers. Nucleic Acids Res. 2015;43:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagle N, Berger MF, Davis MJ, et al. High-throughput detection of actionable genomic alterations in clinical tumor samples by targeted, massively parallel sequencing. Cancer Discov. 2012;2:82–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009;25:1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DePristo MA, Banks E, Poplin RE, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos AH, Lichtenstein L, Gupta M, et al. Oncotator: cancer variant annotation tool. Hum Mutat. 2015;36:E2423–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lastra RR, Park KJ, Schoolmeester JK. Invasive Stratified Mucin-producing Carcinoma and Stratified Mucin-producing Intraepithelial Lesion (SMILE): 15 Cases Presenting a Spectrum of Cervical Neoplasia With Description of a Distinctive Variant of Invasive Adenocarcinoma. Am J Surg Pathol. 2016;40:262–269. [DOI] [PubMed] [Google Scholar]
- 36.Kuragaki C, Enomoto T, Ueno Y, et al. Mutations in the STK11 gene characterize minimal deviation adenocarcinoma of the uterine cervix. Lab Invest. 2003;83:35–45. [DOI] [PubMed] [Google Scholar]
- 37.Takatsu A, Miyamoto T, Fuseya C, et al. Clonality analysis suggests that STK11 gene mutations are involved in progression of lobular endocervical glandular hyperplasia (LEGH) to minimal deviation adenocarcinoma (MDA). Virchows Arch. 2013;462:645–651. [DOI] [PubMed] [Google Scholar]
- 38.Osoegawa A, Kometani T, Nosaki K, et al. LKB1 mutations frequently detected in mucinous bronchioloalveolar carcinoma. Jpn J Clin Oncol. 2011;41:1132–1137. [DOI] [PubMed] [Google Scholar]
- 39.Boudeau J, Kieloch A, Alessi DR, et al. Functional analysis of LKB1/STK11 mutants and two aberrant isoforms found in Peutz-Jeghers Syndrome patients. Hum Mutat. 2003;21:172. [DOI] [PubMed] [Google Scholar]
- 40.Mehenni H, Gehrig C, Nezu J, et al. Loss of LKB1 kinase activity in Peutz-Jeghers syndrome, and evidence for allelic and locus heterogeneity. Am J Hum Genet. 1998;63:1641–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu H, Moriasi CM, Zhang M, et al. Phosphorylation of serine 399 in LKB1 protein short form by protein kinase Cζ is required for its nucleocytoplasmic transport and consequent AMP-activated protein kinase (AMPK) activation. J Biol Chem. 2013;288:16495–16505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017;32:185–203.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu P, Wang Y, Li X. Targeting the untargetable KRAS in cancer therapy. Acta Pharm Sin B 2019. doi: 10.1016/j.apsb.2019.03.002; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.André F, Ciruelos E, Rubovszky G, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2019;380:1929–1940. [DOI] [PubMed] [Google Scholar]
- 45.von Keudell G, Moskowitz AJ. The Role of PI3K Inhibition in Lymphoid Malignancies. Curr Hematol Malig Rep. 2019. doi: 10.1007/s11899-019-00540-w; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


