Abstract
Background
Prolonged androgen deprivation therapy (ADT) is favored over short-term use in patients with localized high-risk prostate cancer (PC).
Objectives
This study sought to compare cardiorespiratory fitness (CRF) and cardiovascular (CV) mortality among patients with PC with and without ADT exposure and to explore how duration of ADT exposure influences CRF and CV mortality.
Methods
Retrospective cohort study of patients referred for exercise treadmill testing (ETT) after a PC diagnosis. PC risk classification was based on Gleason score (GS): high risk if GS ≥8; intermediate risk if GS = 7; and low risk if GS <7. CRF was categorized by metabolic equivalents (METs): METs >8 defined as good CRF and METs ≤8 as reduced CRF. ADT exposure was categorized as short term (≤6 months) versus prolonged (>6 months).
Results
A total of 616 patients underwent an ETT a median of 4.8 years (interquartile range: 2.0, 7.9 years) after PC diagnosis. Of those, 150 patients (24.3%) received ADT prior to the ETT; 99 with short-term and 51 with prolonged exposure. 504 patients (81.8%) had ≥2 CV risk factors. Prolonged ADT was associated with reduced CRF (odds ratio [OR]: 2.71; 95% confidence interval [CI]: 1.31 to 5.61; p = 0.007) and increased CV mortality (hazard ratio [HR]: 3.87; 95% CI: 1.16 to 12.96; p = 0.028) in adjusted analyses. Although the association between short-term ADT exposure and reduced CRF was of borderline significance (OR: 1.71; 95% CI: 1.00 to 2.94; p = 0.052), there was no association with CV mortality (HR: 1.60; 95% CI: 0.51 to 5.01; p = 0.420) in adjusted Cox regression models.
Conclusions
Among patients with PC and high baseline CV risk, prolonged ADT exposure was associated with reduced CRF and increased CV mortality.
Key Words: androgen deprivation therapy, cardio-oncology, cardiorespiratory fitness, cardiovascular mortality, cardiovascular risk, prostate cancer
Abbreviations and Acronyms: ADT, androgen deprivation therapy; BMI, body mass index; CI, confidence interval; CRF, cardiorespiratory fitness; CV, cardiovascular; ETT, exercise treadmill test; HR, hazard ratio; IQR, interquartile range; MET, metabolic equivalent; OR, odds ratio; PC, prostate cancer
Central Illustration
About 1 in 9 men will be diagnosed with prostate cancer (PC) during their lifetime, and it is the second leading cause of cancer death in American men. The population of survivors with PC in the United States is projected to increase from 3.3 million in 2016 to 4.5 million by 2026 (1). Cardiovascular (CV) disease is a leading cause of death in men with a history of PC (2). Androgen deprivation therapy (ADT) is a standard primary treatment for PC with radiation therapy as an alternative to surgery, and is widely used in patients with metastatic, recurrent, and localized high-risk tumors (3,4). ADT regimens of increased intensity and prolonged duration are increasingly applied in clinical practice following several studies that demonstrated superior cancer outcomes compared with those associated with shorter duration therapy in high-risk patients (5).
Whether ADT is associated with increased CV mortality remains controversial. Specifically, some studies report increased risk of CV morbidity (6, 7, 8, 9) and mortality (10) in ADT-exposed men (11,12), whereas others have failed to demonstrate any significant association between ADT exposure and the development of CV disease (13) or mortality (14). Cardiorespiratory fitness (CRF) is a strong independent predictor of all-cause and CV mortality (15, 16, 17). Like the conflicting data on ADT and CV mortality, studies testing the association between ADT exposure and CRF are inconclusive (18, 19, 20). Furthermore, the influence of prolonged ADT exposure versus short-term exposure on CRF requires more comprehensive assessment. Some prior studies exploring ADT-mediated effects on CRF have focused on age-matched healthy individuals as the reference cohort rather than ADT-naive patients with PC (19,20). Therefore, to advance the current understanding of the influence of both ADT exposure and the duration of ADT exposure on CRF and CV mortality, the objectives of this study were: 1) to compare CRF and CV mortality in a large cohort of PC patients with and without ADT exposure; and 2) to explore the influence of prolonged versus short-term ADT usage on both CRF and CV mortality given the increasing use of prolonged ADT regimens in clinical practice.
Methods
Study design
This was a single-center, retrospective cohort study approved by the Partners Healthcare Institutional Review Board. Using ICD-9-CM codes, 1,262 subjects with a diagnosis of PC were identified from 28,959 consecutive patients who underwent exercise treadmill tests (ETTs). Subjects were excluded if PC diagnosis could not be confirmed, if PC diagnosis occurred after ETT, or if ADT exposure occurred only after an ETT. Following additional exclusion of patients with insufficient data available on PC treatment, vital status, and/or CV comorbidities, 616 subjects who underwent ETTs between March 7, 2002, and August 18, 2015, were included in the final cohort (Figure 1).
Figure 1.
Consort Diagram
Consort diagram outlining steps used to identify the final study cohort. Patients were excluded if prostate cancer diagnosis occurred after exercise treadmill test (ETT), if data on prostate cancer treatment/vital status were missing, or if exposure to androgen deprivation therapy (ADT) occurred only after ETT.
Cardiovascular risk factor assessment
Demographics, indication for ETT, medical history, and medication usage were prospectively recorded at the time of the ETT. Ischemic heart disease was defined as any history of myocardial infarction, coronary revascularization, or coronary artery disease. Heart failure was defined as any history of heart failure, cardiomyopathy, left ventricular ejection fraction <40%, or loop diuretic use. Hyperlipidemia was defined as any history of hyperlipidemia or statin use. Diabetes was defined as any history of diabetes or use of insulin or oral hypoglycemic agents. Morise clinical score was calculated based on age, sex, smoking, hyperlipidemia, diabetes, hypertension, estrogen status, body mass index (BMI), family history of coronary artery disease, and symptoms (21). A Morise score of 0 to 8 indicates low pre-test probability of a positive stress test, 9 to 15 indicates an intermediate probability, and 16 to 24 indicates a high probability (21).
Oncology history
Oncologic data were collected, including PC treatment regimens used before and after the ETTs. Gleason score categorized patients as high (score ≥8), intermediate (score = 7), or low (score <7) cancer risk. Details of ADT treatment were recorded, including agent used and duration of ADT exposure prior to the ETT. Short-term and prolonged ADT exposures were defined as use of ADT for ≤6 and >6 months, respectively (22,23).
Exercise protocol
All patients underwent a symptom-limited ETT according to the standard Bruce protocol (24). Heart rate and blood pressure were recorded at rest and periodically during exercise and recovery. ETTs were performed, analyzed, and reported as normal, abnormal, or inconclusive per international standards (25). Resting left ventricular ejection fraction was recorded for ETTs performed with echocardiography (n = 86, 14.0%), nuclear imaging (n = 351, 57.0%), or computed tomography (n = 1, 0.002%).
Cardiorespiratory fitness
CRF was expressed in units of metabolic equivalents (METs), calculated from peak treadmill speed and grade (26). CRF was stratified as reduced (≤8 METs) or good (>8 METs), as established in prior works (15,16,27,28).
Study outcomes
Study outcomes were reduced CRF, as just defined, and CV mortality. Cause-specific mortality data were collected using National Death Index.
Statistical analysis
Categorical demographic variables were compared using Pearson chi-squared tests or Fisher exact tests. Continuous normal data were compared using 2-independent samples Student’s t-tests. Continuous, non-normal variables were compared using Wilcoxon rank-sum tests. Logistic regression was used to assess the relationships among reduced CRF and ADT exposure or ADT duration in crude analyses, and analyses adjusted for age, ETT result, PC risk group, Morise risk score, and BMI. Survival analyses were performed using Cox proportional hazards models. Thirteen patients in the short-term ADT cohort who received additional ADT after their ETTs to bring their cumulative ADT exposure to >6 months were excluded from CV mortality analyses. As CV mortality is measured in the presence of competing risks, cause-specific hazard ratios (HRs) were used. Crude HR with 95% confidence intervals (CIs) were calculated in addition to HRs adjusting for the same set of potential confounders included in the CRF analysis. An inverse probability of treatment-weighted propensity score analysis was performed to check model stability. Martingale residuals were used to assess the proportional hazards assumption. Individuals were right-censored if they did not experience a CV event by the end of follow-up or were lost to follow-up. Cumulative incidence of CV deaths was compared among ADT-naive patients, patients who received ≤6 months of ADT, and those who received >6 months of ADT using Gray test of equality. Analyses were performed using SAS (version 9.4, SAS Institute, Cary, North Carolina). All p values <0.05 were considered statistically significant, and all testing was 2-tailed.
Results
Baseline demographics and CV risk
The mean age at time of ETT was 69.6 ± 7.8 years. The median interval from PC diagnosis to ETT was 4.8 years (interquartile range [IQR]: 2.0 to 7.9 years). The intermediate to high baseline CV risk of these men is highlighted by the following: 504 patients (81.8%) had ≥2 CV risk factors, the mean Morise score was 12.9 ± 1.7, and there was a high frequency of CV medication usage (Table 1).
Table 1.
Baseline Demographics of Study Cohorts
| Entire Cohort (N = 616) | ADT (n = 150) | Non-ADT (n = 466) | p Value | ADT ≤6 Months (n = 99) | ADT >6 Months (n = 51) | p Value | |
|---|---|---|---|---|---|---|---|
| Age at ETT, yrs | 69.6 ± 7.8 | 70.3 ± 7.7 | 69.4 ± 7.8 | 0.232 | 70.2 ± 7.7 | 70.6 ± 7.7 | 0.768 |
| Interval from PC diagnosis to ETT, yrs | 4.8 (2.0–7.9) | 4.0 (1.7–6.9) | 5.0 (2.1–8.2) | 0.056 | 3.2 (1.3–6.3) | 4.6 (2.7–7.9) | 0.015 |
| Cardiovascular history | |||||||
| Body mass index, kg/m2 | 27.7 ± 4.1 | 28.3 ± 4.4 | 27.5 ± 4.0 | 0.040 | 28.2 ± 4.5 | 28.5 ± 4.3 | 0.722 |
| Diabetes mellitus | 127 (20.6) | 36 (24.0) | 91 (19.5) | 0.247 | 23 (23.2) | 13 (25.5) | 0.841 |
| Hypertension | 426 (69.2) | 107 (71.3) | 319 (68.5) | 0.543 | 71 (71.7) | 36 (70.6) | 1.000 |
| Hypercholesterolemia | 454 (73.7) | 113 (75.3) | 341 (73.2) | 0.670 | 76 (76.8) | 37 (72.6) | 0.690 |
| Ischemic heart disease | 208 (33.8) | 57 (38.0) | 151 (32.4) | 0.234 | 39 (39.4) | 18 (36.3) | 0.723 |
| Heart failure | 64 (10.4) | 18 (12.0) | 46 (9.9) | 0.445 | 11 (11.1) | 7 (13.7) | 0.791 |
| LVEF, %∗ | 59 (54–64) | 60 (54–63) | 59 (54-64) | 0.790 | 59 (54–63) | 60 (53–65) | 0.679 |
| Active smoking | 42 (6.8) | 8 (5.3) | 34 (7.3) | 0.462 | 4 (4.0) | 4 (7.8) | 0.444 |
| Morise risk score | 12.9 ± 1.7 | 13.0 ± 1.7 | 12.9 ± 1.8 | 0.399 | 13.0 ± 1.8 | 13.1 ± 1.5 | 0.734 |
| ≥1 Cardiovascular risk factors† | 591 (96.0) | 144 (96.0) | 447 (96.0) | 0.205 | 94 (95.0) | 50 (98.0) | 0.821 |
| ≥2 Cardiovascular risk factors† | 504 (81.8) | 127 (84.7) | 377 (80.9) | 0.085 | 80 (80.8) | 47 (92.2) | 0.210 |
| Cardiovascular medications | |||||||
| Statin | 396 (64.3) | 98 (65.3) | 298 (64.0) | 0.770 | 64 (64.7) | 34 (66.7) | 0.858 |
| Aspirin | 373 (60.6) | 94 (62.7) | 279 (59.9) | 0.566 | 66 (66.7) | 28 (54.9) | 0.212 |
| ACE inhibitor/ARB | 231 (37.5) | 55 (36.7) | 176 (37.8) | 0.847 | 33 (33.3) | 22 (43.1) | 0.284 |
| Atrioventricular nodal blockade | 348 (56.5) | 94 (62.7) | 254 (64.5) | 0.089 | 59 (59.6) | 35 (68.8) | 0.292 |
Values are mean ± SD, median (interquartile range), or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin-receptor blocker; ADT = androgen deprivation therapy; ETT = exercise treadmill test; LVEF = left ventricular ejection fraction; PC = prostate cancer.
Available for 438 patients (71.1%): 330 (70.8%) in non-ADT cohort and 108 (72.0%) in ADT cohort.
Active smoking, diabetes, hypertension, hypercholesterolemia, overweight (body mass index ≥25 kg/m2).
Oncology history and ADT
Almost one-half (n = 304, 49.3%) of the entire cohort were low risk from a PC perspective based on Gleason score <7 at time of diagnosis; 245 (39.8%) were intermediate risk (score = 7) and only 67 (10.9%) were high risk (score ≥8). Prostatectomy, radiation therapy, and adjuvant chemotherapy were employed in 54.1%, 38.2%, and 4.9% of patients, respectively (Table 2).
Table 2.
Oncology Characteristics of the Study Cohorts
| Entire Cohort (N = 616) | ADT (n = 150) | Non-ADT (n = 466) | P Value | ADT ≤6 Months (n = 99) | ADT >6 Months (n = 51) | p Value | |
|---|---|---|---|---|---|---|---|
| Age at diagnosis, yrs | 64.1 ± 7.5 | 65.3 ± 7.6 | 63.7 ± 7.4 | 0.022 | 65.8 ± 7.1 | 64.3 ± 8.5 | 0.268 |
| Nodal status at time of initial treatment | |||||||
| Pathologically positive | 17 (2.8) | 15 (10.0) | 2 (0.4) | <0.001 | 4 (4.1) | 11 (21.6) | 0.002 |
| Pathologically negative | 323 (52.4) | 67 (44.7) | 256 (55.0) | 44 (44.4) | 23 (45.1) | ||
| Clinically negative∗ | 276 (44.8) | 68 (45.3) | 208 (44.6) | 51 (51.5) | 17 (33.3) | ||
| Metastatic status at time of initial treatment | |||||||
| Yes | 39 (6.3) | 8 (5.3) | 31 (6.7) | 0.045 | 1 (1.0) | 7 (13.7) | 0.006 |
| No | 509 (82.7) | 117 (78.0) | 392 (84.1) | 80 (80.8) | 37 (72.6) | ||
| Unknown | 68 (11.0) | 25 (16.7) | 43 (9.2) | 18 (18.2) | 7 (13.7) | ||
| Prostate cancer risk group | |||||||
| High-risk PC (GS ≥8) | 67 (10.9) | 44 (29.3) | 23 (4.9) | <0.001 | 20 (20.2) | 24 (47.1) | 0.001 |
| Intermediate-risk PC (GS = 7) | 245 (39.8) | 90 (60.0) | 155 (33.3) | 66 (66.7) | 24 (47.1) | ||
| Low-risk PC (GS <7) | 304 (49.3) | 16 (10.7) | 288 (61.8) | 13 (13.1) | 3 (5.8) | ||
| Prostatectomy as initial therapy | |||||||
| Yes | 333 (54.1) | 37 (24.7) | 296 (63.5) | <0.001 | 17 (17.2) | 20 (39.2) | 0.001 |
| No | 278 (45.1) | 110 (73.3) | 168 (36.1) | 81 (81.8) | 29 (57.9) | ||
| Unknown | 5 (0.8) | 3 (2.0) | 2 (0.4) | 1 (1.0) | 2 (3.9) | ||
| External radiation/brachytherapy as initial therapy | |||||||
| Yes | 235 (38.2) | 109 (72.7) | 126 (27.0) | <0.001 | 81 (81.8) | 28 (54.9) | 0.001 |
| No | 376 (61.0) | 38 (25.3) | 338 (72.5) | 18 (18.2) | 20 (39.2) | ||
| Unknown | 5 (0.8) | 3 (2.0) | 2 (0.4) | 0 | 3 (5.9) | ||
| Any chemotherapy | |||||||
| Yes | 30 (4.9) | 15 (10.0) | 15 (3.2) | 0.002 | 9 (9.1) | 6 (11.8) | 0.581 |
| No | 583 (94.6) | 135 (90.0) | 448 (96.1) | 90 (90.9) | 45 (88.2) | ||
| Unknown | 3 (0.5) | 0 | 3 (0.7) | 0 | 0 | ||
| Recurrence or progression | |||||||
| Yes | 74 (12.0) | 30 (20.0) | 44 (9.4) | 0.003 | 8 (8.1) | 22 (43.1) | <0.001 |
| No | 532 (86.4) | 120 (80.0) | 412 (88.4) | 91 (91.9) | 29 (56.9) | ||
| Unknown | 10 (1.6) | 0 | 10 (2.2) | 0 | 0 | ||
| Biochemical recurrence | |||||||
| Yes | 99 (16.1) | 52 (34.7) | 47 (10.1) | <0.001 | 20 (20.2) | 32 (62.7) | <0.001 |
| No | 515 (83.6) | 98 (65.3) | 417 (89.5) | 79 (79.8) | 19 (37.3) | ||
| Unknown | 2 (0.3) | 0 | 2 (0.4) | 0 | 0 | ||
Values are mean ± SD or n (%). “Unknown” or “inconclusive” rows excluded from hypothesis tests.
GS = Gleason score; other abbreviations as in Table 1
No evidence of lymph node metastasis on imaging studies or physical examination.
Almost one-quarter (n = 150, 24.4%) of the entire cohort received ADT prior to the ETT. Compared with ADT-naive patients, these patients were older at time of PC diagnosis (65.3 ± 7.6 vs. 63.7 ± 7.4 years; p = 0.022) with higher BMI (28.3 ± 4.4 vs. 27.5 ± 4.0 kg/m2; p = 0.040). Other CV risk factors and medication usage were similar across ADT-naive and ADT-exposed cohorts (Table 1). Treatment of ADT-exposed patients was more likely to have included radiation therapy (72.7% vs. 27.0%, p < 0.001) and chemotherapy (10.0% vs. 3.2%, p = 0.002), but less likely to have included prostatectomy (24.7% vs. 63.5%, p <0.001).
Prolonged ADT exposure
Of 150 ADT-exposed patients, ADT use was prolonged (>6 months) in 51 patients (34.0%) with a median exposure duration of ADT 28 months (IQR: 12 to 52 months). There were no significant differences in baseline demographics, CV risk factors, or medication usage when patients with prolonged ADT exposure were compared with those exposed to ADT for ≤6 months (Table 1). Patients treated with prolonged ADT were more likely to have been treated with prostatectomy (39.2% vs. 17.2%; p = 0.001), but less likely to have received radiation therapy (54.9% vs. 81.8%; p = 0.001) (Table 2).
Exercise treadmill test
Indications for ETT were similar across cohorts (Table 3). ETT was normal in 450 patients (73.1%), abnormal in 103 (16.7%), and inconclusive in 63 (10.2%). The distribution of ETT results did not differ significantly based on ADT exposure or duration of exposure (Table 3).
Table 3.
ETT Indications and Results
| Entire Cohort (N = 616) | ADT (n = 150) | Non-ADT (n = 466) | p Value | ADT ≤6 Months (n = 99) | ADT >6 Months (n = 51) | p Value | |
|---|---|---|---|---|---|---|---|
| Indication for ETT∗ | |||||||
| Chest pain | 166 (27.0) | 40 (26.7) | 126 (27.0) | 1.000 | 25 (25.3) | 15 (29.4) | 0.697 |
| Dyspnea | 94 (15.3) | 24 (16.0) | 70 (15.0) | 0.794 | 16 (16.2) | 8 (15.7) | 1.000 |
| Arrhythmia | 40 (6.5) | 12 (8.0) | 28 (6.0) | 0.445 | 8 (8.1) | 4 (7.8) | 1.000 |
| Other | 316 (51.3) | 74 (49.3) | 242 (51.9) | 0.638 | 50 (50.5) | 24 (47.1) | 0.732 |
| CRF | |||||||
| Exercise duration, mins | 7.9 ± 2.8 | 7.0 ± 2.4 | 8.2 ± 2.9 | <0.001 | 7.3 ± 2.5 | 6.5 ± 2.2 | 0.083 |
| METs completed | 9.5 ± 3.0 | 8.5 ± 2.5 | 9.8 ± 3.1 | <0.001 | 8.7 ± 2.6 | 8.1 ± 2. 3 | 0.127 |
| Good CRF (METs >8) | 391 (63.5) | 77 (51.3) | 314 (67.4) | <0.001 | 54 (54.6) | 23 (45.1) | 0.304 |
| Reduced CRF (METs ≤8) | 225 (36.5) | 73 (48.7) | 152 (32.6) | <0.001 | 45 (45.4) | 28 (54.9) | 0.304 |
| Heart rate response, beats/min | |||||||
| Heart rate at rest | 66 ± 13 | 65 ± 13 | 66 ± 13 | 0.275 | 63 ± 12 | 67 ± 14 | 0.091 |
| Heart rate at peak | 134 ± 23 | 130 ± 22 | 136 ± 24 | 0.018 | 130 ± 22 | 131 ± 22 | 0.936 |
| Blood pressure, mm Hg | |||||||
| SBP at rest | 134 ± 17 | 134 ± 18 | 134 ± 17 | 0.854 | 134 ± 16 | 135 ± 20 | 0.799 |
| DBP at rest | 77 ± 10 | 77 ± 11 | 77 ± 10 | 0.935 | 76 ± 10 | 78 ± 12 | 0.435 |
| SBP at peak exercise | 168 ± 26 | 166 ± 26 | 169 ± 26 | 0.200 | 166 ± 26 | 165 ± 27 | 0.860 |
| DBP at peak exercise | 74 ± 11 | 74 ± 12 | 75 ± 10 | 0.403 | 72 ± 12 | 76 ± 12 | 0.077 |
| ETT result | |||||||
| Normal | 450 (73.1) | 103 (68.7) | 347 (74.5) | 0.359 | 68 (68.7) | 35 (68.6) | 1.000 |
| Abnormal | 103 (16.7) | 29 (19.3) | 74 (15.9) | 19 (19.2) | 10 (19.6) | ||
| Inconclusive | 63 (10.2) | 18 (12.0) | 45 (9.6) | 12 (12.1) | 6 (11.8) | ||
Values are n (%) or mean ± SD. “Unknown” or “inconclusive” rows excluded from hypothesis tests.
CRF = cardiorespiratory fitness; DBP = diastolic blood pressure; ETT = exercise treadmill test; METs = metabolic equivalents; SBP = systolic blood pressure.
Cumulative percentages may exceed 100% as categories are not mutually exclusive.
Reduced cardiorespiratory fitness
The mean METs achieved by the entire cohort were 9.5 ± 3.0 METs, and CRF was reduced (≤8 METs) in 225 patients (36.5%). The frequency of reduced CRF was significantly higher among ADT-exposed patients compared with ADT-naive patients (48.7% vs. 32.6%; p < 0.001) (Figure 2). ADT exposure was associated with an almost 2-fold increased likelihood of reduced CRF among PC patients (odds ratio [OR]: 1.96; 95% CI: 1.35 to 2.85; p < 0.001) (Table 4); this association remained statistically significant after adjusting for potential confounders, including age, ETT result, PC risk group, Morise risk score, and BMI (Table 4). Additional multivariable models that included atrioventricular blocking medications, prostatectomy, or PC nonchemical recurrence and/or progression did not yield significantly different results (data not shown). In exploratory unadjusted analyses, a relative inverse linear dose-response effect of ADT exposure on CRF was observed (Supplemental Figure 1).
Figure 2.
Frequency of Reduced CRF in ADT-Exposed Versus ADT-Naive Patients With PC
Frequency of reduced cardiorespiratory fitness (CRF), defined as failure to achieve >8 metabolic equivalents during exercise treadmill testing, was compared in androgen deprivation therapy (ADT)-exposed versus ADT-naive patients with prostate cancer (PC) using chi-square test. Reduced CRF was more frequent among patients with ADT exposure than among those with no ADT exposure (48.7% vs. 32.6%, p < 0.001).
Table 4.
Associations Among ADT of Any Duration, Short-Term Use, and Prolonged Use With Reduced CRF and CV Mortality
| Reduced CRF (METs ≤8) |
CV Mortality |
|||
|---|---|---|---|---|
| Unadjusted OR (95% CI), p Value |
Adjusted OR∗ (95% CI), p Value |
Unadjusted Cause-Specific HR (95% CI), p Value |
Adjusted Cause-Specific HR∗ (95% CI), p Value |
|
| No ADT (n = 466) | 1.00 | 1.00 | 1.00 | 1.00 |
| ADT (n = 150) | 1.96 (1.35–2.85), <0.001 | 1.97 (1.21–3.20), 0.006 | 2.36 (1.10–5.06), 0.027 | 2.14 (0.83–5.50), 0.115 |
| ADT ≤6 months (n = 99)† | 1.72 (1.11–2.67), 0.016 | 1.71 (1.00–2.94), 0.052 | 1.83 (0.68–4.98), 0.234 | 1.60 (0.51–5.01), 0.420 |
| ADT >6 months (n = 51) | 2.52 (1.40–4.51), 0.002 | 2.71 (1.31–5.61), 0.007 | 3.60 (1.31–9.84), 0.013 | 3.87 (1.16–12.96), 0.028 |
CI = confidence interval; CV = cardiovascular; HR = hazard ratio; OR = odds ratio; other abbreviations as in Tables 1 and 3.
Adjusted for age, ETT result, prostate risk group, Morise risk score, and body mass index (quadratic).
Thirteen patients in the short-term cohort who received additional ADT after ETT to bring their cumulative ADT exposure to >6 months were excluded from CV mortality analyses.
In unadjusted analysis, both short-term ADT exposure (OR: 1.72; 95% CI: 1.11 to 2.67; p = 0.016) and prolonged ADT exposure (OR: 2.52; 95% CI: 1.40 to 4.51; p = 0.002) were each associated with increased risk of reduced CRF among PC patients as compared to those with no exposure to ADT. However, in adjusted analyses, only prolonged ADT exposure (OR: 2.71; 95% CI: 1.31 to 5.61; p = 0.007) remained significantly associated with reduced CRF, whereas the strength of the association with short-term exposure was attenuated and of borderline statistical significance (OR: 1.71; 95% CI: 1.00 to 2.94; p = 0.052) (Table 4).
Cardiovascular mortality
There were 28 CV deaths over a median follow-up of 4.2 years (IQR: 2.3 to 7.1 years) after the ETT; 17 occurred in ADT-naive patients and 11 in ADT-exposed patients. Any ADT therapy was associated with a >2-fold increased risk of CV death (HR: 2.36; 95% CI: 1.10 to 5.06; p = 0.027). However, this association was not statistically significant after adjusting for age, ETT result, PC risk group, Morise risk score, and BMI (HR: 2.14; 95% CI: 0.83 to 5.50; p = 0.115) (Table 4). Prolonged ADT exposure was associated with a significantly higher hazard of CV mortality in unadjusted (HR: 3.60; 95% CI: 1.31 to 9.84; p = 0.013) and adjusted (HR: 3.87; 95% CI: 1.16 to 12.96; p = 0.028) analyses (Table 4, Figure 3). Short-term ADT exposure was not significantly associated with CV mortality in unadjusted or adjusted analyses (Table 4, Figure 3). Across the entire study cohort, reduced CRF (≤8 METs) was associated with a significantly higher risk of CV mortality (HR: 4.60; 95% CI: 2.03 to 10.46; p < 0.001).
Figure 3.
Cumulative Incidence of CV Mortality by ADT Exposure Status
Cumulative incidence for cardiovascular (CV) mortality for patients with prostate cancer and no androgen deprivation therapy (ADT) exposure (green), ADT exposure of ≤6 months (blue), and ADT exposure of >6 months (red) were compared using Gray test of equality. Incidence was similar for patients with no ADT exposure and those with ≤6 months’ exposure (p = 0.077). Incidence was significantly higher among patients with >6 months’ exposure compared with among ADT-naive patients with prostate cancer (p = 0.029).
Discussion
This study investigated the effect of ADT exposure on CRF and CV mortality in 616 patients with PC and a high prevalence of baseline CV risk factors. We observed that any ADT exposure was associated with reduced CRF. Additional analyses stratified by duration of ADT exposure prior to ETT suggest that this observation was driven largely by prolonged ADT use (>6 months). Similarly, prolonged ADT exposure was associated with an almost 4-fold increased adjusted risk of CV mortality.
Our findings advance the current understanding of the influence of both ADT exposure and the duration of ADT exposure on CRF. Use of ADT-naive patients with PC as the control group rather than age-matched healthy individuals to explore ADT-mediated effects on CRF is a key strength of this study. Wall et al. (20) demonstrated lower maximal oxygen uptake (10th to 15th percentiles) during ETTs in a comparison of 112 patients with PC treated with ADT and age-matched healthy individuals. The same group reported significantly lower CRF in those who received ≥3 months of ADT compared with those with <3 months of ADT exposure in analyses that did not control for potential confounders (20,29). Our larger study offers additional insight by demonstrating that whereas prolonged ADT exposure was associated with a 2.7-fold increased likelihood of reduced CRF compared with in ADT-naive patients with PC after adjusting for key confounders, the association with short-term ADT exposure was of borderline significance (p = 0.052) with a smaller effect size (OR: 1.71).
Results of existent studies that have investigated the association between ADT and CV mortality are conflicting. One large observational cohort study showed that gonadotropin-releasing hormone agonist use leads to increased risk of sudden cardiac death following exposure for 5 to 12 months, but not after treatment regimens lasting 1 to 4 months (30). Similarly, an increased risk of sudden cardiac death was observed with ADT use in the veteran population (8); elevated CV mortality was reported with ADT use in patients who received radical prostatectomy as initial treatment (10); and a shorter time to fatal myocardial infarction was noted in patients receiving 6 months of ADT compared with ADT-naive patients from a post hoc analysis combining data from 3 randomized control trials (31). These studies are consistent with our findings that ADT use is associated with increased CV mortality, although we were able to demonstrate this association only for patients exposed to ADT for >6 months.
Conversely, other studies have failed to demonstrate any association between ADT use and CV mortality. A meta-analysis of 8 trials showed no significant difference in CV mortality between patients receiving ADT and control individuals (14). However, the control individuals in some of these trials did receive ADT after the initial treatment phase following randomization, which may have limited the ability to detect a treatment effect on CV mortality (32). A matched cohort study comparing the impact of ≥6 months ADT or bilateral orchiectomy in patients with PC versus in ADT-naive patients with PC did not find any association with increased risk of either myocardial infarction or sudden cardiac death (13). A reanalysis of patients treated with the combination of goserelin and radiation therapy versus radiation therapy alone found no increased risk of CV mortality with goserelin (33). Our finding that CV mortality risk may be a function of duration of ADT exposure might in part explain why some studies failed to demonstrate any association with CV mortality. In our study, ADT exposure within the entire cohort was not independently associated with increased CV mortality (p = 0.115), and it was only after stratifying analyses by duration of ADT exposure that an independent association between prolonged ADT exposure and CV mortality was observed. Whether the CV implications of ADT-associated metabolic perturbations are cumulative over duration of exposure is uncertain. Alternatively, in contrast with large population studies that report incident CV disease within 6 months of starting ADT (11,30), it is important to acknowledge that the absence of significant associations between short-term ADT use and CV mortality in this study may simply reflect the relatively small sample size and number of events. Other reasons why our results may differ from these studies include differences in the definition of CV mortality and in the range of ADT agents considered. Moreover, baseline CV risk was higher in our study and may have modified the association of ADT exposure and CV mortality. In support of this, a retrospective study found that the use of hormonal therapy was associated with increased all-cause mortality in patients with pre-existing heart failure or ischemic heart disease but not in those with <2 risk factors for coronary artery disease (12). In our study, the majority of the patients (81.8%) had ≥2 CV risk factors. Although baseline CV risk may well potentiate the CV risk of ADT exposure, multivariable analyses confirmed an independent association of prolonged ADT exposure with both reduced CRF and CV mortality after controlling for this baseline CV risk using the aggregate Morise score.
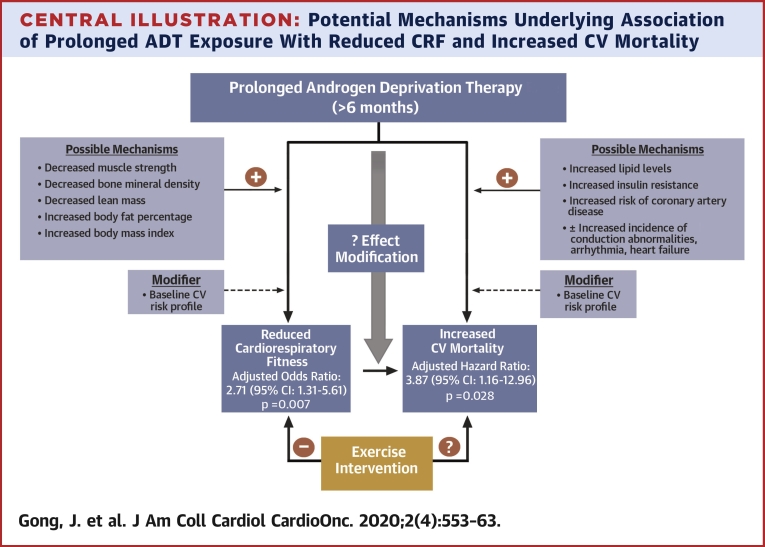
Multiple mechanisms likely mediate the negative association of ADT and CRF observed in this study. ADT has been associated with decreased muscular strength (18,34) that can occur after only 3 months of exposure (18). ADT has also been linked to decreases in bone mineral density and lean muscle mass and to increases in BMI and body fat percentage (34,35); these changes in body composition could also undermine CRF. Similarly, many factors likely contribute to the increased risk of CV mortality associated with prolonged ADT exposure observed in our study. Studies have linked ADT use to increased incidence of conduction disorders (36), arrhythmias (36,37), heart failure (36), and coronary artery disease (8,30,38). Predisposition to CV events is greater among patients with PC and pre-existing CV disease (11,36). Furthermore, metabolic side effects of ADT, including increased lipid levels (38), insulin resistance (39), and higher incidence of diabetes (6,30,38), may also mediate increased CV mortality. Our study proposes an additional mechanism through which ADT use could contribute to increased CV mortality, namely reduced CRF (Central Illustration). The influence of ADT-independent factors prevalent in cancer patients such as chronic fatigue, sleep disorders, depression, pain, anemia, and deconditioning on CRF and associated adverse CV outcomes must also be considered (40,41).
Central Illustration.
Potential Mechanisms Underlying Association of Prolonged ADT Exposure With Reduced CRF and Increased CV Mortality
Association of prolonged androgen deprivation therapy (ADT) exposure with reduced cardiorespiratory fitness (CRF) and increased cardiovascular (CV) mortality are presented together with some proposed mechanisms. Baseline CV risk modifies these associations. Reduced CRF may also in part mediate increased risk of CV mortality. ADT exposure may not only directly influence CRF and CV mortality but may also modify the association between CRF and CV mortality. An exercise intervention concurrent with ADT may mitigate against reduced CRF, and whether this could offset some of the increased risk of CV mortality warrants investigation.
Previous studies have demonstrated strong associations among CRF and all-cause and CV mortality, CV morbidity, and CV risk factors (15, 16, 17). It is possible that ADT exposure might modify this association of CRF with mortality. Two prospective randomized clinical trials (n = 57 and n = 100, respectively) have shown that structured exercise programs can rescue the adverse effects of ADT use including functional performance, muscle strength, lean muscle mass (42, 43, 44), with greater beneficial effects seen with prolonged ADT exposure (44). Whether such exercise interventions can reduce CV morbidity and mortality in ADT-exposed patients requires further evaluation.
Study limitations
The retrospective nature of this study poses limitations. Missing data precluded inclusion of all patients who were referred for ETTs after a diagnosis of PC (Figure 1). However, missing data were considered random and noninformative. This PC cohort had high baseline CV risk reflecting selection bias introduced by studying patients clinically referred for ETTs. This facilitated an assessment of prolonged ADT exposure in a CV risk–enriched cohort but does limit the generalizability of findings to the broader PC population. However, we present multivariable analyses that attempt to control for key potential confounders in a more comprehensive manner than prior similar studies did. We were unable to explore the influence of time from ADT completion on CRF assessment, which limits inference on the relative contribution of duration of ADT exposure versus time from ADT exposure on CRF. The relatively small sample size and number of events could have limited the ability to detect significant associations among short-term ADT use and reduced CRF (a trend was observed) or CV mortality, respectively. This study was not designed to test whether ADT exposure is an effect modifier of the association between CRF and mortality.
Clinical implications
This study highlights that patients with PC and high baseline CV risk are at increased risk of reduced CRF and CV mortality when exposed to prolonged ADT regimens. Whereas prolonged ADT certainly plays a role in the treatment of PC, these findings emphasize the need to consider CV surveillance and risk modification during and after ADT exposure. A clinical trial is warranted to determine whether exercise interventions concurrent with ADT prescription can mitigate CRF impairment and CV risk.
Conclusions
Among patients with PC and high baseline CV risk, prolonged ADT exposure was independently associated with reduced CRF and increased CV mortality. Reduced CRF may in part mediate the increased CV risk that we observed and may represent a therapeutic target. The potential merit of exercise interventions concurrent with prolonged ADT prescription in patients with PC and high CV risk warrants investigation.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Prolonged ADT exposure in patients with high baseline CV risk is associated with reduced CRF and increased CV mortality among patients with PC. Patients with PC treated with prolonged ADT should be advised of increased CV risk and strategies to mitigate CV risk and maintain CRF should be considered.
TRANSLATIONAL OUTLOOK: Mechanisms that underlie reduced CRF and higher CV mortality in the setting of prolonged ADT exposure in patients with PC require additional study. Interventions to preserve CRF and mitigate CV risk in patients with PC exposed to prolonged ADT, adapted before, during, and after ADT, merit further investigation.
Author Disclosures
This work was supported by the Goodman Master Clinician Award from Brigham and Women’s Hospital (to Dr. Groarke) and by the Gelb Master Clinician Award and the Catherine Fitch Fund form Brigham and Women’s Hospital (to Dr. Nohria). Dr. McGregor has served as a consultant for Bayer, Astellas, AstraZeneca, Seattle Genetics, Exelixis, Nektar, Pfizer, Janssen, and Genentech; and has received research support paid to his institution from Bristol Myers Squibb. Dr. Neilan was supported in part through a gift from A. Curt Greer, Pamela Greer, and Kohlberg Foundation, National Institutes of Health/National Heart, Lung, and Blood Institute (grants 1R01HL130539-01A1, 1R01HL137562-01A1, K24HL113128-06), and National Institutes of Health/Harvard Center for AIDS Research (grant P30 AI060354). Dr. Nohria has served as a consultant for Takeda Oncology and Triple Gene Therapy; and has received research support from Amgen, Inc. Dr. Groarke has received research support from Amgen, Inc. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For a supplemental figure, please see the online version of this paper.
Appendix
References
- 1.American Cancer Society . American Cancer Society; Atlanta, GA: 2014. Cancer Treatment and Survivorship Facts and Figures 2014–2015. [Google Scholar]
- 2.Epstein M.M., Edgren G., Rider J.R., Mucci L.A., Adami H.O. Temporal trends in cause of death among Swedish and US men with prostate cancer. J Natl Cancer Inst. 2012;104:1335–1342. doi: 10.1093/jnci/djs299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng M.V., Grossfeld G.D., Sadetsky N., Mehta S.S., Lubeck D.P., Carroll P.R. Contemporary patterns of androgen deprivation therapy use for newly diagnosed prostate cancer. Urology. 2002;60(Suppl 1):7–11. doi: 10.1016/s0090-4295(02)01560-1. discussion 11–2. [DOI] [PubMed] [Google Scholar]
- 4.Fu A.Z., Tsai H.T., Haque R. Mortality and androgen deprivation therapy as salvage treatment for biochemical recurrence after primary therapy for clinically localized prostate cancer. J Urol. 2017;197:1448–1454. doi: 10.1016/j.juro.2016.12.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain M., Fizazi K., Saad F. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N Engl J Med. 2018;378:2465–2474. doi: 10.1056/NEJMoa1800536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen C., Lairson D.R., Swartz M.D., Du X.L. Risks of major long-term side effects associated with androgen-deprivation therapy in men with prostate cancer. Pharmacotherapy. 2018;38:999–1009. doi: 10.1002/phar.2168. [DOI] [PubMed] [Google Scholar]
- 7.Keating N.L., O'Malley A.J., Freedland S.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating N.L., O'Malley A., Freedland S.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2012;104:1518–1523. doi: 10.1093/jnci/djs376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saigal C.S., Gore J.L., Krupski T.L. Androgen deprivation therapy increases cardiovascular morbidity in men with prostate cancer. Cancer. 2007;110:1493–1500. doi: 10.1002/cncr.22933. [DOI] [PubMed] [Google Scholar]
- 10.Tsai H.K., D'Amico A.V., Sadetsky N., Chen M.H., Carroll P.R. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99:1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 11.O'Farrell S., Garmo H., Holmberg L., Adolfsson J., Stattin P., Van Hemelrijck M. Risk and timing of cardiovascular disease after androgen-deprivation therapy in men with prostate cancer. J Clin Oncol. 2015;33:1243–1251. doi: 10.1200/JCO.2014.59.1792. [DOI] [PubMed] [Google Scholar]
- 12.Nanda A., Chen M.H., Braccioforte M.H., Moran B.J., D'Amico A.V. Hormonal therapy use for prostate cancer and mortality in men with coronary artery disease-induced congestive heart failure or myocardial infarction. JAMA. 2009;302:866–873. doi: 10.1001/jama.2009.1137. [DOI] [PubMed] [Google Scholar]
- 13.Alibhai S.M., Duong-Hua M., Sutradhar R. Impact of androgen deprivation therapy on cardiovascular disease and diabetes. J Clin Oncol. 2009;27:3452–3458. doi: 10.1200/JCO.2008.20.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen P.L., Je Y., Schutz F.A. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta-analysis of randomized trials. JAMA. 2011;306:2359–2366. doi: 10.1001/jama.2011.1745. [DOI] [PubMed] [Google Scholar]
- 15.Myers J., Prakash M., Froelicher V., Do D., Partington S., Atwood J.E. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 16.Roger V.L., Jacobsen S.J., Pellikka P.A., Miller T.D., Bailey K.R., Gersh B.J. Prognostic value of treadmill exercise testing: a population-based study in Olmsted County, Minnesota. Circulation. 1998;98:2836–2841. doi: 10.1161/01.cir.98.25.2836. [DOI] [PubMed] [Google Scholar]
- 17.Breneman C.B., Polinski K., Sarzynski M.A. The impact of cardiorespiratory fitness levels on the risk of developing atherogenic dyslipidemia. Am J Med. 2016;129:1060–1066. doi: 10.1016/j.amjmed.2016.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alibhai S.M., Breunis H., Timilshina N. Impact of androgen-deprivation therapy on physical function and quality of life in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28:5038–5045. doi: 10.1200/JCO.2010.29.8091. [DOI] [PubMed] [Google Scholar]
- 19.Joly F., Alibhai S.M., Galica J. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176:2443–2447. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 20.Wall B.A., Galvão D.A., Fatehee N. Maximal exercise testing of men with prostate cancer being treated with androgen deprivation therapy. Med Sci Sports Exerc. 2014;46:2210–2215. doi: 10.1249/MSS.0000000000000353. [DOI] [PubMed] [Google Scholar]
- 21.Morise A.P. Comparison of the Diamond-Forrester method and a new score to estimate the pretest probability of coronary disease before exercise testing. Am Heart J. 1999;138:740–745. doi: 10.1016/s0002-8703(99)70190-0. [DOI] [PubMed] [Google Scholar]
- 22.Bolla M., de Reijke T.M., Van Tienhoven G. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. doi: 10.1056/NEJMoa0810095. [DOI] [PubMed] [Google Scholar]
- 23.Bolla M., Maingon P., Carrie C. Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: results of EORTC Trial 22991. J Clin Oncol. 2016;34:1748–1756. doi: 10.1200/JCO.2015.64.8055. [DOI] [PubMed] [Google Scholar]
- 24.da Silva S.C., Monteiro W.D., Farinatti PdTV. Exercise maximum capacity assessment: a review on the traditional protocols and the evolution to individualized models. Rev Bras Med Esporte. 2011;17:363–369. [Google Scholar]
- 25.Gibbons R.J., Balady G.J., Beasley J.W. ACC/AHA guidelines for exercise testing: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Exercise Testing) J Am Coll Cardiol. 1997;30:260–311. doi: 10.1016/s0735-1097(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 26.Morise A., Evans M., Jalisi F., Shetty R., Stauffer M. A pretest prognostic score to assess patients undergoing exercise or pharmacological stress testing. Heart. 2007;83:200–204. doi: 10.1136/hrt.2006.093799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gulati M., Pandey D.K., Arnsdorf M.F. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 28.Mark D.B., Shaw L., Harrell F.E. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. N Engl J Med. 1991;325:849–853. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 29.Wall B.A., Galvão D.A., Fatehee N. Reduced cardiovascular capacity and resting metabolic rate in men with prostate cancer undergoing androgen deprivation: a comprehensive cross-sectional investigation. Adv Urol. 2015;2015:976235. doi: 10.1155/2015/976235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keating N.L., O'Malley A.J., Smith M.R. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 31.D'Amico A.V., Denham J.W., Crook J. Influence of androgen suppression therapy for prostate cancer on the frequency and timing of fatal myocardial infarctions. J Clin Oncol. 2007;25:2420–2425. doi: 10.1200/JCO.2006.09.3369. [DOI] [PubMed] [Google Scholar]
- 32.Blankfield R.P. Androgen deprivation therapy for prostate cancer and cardiovascular death. JAMA. 2012;307:1252. doi: 10.1001/jama.2012.352. author reply 1252–3. [DOI] [PubMed] [Google Scholar]
- 33.Efstathiou J.A., Bae K., Shipley WU. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85-31. J Clin Oncol. 2009;27:92–99. doi: 10.1200/JCO.2007.12.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galvão D.A., Spry N.A., Taaffe D.R. Changes in muscle, fat and bone mass after 36 weeks of maximal androgen blockade for prostate cancer. BJU Int. 2008;102:44–47. doi: 10.1111/j.1464-410X.2008.07539.x. [DOI] [PubMed] [Google Scholar]
- 35.Haseen F., Murray L.J., Cardwell C.R., O'Sullivan J.M., Cantwell M.M. The effect of androgen deprivation therapy on body composition in men with prostate cancer: systematic review and meta-analysis. J Cancer Surviv. 2010;4:128–139. doi: 10.1007/s11764-009-0114-1. [DOI] [PubMed] [Google Scholar]
- 36.Haque R., UlcickasYood M., Xu X. Cardiovascular disease risk and androgen deprivation therapy in patients with localised prostate cancer: a prospective cohort study. Br J Cancer. 2017;117:1233–1240. doi: 10.1038/bjc.2017.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Hemelrijck M., Garmo H., Holmberg L. Absolute and relative risk of cardiovascular disease in men with prostate cancer: results from the Population-Based PCBaSe Sweden. J Clin Oncol. 2010;28:3448–3456. doi: 10.1200/JCO.2010.29.1567. [DOI] [PubMed] [Google Scholar]
- 38.Kintzel P.E., Chase S.L., Schultz L.M., O'Rourke T.J. Increased risk of metabolic syndrome, diabetes mellitus, and cardiovascular disease in men receiving androgen deprivation therapy for prostate cancer. Pharmacotherapy. 2008;28:1511–1522. doi: 10.1592/phco.28.12.1511. [DOI] [PubMed] [Google Scholar]
- 39.Basaria S., Muller D.C., Carducci M.A., Egan J., Dobs A.S. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- 40.Mustian K.M., Morrow G.R., Carroll J.K., Figueroa-Moseley C.D., Jean-Pierre P., Williams G.C. Integrative nonpharmacologic behavioral interventions for the management of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):52–67. doi: 10.1634/theoncologist.12-S1-52. [DOI] [PubMed] [Google Scholar]
- 41.Ryan J.L., Carroll J.K., Ryan E.P., Mustian K.M., Fiscella K., Morrow G.R. Mechanisms of cancer-related fatigue. Oncologist. 2007;12(Suppl 1):22–34. doi: 10.1634/theoncologist.12-S1-22. [DOI] [PubMed] [Google Scholar]
- 42.Galvão D.A., Spry N., Denham J. A multicentre year-long randomised controlled trial of exercise training targeting physical functioning in men with prostate cancer previously treated with androgen suppression and radiation from TROG 03.04 RADAR. Eur Urol. 2014;65:856–864. doi: 10.1016/j.eururo.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 43.Galvão D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 2010;28:340–347. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 44.Galvão D.A., Taaffe D.R., Spry N., Joseph D., Newton R.U. Acute versus chronic exposure to androgen suppression for prostate cancer: impact on the exercise response. J Urol. 2011;186:1291–1297. doi: 10.1016/j.juro.2011.05.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.