Abstract
Objectives
The purpose of this study was to assess the associations between 3-dimensional echocardiography (3DE)-derived changes in right ventricular (RV) volumes and strains with subsequent RV cardiotoxicity in patients treated with anthracyclines.
Background
Although early detection and prediction of left ventricular (LV) dysfunction has been widely studied in patients receiving anthracyclines, little is known about the early changes in RV size and function in this population.
Methods
A total of 74 patients with diffuse large B-cell lymphoma who received 6 cycles of anthracycline-based treatment were enrolled. Echocardiography was performed at baseline or before chemotherapy (pre-chemotherapy) (T0); after 2 cycles (T1); after 4 cycles (T2); and at the end of 6 cycles of chemotherapy (T3). Right ventricular end-diastolic volume (RVEDV), end-systolic volume (RVESV), ejection fraction (RVEF), longitudinal free wall strain (RVLFS), and longitudinal septal strain (RVLSS) were quantified by 3DE. RV cardiotoxicity was defined as a relative reduction of >10% in 3D RVEF or a relative reduction of >5% to a value of <45%. Volume status was assessed by inferior vena cava diameter (IVCD) and the estimated right atrial pressure (RAP).
Results
Twenty-seven patients developed cardiotoxicity after 6 cycles of chemotherapy (T3). Compared to baseline, increases in 3D RVEDV (58.5 ± 7.7 ml vs. 64.2 ± 7.0 ml; p < 0.001) and RVESV (27.8 ± 4.2 ml vs. 31.3 ± 4.2 ml; p < 0.001) were observed by the end of the fourth cycle of chemotherapy (T2). 3D RVLFS (−27.3 ± 3.1% vs. −24.2 ± 2.6%; p < 0.001) was also decreased at T2 compared to baseline. Statistically significant declines in 3D RVLSS (−26.1 ± 2.5% vs. −22.9 ± 2.7%; p < 0.001) and RVEF (54.0 ± 2.8% vs. 49.8 ± 2.4%; p < 0.001) were only observed at T3. A relative decrease in RVLFS of >12.4% (sensitivity, 78.6%; specificity, 82.6%; area under the curve (AUC), 0.80; p < 0.001); and a relative increase in RVESV of >13.2% (sensitivity, 71.4%; specificity, 71.7%; AUC, 0.76; p <0.001) from baseline to T2 predicted subsequent RV cardiotoxicity at T3. IVCD and RAP did not change significantly over time.
Conclusions
3DE-derived measurements of RV strain and volume were associated with subsequent changes in RVEF. With further study, RVLFS and RVESV could potentially be used to predict subsequent declines in RVEF with anthracyclines.
Key Words: 3-dimensional echocardiography, anthracycline, cardio-oncology, cardiotoxicity, right ventricle
Abbreviations and Acronyms: 2DE, 2-dimensional echocardiography; 3DE, 3-dimensional echocardiography; LV, left ventricle; LVEF, left ventricular ejection fraction; RV, right ventricle; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume; RVLFS, right ventricular longitudinal free-wall strain; RVLSS, right ventricular longitudinal septal strain
Central Illustration
Anthracyclines are widely used in children and adults to treat hematologic malignancies, breast cancer, and sarcoma (1). However, there is a potential for cardiotoxicity, including dose-dependent cardiomyopathy and heart failure. Anthracycline-induced cardiotoxicity has been observed to potentially be irreversible in patients treated for lymphoma, resulting in overall worse outcomes (2). Prior studies suggest that more than one-half of patients treated with anthracyclines exhibit cardiac dysfunction to some extent, and an estimated 5% to 65% develop over heart failure, depending upon dose (3,4).
However, more current studies have indicated that left ventricular ejection fraction (LVEF) recovery and a decrease in adverse cardiac events may be achieved with early institution of angiotensin-converting enzyme inhibitors and beta-blockers (5). These data would imply that it may be important to detect anthracycline-induced cardiotoxicity early. Echocardiography has been recommended by European Society of Cardiology as part of routine surveillance of cardiotoxicity (6). Previous studies have provided a variety of echocardiography parameters to detect and predict anthracycline-induced LV cardiotoxicity (7). However, limited data are available for parameters which could reflect subclinical right ventricular (RV) dysfunction and potential predictors of RV cardiotoxicity. Several animal experiments and clinical studies indicate that the RV may also be susceptible to anthracycline-induced cardiotoxicity (8,9).
Due to the special geometric structure and unique wall motion of the RV, routine techniques for echocardiographic assessment of the RV has challenges (10). However, 3-dimensional echocardiography (3DE) tracks changes in RV volumes in real time, including the inflow and outflow tracts and the apex throughout the cardiac cycle. 3DE evaluation of RV function and morphology has been compared to cardiac magnetic resonance and is accurate, feasible, and reproducible (11,12). Therefore, a prospective cohort study was designed to understand the potential role of 3DE in the early identification and prediction of RV cardiotoxicity induced by anthracycline chemotherapy.
Subjects and Methods
Study population
A total of 74 consecutive patients with pathologically confirmed diffuse large B-cell lymphoma at Fudan University Shanghai Cancer Center were enrolled in the study between May 2014 and May 2015. All patients received 6 cycles of R-CHOP therapy (cyclophosphamide 750 mg/m2; vincristine 1.4 mg/m2 up to a maximum dose of 2 mg/m2; doxorubicin 50 to 70 mg/m2 on day 1; prednisone 100 mg on days 1 to 5; and rituximab 375 mg/m2 every 21 days). All therapy was given intravenously, except for prednisone, which was administered orally. Each patient underwent echocardiography examination before the commencement of chemotherapy (T0) and after the completion of every 2 cycles of chemotherapy (T1), for a total of 6 cycles (T3). Age <18 years, viral myocarditis, hypertension with systolic blood pressure of >140 mm Hg or diastolic pressure of >90 mm Hg, serious arrhythmia (atrial fibrillation, atrial flutter, ventricular tachycardia >3 beats and greater than the second-degree cardiac conduction block), a history of heart failure, and/or coronary artery disease were exclusion criteria for enrollment. All patients were provided informed consent for participation in the study and anthracycline treatment. Fudan University Shanghai Cancer Center (1212117-6) and Zhongshan Hospital Research Ethics Committee approved the protocol (2011-117).
Echocardiography data acquisition
All subjects underwent standard 2DE and 3DE at the baseline (T0), 8 to 15 days after the completion of 2 cycles (T1), 8 to 15 days after the completion of 4 cycles (T2), and at 50 to 60 days after 6 cycles (T3) of chemotherapy. Images were obtained with a commercially available ultrasonography system (iE33, Philips Medical Systems, Andover Massachusetts) equipped with S5-1 (1 to 5 MHz) and X3-1 (1 to 3 MHz), a fully sampled matrix transducer. Standard 2DE and 3DE were performed according to the recommendations of the American Society of Echocardiography (13). Five consecutive cycles for 2DE images and 6 consecutive cardiac cycles for 3DE images were acquired for offline analysis. Image parameters such as depth, sector size, angle, and focus were optimized to achieve a frame rate range of 60 to 80 fps for 2DE and 30 to 45 fps for 3DE analysis. Sonographers and off-line echocardiography readers were blinded to all clinical data.
2D, M-MODE, and Doppler echocardiography analysis
Tricuspid annular plane systolic excursion was acquired through the M-mode approach in the apical 4-chamber view. Pulse wave Doppler variables (tricuspid/mitral inflow velocities: peak E velocity of early diastolic filling [E]; peak A velocity of late diastolic filling [A]; tissue Doppler parameters peak tricuspid/mitral annulus systolic velocity [S′]; RV/LV peak early diastolic myocardial velocity [E′]; and RV/LV peak atrial contraction velocity [A′]) were acquired according to a standard protocol (13). Pulmonary artery systolic pressure was estimated using the continuous wave Doppler of the tricuspid regurgitation jet. Inferior vena cava diameter (IVCD) was measured in the subcostal view with the patient in the supine position at 1.0 to 2.0 cm from the junction of the right atrium. The right atrial pressure was estimated based on IVCD and its collapse after sniff according to the ASE guidelines (13).
3 Dimensional echo data analysis
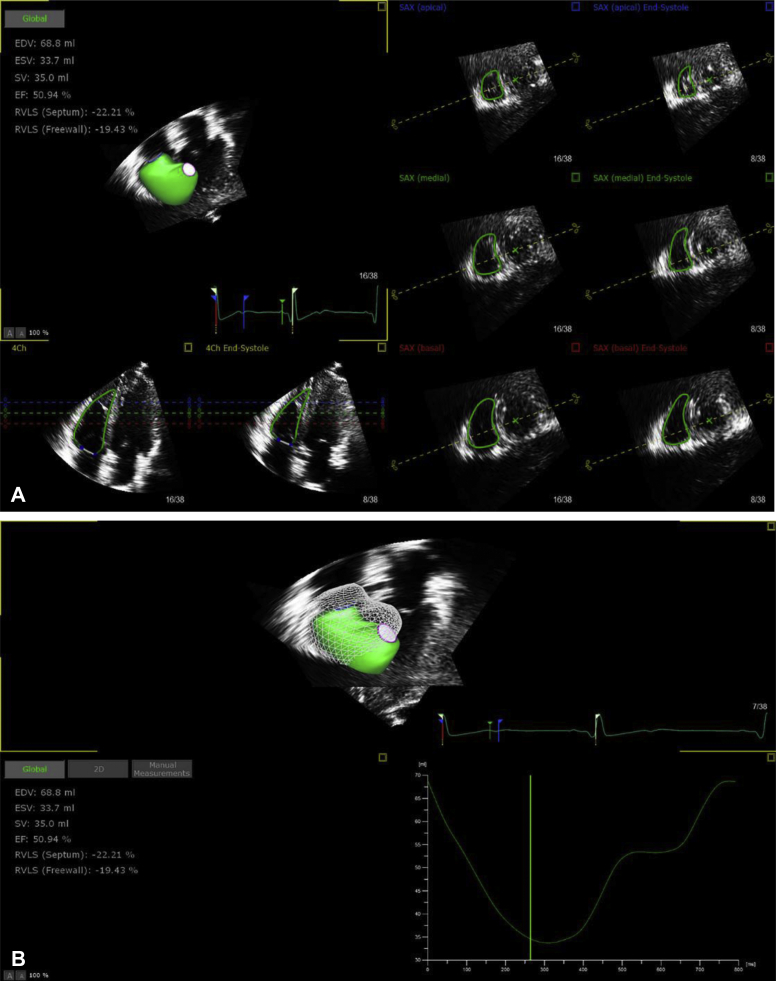
Offline analysis of the right ventricular 3-dimensional echocardiography data was performed using the TomTec 4D RV analysis workstation (version 4.6.0.411, TomTec Imaging Systems, Unterschleißheim, Germany) (Figures 1A and 1B). Care was taken to include trabeculae during measurements. RV end-diastolic volume (RVEDV); RV end-systolic volume (RVESV); RV ejection fraction (RVEF); LV end-diastolic volume (LVEDV); end-systolic volume (LVESV); and LVEF were measured. RV longitudinal free wall strain (RVLFS); RV longitudinal septal strain (RVLSS); LV global longitudinal strain (LVGLS); and LV global circumferential strain (LVGCS) were acquired simultaneously.
Figure 1.
Examples of Echocardiography Studies of 3DE Right Ventricular Parameters Using TomTec Offline Analysis Software
Four planes were displayed for tracing of the endocardium: the apical 4-chamber view, 3 short-axis planes near the apex, the mid-level, and the base of the right ventricle. The right ventricular endocardial contour was traced semiautomatically frame by frame and with refinement by manual adjustment when necessary at end-diastolic and end-systolic phases. 3DE = 3-dimensional echocardiography.
Intraobserver and interobserver variability analysis
Intraobserver and interobserver variability were assessed by randomly selecting 15 patients to be measured by 1 observer twice and by another independent observer. Interobserver measurement showed intraclass correlation coefficients (ICCs) of 0.953 (95% confidence interval [CI]: 0.941 to 0.968) for RVEDV; 0.942 (95% CI: 0.932 to 0.956) for RVESV; 0.926 (95% CI: 0.912 to 0.939) for RVLSS; and 0.945 (95% CI: 0.933 to 0.958) for RVLFS. Intraobserver measurements showed ICCs of 0.961 (95% CI: 0.948 to 0.975) for RVEDV; 0.960 (95% CI: 0.949 to 0.975) for RVESV; 0.933 (95% CI: 0.925 to 0.943) for RVLSS; and 0.937 (95% CI: 0.922 to 0.948) for RVLFS.
Univariate logistic regression analysis of predictors of RV cardiotoxicity
RV cardiotoxicity was defined in this study as a >10% relative reduction in RVEF or a relative reduction of >5% to an absolute value of <45% (7,13). Changes in echocardiographic parameters over time were included in the univariate logistic regression analysis to evaluate possible predictors of subsequent RV cardiotoxicity. Baseline echocardiographic parameters and the relative percentage of changes in echocardiographic parameters between T0 and T1 and between T0 and T2 were included as variables in the univariate logistic regression analysis.
Statistical analysis
Continuous variables were expressed as mean ± SD. Categorical variables were expressed as counts (percentages). Kolmogorov-Smirnov Z tests were used to evaluate the normality of the data. Parametric tests were applied when normality was satisfied, otherwise the Wilcoxon signed rank and chi-square tests were used for nonparametric data. Differences in echocardiographic parameters at baseline and at the end of every 2 cycles of chemotherapy were evaluated using linear mixed-effects models with a random intercept to account for intrapatient correlation of repeated measurements. An independent covariance structure was assumed. The Bonferroni method was used to adjust for multiple pairwise comparisons. Possible predictors (changes over time in echocardiographic parameters) of RV cardiotoxicity were analyzed by univariate logistic regression; odds ratio and 95% CIs were calculated. Receiver operating characteristic (ROC) curves were obtained, and the associated cutoff points with the greatest sensitivity and specificity were selected according to Youden's index. Area under the ROC curve (AUC) was calculated to determine the discriminative ability of various echocardiographic parameters between patients with and without RV cardiotoxicity. Intra- and interobserver variability of RVLSS, RVLFS, RVEDV, and RVESV were assessed using ICCs. SPSS version 22 software (SPSS Inc., Chicago, Illinois) and MedCalc software version 15.6.1.0 (MedCalc, New York City, New York) were used for data processing. A p value of <0.05 was considered statistically significant.
Results
A total of 80 patients were screened. Two patients were excluded due to uncontrolled hypertension and coronary artery disease. Four patients were excluded because of poor image quality. The final analytic cohort included 74 patients, of whom 41 (55%) were men, 33 were women, and ages ranged from 20 to 78 years old (mean age: 48.9 ± 11.8 years). Patient demographics and clinical characteristics are shown in Table 1. The mean cumulative anthracycline dose was 358.20 ± 69.04 mg/m2. No patients required acute treatment for dehydration or additional intravenous fluids beyond the standard chemotherapy. None of the patients received other cardiotoxic therapy, radiation therapy, or cardioprotective medications during the study period, and none developed signs or symptoms of heart failure.
Table 1.
Baseline Clinical Characteristics and Cardiac Risk Factors in Patients With or Without Cardiotoxicity
| All Patients (N = 74) | Cardiotoxicity (n = 27, 36%) | Noncardiotoxicity (n = 47, 64%) | p Value | |
|---|---|---|---|---|
| Age, yrs | 48.9 ± 11.8 | 49.6 ± 10.7 | 48.5 ± 12.1 | 0.112 |
| Height, cm | 166.9 ± 6.1 | 165.7 ± 6.9 | 167.6 ± 5.7 | 0.201 |
| Weight, kg | 65.6 ± 11.6 | 64.4 ± 10.7 | 66.3 ± 11.9 | 0.197 |
| HR, beats/min | 78.6 ± 11.9 | 79.3 ± 11.1 | 78.2 ± 12.4 | 0.226 |
| SBP, mm Hg | 117.4 ± 9.6 | 118.0 ± 9.1 | 117.1 ± 10.3 | 0.109 |
| DBP, mm Hg | 75.2 ± 7.4 | 74.8 ± 6.3 | 75.4 ± 8.1 | 0.318 |
| Women | 33 | 11 | 22 | 0.092 |
| Men | 41 | 16 | 25 | 0.114 |
| Diabetes mellitus | 5 (7) | 2 (7) | 3 (6) | 0.252 |
| Smoker | 20 (27) | 8 (29) | 12 (25) | 0.102 |
| Cumulative anthracycline dose, mg/m2 | 358.20 ± 69.04 | 367.30 ± 63.10 | 352.90 ± 71.14 | 0.097 |
Values are mean ± SD, n, or n (%). The p values compare cardiotoxicity with noncardiotoxicity.
DBP = diastolic blood pressure; HR = heart rate; SBP = systolic blood pressure.
Effects of anthracycline chemotherapy on 2DE and Doppler parameters
2DE and Doppler parameters are shown in Table 2. RV fractional area did not change significantly during follow-up. Pulmonary artery systolic pressure and IVCD remained stable and within normal limits at baseline and at each follow-up point of the chemotherapy. There were no significant changes in Doppler parameters of RV or LV diastolic function during anthracycline chemotherapy.
Table 2.
Vital Signs and 2DE Parameters Before and After Chemotherapy
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| Weight, kg | 65.6 ± 11.6 | 65.2 ± 11.8 | 65.3 ± 12.4 | 65.5 ± 12.2 |
| HR, beats/min | 78.6 ± 11.9 | 79.1 ± 11.3 | 80.5 ± 10.7 | 79.3 ± 11.6 |
| SBP, mm Hg | 117.4 ± 9.6 | 116.8 ± 9.8 | 116.1 ± 11.0 | 115.9 ± 9.5 |
| DBP, mm Hg | 75.2 ± 7.4 | 75.4 ± 7.3 | 74.8 ± 6.8 | 74.0 ± 7.0 |
| RVFAC, % | 43.2 ± 4.6 | 43.4 ± 4.9 | 44.6 ± 4.2 | 44.3 ± 5.5 |
| TAPSE, mm | 21.6 ± 2.3 | 21.4 ± 2.0 | 21.3 ± 1.7 | 20.9 ± 1.9 |
| RV E, cm/s | 55.2 ± 8.8 | 56.4 ± 9.9 | 54.9 ± 7.6 | 56.5 ± 8.3 |
| RV A, cm/s | 50.7 ± 10.8 | 52.5 ± 12.0 | 52.3 ± 12.0 | 48.8 ± 12.0 |
| RV S′, cm/s | 14.5 ± 3.4 | 14.6 ± 3.0 | 14.0 ± 2.6 | 14.6 ± 3.1 |
| RV E′, cm/s | 12.3 ± 4.0 | 11.4 ± 3.1 | 11.2 ± 2.6 | 11.2 ± 3.0 |
| RV A′, cm/s | 14.6 ± 4.2 | 15.4 ± 4.8 | 14.7 ± 3.8 | 14.7 ± 3.4 |
| RV E/E′ ratio | 5.3 ± 1.5 | 5.2 ± 1.4 | 5.1 ± 1.4 | 4.9 ± 1.5 |
| PASP, mm Hg | 30.9 ± 3.8 | 30.7 ± 4.0 | 30.4 ± 3.6 | 30.5 ± 3.9 |
| LV E, cm/s | 64.9 ± 11.8 | 64.1 ± 12.6 | 62.7 ± 10.9 | 60.6 ± 13.3 |
| LV A, cm/s | 63.0 ± 14.9 | 65.6 ± 12.1 | 64.7 ± 16.2 | 66.3 ± 14.1 |
| LV E/A ratio | 1.01 ± 0.21 | 0.99 ± 0.30 | 0.97 ± 0.35 | 0.94 ± 0.25 |
| LV S′, cm/s | 12.2 ± 2.4 | 12.0 ± 2.5 | 11.5 ± 2.9 | 11.6 ± 1.8 |
| LV E′, cm/s | 10.0 ± 1.4 | 9.7 ± 1.3 | 9.6 ± 1.8 | 9.4 ± 1.4 |
| LV A′, cm/s | 8.8 ± 2.4 | 8.7 ± 1.9 | 8.4 ± 1.4 | 8.2 ± 1.6 |
| LV E/E′ ratio | 7.0 ± 1.4 | 6.6 ± 1.7 | 6.4 ± 2.2 | 6.5 ± 1.5 |
| IVCD, mm | 16.7 ± 1.1 | 16.4 ± 1.3 | 17.1 ± 1.5 | 17.7 ± 1.3 |
| RAP, mm Hg | 3.9 ± 1.8 | 4.0 ± 1.7 | 3.9 ± 1.8 | 4.1 ± 2.0 |
Values are mean ± SD.
A = peak inflow velocity of late diastolic filling; A’ = peak late diastolic myocardial velocity; DBP = diastolic blood pressure; E = peak inflow velocity of early diastolic filling; E’ = peak early diastolic myocardial velocity; HR = heart rate; IVCD = inferior vena cava diameter; LV = left ventricle; PASP = pulmonary artery systolic pressure; RAP = right atrial pressure; RV = right ventricle; RVFAC = right ventricular fractional area change; S′ = systolic velocity of tricuspid annulus; SBP = systolic blood pressure; T0 = baseline before chemotherapy; T1 = after 2 cycles of chemotherapy; T2 = after 4 cycles of chemotherapy; T3 = after 6 cycles of chemotherapy; TAPSE = tricuspid annular plane systolic excursion.
Changes in 3DE parameters with anthracycline chemotherapy
3DE-derived RV and LV volumes, EF, and strain parameters are shown in Table 3. Compared to T0 (baseline, pre-chemotherapy), a significant decrease in RVEF was only observed at T3. A total of 27 patients (36%) met the criteria of RV cardiotoxicity at T3 (RVEF decreased from 54.8 ± 3.5% at baseline to 48.3 ± 2.6%). Of the 27 patients, 4 patients experienced a decline in RVEF to <45%. Two of those patients had a decline in RVEF percentage of >10%, and 2 patients had a decline in RVEF between 5% and 10%. RVEDV increased significantly at T2 and T3 compared to T0 or T1.
Table 3.
3DE Parameters Before and at Each Follow-Up Point During Chemotherapy
| T0 | T1 | T2 | T3 | |
|---|---|---|---|---|
| RVEDV, ml | 58.5 ± 7.7 | 60.2 ± 7.7 | 64.2 ± 7.0∗† | 66.0 ± 6.6∗† |
| RVESV, ml | 27.8 ± 4.2 | 28.9 ± 4.3 | 31.3 ± 4.2∗† | 34.1 ± 3.7∗†‡ |
| RVEF, % | 54.0 ± 2.8 | 53.6 ± 2.9 | 52.8 ± 3.1 | 49.8 ± 2.4∗†‡ |
| RVLFS, % | −27.3 ± 3.1 | −25.4 ± 3.3 | −24.2 ± 2.6∗ | −21.9 ± 2.8∗†‡ |
| RVLSS, % | −26.1 ± 2.5 | −25.6 ± 2.9 | −24.7 ± 2.9 | −22.9 ± 2.7∗† |
| LVEDV, ml | 77.6 ± 10.0 | 78.2 ± 11.2 | 79.4 ± 9.7 | 80.4 ± 12.0 |
| LVESV, ml | 30.3 ± 7.6 | 31.5 ± 6.9 | 32.0 ± 6.4 | 33.2 ± 8.0 |
| LVEF, % | 62.1 ± 5.7 | 61.5 ± 5.1 | 60.5 ± 6.2 | 59.3 ± 5.9 |
| LVGLS, % | −24.2 ± 3.6 | −22.3 ± 4.0 | −20.5 ± 3.3∗ | −19.1 ± 4.2∗† |
| LVGCS, % | −26.6 ± 5.3 | −25.7 ± 4.9 | −24.9 ± 4.6 | −23.7 ± 5.6∗ |
Values are mean ± SD.
LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; LVGCS = left ventricular global circumferential strain; LVGLS = left ventricular global longitudinal strain; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVLFS = right ventricular longitudinal free-wall strain; RVLSS = right ventricular longitudinal septal strain; other abbreviations are as in Table 2.
Compared with T0, p < 0.05.
Compared with T1, p < 0.05.
Compared with T2, p < 0.05.
A statistically significant change in RVESV was detected at T2 (p < 0.001) and persisted at T3. Statistically significant strain abnormalities in RVLFS and LVGLS were observed at T2 (p < 0.001). However, RVLSS and LVGCS were significantly decreased only at T3 (p < 0.001), suggestive of a late change. 3DE-derived LVEDV (77.6 ± 10.0 ml vs. 80.4 ± 12.0 ml; p = 0.12), LVESV (30.3 ± 7.6 ml vs. 33.2 ± 8.0 ml; p = 0.078), and LVEF (62.1 ± 5.7% vs. 59.3 ± 5.9%; p = 0.083) were not significantly different from the values at T0 to T3.
Univariable logistic regression and ROC curve analysis of predictors of RV cardiotoxicity
There were no differences in clinical characteristics between patients with and without RV cardiotoxicity (Table 1). Baseline values of RV strain or RV volumes were not predictors of cardiotoxicity, nor were changes in these measurements between T0 and T1. Relative or percent changes (Δ) between T0 and T2 of measurements of RV size and function were also evaluated as potential predictors of RV cardiotoxicity (Table 4).
Table 4.
Univariable Analysis of Potential Predictors of RV Cardiotoxicity
| Odds Ratio | 95% CI | p Value | |
|---|---|---|---|
| ΔRVESV | 1.526 | 1.087–1.972 | 0.002 |
| ΔRVEDV | 0.849 | 0.641–1.173 | 0.225 |
| ΔRVLFS | 1.393 | 1.093–1.692 | 0.001 |
| ΔRVLSS | 1.059 | 0.921–1.182 | 0.120 |
| ΔLVGLS | 1.146 | 0.986–1.671 | 0.092 |
| ΔLVGCS | 0.983 | 0.649–2.143 | 0.116 |
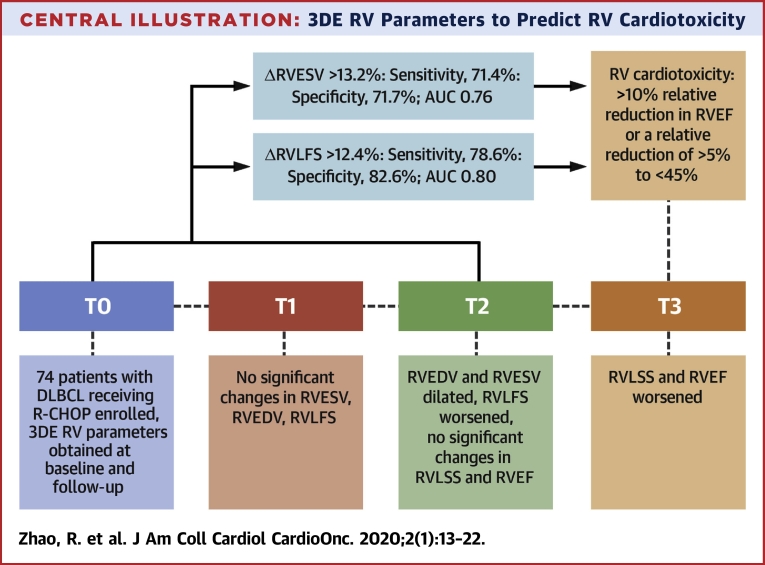
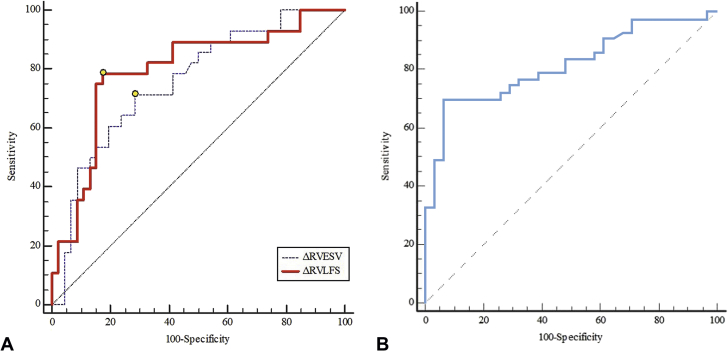
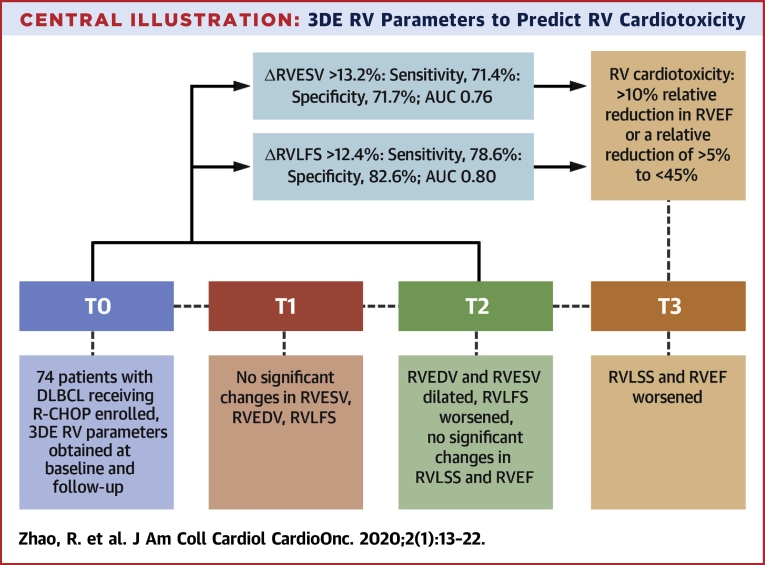
ΔRVESV and ΔRVLFS between T0 and T2 were associated with subsequent RV cardiotoxicity (p = 0.002 and p = 0.001, respectively). However, ΔRVEDV, ΔRVLSS, ΔLVGLS, and ΔLVGCS were not predictors of RV cardiotoxicity. As shown in the ROC curve analysis (Figures 2A and 2B), a relative decrease in ΔRVLFS of >12.4% (sensitivity: 78.6%; specificity: 82.6%; AUC: 0.80; p <0.001) and a relative increase in ΔRVESV of >13.2% (sensitivity: 71.4%; specificity: 71.7%; AUC: 0.76; p <0.001) were able to discriminate between patients with and without RV cardiotoxicity (Figure 2A). When ΔRVLFS and ΔRVESV were combined, the sensitivity decreased to 69.6%, the specificity increased to 93.5%, and the AUC increased to 0.82 (Figure 2B). The Central Illustration displays the change sequence of 3DE-based RV parameters during the chemotherapy. RV volumes and strains changed earlier than RVEF. Of these volume and strain indices, RVESV and RVLFS could be predictors of RV cardiotoxicity defined as the decline of RVEF at the end of the follow-up.
Figure 2.
ROC Curves for 3-Dimensional Echocardiographic Parameters in Predicting Right Ventricular Cardiotoxicity
(A) ROC curve analysis of ΔRVLFS and ΔRVESV respectively. The point corresponding to the cutoff value were marked as yellow circles on each curve respectively. There were no significant differences between the area under each curve (p = 0.45). (B) ROC curve analysis of combined ΔRVLFS and ΔRVESV (curve). When ΔRVLFS and ΔRVESV were combined in the ROC curve analysis, the sensitivity declined while the specificity increased, and the AUC increased. Δ = percentage change of measure between T0 (pre-chemotherapy) and T2 (after 4 cycles); AUC = area under the curve; ROC = receiver operating characteristic; RVESV = right ventricular end-systolic volume; RVLFS = right ventricular longitudinal free wall strain.
Central Illustration.
3DE RV Parameters to Predict RV Cardiotoxicity
RV volumes, RV strain parameters and RVEF were determined during anthracycline chemotherapy. RVESV, RVEDV, and RVLFS changed significantly by T2. RVEF declined significantly at T3. RV cardiotoxicity was defined as a relative reduction in RVEF of >10% or a 5% relative reduction in RVEF to <45%. A relative decrease in RVLFS of >12.4% and a relative increase in RVESV of >13.2% from T0 to T2 was associated with subsequent RV cardiotoxicity. 3DE = 3-dimensional echocardiography; AUC = area under the curve; DLBCL = diffuse large B-cell lymphoma; R-CHOP = cyclophosphamide, 750 mg/m2; vincristine, 1.4 mg/m2 up to a maximum dose of 2 mg/m2; doxorubicin, 50 to 70 mg/m2 on day 1; prednisone, 100 mg on days 1 to 5; and rituximab, 375 mg/m2; RV = right ventricular; RVEDV = right ventricular end-diastolic volume; RVEF = right ventricular ejection fraction; RVESV = right ventricular end-systolic volume; RVLFS = right ventricular longitudinal free-wall strain; RVLSS = right ventricular longitudinal septal strain; T0 = before chemotherapy; T1 = after the completion of 2 cycles of chemotherapy; T2 = after the completion of 4 cycles of chemotherapy; T3 = after the completion of 6 cycles of chemotherapy.
Discussion
In this study, changes in RV structure and function in lymphoma patients treated with anthracycline-based chemotherapy were carefully detailed using 3DE. Compared with 3DE-derived LVEF, 3DE-derived RVEF was impaired at an earlier stage. Of the subclinical changes in RV structure and function, the changes in RVLFS and RVESV occurred after the completion of the fourth cycle of chemotherapy (T2). These changes were associated with a subsequent decline of RVEF at the end of the entire chemotherapy (T3).
RVEF, LVEF, and anthracycline-induced cardiotoxicity
The definition of LV cardiotoxicity in previously published studies has been variable, which has been a limitation for the field of cardio-oncology. Some studies defined LV cardiotoxicity as a reduction of the LVEF of ≥5% to <55% with symptoms of heart failure or an asymptomatic reduction of the LVEF of ≥10% to <55% (7), whereas other studies have chosen an LVEF decline of <45% or 15 points from baseline as LV cardiotoxicity (14). The 2016 European Society of Cardiology position paper on cancer treatments and cardiovascular toxicity recommends an absolute decrease of 10% to a value below a lower limit of normal (6). Due to the paucity of data, the definition RV cardiotoxicity is not completely established in cardio-oncology. Based on the aforementioned guidance in defining LV cardiotoxicity from previous studies, the present authors found it reasonable to use a percentage or relative decline in RVEF as a definition of cardiotoxicity. The lower limit of normal in the present study was defined as 45%, which has been recommended by the ASE guidelines (13).
Of note, 3DE-based LVEF did not show a significant change during chemotherapy, whereas RVEF decreased significantly at T3. The reasons accounting for this phenomenon may be the structural differences between the 2 ventricles and time period in which cardiotoxicity may manifest. Anthracycline-induced cardiotoxicity tends to display a regional pattern in which the subendocardial component of the ventricle is most susceptible to the toxicity (15). At an early stage, the impaired contractility of the subendocardial layer could be compensated by the relatively normal epicardial layer, thus leading to a preserved LVEF. If the toxic effects of chemotherapy persist, cardiac decompensation and symptomatic heart failure could occur. It is possible that the thinner RV wall has less reserve for compensation which might cause the earlier manifestation of a decline in RVEF (16). Given that RV dysfunction has been associated with worse outcomes in other populations (17), it is important to further define and understand the changes in RV function with anthracyclines.
Changes in RV strain parameters and associations with RV cardiotoxicity
EF is an index frequently used to reflect chemotherapy-related systolic dysfunction. However, due to its lack of sensitivity in early detection of subclinical cardiac injury in cancer patients receiving chemotherapy, the assessment of EF alone may be limited (7). Strain derived from speckle tracking imaging is a technique which has been used to evaluate subclinical cardiac dysfunction across multiple disease states (18). Several studies have demonstrated the use of LV strain parameters in the early detection and prediction of LV cardiotoxicity induced by cancer (19,20). However, there are few studies in RV strain as it relates to cancer cardiotoxicity.
Boczar et al. (21) reported that longitudinal strain-RV free wall decreased significantly in patients with breast cancer treated with anthracyclines, which was consistent with the results in the present study. They also found that longitudinal strain-RV free wall was impaired 3 months after the initiation of chemotherapy, which was similar to the time frame observed in the present study. The present longitudinal data, however, uniquely demonstrates the temporal change in RV strain, RV volumes, and RVEF. After analyzing the relationship between the changes of RV strain indices and RVEF, it was found that a relative decrease of >12.4% in RVLFS between baseline and the completion of 4 cycles of anthracycline chemotherapy (T2) was associated with a subsequent RVEF decline after 6 cycles (T3). Our data may suggest that, with further study, RVLFS could be a useful index to aid in the prediction of subsequent RV cardiotoxicity.
However, whether there is an earlier time point at which RVLFS could predict subsequent declines in RVEF is still unknown. The changes in RVLSS, the longitudinal septal strain of RV, demonstrated a delayed response compared with that in RVLFS. The present authors believe that the irregular geometry and the potential resultant uneven stress of the RV may have contributed to the asynchronous pattern in the changes in strain (22). RVLSS mainly reflects the longitudinal strain of the septal myocardium, whereas RVLFS reflects the longitudinal strain of the free wall. According to Laplace’s law, wall stress correlates positively with pressure and radius and inversely with wall thickness. It is tempting to speculate that, given a free wall that is thinner in comparison to the septum, there was a greater degree of wall stress and thus worse strain (22,23) Moreover, as the septum is shared by both LV and RV, LV deformation may result in some compensatory effects.
RV volume parameters in predicting RV cardiotoxicity
The volumetric alterations in 3DE RV were assessed, and it was determined that both RVESV and RVEDV increased significantly after 4 cycles of chemotherapy (T2) compared to baseline (T0). Analogous to studies of LV size (24), RVESV increased before RVEF decline. The percent change in RVESV between T0 and T2 was associated with a decrease in RVEF.
Because the RV is more dependent on preload than the LV, one might assume that preload resulted in the observed changes in RV function. However, the patient’s body weight, blood pressure, pulmonary arterial systolic pressures, IVCD, and estimated right atrial pressure did not change significantly during the course of the study. Moreover, each echocardiogram was obtained 8 to 15 days after T1 and T2 and 50 to 60 days after T3 to avoid the influence of acute preload changes. Of note, LV volumes did not change significantly during follow-up. It remains unknown why the RV may be more susceptible than the LV in the setting. At least to some extent, this may be related to differences in LV wall thickness and wall stress.
Study limitations
One limitation of the present study was the absence of a comparison between 3DE and cardiac magnetic resonance imaging or computed tomography. Additionally, due to the small size and short follow-up period of our study, a larger sample with longer follow-up period is required in future studies and to draw more definitive conclusions. Moreover, the sample size was relatively small, which also limited the ability to adjust for confounders. Admittedly, the present definition of RV cardiotoxicity was somewhat arbitrary and based on relative, instead of absolute changes of small magnitude. Standardized, clinically meaningful definitions of RV cardiotoxicity need to be developed. The specific inclusion and exclusion criteria also affect the generalizability of the study. Although we tried to maintain stable volume status within patients and carefully monitor preload conditions, parameters such as RV volumes and strain are preload-dependent. Finally, the reference cutoff points presented in our AUC analyses need to be externally validated and studied in much larger datasets.
Conclusions
Subclinical changes in RV function and size, including in 3DE-derived RV strain and volume, occur after the anthracycline-based chemotherapy. With further study, RV longitudinal free-wall strain and RV end-systolic volumes could be useful indices to predict subsequent declines in RVEF with anthracyclines. In the present study, the decrease in RVEF preceded LVEF changes. If confirmed in external cohorts, future monitoring of anthracycline toxicity may potentially include parameters based on the 3DE assessment of the right ventricle.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In patients treated with anthracycline-based chemotherapy, the decrease in 3DE RVEF during anthracycline treatment occurred earlier than changes in LVEF. With further study, early changes in 3DE RV longitudinal free-wall strain and RV end-systolic volumes could be promising predictors of cardiotoxicity.
TRANSLATIONAL OUTLOOK: More studies are needed to determine the relationship between RVEF declines and adverse clinical outcomes in patients exposed to anthracyclines. There is also an important need for standardized definitions of cardiotoxicity. This study provides early data to support the potential use of 3DE-derived measurements of RV function and size in cardio-oncology.
Footnotes
Supported by National Nature Science Foundation of China grant N0.81771840 and Excellent Talent Training Program of Shanghai Health System grant NO.2017BR027. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Contributor Information
David H. Hsi, Email: dhsi@stamhealth.org.
Leilei Cheng, Email: cheng.leilei@zs-hospital.sh.cn.
References
- 1.Early Breast Cancer Trialists Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 2.Hershman D.L., McBride R.B., Eisenberger A. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B-cell non-Hodgkin's lymphoma. J Clin Oncol. 2008;26:3159–3165. doi: 10.1200/JCO.2007.14.1242. [DOI] [PubMed] [Google Scholar]
- 3.Pai V.B., Nahata M.C. Cardiotoxicity of chemotherapeutic agents. Incidence, treatment and prevention. Drug Safety. 2000;22:263–302. doi: 10.2165/00002018-200022040-00002. [DOI] [PubMed] [Google Scholar]
- 4.Swain S.M., Whaley F.S., Ewer M.S. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D., Colombo A., Lamantia G. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 6.Zamorano J.L., Lancellotti P., Rodriguez Muñoz D. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 7.Sawaya H., Sebag I.A., Plana J.C. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am J Cardiol. 2011;107:1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bing R., Naoum C., Kritharides L. The vulnerable right ventricle: recurrent, transient right ventricular failure on a background of systemic sclerosis and previous anthracycline exposure. Int J Cardiol. 2015;15:223–225. doi: 10.1016/j.ijcard.2014.10.078. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira G.H., Dupont M., Naftel D. Increased need for right ventricular support in patients with chemotherapy-induced cardiomyopathy undergoing mechanical circulatory support. J Am Coll Cardiol. 2014;63:240–248. doi: 10.1016/j.jacc.2013.09.040. [DOI] [PubMed] [Google Scholar]
- 10.Surkova E., Muraru D. The use of multimodality cardiovascular imaging to assess right ventricular size and function. Int J Cardiol. 2016;214:54–69. doi: 10.1016/j.ijcard.2016.03.074. [DOI] [PubMed] [Google Scholar]
- 11.Grewal J., Majdalany D., Syed I. Three-dimensional echocardiographic assessment of right ventricular volume and function in adult patients with congenital heart disease: comparison with magnetic resonance imaging. J Am Soc Echocardiogr. 2010;23:127–133. doi: 10.1016/j.echo.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 12.vanderZwaan H.B., Helbing W.A., McGhie J.S. Clinical value of real-time three-dimensional echocardiography for right ventricular quantification in congenital heart disease: validation with cardiac magnetic resonance imaging. J Am Soc Echocardiogr. 2010;23:134–140. doi: 10.1016/j.echo.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Rudski L.G., Lai W.W., J Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Ryberg M., Nielsen D., Cortese G. New insight into epirubicin cardiac toxicity: competing risks analysis of 1097 breast cancer patients. J Natl Cancer Inst. 2008;100:1058–1067. doi: 10.1093/jnci/djn206. [DOI] [PubMed] [Google Scholar]
- 15.Ho E., Brown A., Barrett P. Subclinical anthracycline- and trastuzumab-induced cardiotoxicity in the long-term follow up of asymptomatic breast cancer survivors: a speckle tracking echocardiographic study. Heart. 2010;96:701–707. doi: 10.1136/hrt.2009.173997. [DOI] [PubMed] [Google Scholar]
- 16.Perel R.D., Slaughter R.E., Strugnell W.E. Subendocardial late gadolinium enhancement in two patients with anthracycline following treatment for Ewing’s sarcoma. J Cardiovasc Magn Reson. 2006;8:789–791. doi: 10.1080/10976640600737664. [DOI] [PubMed] [Google Scholar]
- 17.Nagata Y., Wu V.C., Kado Y. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging. 2017;10:1–11. doi: 10.1161/CIRCIMAGING.116.005384. [DOI] [PubMed] [Google Scholar]
- 18.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoodley P.W., Boyd A., Hui R. Left ventricular systolic function in HER2/neu negative breast cancer patients treated with anthracycline chemotherapy: a comparative analysis of left ventricular ejection fraction and myocardial strain imaging over 12 months. Eur J Cancer. 2013;49:3396–3403. doi: 10.1016/j.ejca.2013.06.046. [DOI] [PubMed] [Google Scholar]
- 20.Negishi K., Negishi T., Hare J.L. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. 2013;26:493–498. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Emery Boczar K., Aseyev O., Sulpher J. Right heart function deteriorates in breast cancer patients undergoing anthracycline-based chemotherapy. Echo Res Pract. 2016;2:79–84. doi: 10.1530/ERP-16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haddad F., Hunt S.A., Rosenthal D.N. Right ventricular function in cardiovascular disease, part I anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 23.Regen D.M. Calculation of left ventricular wall stress. Circ Res. 1990;67:245–252. doi: 10.1161/01.res.67.2.245. [DOI] [PubMed] [Google Scholar]
- 24.Mousavi N., Tan T.C., Ali M. Echocardiographic parameters of left ventricular size and function as predictors of symptomatic heart failure in patients with a left ventricular ejection fraction of 50-59% treated with anthracyclines. Eur Heart J Cardiovasc Imaging. 2015;16:977–984. doi: 10.1093/ehjci/jev113. [DOI] [PubMed] [Google Scholar]