Anthracycline-based chemotherapy (AC) is a common treatment for patients with breast cancer and has been associated with a dramatic improvement in breast cancer survivorship. Among patients with early-stage breast cancer, cardiovascular diseases represent the most common cause of mortality, and there is a growing emphasis on strategies for minimizing the toxic effects of breast cancer treatments on the cardiovascular system (1).
The primary therapeutic approach for preventing heart failure following anthracycline exposure is to intervene with heart failure pharmacotherapy in patients with cardiac dysfunction (1,2). However, patients may be less responsive to this approach if dysfunction is detected late (3), and so there is interest in primary preventive approaches. Exercise training has been proposed as 1 such approach (4) because it is safe, inexpensive, and already recommended as a strategy for counteracting other adverse effects of cancer treatment. However, little is known about whether exercise training can effectively counteract the cardiotoxic effects of AC.
Cardiac magnetic resonance (CMR) is an increasingly available imaging method for assessing cardiac function. The excellent image resolution makes CMR assessment of ventricular volume and function the gold standard noninvasive technique, and sequences such as T1 mapping may allow early detection of chemotherapy-related inflammation, edema, and fibrosis.
In this study, we evaluated native T1 mapping at 3-T using inversion recovery (ShMOLLI) and saturation recovery (SASHA) techniques and measured global longitudinal strain (GLS) using feature tracking of cine images, before and after completion of AC-based chemotherapy. A subset of the study group underwent exercise training. We hypothesized that: 1) anthracyclines would increase myocardial inflammation (T1 mapping) and impair systolic function measured by GLS; and 2) exercise training would attenuate these changes.
This was a single-center, nonrandomized clinical trial. All research was performed at the Baker Heart and Diabetes Institute, Melbourne, Australia, between May 2016 and December 2017. The experimental procedures were explained to all participants, with informed consent obtained as approved by the institutional review boards of the Alfred Health Research Ethics Committee and registered with Australia and New Zealand Clinical Trials Registry (ACTRN12616001602415). All procedures conformed to the standards set by the Declaration of Helsinki.
A total of 27 participants with recently diagnosed breast cancer who were scheduled for AC-based chemotherapy were included. At baseline all participants were assessed using CMR, repeated 4 months later (3 weeks after the final cycle of AC). After baseline assessment, participants pragmatically selected to usual care or an exercise training intervention. Details of the exercise program have been published previously (5). Participants in the exercise group underwent a nonlinear, combined moderate- to vigorous-intensity aerobic and resistance training regimen (3/week, 60 min/session, 2 supervised, 1 home-based) that was designed to synchronize with each participant’s individual chemotherapy cycle.
We performed all CMR examinations on a clinical 3.0-T magnetic resonance imaging scanner (Magnetom Prisma, Siemens Healthineers, Erlangen, Germany). All image post-processing was performed using a dedicated software package (CVI42, Circle Cardiovascular Imaging, Calgary, Canada). Each study was analyzed independently by an experienced CMR reader blinded to the patient group.
Myocardial T1 times were estimated of by means of prototype ShMOLLI and SASHA sequences (Siemens Healthineers). This sequence automatically generated pixel maps of T1 times that were used during post-processing, with a motion correction algorithm applied to the raw images.
Each sequence was acquired within an end-expiration breath-hold using an electrocardiogram-triggered single-shot acquisition with a balanced steady-state free precession readout in a single mid–short-axis slice. Native T1 (pre-contrast) and post-contrast T1 times were measured in the myocardium and left ventricular (LV) blood pool by using a region of interest on the T1 pixel map. T1 measurements were taken at the mid–short-axis level by taking a region within the septum.
GLS was calculated using feature tracking on the cine images on CVI42 software. The myocardium was defined according to American Heart Association segments by placing a marker across the mitral valve annulus and from the annulus to the apex on long-axis images and by marking endocardial and epicardial borders in the short-axis volumetric stack and 3 apical cine images (4-chamber, 2-chamber, 3-chamber). Markers were placed at both right ventricular (RV) insertion points on the short-axis images.
All data were analyzed using SPSS Statistics software version 23.0 (IBM Corp, Armonk, New York). Data are presented as mean ± SD unless otherwise stated, and p <0.05 was considered statistically significant. Paired Student’s t-tests were used to compare continuous data. Linear relationships were assessed using Pearson's correlation coefficients. Two-way mixed analysis of variance evaluated the interaction between groups according to exercise status.
The 2 groups were well matched, with no significant differences in age (46 ± 9 years vs. 51 ± 12 years; p = 0.19), body size (body surface area 1.76 ± 0.26 m2 vs. 1.83 ± 0.24 m2; p = 0.51), LV size (118 ± 19 ml vs. 113 ± 21 ml; p = 0.50), and LV ejection fraction (LVEF; 62 ± 4% vs. 64 ± 5%; p = 0.25), or T1 mapping parameters (ShMOLLI 1,170 ± 27 ms vs. 1,179 ± 44 ms; p = 0.55; and SASHA 1,538 ± 32 ms vs. 1,538 ± 40 ms; p = 0.99) and GLS (−20.11 ± 1.47 vs. −20.48 ± 1.66; p = 0.55). No participants were treated for hypertension, diabetes mellitus, or dyslipidemia.
The primary breast cancer diagnosis, prescribed chemotherapy regimen, and dose were similar between the groups. No participants received trastuzumab during the study period. Adherence to the exercise intervention was modest, with one-half of the participants completing 80% of the 24 prescribed sessions (group average 76%; range 38% to 88%).
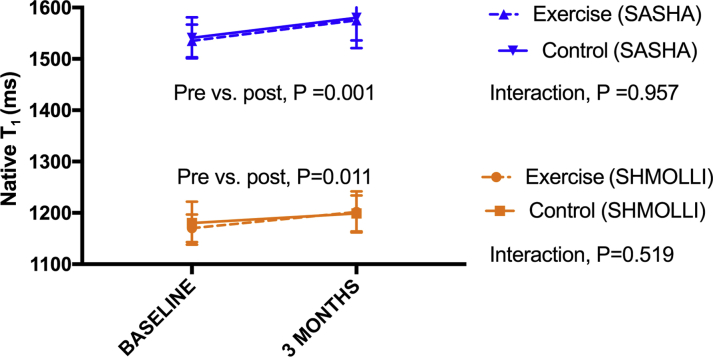
There was a significant increase in native T1 time following AC chemotherapy using SASHA and ShMOLLI methods across all participants (1,538 ± 36 ms vs. 1,581 ± 50 ms; p = 0.001; and 1,174 ± 36 ms vs. 1,203 ± 37 ms; p = 0.011, respectively) (Figure 1).
Figure 1.
Change in Native T1 Mapping Time
There was an increase in native T1 time using 2 T1 methods—SASHA (blue) and ShMOLLI (orange)—after anthracycline-based chemotherapy. The increase was not ameliorated by exercise (solid line) compared with sedentary control subjects (dashed line).
The significant increase in T1 times were associated with a trend to a small reduction in LVEF (63.1 ± 4.3% vs. 61.3 ± 3.5%; p = 0.066) but no change in LV end-diastolic volume (p = 0.829), left atrial volume (p = 0.844), or LV mass (p = 0.856). There was no change in RV end-diastolic volume (p = 0.852) but a significant 4.6% decline in RV ejection fraction with AC (RVEF; 62.7 ± 5.7% vs. 58.6 ± 6.1%; p = 0.004).
There was a modest negative correlation between the change in SASHA and the decline in LVEF (R = −0.424; p = 0.04), but not with ShMOLLI (p = 0.788). There was also no correlation between the change in T1 mapping times and the decline in RVEF.
An exercise program did not influence the increase in native T1 times (interaction p = 0.519 for ShMOLLI; p = 0.957 for SASHA), nor did it attenuate the change in LVEF (interaction p = 0.557) and RVEF (interaction p = 0.631).
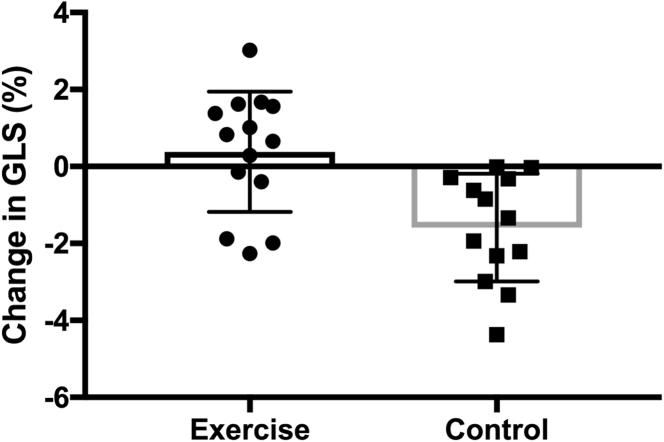
Exercise had a significant impact on GLS (interaction for exercise effect; p < 0.001) with a significant reduction in strain seen in the control group (Δ −1.6 ± 1.4%; p = 0.002) as opposed to preservation of strain in the exercise group (Δ +0.56 ± 1.5%; p = 0.377) (Figure 2).
Figure 2.
Change in GLS
All participants in the sedentary control group (right, squares) had a decrease in global longitudinal strain (GLS) after anthracycline-based chemotherapy (Δ − 1.6 ± 1.4%; p = 0.002), whereas global longitudinal strain was preserved in the exercise group (left, circles) (Δ + 0.56 ± 1.5%, p = 0.377; interaction for exercise effect; p < 0.001). Mean ± SD are marked on the figure.
There were no significant correlations between the change in GLS and changes in LVEF (p = 0.285) or native T1 times (p > 0.50).
Investigating the hypothesis that novel CMR measures would be a sensitive means of identifying anthracycline-induced cardiotoxicity, we identified a significant increase in T1 times and a reduction in GLS representing potential surrogates of early myocardial inflammation and dysfunction, respectively. Intriguingly, we also observed a significant attenuation of myocardial dysfunction associated with a structured exercise program but no effect on inflammation.
Previous studies have evaluated T1 mapping in the context of AC chemotherapy (6). In a cross-sectional analysis of cancer survivors, Jordan et al. (7) observed an elevation in myocardial native T1 and extracellular volume (ECV) that was independent of the underlying cancer and cardiovascular comorbidities. With the larger time interval between treatment and assessment, these findings suggest that imaging biomarkers may represent the development of interstitial fibrosis in cancer survivors and reflect a causal link between cardiotoxic cancer therapies and myocardial dysfunction.
The findings suggest an acute process in the myocardium in response to AC chemotherapy, detectable by T1 mapping, that may represent inflammation and/or early fibrosis. The increased native T1 time may be similar to that seen in acute myocarditis (8) and may be a result of increased free water associated with inflammation and edema. A total of 50% of participants had a rise in T1 time >1 SD, a much higher percentage than that expected to develop long-term cardiotoxicity related to breast cancer treatment.
Alterations of myocardial deformation (GLS) have been shown to precede significant change in LVEF (9). The decrease in GLS in the control group of our study is consistent with previous findings in published echocardiography reports, with the additional finding of an attenuation with a dedicated exercise program. This benefit in cardiovascular performance associated with exercise training during chemotherapy is consistent with multiple studies that have demonstrated an improvement in maximal oxygen consumption as a less specific marker of preserved cardiovascular function (4,5). Although our previously reported findings did not determine an association with echocardiography-derived strain, we believe that echocardiography and CMR may measure slightly different aspects of myocardial function—endocardial with feature tracking versus midmyocardial with speckle tracking.
Our findings suggest a consistent, early toxic effect on the myocardium. Despite nearly ubiquitous evidence of subclinical cardiotoxicity, the exercise program did reduce the negative impact of this AC on GLS, thus indicating a beneficial and potentially cardioprotective effect of exercise during treatment. Exercise constitutes a holistic therapy and improves functional capacity through a combination of enhanced cardiovascular, skeletal muscle, and metabolic processes. It has the potential to attenuate chemotherapy-induced injury caused by any, or all, of these factors.
It is important to determine whether these changes in T1 mapping persist and whether they predict long-term cardiotoxicity, cardiovascular morbidity, and mortality. Moreover, it will be important to determine whether these surrogate measures of preserved myocardial function associated with exercise training also predict a reduction in long-term clinical events.
The greatest limitation in the interpretation of our data is the nonrandomized nature of the trial. We were unable to randomize the participants because of geographic constraints. It is not possible to exclude the fact that this selection bias may have influenced the observed differences in functional capacity.
In patients with breast cancer, native T1 times were significantly increased immediately following AC chemotherapy, possibly reflecting myocardial inflammation. A structured exercise program undertaken during chemotherapy was associated with preservation of myocardial function, as opposed to a reduction in GLS in nonexercising patients, but did not influence T1 times. The significance of these changes on long-term cardiac function and heart failure is yet to be determined.
Footnotes
Please note: This project was supported by a grant from the Jack Brockhoff Foundation, Australia. Dr. La Gerche is supported by a Career Development Fellowship from the National Health and Medical Research Council (NHMRC 1089039) and a Future Leaders Fellowship from the National Heart Foundation (NHF 100409) of Australia. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 2.Zamorano J.L., Lancellotti P., Rodriguez Munoz D. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 3.Cardinale D., Colombo A., Bacchiani G. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–1988. doi: 10.1161/CIRCULATIONAHA.114.013777. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrist S.C., Barac A., Ades P.A. Cardio-oncology rehabilitation to manage cardiovascular outcomes in cancer patients and survivors: a scientific statement from the American Heart Association. Circulation. 2019;139:e997–e1012. doi: 10.1161/CIR.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howden E.J., Bigaran A., Beaudry R. Exercise as a diagnostic and therapeutic tool for the prevention of cardiovascular dysfunction in breast cancer patients. Eur J Prev Cardiol. 2019;26:305–315. doi: 10.1177/2047487318811181. [DOI] [PubMed] [Google Scholar]
- 6.Tham E.B., Haykowsky M.J., Chow K. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan J.H., Vasu S., Morgan T.M. Anthracycline-associated T1 mapping characteristics are elevated independent of the presence of cardiovascular comorbidities in cancer survivors. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.115.004325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira V.M., Piechnik S.K., Dall'Armellina E. Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson. 2014;16 doi: 10.1186/1532-429X-16-36. 36–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thavendiranathan P., Poulin F., Lim K.D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]