Cardiovascular (CV) disease is a leading cause of death among 15 million cancer survivors in the United States today (1). Mitigating CV risk in this population should be a priority for both oncologists and cardiologists. Most patients with cancer undergo nongated chest computed tomography (NGCCT) imaging for diagnosis, staging, and/or surveillance. Incidental detection of coronary artery calcification (CAC) on NGCCT in oncology patients may present an opportunity to detect and modify CV risk through lifestyle interventions, or pharmacological therapy as appropriate (e.g., use of statin and/or aspirin), especially for those without known atherosclerotic CV disease (ASCVD). We hypothesize that knowledge of CAC rarely influences preventive practices in the cancer population in clinical practice, and it may constitute a missed opportunity for reducing CV risk.
Five-year survival rates for non–small cell lung cancer (NSCLC) now reach 70% to 90% for small, localized tumors 2, 3. Because of shared risk factors, adverse CV events are frequently observed among NSCLC survivors (4). In this retrospective study, we sought to assess the prevalence of CAC on NGCCT performed at diagnosis of early-stage NSCLC, and to determine whether the incidental finding of CAC influenced subsequent prescription of statin and/or aspirin.
Noncontrast NGCCT scans performed across 25 hospitals in 164 patients at diagnosis of early-stage NSCLC were assessed for CAC (Figure 1). The mean age was 68 ± 10 years, 64 (39.0%) were men, and 132 (80.5%) had stage 1 disease. A radiologist and a cardiologist with advanced training in CV imaging independently and blindly reviewed CT images. The readers provided a simple, overall visual assessment of none, mild, moderate, or severe CAC for the entire coronary arterial circulation. A third reader provided consensus in cases of disagreement. CAC was classified as mild if there were only isolated flecks of calcification; severe if there was continuous CAC within one or more coronary artery; and moderate if there was more than mild calcification but less than the description of severe calcification. This overall visual assessment approach to CAC quantification on NGCCT scans was validated by the National Lung Screening Trial investigators who reported good agreement with Agatston scoring, good inter-reader agreement among different radiologists, and that this approach was sufficient for CV risk classification (5).
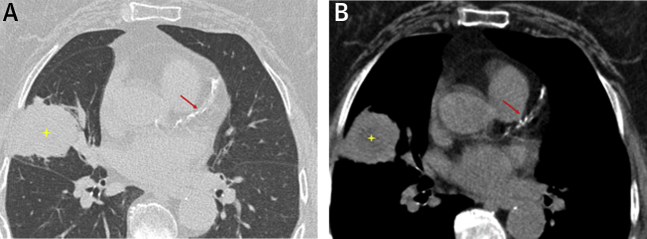
Figure 1.
Incidental Coronary Artery Calcification on Chest CT Performed in a Patient With Non-Small Cell Lung Cancer
(A) Lung window from a nongated, noncontrast chest computed tomography scan demonstrates a large mass in the right middle lobe (yellow star), subsequently proven to be non–small cell lung carcinoma. (B) Corresponding mediastinal window demonstrates severe atherosclerotic calcification affecting the left anterior descending artery (red arrow).
CAC was present in 50 (98.0%) of 51 patients with known ASCVD and in 78 (69.0%) of 113 patients without pre-existing ASCVD. Of these 78 patients with CAC and no known ASCVD, CAC was graded as mild in 40 (51.3%), moderate in 24 (30.8%), and severe in 14 (17.9%); 51 (65.4%) were not on aspirin, 48 (61.5%) were not on statin therapy, and 36 (46.2%) were not on either therapy. Medication usage was reviewed again at a median of 198 (172 to 237) days after this index scan. Over this intervening period, among patients with CAC and no known ASCVD, aspirin was initiated in only 4 of 51 (7.8%) aspirin-naive patients and statin therapy was initiated in only 1 of 48 (2.1%) statin-naïve patients. Allergy or intolerance to either therapy was recorded for 3 patients. Aspirin was declined by 1 patient, and concurrent anticoagulation or gastrointestinal bleeding may have precluded aspirin prescription in 3 additional patients. No patients had abnormal liver function tests to deter statin therapy or severe thrombocytopenia to deter aspirin usage.
We demonstrate that CAC is prevalent (69%) among patients with early-stage NSCLC and no known ASCVD at time of cancer diagnosis, and that the incidental finding of CAC on NGCCT rarely results in prescription of either aspirin or statin therapy despite lack of contraindications to either therapy. This represents a missed opportunity to modify CV risk for a cohort of patients predisposed to adverse CV events. Similarly, existing data suggest underutilization of guideline-directed medical therapies including aspirin and statin therapy for secondary prevention in patients with cancer following acute myocardial infarction (AMI) 6, 7. We recognize that safety concerns related to a higher prevalence of significant thrombocytopenia or hepatic dysfunction among patients with cancer compared with nononcology cohorts may negatively influence prescription of aspirin and statin therapies, respectively. In a multivariable analysis of 456 patients with cancer and a discharge diagnosis of AMI, however, aspirin use was associated with a 23% decreased risk of death (7). Aspirin conferred a survival benefit in a small study of 70 patients with cancer with and without thrombocytopenia following an acute coronary syndrome (8). In another study of 118 patients with hematologic malignancies diagnosed with AMI, aspirin was associated with improved survival without increase in major bleeding, even in patients with severe thrombocytopenia (6). These data inform a consensus statement from the Society for Cardiovascular Angiography and Interventions that advocates to continue aspirin in patients with cancer who have an indication for antiplatelet therapy and a platelet count above 10,000/ml (9). Larger prospective multicenter studies to clarify safety of aspirin and statin therapy in patients undergoing active cancer care, particularly in the setting of thrombocytopenia, are needed to challenge the apparent underutilization of these therapies for primary and secondary prevention of adverse CV events in this cohort.
We observed a key obstacle that may have hindered an appropriate clinical response, in that the presence of CAC was included in NGCCT reports for only 27 (34.6%) patients with CAC and no pre-existing ASCVD. Radiologists need to recognize the opportunity to highlight the presence of coronary atherosclerosis in scans performed for cancer-specific indications. Similar attention should be awarded to atherosclerosis identified in other arterial territories. In addition to specific commentary on the presence and location of atherosclerosis in imaging reports, radiologists could specify the need for clinical correlation for these findings. This might reduce the likelihood that atherosclerosis is overlooked by clinicians more focused on cancer care. Cardiologists can also take advantage of existing cancer imaging when reviewing oncology patients by routinely conducting their own review of images for the presence, location, and burden of atherosclerotic calcification. When interpreting the clinical relevance of CAC identified during cancer imaging, cardiologists may underestimate cancer survival, whereas oncologists may underestimate the risk of adverse CV outcomes during survivorship. Better communication between oncologists and cardiologists might help improve and further optimize clinical management of cancer patients and survivors. The findings of this study performed in patients with NSCLC may also apply to patients with other malignancies who have NGCCT performed as part of their cancer care.
In an era when improving CV outcomes for cancer survivors is an important public health goal, the incidental finding of CAC on cancer imaging warrants specific recognition by reporting radiologists and careful consideration by clinicians, who should weigh potential merits of preventative pharmacological therapy in mitigation of CV risk throughout the period of cancer therapy and eventual survivorship.
Footnotes
Please note: This work was supported by the Goodman Master Clinician Award, Brigham and Women’s Hospital, granted to Dr. Groarke. This work was also supported by the Gelb Master Clinician Award, Brigham and Women’s Hospital, granted to Dr. Nohria. Dr. Groarke has received research support from Amgen, Inc. Dr. Nohria is a consultant for Takeda; and has received research support from Amgen, Inc. Dr. Mehra is a consultant to Abbott, Medtronic, Janssen, Portola, Xogenex, Bayer, Mesoblast, NupulseCV, and Fineheart. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Abdel-Rahman O. Risk of cardiac death among cancer survivors in the United States: a SEER database analysis. Expert Rev Anticancer Ther. 2017;17:873–878. doi: 10.1080/14737140.2017.1344099. [DOI] [PubMed] [Google Scholar]
- 2.Miller K.D., Siegel R.L., Lin C.C. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Blandin Knight S., Crosbie P.A., Balata H., Chudziak J., Hussell T., Dive C. Progress and prospects of early detection in lung cancer. Open Biol. 2017;7 doi: 10.1098/rsob.170070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kocher F., Fiegl M., Mian M., Hilbe W. Cardiovascular comorbidities and events in NSCLC: often underestimated but worth considering. Clin Lung Cancer. 2015;16:305–312. doi: 10.1016/j.cllc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 5.Chiles C., Duan F., Gladish G.W. Association of coronary artery calcification and mortality in the National Lung Screening Trial: a comparison of three scoring methods. Radiology. 2015;276:82–90. doi: 10.1148/radiol.15142062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feher A., Kampaktsis P.N., Parameswaran R., Stein E.M., Steingart R., Gupta D. Aspirin is associated with improved survival in severely thrombocytopenic cancer patients with acute myocardial infarction. Oncologist. 2017;22:213–221. doi: 10.1634/theoncologist.2016-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf S.W., Daraban N., Abbasi N., Lei X., Durand J.B., Daher I.N. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35:443–450. doi: 10.1002/clc.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkiss M.G., Yusuf S.W., Warneke C.L. Impact of aspirin therapy in cancer patients with thrombocytopenia and acute coronary syndromes. Cancer. 2007;109:621–627. doi: 10.1002/cncr.22434. [DOI] [PubMed] [Google Scholar]
- 9.Iliescu C., Grines C.L., Herrmann J. SCAI expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (Endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista) Catheter Cardiovasc Interv. 2016;87:895–899. doi: 10.1002/ccd.26375. [DOI] [PubMed] [Google Scholar]