Abstract
Background
Patients with cancer have an increased risk of atrial fibrillation (AF). However, there is a paucity of information regarding the association between cancer type and risk of AF.
Objectives
This study sought to evaluate the risk of AF according to the type of cancer.
Methods
We enrolled 816,811 patients who were diagnosed with cancer from the Korean National Health Insurance Service database between 2009 and 2016. Age- and sex-matched noncancer control subjects (1:2; n = 1,633,663) were also selected. Newly diagnosed AF was identified based on the type of cancer.
Results
During a median follow-up of 4.5 years, AF was newly diagnosed in 25,356 patients with cancer (6.6 per 1,000 person-years). In multivariable Fine and Gray’s regression analysis, cancer was an independent risk factor for incident AF (adjusted subdistribution hazard ratio [aHR]: 1.63; 95% confidence interval [CI]: 1.61 to 1.66). Multiple myeloma showed a higher association with incident AF (aHR: 3.34; 95% CI: 2.98 to 3.75). Esophageal cancer showed the highest risk among solid cancers (aHR: 2.69; 95% CI: 2.45 to 2.95), and stomach cancer showed the lowest association with AF risk (aHR: 1.27; 95% CI 1.23 to 1.32).
Conclusions
Although patients with cancer were found to have a higher risk of AF, the impact on AF development varied by cancer type.
Key Words: atrial fibrillation, cancer, epidemiology, type of cancer
Abbreviations and Acronyms: AF, atrial fibrillation; CI, confidence interval; CKD, chronic kidney disease; CNS, central nervous system; CVD, cardiovascular disease; DM, diabetes mellitus; HR, hazard ratio; ICD-10, International Classification of Diseases–10th Revision; IQR, interquartile range; NHIS, National Health Insurance Service
Central Illustration
The life expectancy of patients with cancer is increasing as a result of recent advances in screening, diagnosis, and treatment of cancer (1). The number of patients with a history of cancer in the Unites States is believed to reach more than 26 million by 2040 (2). Cardiovascular disease is the second most common cause of late morbidity and death among cancer survivors (3). In addition to the recurrence, progression, and development of secondary malignancies, cardiovascular disease is a major concern in cancer survivors (4,5).
Atrial fibrillation (AF) is the most common sustained arrhythmia, and is increasing in both prevalence and incidence (6). The prevalence of AF increases with age, and approximately 10% of patients >80 years of age have AF (7). AF is also known to increase the risk of stroke, heart failure, and death (8). Previous studies have reported that cancer is an independent risk factor for AF (9, 10, 11). Inversely, an increased risk of cancer after AF diagnosis has also been reported (12). However, there is a paucity of information regarding the association between cancer type and risk of AF. Cancer is a heterogeneous disease, and the impact of cancer on AF risk may vary depending on the cancer type. In this study, we aimed to examine the risk of AF according to the type of cancer using a nationwide population-based study.
Methods
Data sources
We used the database of the National Health Insurance Service (NHIS) of Korea (13). The Korean NHIS is a compulsory health insurance program administered by the Korean government, which covers almost the entire Korean population (approximately 52 million people). The Korean NHIS database includes sociodemographic information, diagnoses, use of inpatient and outpatient services, and prescription claims. Diagnoses are recorded using International Classification of Diseases–10th Revision (ICD-10) codes. Individuals in the NHIS are recommended to receive a standardized health screening program biannually, and the health behaviors, physical examination, vital statistics, and laboratory tests are recorded for each subject. Although the resident registration number of each subject in the NHIS was de-identified to ensure privacy, it remains possible to follow all of the claims of the same subject continuously (13, 14, 15). This study was exempt from the Institutional Review Board of Seoul National University Hospital (E-1912-086-1089). Informed consent was not required because the patient records and information were anonymized and de-identified before analysis.
Characteristics of the study population
The patient selection is summarized in Figure 1. We identified patients who had been diagnosed with cancer and had undergone health examinations in the 2 years prior to cancer diagnosis between 2009 and 2016. A cancer diagnosis is defined when both ICD-10 codes and cancer-specific insurance claim code (V193 code) are satisfied. In Korea, patients with newly diagnosed cancer were registered with cancer-specific insurance claim code (code V193) in order to receive financial support. The reliability of data on cancer diagnosis was assumed because the Korean government provides financial support for patients with cancer-related ICD-10 codes on the basis of clinicopathologic assessments (16). Among these patients, we excluded those who had previously been diagnosed with AF prior to a cancer diagnosis. The index date of the patients with cancer was defined as the first day on which the definition of cancer diagnosis was satisfied. We included an age- and sex-matched comparator group (referred to as the control group) at a 1:2 ratio based on the index date of the matched cancer patients.
Figure 1.
Flowchart of the Cohort Establishment and Follow-Up
Patients who were newly diagnosed with cancer between 2009 and 2016 and had a health examination in the 2 years prior to cancer diagnosis were identified. Age- and sex-matched noncancer control subjects without prior history of atrial fibrillation (AF) were also selected. Incident AF events were monitored until December 2017.
We obtained baseline characteristics of study subjects, including age, sex, comorbidities, and health checkup data. Demographic data, including smoking status, alcohol consumption, and physical activity, were collected based on a self-reported questionnaire. Socioeconomic status was determined based on the health insurance premiums paid. Detailed definitions of comorbidities and demographic data are provided in Supplemental Table 1. To compare the incidence of AF according to cancer type, we classified 19 types of cancer as follows: esophageal cancer (C15), stomach cancer (C16), colorectal cancer (C18 to C20), liver cancer (C22), biliary cancer (C23, C24), pancreatic cancer (C25), head and neck cancer (C00 to C14, C30 to C32), lung cancer (C33 to 34), melanoma (C43), breast cancer (C50), gynecologic cancer (C53 to C57), prostate cancer (C61), renal cancer (C64), bladder cancer (C67), central nervous system (CNS) cancer (C70 to C72), thyroid cancer (C73), non-Hodgkin lymphoma (C82 to C86), multiple myeloma (C90), and leukemia (C91 to C95). The cancer ICD codes are listed in Supplemental Table 2.
Patient follow-up and outcomes
The study population was followed from the index year until new onset AF, death, or censoring at the end of the study period (December 31, 2017), whichever came first. AF was defined using ICD-10 codes (I48.0 to I48.4, I.48.9). Atrial flutter was also included in the definition of AF. To ensure diagnostic accuracy and exclude patients with transient AF, we only defined patients as having AF when it was a discharge diagnosis or was confirmed more than twice in an outpatient clinic (13). Additionally, we set 3 landmark points, 90 days, 1 year, and 5 years after cancer diagnosis, and performed landmark analysis to minimize the impact of postoperative AF and evaluate the long-term effects of cancer on the incidence of AF.
Statistical analysis
The baseline characteristics are presented as the mean ± SD or median (interquartile range [IQR]) for continuous variables and number and percentage for categorical variables. The incidence rates of AF were calculated from the total number of new onset AF events divided by the total person-years during the follow-up period. The cumulative hazard of AF between patients with cancer and the noncancer control cohort was compared using Kaplan–Meier estimates with the log-rank test. The hazard ratio (HR) and 95% confidence interval (CI) were calculated from the Fine and Gray’s competing risk regression model, and death was considered as a competing risk. First, the incidence of AF in patients with cancer was compared with that in healthy subjects. Second, 19 types of cancers were analyzed separately. We adjusted the following known AF risk factors to control for confounding factors: hypertension, diabetes mellitus (DM), dyslipidemia, obesity, chronic kidney disease (CKD), smoking, drinking, physical exercise status, and socioeconomic status (low income) (17,18). Subgroup analysis was performed, divided by age, sex, and comorbidities. Landmark analyses were performed with the 3 landmark points (90 days, 1 year, and 5 years after cancer diagnosis). Landmark analyses were performed with the 3 landmark points in patients who were event-free at the landmark time. All p values were 2-sided, and p < 0.05 was considered statistically significant. Statistical tests were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina).
Results
Baseline characteristics
As shown in Figure 1, 816,811 cancer patients and 1,633,663 healthy control subjects were evaluated. The mean age of the participants was 57.5 ± 12.5 years, and 46.9% were men in both groups. The baseline demographic data and comorbidities are presented in Table 1. The median follow-up duration was 4.5 (IQR: 2.7 to 6.6) years in the cancer cohort and 5.5 (IQR: 3.6 to 7.2) years in the noncancer control group. The characteristics of the study population according to cancer type are summarized in Supplemental Table 3. Thyroid cancer was the most common diagnosis, followed by stomach cancer, colorectal cancer, breast cancer, and lung cancer. Lymphoma was the most common diagnosis of patients with hematologic malignancies.
Table 1.
Baseline Clinical Characteristics of the Study Participants
| Control Subjects (n = 1,633,663) | Cancer Patients (n = 816,811) | p Value | |
|---|---|---|---|
| Age, yrs | 57.50 ± 12.47 | 57.51±12.48 | 0.601 |
| Age ≥65 yrs | 481,451 (29.47) | 241,026 (29.51) | 0.544 |
| Male | 765,350 (46.85) | 382,648 (46.85) | 0.975 |
| Body mass index, kg/m2 | 23.89 ± 3.26 | 23.93 ± 3.23 | <0.001 |
| Hypertension | 636,413 (38.96) | 323,982 (39.66) | <0.001 |
| Dyslipidemia | 453,246 (27.74) | 217,528 (26.63) | <0.001 |
| Diabetes mellitus | 250,953 (15.36) | 134,502 (16.47) | <.0001 |
| Chronic kidney disease | 125,722 (7.70) | 59,905 (7.33) | <0.001 |
| Smoking | <0.001 | ||
| Never smoker | 1,059,791 (64.87) | 524,073 (64.16) | |
| Former smoker | 245,465 (15.03) | 131,219 (16.06) | |
| Current smoker | 328,407 (20.1) | 161,519 (19.77) | |
| Alcohol use∗ | <0.001 | ||
| No drinker | 1,013,635 (62.05) | 499,744 (61.18) | |
| Mild drinker | 525,059 (32.14) | 267,704 (32.77) | |
| Heavy drinker | 94,969 (5.81) | 49,363 (6.04) | |
| Regular exercise† | 720,134 (44.08) | 389,882 (47.73) | <0.001 |
| Low income‡ | 418,496 (25.62) | 172,675 (21.14) | <0.001 |
Values are mean ± SD or n (%).
Alcohol consumption is denoted as the following: nondrinker (alcohol consumption 0 g), mild to moderate drinker (alcohol consumption >0 g to <30 g/day), and heavy drinker (alcohol consumption ≥30 g/day).
Regular exercise denotes performing >30 min of moderate-intensity exercise (e.g., brisk pace walking, tennis doubles, bicycling leisurely) ≥5 times a week or >20 min of vigorous-intensity exercise (e.g., running, climbing, fast cycling, aerobics) ≥3 times a week.
Low income denotes income belongs to lower 20% among the entire Korean population of subjects supported by the Medical Aid program.
Incidence of AF in cancer patients
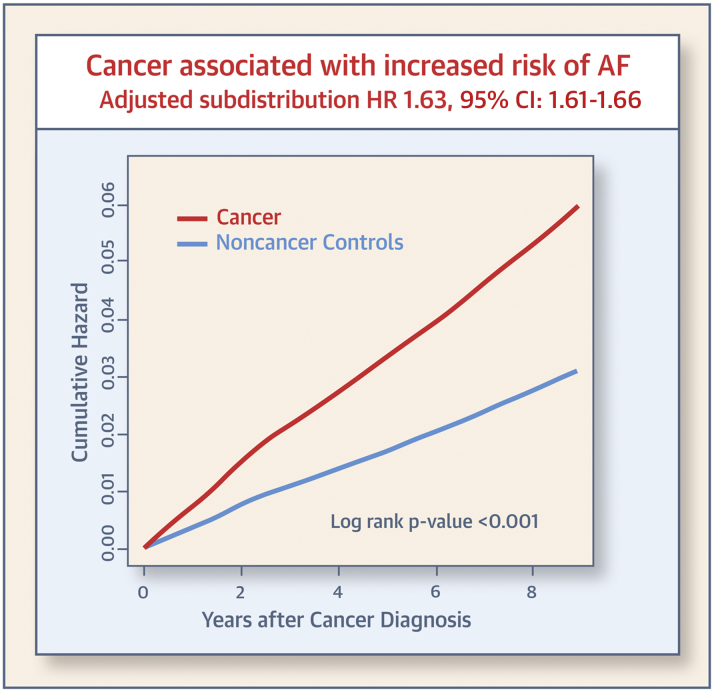
Table 2 presents the number of events, incidence rate, and crude and adjusted subdistribution HRs for AF incidence in cancer and control cohorts. During the follow-up period, 25,356 (3.1%) patients developed AF in the cancer cohort, and 31,801 (1.9%) subjects developed AF in the control group. In patients with AF, the median time from cancer diagnosis to AF was 1.7 (IQR: 0.9 to 3.2) years in the cancer cohort and 3.2 (IQR: 1.6 to 5.1) years in the control group. Patients with cancer showed a higher AF incidence than the general population (6.6 per 1,000 person-years in patients vs. 3.6 per 1,000 person-years in control subjects). In Fine and Gray’s regression analysis, cancer diagnosis was associated with a 1.6-fold higher risk of AF development (subdistribution HR: 1.64; 95% confidence interval [CI]: 1.61 to 1.66). After adjusting for hypertension, DM, dyslipidemia, obesity, CKD, smoking, drinking, physical exercise status, and income level, the association between a cancer diagnosis and AF was similar (adjusted subdistribution HR: 1.63; 95% CI: 1.61 to 1.66). Figure 2 shows the age- and sex-adjusted cumulative hazard curves for AF in the cancer and control groups.
Table 2.
Risk of Atrial Fibrillation in Patients With Cancer and the General Population
| Groups | n | Events | Duration (Person-Years) | Time to Event (Years)∗ | Incidence∗ | Subdistribution HR (95% CI) |
|
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | ||||||
| Noncancer control subjects | 1,633,663 | 31,801 | 8,795,076 | 3.2 (1.6–5.1) | 3.62 | Reference | Reference |
| Cancer patients | 816,811 | 25,356 | 3,813,800 | 1.7 (0.9–3.2) | 6.65 | 1.64 (1.61–1.66) | 1.63 (1.61–1.66) |
Values are median (interquartile range), unless otherwise indicated. Death was considered a competing risk in Fine and Gray’s competing risk regression models. Model 1: age- and sex-matched cohort; model 2: adjusted for age, sex, smoking, drinking, regular exercise, socioeconomic status, diabetes mellitus, hypertension, dyslipidemia, body mass index, and chronic kidney disease.
CI = confidence interval; HR = hazard ratio.
Per 1,000 person-years.
Figure 2.
Cumulative Hazard of AF According to the Diagnosis of Cancer
Age- and sex-adjusted Kaplan–Meier curves with cumulative hazard of atrial fibrillation (AF). Patients with cancer show a consistently higher incidence of AF compared with noncancer control subjects. CI = confidence interval; HR = hazard ratio.
Incidence of AF depending on the type of cancers
The Central Illustration demonstrates the number of events, the median time to AF, and the incidence and risk of AF according to the type of cancer. All types of cancer contributed to the incidence of AF. However, the incidence rate of AF varied according to the cancer type. Patients with multiple myeloma showed the highest risk of AF compared with the noncancer control group (adjusted subdistribution HR: 3.34; 95% CI: 2.98 to 3.75), and patients with stomach cancer showed the lowest risk of AF (adjusted subdistribution HR: 1.27; 95% CI: 1.23 to 1.32). All hematologic malignancies, including lymphoma, leukemia, and multiple myeloma, showed a high risk of AF development (for leukemia, adjusted subdistribution HR: 2.64; 95% CI: 2.38 to 2.92; for lymphoma, adjusted subdistribution HR: 2.29; 95% CI: 2.10 to 2.51). Among solid cancers, intrathoracic malignancies, including lung cancer, esophageal cancer, and CNS cancer, were associated with a high risk of AF development (for patients with esophageal cancer, adjusted subdistribution HR: 2.69; 95% CI: 2.45 to 2.95; for CNS cancer, adjusted subdistribution HR: 2.62; 95% CI: 2.35 to 2.91; for lung cancer, adjusted subdistribution HR: 2.39; 95% CI: 2.30 to 2.48).
Central Illustration.
AF Risk According to Cancer, as Compared With Noncancer Control Subjects, and Cancer Type
816,811 patients who were diagnosed with cancer from the Korean National Health Insurance Service database between 2009 and 2016 were compared to 1,633,663 age-and sex-matched non-cancer control subjects (1:2). In multivariable Fine and Gray’s regression analysis, cancer was an independent risk factor for incident AF (adjusted subdistribution hazard ratio [aHR]: 1.63; 95% confidence interval [CI]: 1.61 to 1.66). All types of cancer show an increased risk of AF compared with the control group, but the risk of AF varied depending on the type of cancer. Death was considered a competing risk in Fine and Gray’s competing risk regression models. The time to event for subjects having AF presented as the median (years). ∗Per 1000 person-years. †Adjusted for age, sex, smoking, drinking, regular exercise, socioeconomic status, diabetes mellitus, hypertension, dyslipidemia, BMI, and chronic kidney disease.
Subgroup analysis and landmark analysis
Patients with cancer showed a consistently higher risk of AF development across all subgroups (Figure 3). Although subgroup analyses demonstrated that the absolute incidence of AF was higher in those with established AF risk factors (older age, DM, hypertension, CKD, obesity, and smoking) compared with patients without cancer, the relative hazard of AF in cancer patients was greater in those without AF risk factors.
Figure 3.
Subgroup Analysis for AF Risk in Patients With Cancer
Patients with cancer showed a consistently higher risk of developing AF, regardless of the subgroup statement. Death was considered a competing risk in Fine and Gray’s competing risk regression models. ∗Per 1,000 person-years. †Adjusted for age, sex, smoking, drinking, regular exercise, socioeconomic status, diabetes mellitus (DM), hypertension, dyslipidemia, body mass index (BMI), and chronic kidney disease (CKD). CI = confidence interval; other abbreviations as in Figure 2.
Figure 4 shows the incidence of AF stratified by age into 5 groups and major cancer types. Multiple myeloma showed the highest incidence of AF in the >35 years of age group among hematologic malignancies. Among the major types of solid malignancies, lung cancer showed the highest AF incidence in the >50 years of age group, and liver cancer showed the highest incidence of AF in the <50 years of age group. Other types of cancer stratified by age are presented in Supplemental Table 4.
Figure 4.
AF Incidence According to the Type of Cancer and Age
(A) Atrial fibrillation (AF) incidence according to age in patients with hematologic malignancies; (B) AF incidence according to age in patients with major solid malignancies. In A, multiple myeloma showed the highest incidence of AF in the >35 years of age group and a steep rise with increasing age. In B, lung cancer showed the highest AF incidence in the >50 years of age group, and liver cancer showed the highest AF incidence in the <50 years of age group.
Table 3 shows the results of the landmark analyses. In the landmark analysis performed 90 days after cancer diagnosis, the cancer cohort consistently showed a higher risk of AF than the noncancer control cohort (adjusted subdistribution HR: 1.56; 95% CI: 1.54 to 1.59). The impact of the risk of AF declined with time from cancer diagnosis but remained significant. Patients with cancer showed a 44% higher risk of AF development (adjusted subdistribution HR: 1.44; 95% CI: 1.42. to 1.47) 1 year after cancer diagnosis and an 8% higher risk of AF (adjusted subdistribution HR: 1.08; 95% CI: 1.03 to 1.12) 5 years after cancer diagnosis. Table 4 shows the results of the landmark analysis according to the type of cancer. In all types of cancer, the impact of cancer on AF incidence attenuated with time after cancer diagnosis. Moreover, many cancers were not significantly associated with an increased incidence of AF 5 years after cancer diagnosis, except for patients with hematologic malignancies (multiple myeloma, leukemia, and lymphoma), lung, liver, renal, and gynecologic cancers.
Table 3.
Landmark Analysis: Risk of Atrial Fibrillation at 90 Days, 1 Year, and 5 Years After a Cancer Diagnosis
| Noncancer Control Subjects | Cancer Patients | |
|---|---|---|
| 90 days | ||
| Number | 1,631,826 | 813,650 |
| Events | 30,326 | 23,005 |
| Duration, person-years | 8,392,491 | 3,612,754 |
| Time to event, yrs | 3.11 (1.58–4.92) | 1.61 (0.88–3.21) |
| Incidence∗ | 3.61 | 6.37 |
| Model 1: Subdistribution HR (95% CI) | Reference | 1.57 (1.54–1.59) |
| Model 2: Subdistribution HR (95% CI) | Reference | 1.56 (1.54–1.59) |
| 1 yr | ||
| Number | 1,623,932 | 790,183 |
| Events | 26,800 | 18,180 |
| Duration, person-years | 7,165,685 | 3,006,675 |
| Time to event, yrs | 2.74 (1.32–4.40) | 1.25 (0.60–3.08) |
| Incidence∗ | 3.74 | 6.05 |
| Model 1: Subdistribution HR (95% CI) | Reference | 1.45 (1.42–1.48) |
| Model 2: Subdistribution HR (95% CI) | Reference | 1.44 (1.42–1.47) |
| 5 yrs | ||
| Number | 952,163 | 374,557 |
| Events | 8,187 | 2,982 |
| Duration, person-years | 1,802,692 | 683,875 |
| Time to event, yrs | 1.21 (0.56–2.05) | 1.16 (0.53–1.98) |
| Incidence∗ | 4.54 | 4.36 |
| Model 1: Subdistribution HR (95% CI) | Reference | 1.09 (1.04–1.14) |
| Model 2: Subdistribution HR (95% CI) | Reference | 1.08 (1.03–1.12) |
Values are median (interquartile range), unless otherwise indicated. Death was considered a competing risk in Fine and Gray’s competing risk regression model. Model 1: adjusted for age and sex; model 2: adjusted for age, sex, smoking, drinking, regular exercise, socioeconomic status, diabetes mellitus, hypertension, dyslipidemia, body mass index, and chronic kidney disease.
Abbreviations as in Table 2.
Per 1,000 person-years.
Table 4.
Landmark Analysis: Risk of Atrial Fibrillation 90 Days, 1 Year, and 5 Years After a Cancer Diagnosis Compared With Noncancer Control Subjects
| 90 Days After a Cancer Diagnosis |
1 Year After a Cancer Diagnosis |
5 Years After a Cancer Diagnosis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Event/No. | Incidence∗ | Subdistribution HR (95% CI) Model 1 |
Subdistribution HR (95% CI) Model 2 |
Event/No. | Incidence∗ | Subdistribution HR (95% CI) Model 1 |
Subdistribution HR (95% CI) Model 2 |
Event/No. | Incidence∗ | Subdistribution HR (95% CI) Model 1 |
Subdistribution HR (95% CI) Model 2 |
|
| All | 30,326/1,631,826 | 3.61 | Reference | Reference | 790,183/1,623,932 | 3.74 | Reference | Reference | 8,187/952,163 | 4.54 | Reference | Reference |
| Stomach | 3,111/124,058 | 5.28 | 1.14 (1.10–1.18) | 1.14 (1.10–1.19) | 2,566/121,244 | 5.17 | 1.08 (1.04–1.13) | 1.08 (1.04–1.13) | 603/64,024 | 4.97 | 0.97 (0.89–1.05) | 0.96 (0.88–1.04) |
| Colorectal | 3,010/101,220 | 6.26 | 1.40 (1.35–1.45) | 1.38 (1.33–1.43) | 2,379/99,580 | 5.88 | 1.26 (1.21–1.32) | 1.24 (1.19–1.29) | 496/51,654 | 5.21 | 1.02 (0.93–1.12) | 0.99 (0.91–1.09) |
| Liver | 1,741/46,255 | 11.14 | 1.82 (1.74–1.92) | 1.79 (1.70–1.88) | 1,359/42,841 | 11.10 | 1.75 (1.65–1.85) | 1.70 (1.61–1.8) | 155/12,676 | 7.21 | 1.48 (1.26–1.74) | 1.44 (1.23–1.70) |
| Pancreatic | 535/14,930 | 14.30 | 1.59 (1.46–1.74) | 1.58 (1.45–1.73) | 387/13,408 | 14.54 | 1.47 (1.33–1.63) | 1.46 (1.32–1.62) | 18/1,893 | 5.52 | 1.10 (0.69–1.75) | 1.09 (0.68–1.72) |
| Lung | 2,785/49,115 | 17.48 | 2.27 (2.18–2.36) | 2.32 (2.23–2.41) | 2,154/46,033 | 17.48 | 2.13 (2.03–2.23) | 2.17 (2.07–2.27) | 168/11,795 | 8.59 | 1.46 (1.25–1.70) | 1.47 (1.26–1.71) |
| Breast | 1,068/80,920 | 2.82 | 1.46 (1.38–1.56) | 1.48 (1.39–1.58) | 868/80,577 | 2.73 | 1.38 (1.29–1.48) | 1.40 (1.30–1.50) | 145/38,869 | 2.08 | 0.99 (0.84–1.17) | 1.00 (0.84–1.18) |
| Gynecologic | 519/31,420 | 3.59 | 1.53 (1.40–1.67) | 1.52 (1.40–1.66) | 419/31,079 | 3.46 | 1.44 (1.31–1.59) | 1.44 (1.30–1.58) | 92/14,650 | 3.40 | 1.40 (1.13–1.72) | 1.38 (1.12–1.70) |
| Thyroid | 1,779/153,750 | 2.17 | 1.26 (1.20–1.32) | 1.27 (1.21–1.33) | 1,389/153,277 | 1.97 | 1.13 (1.07–1.19) | 1.13 (1.07–1.20) | 363/98,020 | 2.02 | 1.06 (0.95–1.18) | 1.05 (0.94–1.18) |
| Non-Hodgkin lymphoma | 480/12,130 | 9.55 | 2.29 (2.09–2.50) | 2.30 (2.10–2.52) | 380/11,681 | 9.22 | 2.18 (1.96–2.41) | 2.19 (1.97–2.42) | 50/4,868 | 5.58 | 1.51 (1.14–1.99) | 1.51 (1.14–1.99) |
| Prostate | 1,271/28,930 | 8.70 | 1.31 (1.24–1.39) | 1.31 (1.23–1.38) | 1,041/28,570 | 8.36 | 1.19 (1.11–1.26) | 1.18 (1.11–1.26) | 247/17,137 | 8.07 | 0.93 (0.81–1.05) | 0.92 (0.81–1.05) |
| Head & Neck | 420/11,613 | 8.35 | 1.74 (1.58–1.92) | 1.74 (1.58–1.92) | 337/11,366 | 8.10 | 1.60 (1.43–1.78) | 1.59 (1.43–1.78) | 50/4,957 | 5.63 | 1.11 (0.84–1.47) | 1.09 (0.83–1.45) |
| Esophagus | 417/6,503 | 18.18 | 2.46 (2.23–2.71) | 2.49 (2.25–2.75) | 314/6,021 | 17.26 | 2.23 (1.99–2.50) | 2.24 (2.00–2.51) | 31/2,007 | 8.90 | 1.39 (0.97–1.97) | 1.36 (0.96–1.94) |
| Biliary | 513/12,070 | 12.90 | 1.66 (1.52–1.82) | 1.65 (1.51–1.82) | 398/11,259 | 12.88 | 1.55 (1.40–1.71) | 1.54 (1.39–1.70) | 36/3,026 | 6.79 | 1.08 (0.77–1.51) | 1.07 (0.76–1.49) |
| Renal | 461/14,549 | 6.87 | 1.94 (1.77–2.13) | 1.81 (1.65–1.99) | 359/14,333 | 6.39 | 1.74 (1.57–1.94) | 1.64 (1.47–1.82) | 74/6,984 | 5.89 | 1.46 (1.15–1.84) | 1.36 (1.08–1.72) |
| Bladder cancer | 611/13,999 | 9.17 | 1.58 (1.46–1.72) | 1.57 (1.45–1.70) | 502/13,787 | 8.94 | 1.47 (1.35–1.61) | 1.46 (1.33–1.60) | 91/7,292 | 6.86 | 1.00 (0.81–1.23) | 0.99 (0.80–1.22) |
| CNS cancer | 342/8,218 | 11.04 | 2.64 (2.37–2.94) | 2.62 (2.35–2.92) | 247/7,867 | 9.92 | 2.29 (2.01–2.60) | 2.28 (2.00–2.59) | 20/2,696 | 4.10 | 1.25 (0.80–1.96) | 1.25 (0.80–1.96) |
| Multiple myeloma | 282/4,034 | 19.95 | 3.36 (2.99–3.79) | 3.29 (2.92–3.70) | 227/3,843 | 20.36 | 3.19 (2.79–3.65) | 3.12 (2.73–3.57) | 34/1,087 | 20.14 | 3.76 (2.68–5.30) | 3.67 (2.61–5.16) |
| Leukemia | 374/8,531 | 12.92 | 2.69 (2.43–2.98) | 2.69 (2.42–2.98) | 298/7,996 | 13.15 | 2.66 (2.37–2.99) | 2.65 (2.36–2.98) | 23/2,343 | 5.47 | 1.74 (1.14–2.63) | 1.72 (1.13–2.61) |
| Melanoma | 68/1,961 | 8.23 | 1.75 (1.37–2.22) | 1.73 (1.36–2.19) | 53/1,928 | 7.80 | 1.57 (1.20–2.06) | 1.56 (1.19–2.04) | 9/796 | 6.89 | 1.17 (0.59–2.35) | 1.14(0.57–2.29) |
Death was considered a competing risk in Fine and Gray’s competing risk regression model. Model 1: adjusted for age and sex; model 2: adjusted for smoking, drinking, regular exercise, socioeconomic status, diabetes mellitus, hypertension, dyslipidemia, body mass index, and chronic kidney disease.
Abbreviations as in Table 2.
Per 1,000 person-years.
Discussion
In this large population-based study, we found that: 1) patients with a history of cancer had a higher risk of AF compared with those without; 2) the risk of AF varies depending on the type of cancer (Central Illustration); 3) among various types of cancer, hematologic malignancies, including lymphoma, leukemia, and multiple myeloma, and intrathoracic malignancies, including lung cancer and esophageal cancer, and CNS cancer were associated with a more than 2-fold increased risk of AF compared with the noncancer control group; and 4) the association between cancer and the risk of AF declines with time after a cancer diagnosis, although hematologic malignancies (multiple myeloma, leukemia, and lymphoma), lung, liver, renal, and gynecologic cancers showed a persistently increased risk of AF 5 years after cancer diagnosis.
AF is important in the context of cancer. Patients with cancer and new onset AF showed a 2-fold increased risk of thromboembolism and a 6-fold increased risk of heart failure (19). Therefore, AF is an important comorbidity that needs to be detected and controlled in patients with cancer. Previous studies have reported that patients with cancer have an increased risk of AF (9, 10, 11). Indeed, a recent meta-analysis reported a 47% increased risk of AF in patients with cancer (odds ratio: 1.47; 95% CI: 1.31 to 1.66) (9). Furthermore, a population-based study in Denmark reported a 1.4-fold increase in incident AF in patients with cancer compared with the general population (10). In addition, patients with cancer but without active treatment were associated with a 20% increased risk of AF (odds ratio: 1.19; 95% CI: 1.02 to 1.38) (11). In line with previous studies, we found that patients diagnosed with cancer had a 63% higher risk of AF compared with the age- and sex-matched noncancer control subjects. To the best of our knowledge, we analyzed the largest number of patients with a history of cancer and provide definitive data regarding the association between cancer and the risk of AF. Notably, we found that the risk of incident AF varies depending on the type of cancer. Hematologic malignancies, such as lymphoma, leukemia, and multiple myeloma, have a higher risk of incident AF. In contrast, breast, colorectal, stomach, thyroid, and prostate cancers showed a relatively lower risk of AF. Recently, a Danish population-based study also showed varying risks of AF according to cancer type (10). In this study, lung cancer showed the highest risk of AF, with an HR of 3.16 (95% CI: 3.04 to 3.30). Patients with upper gastrointestinal cancer or CNS cancer had a more than 2-fold increased risk of AF.
There are several explanations for the high risk of AF observed in patients with cancer. First, cancer and AF share common risk factors, with age shown to be the strongest risk factor for both AF and cancer (17,20). Smoking, alcohol use, and obesity are also risk factors for both diseases (5,17). However, we found that patients with a history of cancer had a higher risk of AF even after adjustment for these factors, which suggests that another mechanism may be responsible for the increased risk of AF in patients with cancer. Second, many cancer therapies, including surgery and systemic treatment, are associated with new onset AF. Considering that most postoperative AF occurred during the first postoperative week, we used landmark analysis at 90 days to exclude the impact of postoperative AF (21). In addition to postoperative AF, anti-cancer drugs such as anthracyclines, melphalan, and ibrutinib are associated with atrial remodeling acting as an AF substrate (22,23). Hematopoietic stem cell transplantation, a key treatment strategy for hematologic malignancies, is associated with the development of AF (24). With infusion, patients may also receive large volume loads with cancer therapy. Third, inflammation may be another possible mechanism. Indeed, chronic inflammation may lead to carcinogenesis, and inflammation is also thought to be associated with the development of AF (5). Moreover, C-reactive protein, a representative inflammatory marker, is increased in patients with AF, and its level is correlated with AF burden (25). Other inflammatory markers, such as interleukin-6 and tumor necrosis factor α, are also known to be related to AF (26,27). Previous studies have examined the AF risk in chronic inflammatory disease and have found a significant relationship with a higher risk of AF (28, 29, 30, 31, 32). Fourth, the autonomic nervous system imbalance, paraneoplastic syndromes, or direct invasion of tumors into cardiac structures may also be possible causes for this association (33). These multifactorial factors may also be responsible for the varying risks of developing AF according to the type of cancer. In our study, hematologic malignancies tended to have a higher risk of AF than nonhematologic malignancies. Several possible mechanisms could explain this finding. It is likely that the difference in treatment modalities will have affected the occurrence of AF (24). Doxorubicin, melphalan, and ibrutinib are associated with incident AF, and these anticancer drugs are more commonly prescribed for hematologic malignancies (34, 35, 36). Moreover, inflammation is likely to have an impact on the risk of AF in patients with hematologic malignancies (37).
In previous studies, the long-term effect of cancer on the development of AF has been controversial. In a large population-based Danish study, patients with a diagnosis of colorectal cancer were more likely to develop AF within 90 days of diagnosis than the general population, although this was not the case beyond this initial 90-day period (38). Another Danish population-based study reported that the association between overall cancer and AF was highest within the first 90 days, but it remained significant over time (10). Our study showed a significant AF risk even excluding AF that occurred within 90 days of diagnosis. The impact of cancer on the incidence of AF declined but remained significant over time. Some types of cancer, including hematologic malignancies (multiple myeloma, leukemia, and lymphoma), lung, liver, renal, and gynecologic cancers, are a persistent risk of AF even 5 years after a cancer diagnosis, but other types of cancer are not. It may be that other risk factors influence AF risk in the longer term.
Considering the high risk of AF in patients with hematologic malignancies, intrathoracic malignancies, and CNS cancer, physicians might consider more intensive screening in these subgroups. However, it is unclear whether routine screening could improve outcomes. Additional study is needed to clarify this. Knowledge gaps also include the association between AF and risk of subsequent adverse cardiovascular outcomes, including death, as well as optimal management strategies.
Study limitations
First, specific information on the stage of cancer, responsiveness to treatment, treatment strategy, and biomarkers were not accessible in the claims database. This may have impacted our results, given that treatment strategies, such as chemotherapeutic agents, radiation, and surgery, can affect the risk of AF. Although we performed a landmark analysis using 90 days after the cancer diagnosis, all postoperative AF could not be ruled out because of the limitations of claims data. We have also not identified the association between each treatment type and AF in patients with cancer. Further research could be helpful to identify whether specific treatment groups affect the development of AF, especially in patients with hematologic malignancies. Second, misclassification of the diagnosis based on the ICD code is possible. However, the diagnosis of cancer is based on both the ICD-10 code and V193 (cancer-specific insurance claim code), and any misclassification bias on cancer diagnosis is considered to be small. Moreover, the diagnosis of AF was validated in a previous study, which reported a positive predictive value of 94.1% when using ICD-10 codes in the Korean NHIS database (39). Third, the cumulative incidence curves were plotted without considering death as a competing risk. However, death was considered a competing risk in Fine and Gray’s competing risk regression model. Fourth, the study included only the Korean population; therefore, extrapolation to other races should be performed with caution. Nevertheless, this study includes the largest number of patients with cancer and revealed an association between cancer and the risk of AF. Last, we did not observe outcomes such as mortality, heart failure, or thromboembolic events in patients with cancer and AF. Further studies are needed to evaluate the impact of AF on the outcomes of patients with cancer. Two prospective cohort studies are ongoing to verify the outcome and effectiveness of using anticoagulants in patients with cancer and AF (NCT03909386 and NCT04508855).
Conclusions
Patients with cancer showed a higher risk of AF than the general population, and the risk on AF development varied according to cancer type. An increased risk of AF should be considered when treating patients with cancer.
Perspectives.
COMPETENCY IN PATIENT CARE: Patients with cancer are at a higher risk of incident AF than patients without cancer. The impact of cancer on the incidence of AF incidence varies according to the type of cancer. Hematologic malignancies (multiple myeloma, leukemia, and lymphoma), intrathoracic malignancies (lung cancer and esophageal cancer), and CNS cancers showed a higher incidence of AF than the general population. Physicians and patients should be aware of these risks.
TRANSLATIONAL OUTLOOK: Further studies are needed to identify the predictors of AF, especially in patients with hematologic malignancies. Future studies are also needed to determine whether routine AF screening results in improved outcomes in specific populations.
Funding Support and Author Disclosures
This work was supported by the Korea Medical Device Development Fund grant funded by the Korean government (the Ministry of Science and ICT; the Ministry of Trade, Industry and Energy; the Ministry of Health and Welfare; the Ministry of Food and Drug Safety) (Project Number: 202013B14) and by the Korea National Research Foundation funded by the Ministry of Education, Science and Technology (grant 2020R1F1A106740). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental tables, please see the online version of this paper.
Appendix
References
- 1.Jemal A., Ward E.M., Johnson C.J. Annual Report to the Nation on the Status of Cancer, 1975-2014, Featuring Survival. J Natl Cancer Inst. 2017;109:djx030. doi: 10.1093/jnci/djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluethmann S.M., Mariotto A.B., Rowland J.H. Anticipating the “silver tsunami”: prevalence trajectories and comorbidity burden among older cancer survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25:1029–1036. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens A.C., Liu Q., Neglia J.P. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okwuosa T.M., Anzevino S., Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J. 2017;93:82–90. doi: 10.1136/postgradmedj-2016-134417. [DOI] [PubMed] [Google Scholar]
- 5.Vincent L., Leedy D., Masri S.C., Cheng R.K. Cardiovascular disease and cancer: is there increasing overlap? Curr Oncol Rep. 2019;21:47. doi: 10.1007/s11912-019-0796-0. [DOI] [PubMed] [Google Scholar]
- 6.Schnabel R.B., Yin X., Gona P. 50 year trends in atrial fibrillation prevalence, incidence, risk factors, and mortality in the Framingham Heart Study: a cohort study. Lancet. 2015;386:154–162. doi: 10.1016/S0140-6736(14)61774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Go A.S., Phillips K.A., Chang Y.C. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 8.Joung B. Risk factor management for atrial fibrillation. Korean Circ J. 2019;49:794–807. doi: 10.4070/kcj.2019.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan M., Zhang Z., Tse G. Association of cancer and the risk of developing atrial fibrillation: a systematic review and meta-analysis. Cardiol Res Pract. 2019;2019:8985273. doi: 10.1155/2019/8985273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jakobsen C.B., Lamberts M., Carlson N. Incidence of atrial fibrillation in different major cancer subtypes: a nationwide population-based 12 year follow up study. BMC Cancer. 2019;19:1105. doi: 10.1186/s12885-019-6314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Neal W.T., Lakoski S.G., Qureshi W. Relation between cancer and atrial fibrillation (from the REasons for Geographic And Racial Differences in Stroke Study) Am J Cardiol. 2015;115:1090–1094. doi: 10.1016/j.amjcard.2015.01.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conen D., Wong J.A., Sandhu R.K. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. 2016;1:389–396. doi: 10.1001/jamacardio.2016.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi E.K. Cardiovascular research using the Korean National Health Information Database. Korean Circ J. 2020;50:754–772. doi: 10.4070/kcj.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheol Seong S., Kim Y.Y., Khang Y.H. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seong S.C., Kim Y.Y., Park S.K. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2017-016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahn S.Y., Choi Y.J., Han K., Ko G.J., Kwon Y.J., Park Y.G. Dipstick proteinuria and cancer incidence: a nationwide population-based study. J Nephrol. 2020;33:1067–1077. doi: 10.1007/s40620-020-00740-1. [DOI] [PubMed] [Google Scholar]
- 17.Hindricks G., Potpara T., Dagres N. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.R., Choi E.K., Han K., Cha M.J., Oh S. Prevalence of non-valvular atrial fibrillation based on geographical distribution and socioeconomic status in the entire Korean Population. Korean Circ J. 2018;48:622–634. doi: 10.4070/kcj.2017.0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu Y.F., Liu C.J., Chang P.M. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355–357. doi: 10.1016/j.ijcard.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 21.Dobrev D., Aguilar M., Heijman J., Guichard J.B., Nattel S. Postoperative atrial fibrillation: mechanisms, manifestations and management. Nat Rev Cardiol. 2019;16:417–436. doi: 10.1038/s41569-019-0166-5. [DOI] [PubMed] [Google Scholar]
- 22.Alexandre J., Moslehi J.J., Bersell K.R., Funck-Brentano C., Roden D.M., Salem J.E. Anticancer drug-induced cardiac rhythm disorders: current knowledge and basic underlying mechanisms. Pharmacol Ther. 2018;189:89–103. doi: 10.1016/j.pharmthera.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Kaakeh Y., Overholser B.R., Lopshire J.C., Tisdale J.E. Drug-induced atrial fibrillation. Drugs. 2012;72:1617–1630. doi: 10.2165/11633140-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathur P., Paydak H., Thanendrarajan S., van Rhee F. Atrial fibrillation in hematologic malignancies, especially after autologous hematopoietic stem cell transplantation: review of risk factors, current management, and future directions. Clin Lymphoma Myeloma Leuk. 2016;16:70–75. doi: 10.1016/j.clml.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Chung M.K., Martin D.O., Sprecher D. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001;104:2886–2891. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 26.Patel P., Dokainish H., Tsai P., Lakkis N. Update on the association of inflammation and atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:1064–1070. doi: 10.1111/j.1540-8167.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 27.Guo Y., Lip G.Y., Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. 2012;60:2263–2270. doi: 10.1016/j.jacc.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 28.Choi Y.J., Choi E.K., Han K.D. Increased risk of atrial fibrillation in patients with inflammatory bowel disease: a nationwide population-based study. World J Gastroenterol. 2019;25:2788–2798. doi: 10.3748/wjg.v25.i22.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee E., Choi E.K., Jung J.H. Increased risk of atrial fibrillation in patients with Behcet's disease: a nationwide population-based study. Int J Cardiol. 2019;292:106–111. doi: 10.1016/j.ijcard.2019.06.045. [DOI] [PubMed] [Google Scholar]
- 30.Moon I., Choi E.K., Jung J.H. Ankylosing spondylitis: A novel risk factor for atrial fibrillation - a nationwide population-based study. Int J Cardiol. 2019;275:77–82. doi: 10.1016/j.ijcard.2018.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Rhee T.M., Lee J.H., Choi E.K. Increased risk of atrial fibrillation and thromboembolism in patients with severe psoriasis: a nationwide population-based study. Sci Rep. 2017;7:9973. doi: 10.1038/s41598-017-10556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ungprasert P., Srivali N., Kittanamongkolchai W. Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. 2017;20:434–441. doi: 10.1111/1756-185X.12820. [DOI] [PubMed] [Google Scholar]
- 33.Farmakis D., Parissis J., Filippatos G. Insights into onco-cardiology: atrial fibrillation in cancer. J Am Coll Cardiol. 2014;63:945–953. doi: 10.1016/j.jacc.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 34.Phillips G.L., Meisenberg B., Reece D.E. Amifostine and autologous hematopoietic stem cell support of escalating-dose melphalan: a phase I study. Biol Blood Marrow Transplant. 2004;10:473–483. doi: 10.1016/j.bbmt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 35.Burger J.A., Tedeschi A., Barr P.M. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373:2425–2437. doi: 10.1056/NEJMoa1509388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amioka M., Sairaku A., Ochi T. Prognostic significance of new-onset atrial fibrillation in patients with non-Hodgkin's lymphoma treated with anthracyclines. Am J Cardiol. 2016;118:1386–1389. doi: 10.1016/j.amjcard.2016.07.049. [DOI] [PubMed] [Google Scholar]
- 37.Craver B.M., El Alaoui K., Scherber R.M., Fleischman A.G. The critical role of inflammation in the pathogenesis and progression of myeloid malignancies. Cancers (Basel) 2018;10:104. doi: 10.3390/cancers10040104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erichsen R., Christiansen C.F., Mehnert F., Weiss N.S., Baron J.A., Sorensen H.T. Colorectal cancer and risk of atrial fibrillation and flutter: a population-based case-control study. Intern Emerg Med. 2012;7:431–438. doi: 10.1007/s11739-011-0701-9. [DOI] [PubMed] [Google Scholar]
- 39.Lee S.S., Kong K.A., Kim D. Clinical implication of an impaired fasting glucose and prehypertension related to new onset atrial fibrillation in a healthy Asian population without underlying disease: a nationwide cohort study in Korea. Eur Heart J. 2017;38:2599–2607. doi: 10.1093/eurheartj/ehx316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.