Abstract
AL amyloidosis results from clonal production of immunoglobulin light chains, most commonly arising from a clonal plasma cell disorder. Once considered a nearly uniformly fatal disease, prognosis has improved markedly over the past 15 years, predominantly because of advances in light chain suppressive therapies. Cardiac deposition of amyloid fibrils is common, and the severity of cardiac involvement remains the primary driver of prognosis. Improvements in chemotherapy/immunotherapy have prompted a reassessment of the role of advanced cardiac therapies previously considered contraindicated in most patients, including the role of implantable cardioverter-defibrillators and cardiac transplantation. This state-of-the-art review highlights the current state of the field, including diagnosis, prognosis, and hematologic- and cardiac-specific therapies.
Key Words: AL amyloidosis, amyloidosis, diagnosis, drug therapy, heart failure, imaging, treatment
Abbreviations and Acronyms: ASCT, autologous stem cell transplantation; BNP, B-type natriuretic peptide; CyBorD, cyclophosphamide, bortezomib, and dexamethasone; FLC, free light chain; ICD, implantable cardioverter-defibrillator; MGUS, monoclonal gammopathy of undetermined significance; NT-proBNP, N-terminal pro–B-type natriuretic peptide; SAP, serum amyloid P; SPIE, serum protein electrophoresis with immunofixation; UPIE, urine protein electrophoresis with immunofixation
Central Illustration
Highlights
-
•
Systemic light chain amyloidosis usually arises from a clonal plasma cell disorder.
-
•
Cardiac involvement in light chain amyloidosis is common and is the main driver of prognosis.
-
•
Improvements in light chain suppressive therapy have led to markedly improved outcomes over the past decade.
-
•
With improved outcomes, implantable cardioverter-defibrillator placement and cardiac transplantation are likely appropriate for select patients.
AL amyloidosis is an increasingly diagnosed disorder (1) involving deposition of misfolded proteins (monoclonal immunoglobulin light chains) in 1 or more tissues in the body (2). The disease can be localized or systemic, a nuisance or life-threatening, and requires specialized expertise to best diagnose and treat. Along with transthyretin amyloidosis (ATTR), AL amyloidosis represents 1 of the 2 most common forms of amyloidosis with frequent cardiac involvement. This article will review the clinical aspects of AL amyloidosis, including diagnosis, prognosis, and state-of-the-art treatment.
Pathophysiology
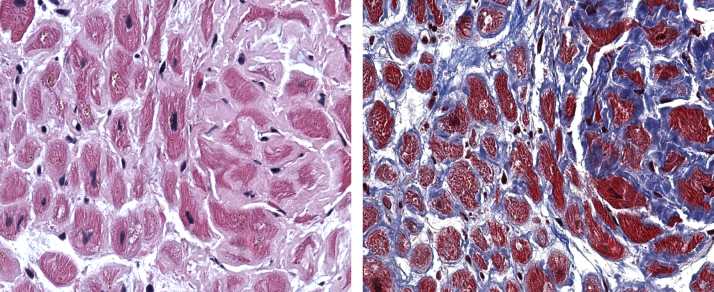
Although a detailed discussion of the pathophysiology of the disease is beyond the scope of this review, the basic principles underlying the mechanisms of injury are critical for understanding the disease and treatment goals. Amyloidosis refers to a group of diseases in which there is extracellular deposition of amyloid fibrils composed of precursor proteins that self-assemble in a β-sheet conformation (3). The “L” in AL amyloidosis refers to the dominant protein that is depositing in AL amyloid fibrils, a clonal immunoglobulin light chain (most commonly secreted by a clonal plasma cell disorder). Because the clonal light chains circulate in the bloodstream, they can theoretically deposit as amyloid fibrils in almost any organ in the body; therefore, most patients have multiorgan deposition, although in each individual with the disease, the pattern of organ involvement varies (3). When amyloid fibrils infiltrate the heart, deposition is most commonly diffusely present throughout the myocardium, with amyloid deposits surrounding each cardiomyocyte (Figure 1).
Figure 1.
Cardiac Amyloidosis Pathology
(Left) Hematoxylin-eosin stain of an endomyocardial biopsy sample demonstrating the characteristic pale eosinophilic amyloid deposits surrounding each cardiomyocyte. (Right) Trichome staining highlights amyloid deposits in blue. Images courtesy of Gerald Berry, MD, Stanford Amyloid Center.
When amyloid fibrils deposit, they can cause organ dysfunction by multiple mechanisms. Disruption of organ architecture routinely occurs; for example, with cardiac amyloidosis, increased wall thickness (due to amyloid fibril infiltration) directly leads to small end-diastolic volumes and can be a contributor to poor stroke volume/cardiac output. However, the primary mechanism of injury is likely to be from direct cellular toxicity from amyloid precursors 4, 5, 6; therefore, cardiac amyloidosis due to AL amyloidosis has been termed a “toxic-infiltrative cardiomyopathy” (7). Organ injury and prognosis are dependent not only on cumulative amyloid fibril deposition but also on the type of fibril being deposited; this has been clearly demonstrated with worse prognosis in cardiac amyloidosis due to AL fibrils than ATTR fibrils, despite greater cumulative fibril deposition in most patients with ATTR amyloidosis 8, 9, 10, 11, 12. Therefore, preventing amyloid precursor/fibril deposition (e.g., by lowering circulating pathologic light chains in AL amyloidosis) may have a greater therapeutic impact than removing already-formed amyloid deposits. Perhaps not surprisingly, prognosis has been mainly dependent on earlier diagnosis and the successful reduction of circulating pathologic light chains after diagnosis, with limited success achieved so far with strategies of amyloid fibril removal.
Diagnosis
Localized versus systemic disease
The fundamental characteristic required for AL amyloid deposition is the production of monoclonal immunoglobulin light chains. Most commonly, this occurs with the proliferation of a plasma cell clone in the bone marrow, with resultant secretion of monoclonal light chains (with or without secretion of monoclonal immunoglobulins) into the bloodstream (2). However, clonal light chain production can occur from other sources as well, including the following:
-
•
Production by clonal B-lymphocytes in lymphoma
-
•
Production by clonal plasma cells in a location outside of the bone marrow (plasmacytoma)
-
•
Production by clonal plasma cells or B-lymphocytes within tissue, often in the setting of inflammation
If amyloid deposits are only found at the site of production itself (e.g., within a lymphoma/plasmacytoma), this represents localized AL amyloidosis. If amyloid deposits are found in other organs after being secreted into the bloodstream, this represents systemic AL amyloidosis (Table 1).
Table 1.
Typical Characteristics of Localized Versus Systemic AL Amyloidosis∗
| Localized | Systemic | |
|---|---|---|
| SPIE or UPIE | Normal | Abnormal |
| Serum FLC ratio | Normal | Abnormal |
| Common locations for deposits | Skin, larynx, sites of chronic inflammation | Heart, kidneys, liver, GI tract, nerves |
| Bone marrow biopsy | Normal | Clonal plasma cell population |
| Presentation | Rash, voice change, incidental finding on biopsy | Vital organ dysfunction |
| Treatment | None, or localized treatment if symptoms | Systemic chemotherapy or immunotherapy |
FLC = free light chain; GI = gastrointestinal; SPIE = serum protein electrophoresis with immunofixation; UPIE = urine protein electrophoresis with immunofixation.
Although these values represent the most common findings, patients with localized AL amyloidosis can have abnormal free light chain ratios or the presence of a monoclonal protein, and some patients with systemic AL amyloidosis can have normal SPIE, UPIE, or FLC.
Localized AL amyloid deposits are typically found either incidentally on a biopsy being performed for another reason (e.g., gastritis with severe inflammation and associated AL amyloid deposits) or because of localized symptoms (e.g., rash from skin deposition or voice changes from laryngeal deposition). Localized deposits typically require either no treatment or local excision for symptom relief (e.g., excision of laryngeal deposits leading to voice changes). Most patients with localized AL amyloidosis do not have evidence of a circulating clonal light chain or immunoglobulin on serum or urine testing, although abnormalities can be detected in 7% to 20% of patients 13, 14. Clonal plasma cell or B-cell populations on bone marrow biopsy are less commonly seen, occurring in only 0% to 2% of patients in 2 large series 13, 14. This article focuses primarily on systemic AL amyloidosis, which is both more commonly diagnosed and a much more medically serious disorder.
Monoclonal gammopathy of undetermined significance versus myeloma versus AL amyloidosis
As noted previously, systemic AL amyloidosis most commonly arises from clonal light chains produced by a clonal plasma cell proliferation in the bone marrow. Because 2 other more common conditions (monoclonal gammopathy of undetermined significance [MGUS] and myeloma) also arise from clonal plasma cell proliferation, this can lead to confusion as to how the diseases relate to one another (Table 2).
Table 2.
Spectrum of Clonal Plasma Cell Disorders
| MGUS | Asymptomatic Myeloma | Symptomatic Myeloma | AL Amyloidosis | |
|---|---|---|---|---|
| How common is it?∗ | +++++++ | +++++ | +++ | + |
| Typical clonal plasma cell percentage | <10% | 10%-60% | >10% | Any |
| Typical concentration of paraprotein on SPIE/UPIE/serum FLC | Low | Moderate | High | Any |
| Treatment required? | No | No | Yes | Yes |
MGUS = monoclonal gammopathy of undetermined significance; other abbreviations as in Table 1.
More + refers to being more common.
When a plasma cell clonally proliferates, it can cause illness in 2 distinct ways:
-
•
The plasma cell clone itself can be proliferative, such that it occupies much of the marrow space (crowding out normal marrow), causing complications such as cytopenias, lytic bone lesions, or hypercalcemia.
-
•
The plasma cell clone can produce monoclonal immunoglobulin and/or monoclonal light chains, which circulate in the bloodstream and cause damage.
Importantly, most plasma cell clones do not cause illness; frequently, the clone is not overly proliferative (taking over <10% of the marrow and not affecting the bone tissue), and the immunoglobulins and/or light chains are eliminated harmlessly in the urine. This scenario, called MGUS, is increasingly common as people age and itself does not require treatment 15, 16. Although patients with MGUS should be monitored to make sure they do not develop myeloma or AL amyloidosis, most will never require treatment.
When the plasma cell clone is itself more malignant, proliferating to >10% of marrow cellularity, it is now called “myeloma.” Depending on whether or not the proliferation is causing certain laboratory abnormalities/organ dysfunction, it is considered “asymptomatic myeloma” (typically not requiring treatment) or “symptomatic myeloma” (typically requiring treatment). Traditionally, the criteria used to define symptomatic myeloma were the CRAB criteria (hyperCalcemia, Renal insufficiency, Anemia and lytic Bone lesions). However, more recently, additional myeloma-defining events have been identified including serum free light chain (FLC) ratio (involved:noninvolved light chain) ≥100, clonal bone marrow plasma cells ≥60%, and lytic lesion detection by more sensitive imaging techniques (whole-body low-dose computed tomography, whole-body magnetic resonance imaging [MRI], or positron emission tomography/computed tomography) (17). For patients meeting these so-called “SLiM-CRAB criteria,” plasma cell–directed treatment is recommended (17).
Regardless of the local aggressiveness of the plasma cell clone, as long as it produces a monoclonal light chain, it is capable of causing AL amyloidosis. It is likely that the primary reason why one clone causes AL amyloidosis and another does not is the specific conformation of the light chain, which is different with every clone (and therefore in every patient with the disease). In particular, light chains that are more “kinetically unstable” (requiring less energy to transition from the native folded state to a transitional unfolded state) are more susceptible to endoproteolysis, resulting in the release and circulation of amyloidogenic light chain fragments (18). If the light chain’s conformation is such that it is prone to misfold, AL amyloid deposits can occur, regardless of whether the light chain is produced in large quantities or if the plasma cell clone itself is particularly aggressive.
Because small clones are more common than large clones (e.g., MGUS is more common than myeloma) (15), it is common that patients with AL amyloidosis do not have concomitant myeloma, although nothing prevents the 2 from coexisting (because they both fundamentally arise from a clonal plasma cell population), and they do coexist in a sizable percentage of patients.
Clinical suspicion and laboratory testing
AL amyloidosis is most commonly diagnosed when end-organ dysfunction prompts an evaluation. Ultimately, the diagnosis can only be made by biopsy, with the identification of extracellular amyloid deposits that are subtyped by either immunohistochemistry or mass spectrometry as arising from the deposition of monoclonal kappa or lambda light chains 19, 20. Therefore, clinical presentation depends on which organs have significant organ deposition (Table 3).
Table 3.
Common Organ Involvement of Systemic AL Amyloidosis With Associated Symptoms/Laboratory Abnormalities
| Signs/Symptoms | Laboratory/Imaging Abnormalities | |
|---|---|---|
| Heart | Diastolic > systolic dysfunction Arrhythmias (tachy/brady) |
Increased wall thickness EKG abnormalities (low voltages, Q waves) Elevated troponin Elevated BNP/NT-proBNP |
| Kidneys | Edema | Albuminuria/hypoalbuminemia GFR reduction |
| Gastrointestinal tract | Dysphagia Macroglossia Diarrhea Constipation GI bleeding |
|
| Liver | Hepatomegaly | Elevated alkaline phosphatase |
| Nervous system | Peripheral neuropathy Autonomic dysfunction Carpal tunnel syndrome (especially bilateral)∗ |
|
| Lungs | Dyspnea | Pleural effusions, pulmonary nodules/infiltrates |
AL = light chain; BNP = B-type natriuretic peptide; EKG = electrocardiographic; GFR = glomerular filtration rate; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
Carpal tunnel syndrome commonly occurs from soft tissue deposits within the carpal tunnel space, rather than from direct nerve infiltration.
Multiple clues can exist that should raise suspicion of a diagnosis of cardiac amyloidosis. Besides clues on imaging or electrocardiography (discussed later), clinical/laboratory clues include the following:
-
1.
Persistently elevated cardiac-specific troponin or multiple presentations with elevated cardiac troponin without adequate explanation on coronary angiography
-
2.
Heart failure combined with other characteristic organ findings (e.g., macroglossia, nephrotic syndrome, elevated alkaline phosphatase, or dysphagia)
-
3.
Unexplained heart block, atrial arrhythmias, or ventricular arrhythmias in a patient with increased ventricular wall thickness
If AL amyloidosis is suspected, laboratory testing should be initiated to evaluate for both monoclonal protein production and evidence of vital organ infiltration/dysfunction (Table 4). The combination of normal serum protein electrophoresis with immunofixation (SPIE), normal urine protein electrophoresis with immunofixation (UPIE), and a normal serum FLC ratio nearly rules out systemic AL amyloidosis 2, 21. However, the presence of an abnormality in any of these tests is insufficient for a diagnosis of AL amyloidosis, because MGUS or myeloma without AL amyloidosis are possibilities. As such, biopsy evidence of amyloid deposits (with amyloid subtyping) is always needed (19).
Table 4.
Laboratory Testing in AL Amyloidosis
| Test | Ordered to Test for | Normal Range∗ |
|---|---|---|
| SPIE† | Clonal immunoglobulin production | No M spike present |
| UPIE† | Clonal light chain production | No M spike present |
| Serum FLC assay | Detecting low-level clonal light chain production; clonality assumed if ratio is far from 1:1 | Kappa:lambda ratio = 0.26-1.65‡ |
| Cardiac troponin | Cardiomyocyte injury | Troponin I: <0.055 ng/ml Troponin T: <0.025 ng/ml |
| BNP or NT-proBNP | Abnormal cardiac function/heart failure (clinical or subclinical) | NT-proBNP: <300 pg/ml BNP: <100 pg/ml |
| Urine albumin:creatinine ratio§ | Renal injury/proteinuria | <30 mg albumin/g creatinine |
| Alkaline phosphatase | Hepatic infiltration | 35-105 U/l |
Normal ranges may vary slightly according to local laboratory standards.
SPIE and UPIE are more sensitive than protein electrophoresis without immunofixation and should be ordered as the preferred tests.
In patients with kidney disease, mild elevations in the kappa:lambda ratio are frequently encountered. In the setting of a normal SPIE/UPIE, a kappa:lambda ratio up to 2.5 can typically be considered normal.
Urine albumin:creatinine ratio is preferred over urine protein:creatinine ratio because the latter can detect light chain excretion, which is not itself a sign of renal injury; 24-h urine collection for albuminuria is a reasonable alternative test.
During treatment with chemotherapy and/or immunotherapy, SPIE/UPIE/FLC can be followed to track treatment response; in general, FLC ratios are the main marker followed because they are more sensitive for low-level abnormalities, and they are measuring the protein that is depositing (light chains) rather than whole immunoglobulins.
Biopsy sites and bone scintigraphy
The biopsy site can be chosen in 1 of 2 ways: minimizing false negatives or minimizing biopsy-associated risk. When aiming for the latter, biopsies can be attempted of the abdominal fat pad (most commonly), rectum, or skin. Reported sensitivities for abdominal fat pad biopsies in AL amyloidosis at high-volume centers are as high as 84%, with higher and lower rates observed, respectively, in patients with higher and lower total body amyloid burden 22, 23. The sensitivity of fat pad biopsy is much lower in patients with ATTR amyloidosis, making fat pad biopsy a generally poor test for evaluation of that disease 22, 23, 24. Bone marrow biopsy, which is often being performed anyway to evaluate a patient with monoclonal immunoglobulin and/or light chain production, can also yield a diagnosis of amyloidosis if there are Congo red–positive deposits present. However, even when deposits are observed in the bone marrow, it is essential to confirm subtyping because other amyloid deposits (e.g., ATTR) can be observed in the bone marrow.
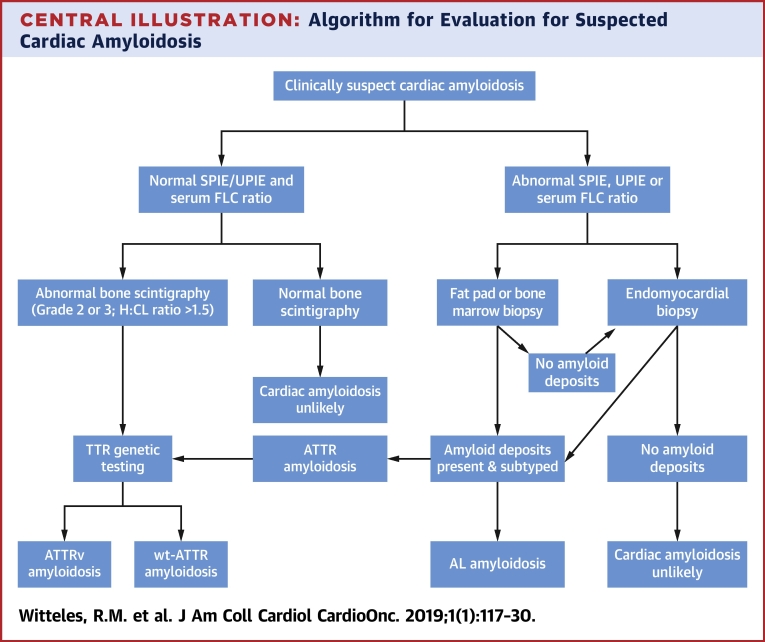
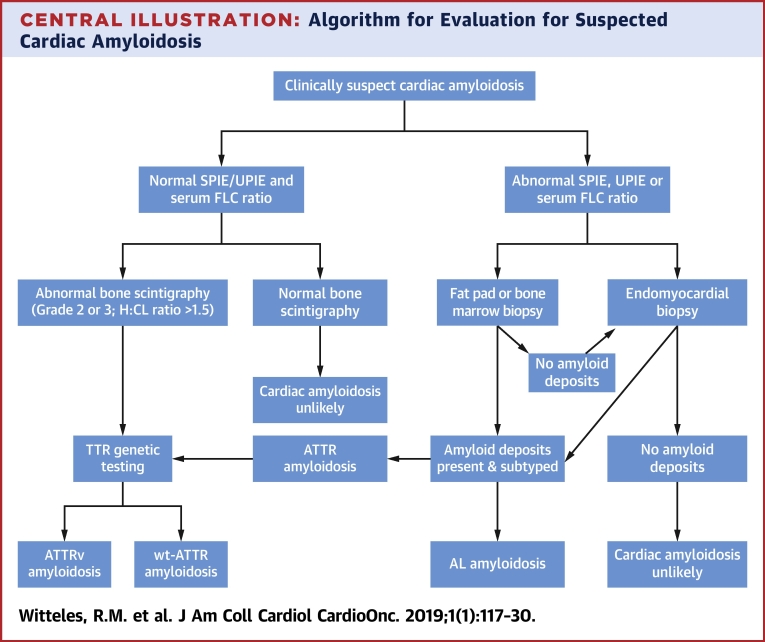
The alternative approach is to biopsy the clinically involved organ (e.g., endomyocardial biopsy if cardiac involvement is suspected, renal biopsy if renal involvement is suspected, and so on). This approach yields nearly 100% sensitivity and specificity and has the further advantage of yielding adequate deposits to accurately subtype the amyloid fibrils (19). Because of this higher yield and the importance of making a timely diagnosis and starting patients with AL amyloidosis on light chain suppressive therapy, proceeding to organ biopsy without first performing a fat pad biopsy is a reasonable strategy. Given that there is still a significant false-negative rate with fat pad biopsy even in experienced hands, organ biopsy should almost always be pursued after a negative fat pad biopsy if clinical suspicion remains (Central Illustration).
Central Illustration.
Algorithm for Evaluation for Suspected Cardiac Amyloidosis
If SPIE, UPIE, or FLC ratio are abnormal, it is reasonable to proceed with fat pad or bone marrow biopsy or to proceed directly to endomyocardial biopsy depending on institutional preference. However, if fat pad and/or bone marrow biopsy do not reveal amyloid deposits, endomyocardial biopsy should be pursued. H:CL = heart to contralateral lung ratio; other abbreviations in Table 1.
When biopsying, it is crucial to not only determine the presence of amyloid deposits but also to subtype the amyloid deposits (e.g., AL, ATTR, etc.). This can be performed with either immunohistochemistry with custom-made antibodies or mass spectrometry; because immunohistochemistry interpretation is dependent on the preparation of the sample and the experience of the pathologist, we generally recommend mass spectrometry (performed at specialty centers) as the preferred approach unless immunohistochemistry is performed by a very experienced pathologist 20, 25.
Imaging
Most commonly, cardiac amyloidosis is suspected based on echocardiographic findings. The classic appearance of increased wall mass is observed in most cases, with particular clues including the following 26, 27:
-
•
Increased wall mass for both the left and right ventricles in a concentric/diffuse pattern
-
•
Thickening of the interatrial septum and/or valves
-
•
Presence of a pericardial effusion (most often small/not hemodynamically significant)
An abnormal echotexture (echobright or “sparkling” appearance) has been frequently associated with amyloidosis, although it is not sensitive for the disease and its absence should not dissuade one from pursuing the diagnosis 26, 27. A characteristic pattern of abnormal longitudinal strain is commonly encountered in cardiac amyloidosis, in which there is relative sparing of strain abnormalities at the left ventricular apex compared with the base; this pattern has been referred to as a “cherry on top” pattern based on the bull’s-eye plot of global longitudinal strain 28, 29, 30, 31, 32. Some evidence also points to strain being useful as a marker of disease response to light chain therapy 31, 32. Importantly, the abnormal strain pattern described does not differentiate AL amyloidosis from other forms of cardiac amyloidosis 31, 32. Stroke volume index (stroke volume divided by body surface area) is similarly predictive of survival in AL amyloidosis compared with left ventricular strain and is a simple and straightforward value that can be obtained with echocardiography (33).
One of the most important clues to the diagnosis of amyloidosis can be the presence of a low “voltage:mass” ratio, comparing electrocardiographic voltages with ventricular mass as assessed by imaging (34). Much like is the case for echotexture, relying on the presence of low voltages to make a diagnosis (or being dissuaded from making a diagnosis because of the absence of low voltages) is a mistake. Many patients with cardiac amyloidosis do not have low voltages; this is particularly true for ATTR amyloidosis but can be true for many patients with AL amyloidosis as well. Normalizing the actual voltage to the “expected” voltage based on left ventricular mass raises the sensitivity and specificity of using voltage as a diagnostic tool; specifically, the presence of “normal” electrical voltages in the setting of ventricular “hypertrophy” on echocardiography should raise a red flag for infiltrative disorders (34).
Cardiac MRI is frequently used in patients with suspected infiltrative cardiomyopathies or more generally in patients with unclassified nonischemic cardiomyopathies. The use of cardiac MRI can be helpful in suggesting a diagnosis of cardiac amyloidosis, including both nonspecific signs (e.g., increased concentric biventricular wall mass) and more specific signs (e.g., diffuse nonischemic pattern of delayed gadolinium uptake and difficulty nulling the myocardium) 27, 35, 36. However, because cardiac MRI is unable to definitively differentiate between AL and ATTR amyloidosis (or to differentiate them from less common forms of systemic amyloidosis) 37, 38, it is almost always an intermediate step in the evaluation of patients. Therefore, although cardiac MRI can play an important role in raising suspicion, it is not the best diagnostic test to order if one is already concerned about evaluating for a diagnosis of cardiac amyloidosis specifically.
In recent years, bone scintigraphy (99mTechnetium pyrophosphate in the United States, 99mTechnetium 3,3-diphosphono-1,2-propanodicarboxylic acid in Europe) has been determined to be sensitive and specific for cardiac ATTR amyloid deposits, offering many patients a noninvasive option for establishing a diagnosis 39, 40, 41. Importantly, although specificity approaches 100% with this technique, this only holds true once a monoclonal protein has been ruled out. Bone scintigraphy is not specific enough for ATTR amyloidosis to exclude AL amyloidosis as a cause of tracer uptake if a monoclonal protein is present; in the largest study of bone scintigraphy, 27% of patients with AL amyloidosis proven by endomyocardial biopsy had positive (grade 2 or 3) technetium pyrophosphate scans (41). As such, bone scintigraphy should not be performed without first confirming normal results for SPIE, UPIE, and serum FLC; if these tests are abnormal, biopsy must be pursued to confirm a diagnosis. A basic diagnostic flowchart is displayed in the Central Illustration.
Prognosis
The nearly universal factor in early trials of amyloidosis was a dismal overall survival. In the first major clinical trial to demonstrate an effective light chain suppressive therapy strategy, patients treated with melphalan and prednisone had more than twice the survival of patients in the nonchemotherapy arm, but unfortunately this doubling was from only 8 to 18 months (42).
Despite AL amyloidosis being a disease that fundamentally arises as a result of a hematologic malignancy, the prognosis is primarily driven not by tumor characteristics but by extent of cardiac involvement. The first major attempt at a staging system was undertaken by Mayo Clinic and published in 2004 and was remarkable because the entire staging system was based solely on the presence/absence of elevations in cardiac biomarkers (troponin and N-terminal pro–B-type natriuretic peptide [NT-proBNP]) (8). This system produced 3 stages with separate survival curves, but perhaps the most notable aspect was the overall poor prognosis throughout the cohort, with median survivals of 2.2, 0.9, and 0.3 years, respectively, for Stage 1, 2, and 3 patients. The same group subsequently updated the staging system 8 years later, adjusting their biomarker thresholds (including increasing the threshold for NT-proBNP elevation from 332 to 1,800 pg/ml) and adding a third variable, whether or not the difference in serum free light chain at diagnosis (pathologic light chain concentration − nonpathologic light chain concentration) was ≥18 mg/dl (10). These 3 dichotomous variables now defined 4 stages, but the most notable finding was the significantly improved survival across most stages (7.8, 3.4, 1.2, and 0.5 years for Stages 1, 2, 3, and 4, respectively) (10). Almost certainly, the primary reason for the improved survival between the cohorts was the improvement in chemotherapy over the same time period.
A European collaborative examined 230 patients treated with a combination of cyclophosphamide, bortezomib, and dexamethasone, still one of the most commonly used up-front chemotherapy regimens, and stratified results according to the 2004 Mayo Clinic staging system (12). For this study, particularly high-risk patients were separated in Stage 3 as having Stage 3a or Stage 3b disease, the latter defined as those with NT-proBNP >8,500 ng/l. The cumulative probability of survival at 3 years was 100% in Stage I, 52% in Stage II, 55% in Stage 3a, and 19% in Stage 3b. Importantly, this study included patients between 2006 and 2013, before the availability of some of the most recent light chain suppressive therapies (12).
A more recent staging system from Boston University was published in 2019 (11). The primary purpose of this system was to develop cutoffs for B-type natriuretic peptide (BNP) rather than NT-proBNP. In this system, the authors used a troponin I cutoff of 0.1 ng/ml and a BNP value of 81 pg/ml to divide patients into Stages 1, 2, and 3 and added a cohort of higher-risk Stage 3 patients (Stage 3b) as those with BNP >700 pg/ml. Most notably, the median overall survival has clearly continued to improve, with median survivals not reached in Stage 1 at 10 years (>80% survival), 9.4 years for Stage 2, 4.3 years for Stage 3, and 1 year for Stage 3b. Difference in free light chains was no longer used as a prognostic factor in the Boston University system, possibly reflecting the improved ability to control abnormal light chain production in the present era.
Therefore, the main message from staging systems is as follows:
-
1.
The severity of cardiac involvement remains the primary prognostic factor in AL amyloidosis.
-
2.
Prognosis can be well stratified by 2 cardiac biomarkers: cardiac-specific troponin and NT-proBNP (or BNP).
-
3.
Prognosis has markedly improved over the last 2 decades, with patients in all but the highest-risk groups having a median survival of >4 years, with some groups having a median survival of >10 years. Because all longitudinal survival studies include patients who began treatment before the advent of more modern light chain suppressive therapies, it is likely that the true life expectancy of a newly diagnosed patient is even higher than is reflected in even the most recent prognostic algorithms.
Treatment
Light chain suppressive therapy
Light chain amyloidosis is unique among malignancies in that the primary marker of disease (abnormal serum light chains) reflects the actual pathogenic driver of disease progression. Therefore, the primary goal of therapy in AL amyloidosis is to decrease the pathologic light chain levels as much as possible and to do so with the least treatment toxicity possible.
The first regimen demonstrated to improve outcomes in AL amyloidosis was a combination of an alkylator (melphalan) with a steroid (prednisone) 42, 43. Although this regimen resulted in more than a doubling in the median survival in the largest trial, the overall outcomes were still dismal, with a median survival of only 18 months (42). Modestly improved outcomes were subsequently seen combining melphalan with high-dose dexamethasone rather than prednisone 44, 45. Because of the poor outcomes with standard chemotherapy, high-dose melphalan followed by autologous stem cell transplantation (ASCT) was investigated as a treatment alternative. One study of 312 patients who received ASCT demonstrated a median survival of 4.6 years, with clear stratification of survival based on whether or not cardiac involvement was present (6.4 vs. 1.6 years); individuals who did not receive ASCT due to exclusion criteria (typically severe cardiac involvement) had a particularly poor median survival of 4 months (46). Two studies with long-term follow-up data of ASCT patients reported that up to 30% of patients are long-term survivors, with up to a 15- to 20-year follow-up 47, 48.
Notably, the previously mentioned data (and other studies of ASCT) were nonrandomized, raising the potential that patients who received ASCT were overall healthier than those who did not. In addition, even at large referral centers that routinely perform ASCT in eligible patients, ASCT is not an option for most patients due to tight selection criteria; in 1 study, only 20% of patients evaluated within 1 year of diagnosis ended up receiving ASCT (49). One case-control study reported 1-, 2-, and 4-year survival rates of 89%, 81%, and 71%, respectively, for ASCT-treated patients versus 71%, 55%, and 41%, respectively, for patients who did not undergo ASCT (50). However, although “control” patients in the study were chosen to match specific clinical/laboratory variables, it is impossible to account for all clinical factors that can potentially affect the decision of both provider/patient to offer/undergo ASCT, raising the possibility that these results remain significantly confounded.
Only 1 randomized trial has addressed the question of standard chemotherapy versus ASCT, randomizing 100 patients in France to standard chemotherapy (melphalan and dexamethasone) or high-dose melphalan with ASCT. This study showed significantly better overall survival in the standard chemotherapy arm compared with the ASCT arm (51). Outcomes for low-risk patients (without significant cardiac disease) and high-risk patients (with significant cardiac disease) both trended toward benefit with standard chemotherapy. Although the trial has been critiqued because of relatively high ASCT-related mortality, a prespecified landmark analysis (only considering patients who received their treatment and lived at least 6 months from randomization) also trended toward benefit of standard chemotherapy over ASCT (51).
Therefore, based on the available data to date, there is conflicting evidence regarding the role of ASCT for the treatment of amyloidosis. On one hand, there are multiple retrospective case-control studies and case series suggesting potential superiority for ASCT over standard chemotherapy. On the other hand, the only randomized trial showed superiority for standard chemotherapy over ASCT. Furthermore, because the trial was published in 2007, the “standard chemotherapy” arm did not include any newer chemotherapy/immunotherapy regimens, raising the potential that the “standard therapy” arm would perform even better versus ASCT if the trial was repeated in the present era. As such, based on current data, both approaches are reasonable, and we would recommend an approach of shared, informed decision making between the patient and the treatment team.
The modern era offers many newer options for light chain suppressive therapies (Table 5). Although these therapies were developed and approved for the treatment of myeloma, they are unsurprisingly useful for treating AL amyloidosis as well, given that both conditions arise from a clonal plasma cell disorder. Most commonly, patients with a new diagnosis of AL amyloidosis are begun on a regimen combining the alkylator cyclophosphamide, the proteasome inhibitor bortezomib, and the steroid dexamethasone (CyBorD). One European study of 230 patients treated with frontline CyBorD yielded overall hematologic response rates of 60%, including 43% achieving at least very good partial responses (12). Multiple smaller studies showed even higher hematologic response rates, up to 81% to 94% 52, 53. Other proteasome inhibitors such as carfilzomib (54) and the oral agent ixazomib (55) have also shown promise, although both have their own treatment toxicities and are not typically chosen as first-line therapies.
Table 5.
Common Chemotherapy/Immunotherapy Agents in AL Amyloidosis
| Examples | First Line? | Notable Toxicities | |
|---|---|---|---|
| Steroids | Dexamethasone, prednisone | Yes | Hyperglycemia, neuropsychiatric effects, edema, immunosuppression |
| Alkylators | Melphalan, cyclophosphamide | Commonly | Myelosuppression, stomatitis |
| Proteasome inhibitors | Bortezomib, carfilzomib, ixazomib | Commonly | Neuropathy, thrombocytopenia, shingles reactivation, thrombosis/hypertension/cardiotoxicity (carfilzomib) |
| Immunomodulators | Lenalidomide, pomalidomide | Occasionally | Myelosuppression, rash, neuropathy, thrombosis, birth defects |
| Anti-CD38 Antibody | Daratumumab | No (ANDROMEDA trial investigating) (66) | Infusion reaction, hypogammaglobulinemia |
| Anti-SLAMF7 antibody | Elotuzumab | No | Infusion reaction, hypogammaglobulinemia |
Immunomodulators structurally related to thalidomide represent another commonly used group of agents used in AL amyloidosis. Lenalidomide has been used most commonly 56, 57, 58, 59, 60, 61, but in recent years pomalidomide has also served as a useful agent (62), particularly in patients refractory to lenalidomide. Other agents, including venetoclax (63) and elotuzumab (64), have been tried in some patients as second- or third-line therapy, often in conjunction with other standard light chain suppressive therapies.
Perhaps the most promising agent from recent years is daratumumab, an antibody directed against CD38, a cell surface protein expressed on clonal plasma cells. In 1 study of 25 consecutive patients refractory to multiple other lines of treatment (median of 3 prior therapies), every patient had a decrease in pathologic light chains, with an overall hematologic response rate of 76% (65). Daratumumab as an up-front therapy (added to CyBorD) is currently under investigation in the phase 3 ANDROMEDA clinical trial (66).
The most notable toxicities of the commonly used chemotherapy/immunotherapy agents are summarized in Table 5. Of all of the agents, the greatest concern for cardiovascular toxicity is with the proteasome inhibitor carfilzomib, based mainly on clinical trials in myeloma patients 67, 68, 69, 70, 71. Although increased heart failure adverse events have been consistently reported with carfilzomib in myeloma trials 68, 69, 70, 71, left ventricular systolic dysfunction is not commonly seen, with virtually no left ventricular ejection fraction declines observed in either of the 2 largest studies that used routine echocardiographic screening 68, 71. The clearest cardiovascular safety signals, at least in myeloma trials, have been increased rates of thrombosis, hypertension, and heart failure with preserved ejection fraction 68, 69, 70, 71. Therefore, differentiating whether the cause of worsening heart failure symptoms in AL amyloidosis patients treated with carfilzomib is from the disease or disease therapy can be challenging. Other proteasome inhibitors are not clearly associated with direct cardiovascular toxicity; a meta-analysis of 5,718 patients treated with bortezomib in myeloma trials showed no increased cardiovascular toxicity compared with controls (72).
Other important potential cardiovascular toxicities from AL amyloidosis therapies include steroid-related fluid retention, and increased rates of thrombosis in patients treated with thalidomide analogs such as lenalidomide and pomalidomide (particularly in patients with myeloma) 73, 74, 75. Two early studies of lenalidomide in AL amyloidosis raised the question of a correlation between lenalidomide therapy and elevations in BNP/NT-proBNP levels, although it was difficult to differentiate if this was a direct treatment effect or represented disease progression during early therapy; notably, neither study demonstrated a clear increase in clinical heart failure with lenalidomide therapy 76, 77.
Importantly, in virtually all studies, overall survival is tightly correlated with the degree of hematologic response, emphasizing the importance of moving to a different line of therapy expeditiously if a current treatment regimen is not adequately effective in reducing light chain production.
Other pharmacological approaches
All therapies described previously aim to eliminate the clonal cells that produce pathologic light chains. This is expected to reduce or eliminate ongoing amyloid deposition but will not directly lead to the removal of amyloid deposits; as such, significant interest has therefore existed for alternative pharmacological approaches.
One approach targeted serum amyloid P (SAP, a component of all amyloid fibril deposits) 78, 79. In a phase 1 trial that administered both an agent to deplete circulating SAP and a monoclonal antibody targeting SAP, there was modest evidence of decreased amyloid deposits as assessed by SAP scintigraphy and MRI (80). Although 8 of the 16 patients included in this trial had AL amyloidosis, it is notable that patients with cardiac involvement were excluded for safety reasons (80). The same group later included 6 cardiac patients in a follow-up study (81), but clear efficacy data for this approach in cardiac amyloidosis have yet to be demonstrated. A phase 2 trial of an anti-SAP antibody specifically focusing on cardiac amyloidosis was recently terminated for a “change in benefit/risk profile” (82).
Another approach involved an antibody (NEOD001) aimed at the amyloid moiety itself, with the potential to both bind to soluble aggregates of amyloid protein and to aid in the clearance of amyloid deposits in tissues (83). However, despite promising results in a phase 1/2 study (84), a phase 3 study failed to meet its endpoints, prompting a review of a second phase 3 study, which was subsequently also halted for futility (85).
Other approaches have included the administration of agents that can potentially interfere with amyloid fibril formation, including doxycycline or the combination of doxycycline/ursodiol, for both AL and other systemic amyloidosis. Although some early studies have been encouraging (86), rigorous clinical trial data to date are lacking (87).
Cardiac-Specific Treatments
Although light chain reduction therapy is the backbone to AL amyloidosis treatment, treating organ dysfunction in parallel is of great importance, particularly cardiac dysfunction, given that cardiac involvement is the primary driver of mortality in AL amyloidosis. Therapies can be grouped into medical therapy, arrhythmia management, and cardiac transplantation.
Medical therapy
Although no high-grade evidence exists to guide medical therapy in cardiac amyloidosis, generally accepted treatment principles are widely acknowledged (20). The mainstay of symptomatic therapy is volume management. Patients with systemic AL amyloidosis can be particularly prone to the challenging combination of hypotension with concomitant volume overload for the following reasons:
-
1.The presence of significant cardiac impairment due to:
-
a.Poor ventricular compliance/diastolic dysfunction
-
b.Reduced end-diastolic volume due to increased wall thickness
-
a.
-
2.
Coexisting renal (common) or hepatic (less common) amyloid deposition leading to reduced serum albumin concentrations, which can be severe
-
3.
Concomitant autonomic dysfunction from amyloid deposition, leading to postural hypotension
Salt restriction and appropriate diuretic doses are essential for most patients, often best achieved by combining loop diuretics and aldosterone antagonists as allowed by serum potassium levels; in some cases, thiazide diuretics are necessary for sequential nephron channel blockade. If symptomatic hypotension limits the ability to achieve euvolemia, midodrine can be useful (particularly for patients with autonomic dysfunction); in select patients with severe nephrotic syndrome, intermittent albumin infusions may be necessary.
Standard systolic heart failure therapies are often poorly tolerated in patients with AL amyloidosis with cardiac involvement (20). Both β-blockers and vasodilators (e.g., angiotensin-converting enzyme inhibitors and angiotensin receptor blockers), in particular, can exacerbate the hypotension that is usually present for the reasons noted previously. Beta-blockers can also worsen bradycardia/heart block and blunt the compensatory tachycardic response to low stroke volume and/or vasodilatation from autonomic dysfunction. Digoxin has traditionally been considered contraindicated in cardiac amyloidosis because of the potential for tissue toxicity (from direct binding of digoxin to amyloid fibrils), although recent studies suggest that digoxin can be cautiously used in appropriate patients, particularly when needed for rate control of atrial fibrillation (88).
Arrhythmia management
Atrial arrhythmias are common in patients with amyloidosis 89, 90, 91 and, because of the degree of baseline cardiac impairment, can be poorly tolerated. Anticoagulation is critical because patients with cardiac amyloidosis are at particularly high risk for thrombus formation. In 1 recent study of patients with cardiac amyloidosis undergoing planned transesophageal echocardiogram/cardioversion (50% of whom had AL amyloidosis), 28% had thrombus identified on echocardiogram compared with 2.5% of control patients without amyloidosis (92). For this reason, we and others recommend transesophageal echocardiography before elective cardioversion in all patients with amyloidosis, even if they have been on consistent therapeutic anticoagulation (92).
Bradyarrhythmias (particularly heart block) are also common in cardiac amyloidosis, although they are overall more common in ATTR than AL amyloidosis. Symptomatic bradycardia is an indication for pacemaker placement as in any form of heart disease; given the high rates of bradyarrhythmias in amyloidosis patients with syncope or presyncope, a low threshold should be considered for device placement in such patients (91).
Traditionally, implantable cardioverter-defibrillator (ICD) placement was considered contraindicated in patients with AL amyloidosis. The generally stated reasons included the poor overall prognosis in AL amyloidosis with cardiac involvement and a purported lack of success in resuscitating AL amyloidosis patients with ventricular arrhythmias with ICD shocks. In addition, many episodes of sudden death in AL amyloidosis can be caused by bradyarrhythmias or pulseless electrical activity rather than ventricular tachyarrhythmias (91). In 1 older study of patients who received ICDs before ASCT, 2 of 19 patients received successful/appropriate ICD shocks, with 1 patient still alive 30 months after receiving multiple therapies for ventricular tachycardia/fibrillation (93). Another study from a similar time frame (before the availability of newer AL suppressive therapies) found that 12 of 33 (36%) of AL amyloidosis patients received appropriate ICD shocks in the first year, but there was no overall survival advantage noted (94). It is notable that many patients in this cohort met “traditional” ICD indications (left ventricular ejection fraction <35%), implying later stages of disease, and the studies may have been underpowered to detect an overall survival difference. Two other studies from a later time frame (after approval of more modern light chain suppressive therapies), each with 12 patients with AL amyloidosis, showed high rates of successful resuscitation with appropriate ICD shocks for ventricular arrhythmias 95, 96.
Although high-quality evidence does not currently exist for guiding decisions of whether or not to place ICDs in AL amyloidosis patients, the preponderance of the data support the effectiveness of ICDs in successfully resuscitating AL amyloidosis patients with ventricular arrhythmias in most cases. Because current guidelines favor primary prevention ICD placement in high-risk heart failure patients with a life expectancy >1 year (97), we generally favor ICD placement in patients with AL amyloidosis who meet this life expectancy threshold and who either have a history highly suspicious for arrhythmia-associated syncope or who have evidence of ventricular arrhythmias on ambulatory telemetry (96).
Cardiac transplantation
Early reports of cardiac transplantation for amyloidosis were overall discouraging. Of 24 transplants for amyloidosis in the United Kingdom from 1982 to 2002, 17 were for AL amyloidosis. The median survival in the whole cohort was only 29 months, and the 1-year survival for AL amyloidosis patients was an unacceptably low 59% (98). A subsequent report out of the United States covering a similar time period (1987 to 2002) of 69 patients with amyloidosis showed slightly better but still discouraging results; the 1-year survival rate was 75% with a 5-year survival of 54%, both statistically worse than for nonamyloidosis transplant patients (99). Notably, the United States does not differentiate between ATTR and AL amyloidosis in its national transplant registry. Men (overrepresented among ATTR amyloidosis patients) survived longer than women in this cohort, suggesting that results may have been worse if restricted to AL amyloidosis patients (99).
The Mayo Clinic subsequently published its experience transplanting 11 AL amyloidosis patients over 11 years, emphasizing strict screening to exclude clinically significant extracardiac amyloid deposition and following up each heart transplant with an ASCT (100). The median survival in this cohort was 76 months—still less than nonamyloidosis transplants but improved compared with prior cohorts. A subsequent report from the same institution examined 23 patients over 20 years, with a median post-transplant survival of only 3.5 years; however, for those patients who achieved a complete hematologic response, the median survival was 10.8 years (101). Our experience at the Stanford Amyloid Center transplanting patients in a later time frame (after the availability of more effective light chain suppressive therapies) has been further encouraging. A report in 2015 of 19 amyloidosis patients (9 with AL amyloidosis) noted a similar overall survival to the overall transplant population; similar screening criteria to Mayo Clinic were used, but many patients were not treated with ASCT after heart transplantation (102). Our subsequent experience has continued to be positive, and our institution now does not routinely perform ASCT after heart transplantation, although we do emphasize pre-transplant and early post-transplant light chain suppressive therapy with chemotherapy and/or immunotherapy.
U.S. national data are also reflective of a large increase in heart transplantations for cardiac amyloidosis, with improved overall outcomes. Using 2008 as a landmark year based on the approval of newer light chain suppressive therapies, 1 study examined all heart transplants in the United States in era 1 (1987 to 2007) and era 2 (2008 to 2013). Transplantations for amyloidosis increased from 0.3% of all transplants in era 1 to 1.2% in era 2. Although transplant survival was worse in amyloidosis patients compared with all other restrictive cardiomyopathy patients in era 1, outcomes were similar to one another in era 2, likely reflecting better light chain suppressive therapy and a higher proportion of transplants for ATTR amyloidosis (103).
Importantly, AL amyloidosis patients needing a heart transplant have a much higher transplant waiting list mortality than other patients, with 35% dying while on the wait list in 1 study (104). As an acknowledgment of the high transplant waiting list mortality, the 2018 revision to the U.S. heart transplant policy listed amyloidosis as a higher status than most other diagnoses (status 4) (105).
Summary
AL amyloidosis most commonly arises from a clonal plasma cell disorder, with deposition of pathologic light chains usually occurring in multiple vital organs. Cardiac involvement is common, and is the primary determinant of prognosis in the disease. Optimal care for patients involves close coordination between hematologists, cardiologists, and other subspecialists; patients should ideally be evaluated in a specialty center for the disease soon after a diagnosis is considered or has been made.
Although the disease remains life-threatening, advances in chemotherapy and immunotherapy have resulted in dramatically improved outcomes across all patient subgroups, although patients with severe cardiac involvement at diagnosis continue to have an overall poor prognosis. As such, early diagnosis and prompt initiation of effective light chain suppressive therapy as soon as a diagnosis is made is critical. With improved prognosis, old paradigms of cardiac-specific therapy are being reconsidered, including the role of ICDs and cardiac transplantation for select patients.
Footnotes
Dr. Liedtke has served on advisory committees for Amgen/Onyx, Takeda, Pfizer, Caelum, Prothena, Gilead, Adaptive, and IQVIA/Jazz Pharmaceuticals; and has received research funding from Amgen/Onyx, Takeda, Pfizer, Celgene, Prothena, Genentech/Roche, Gilead, Janssen, Agios, and Celator. Dr. Witteles has received research funding from and served on advisory committees for Pfizer, Alnylam, and Eidos; and has been an adjudicator for cardiac events for trials by Genentech/Roche. Matthew Maurer, MD, served as Guest Editor for this paper. Anju Nohria, MD, served as Guest Editor-in-Chief for this paper.
References
- 1.Gilstrap L.G., Dominici F., Wang Y. Epidemiology of cardiac amyloidosis-associated heart failure hospitalizations among fee-for-service Medicare beneficiaries in the United States. Circ Heart Fail. 2019;12 doi: 10.1161/CIRCHEARTFAILURE.118.005407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gertz M.A. Immunoglobulin light chain amyloidosis: 2016 update on diagnosis, prognosis, and treatment. Am J Hematol. 2016;91:947–956. doi: 10.1002/ajh.24433. [DOI] [PubMed] [Google Scholar]
- 3.Wechalekar A.D., Gillmore J.D., Hawkins P.N. Systemic amyloidosis. Lancet. 2016;387:2641–2654. doi: 10.1016/S0140-6736(15)01274-X. [DOI] [PubMed] [Google Scholar]
- 4.Brenner D.A., Jain M., Pimentel D.R. Human amyloidogenic light chains directly impair cardiomyocyte function through an increase in cellular oxidant stress. Circ Res. 2004;94:1008–1010. doi: 10.1161/01.RES.0000126569.75419.74. [DOI] [PubMed] [Google Scholar]
- 5.Liao R., Jain M., Teller P. Infusion of light chains from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse hearts. Circulation. 2001;104:1594–1597. [PubMed] [Google Scholar]
- 6.Shi J., Guan J., Jiang B. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. 2010;107:4188–4193. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falk R.H., Alexander K.M., Liao R., Dorbala S. AL (light-chain) cardiac amyloidosis: a review of diagnosis and therapy. J Am Coll Cardiol. 2016;68:1323–1341. doi: 10.1016/j.jacc.2016.06.053. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A., Gertz M.A., Kyle R.A. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: a staging system for primary systemic amyloidosis. J Clin Oncol. 2004;22:3751–3757. doi: 10.1200/JCO.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 9.Gillmore J.D., Damy T., Fontana M. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 10.Kumar S., Dispenzieri A., Lacy M.Q. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012;30:989–995. doi: 10.1200/JCO.2011.38.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lilleness B., Ruberg F.L., Mussinelli R., Doros G., Sanchorawala V. Development and validation of a survival staging system incorporating BNP in patients with light chain amyloidosis. Blood. 2019;133:215–223. doi: 10.1182/blood-2018-06-858951. [DOI] [PubMed] [Google Scholar]
- 12.Palladini G., Sachchithanantham S., Milani P. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126:612–615. doi: 10.1182/blood-2015-01-620302. [DOI] [PubMed] [Google Scholar]
- 13.Kourelis T.V., Kyle R.A., Dingli D. Presentation and outcomes of localized immunoglobulin light chain amyloidosis: the Mayo Clinic experience. Mayo Clin Proc. 2017;92:908–917. doi: 10.1016/j.mayocp.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Mahmood S., Bridoux F., Venner C.P. Natural history and outcomes in localised immunoglobulin light-chain amyloidosis: a long-term observational study. Lancet Haematol. 2015;2:e241–e250. doi: 10.1016/S2352-3026(15)00068-X. [DOI] [PubMed] [Google Scholar]
- 15.Landgren O., Gridley G., Turesson I. Risk of monoclonal gammopathy of undetermined significance (MGUS) and subsequent multiple myeloma among African American and white veterans in the United States. Blood. 2006;107:904–906. doi: 10.1182/blood-2005-08-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Go R.S., Rajkumar S.V. How I manage monoclonal gammopathy of undetermined significance. Blood. 2018;131:163–173. doi: 10.1182/blood-2017-09-807560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rajkumar S.V., Dimopoulos M.A., Palumbo A. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15:e538–e548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 18.Morgan G.J., Kelly J.W. The kinetic stability of a full-length antibody light chain dimer cetermines whether endoproteolysis can release amyloidogenic variable domains. J Mol Biol. 2016;428:4280–4297. doi: 10.1016/j.jmb.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leung N., Nasr S.H., Sethi S. How I treat amyloidosis: the importance of accurate diagnosis and amyloid typing. Blood. 2012;120:3206–3213. doi: 10.1182/blood-2012-03-413682. [DOI] [PubMed] [Google Scholar]
- 20.Palladini G., Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128:159–168. doi: 10.1182/blood-2016-01-629790. [DOI] [PubMed] [Google Scholar]
- 21.Palladini G., Russo P., Bosoni T. Identification of amyloidogenic light chains requires the combination of serum-free light chain assay with immunofixation of serum and urine. Clin Chem. 2009;55:499–504. doi: 10.1373/clinchem.2008.117143. [DOI] [PubMed] [Google Scholar]
- 22.Garcia Y., Collins A.B., Stone J.R. Abdominal fat pad excisional biopsy for the diagnosis and typing of systemic amyloidosis. Hum Pathol. 2018;72:71–79. doi: 10.1016/j.humpath.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Quarta C.C., Gonzalez-Lopez E., Gilbertson J.A. Diagnostic sensitivity of abdominal fat aspiration in cardiac amyloidosis. Eur Heart J. 2017;38:1905–1908. doi: 10.1093/eurheartj/ehx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fine N.M., Arruda-Olson A.M., Dispenzieri A. Yield of noncardiac biopsy for the diagnosis of transthyretin cardiac amyloidosis. Am J Cardiol. 2014;113:1723–1727. doi: 10.1016/j.amjcard.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 25.Gilbertson J.A., Theis J.D., Vrana J.A. A comparison of immunohistochemistry and mass spectrometry for determining the amyloid fibril protein from formalin-fixed biopsy tissue. J Clin Pathol. 2015;68:314–317. doi: 10.1136/jclinpath-2014-202722. [DOI] [PubMed] [Google Scholar]
- 26.Di Nunzio D., Recupero A., de Gregorio C., Zito C., Carerj S., Di Bella G. Echocardiographic findings in cardiac amyloidosis: inside two-dimensional, Doppler, and strain imaging. Curr Cardiol Rep. 2019;21:7. doi: 10.1007/s11886-019-1094-z. [DOI] [PubMed] [Google Scholar]
- 27.Tuzovic M., Yang E.H., Baas A.S. Cardiac amyloidosis: diagnosis and treatment strategies. Curr Oncol Rep. 2017;19:46. doi: 10.1007/s11912-017-0607-4. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.P., Park J.B., Kim H.K., Kim Y.J., Grogan M., Sohn D.W. Contemporary imaging diagnosis of cardiac amyloidosis. J Cardiovasc Imaging. 2019;27:1–10. doi: 10.4250/jcvi.2019.27.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu D., Hu K., Niemann M. Effect of combined systolic and diastolic functional parameter assessment for differentiation of cardiac amyloidosis from other causes of concentric left ventricular hypertrophy. Circ Cardiovasc Imaging. 2013;6:1066–1072. doi: 10.1161/CIRCIMAGING.113.000683. [DOI] [PubMed] [Google Scholar]
- 30.Phelan D., Collier P., Thavendiranathan P. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012;98:1442–1448. doi: 10.1136/heartjnl-2012-302353. [DOI] [PubMed] [Google Scholar]
- 31.Salinaro F., Meier-Ewert H.K., Miller E.J. Longitudinal systolic strain, cardiac function improvement, and survival following treatment of light-chain (AL) cardiac amyloidosis. Eur Heart J Cardiovasc Imaging. 2017;18:1057–1064. doi: 10.1093/ehjci/jew298. [DOI] [PubMed] [Google Scholar]
- 32.Tuzovic M., Kobayashi Y., Wheeler M. Functional cardiac recovery and hematologic response to chemotherapy in patients with light-chain amyloidosis (from the Stanford University Amyloidosis Registry) Am J Cardiol. 2017;120:1381–1386. doi: 10.1016/j.amjcard.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 33.Milani P., Dispenzieri A., Scott C.G. Independent prognostic value of stroke volume index in patients with immunoglobulin light chain amyloidosis. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sperry B.W., Vranian M.N., Hachamovitch R. Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int J Cardiol. 2016;214:477–481. doi: 10.1016/j.ijcard.2016.04.030. [DOI] [PubMed] [Google Scholar]
- 35.Boynton S.J., Geske J.B., Dispenzieri A. LGE provides incremental prognostic information over serum biomarkers in AL cardiac amyloidosis. J Am Coll Cardiol Img. 2016;9:680–686. doi: 10.1016/j.jcmg.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Syed I.S., Glockner J.F., Feng D. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. J Am Coll Cardiol Img. 2010;3:155–164. doi: 10.1016/j.jcmg.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 37.Dungu J.N., Valencia O., Pinney J.H. CMR-based differentiation of AL and ATTR cardiac amyloidosis. J Am Coll Cardiol Img. 2014;7:133–142. doi: 10.1016/j.jcmg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Fontana M., Pica S., Reant P. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–1579. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bokhari S., Castano A., Pozniakoff T., Deslisle S., Latif F., Maurer M.S. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013;6:195–201. doi: 10.1161/CIRCIMAGING.112.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castano A., Haq M., Narotsky D.L. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880–889. doi: 10.1001/jamacardio.2016.2839. [DOI] [PubMed] [Google Scholar]
- 41.Gillmore J.D., Maurer M.S., Falk R.H. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133:2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 42.Kyle R.A., Gertz M.A., Greipp P.R. A trial of three regimens for primary amyloidosis: colchicine alone, melphalan and prednisone, and melphalan, prednisone, and colchicine. N Engl J Med. 1997;336:1202–1207. doi: 10.1056/NEJM199704243361702. [DOI] [PubMed] [Google Scholar]
- 43.Skinner M., Anderson J., Simms R. Treatment of 100 patients with primary amyloidosis: a randomized trial of melphalan, prednisone, and colchicine versus colchicine only. Am J Med. 1996;100:290–298. doi: 10.1016/s0002-9343(97)89487-9. [DOI] [PubMed] [Google Scholar]
- 44.Palladini G., Perfetti V., Obici L. Association of melphalan and high-dose dexamethasone is effective and well tolerated in patients with AL (primary) amyloidosis who are ineligible for stem cell transplantation. Blood. 2004;103:2936–2938. doi: 10.1182/blood-2003-08-2788. [DOI] [PubMed] [Google Scholar]
- 45.Palladini G., Milani P., Foli A. Oral melphalan and dexamethasone grants extended survival with minimal toxicity in AL amyloidosis: long-term results of a risk-adapted approach. Haematologica. 2014;99:743–750. doi: 10.3324/haematol.2013.095463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skinner M., Sanchorawala V., Seldin D.C. High-dose melphalan and autologous stem-cell transplantation in patients with AL amyloidosis: an 8-year study. Ann Intern Med. 2004;140:85–93. doi: 10.7326/0003-4819-140-2-200401200-00008. [DOI] [PubMed] [Google Scholar]
- 47.Sanchorawala V., Sun F., Quillen K., Sloan J.M., Berk J.L., Seldin D.C. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015;126:2345–2347. doi: 10.1182/blood-2015-08-662726. [DOI] [PubMed] [Google Scholar]
- 48.Sidana S., Sidiqi M.H., Dispenzieri A. Fifteen year overall survival rates after autologous stem cell transplantation for AL amyloidosis. Am J Hematol. 2019 Jun 28 doi: 10.1002/ajh.25566. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 49.Dispenzieri A., Seenithamby K., Lacy M.Q. Patients with immunoglobulin light chain amyloidosis undergoing autologous stem cell transplantation have superior outcomes compared with patients with multiple myeloma: a retrospective review from a tertiary referral center. Bone Marrow Transplant. 2013;48:1302–1307. doi: 10.1038/bmt.2013.53. [DOI] [PubMed] [Google Scholar]
- 50.Dispenzieri A., Kyle R.A., Lacy M.Q. Superior survival in primary systemic amyloidosis patients undergoing peripheral blood stem cell transplantation: a case-control study. Blood. 2004;103:3960–3963. doi: 10.1182/blood-2003-12-4192. [DOI] [PubMed] [Google Scholar]
- 51.Jaccard A., Moreau P., Leblond V. High-dose melphalan versus melphalan plus dexamethasone for AL amyloidosis. N Engl J Med. 2007;357:1083–1093. doi: 10.1056/NEJMoa070484. [DOI] [PubMed] [Google Scholar]
- 52.Venner C.P., Lane T., Foard D. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012;119:4387–4390. doi: 10.1182/blood-2011-10-388462. [DOI] [PubMed] [Google Scholar]
- 53.Mikhael J.R., Schuster S.R., Jimenez-Zepeda V.H. Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012;119:4391–4394. doi: 10.1182/blood-2011-11-390930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cohen A.D., Landau H., Scott E.C. Safety and efficacy of carfilzomib (CFZ) in previously-treated systemic light-chain (AL) amyloidosis. Blood. 2016;128:645. [Google Scholar]
- 55.Sanchorawala V., Palladini G., Kukreti V. A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017;130:597–605. doi: 10.1182/blood-2017-03-771220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dinner S., Witteles W., Afghahi A. Lenalidomide, melphalan and dexamethasone in a population of patients with immunoglobulin light chain amyloidosis with high rates of advanced cardiac involvement. Haematologica. 2013;98:1593–1599. doi: 10.3324/haematol.2013.084574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kumar S.K., Hayman S.R., Buadi F.K. Lenalidomide, cyclophosphamide, and dexamethasone (CRd) for light-chain amyloidosis: long-term results from a phase 2 trial. Blood. 2012;119:4860–4867. doi: 10.1182/blood-2012-01-407791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cibeira M.T., Oriol A., Lahuerta J.J. A phase II trial of lenalidomide, dexamethasone and cyclophosphamide for newly diagnosed patients with systemic immunoglobulin light chain amyloidosis. Br J Haematol. 2015;170:804–813. doi: 10.1111/bjh.13500. [DOI] [PubMed] [Google Scholar]
- 59.Palladini G., Russo P., Milani P. A phase II trial of cyclophosphamide, lenalidomide and dexamethasone in previously treated patients with AL amyloidosis. Haematologica. 2013;98:433–436. doi: 10.3324/haematol.2012.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dispenzieri A., Lacy M.Q., Zeldenrust S.R. The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007;109:465–470. doi: 10.1182/blood-2006-07-032987. [DOI] [PubMed] [Google Scholar]
- 61.Sanchorawala V., Wright D.G., Rosenzweig M. Lenalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 2 trial. Blood. 2007;109:492–496. doi: 10.1182/blood-2006-07-030544. [DOI] [PubMed] [Google Scholar]
- 62.Sanchorawala V., Shelton A.C., Lo S., Varga C., Sloan J.M., Seldin D.C. Pomalidomide and dexamethasone in the treatment of AL amyloidosis: results of a phase 1 and 2 trial. Blood. 2016;128:1059–1062. doi: 10.1182/blood-2016-04-710822. [DOI] [PubMed] [Google Scholar]
- 63.Vaxman I., Gertz M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019;141:93–106. doi: 10.1159/000495455. [DOI] [PubMed] [Google Scholar]
- 64.Iqbal S.M., Stecklein K., Sarow J., Krabak M., Hillengass J., McCarthy P. Elotuzumab in combination with lenalidomide and dexamethasone for treatment-resistant immunoglobulin light chain amyloidosis with multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19:e33–e36. doi: 10.1016/j.clml.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 65.Kaufman G.P., Schrier S.L., Lafayette R.A., Arai S., Witteles R.M., Liedtke M. Daratumumab yields rapid and deep hematologic responses in patients with heavily pretreated AL amyloidosis. Blood. 2017;130:900–902. doi: 10.1182/blood-2017-01-763599. [DOI] [PubMed] [Google Scholar]
- 66.A study to evaluate the efficacy and safety of daratumumab in combination with cyclophosphamide, bortezomib and dexamethasone (CyBorD) compared to CyBorD alone in newly diagnosed systemic amyloid light chain (AL) amyloidosis. https://clinicaltrials.gov/ct2/show/NCT03201965?term=daratumumab&cond=Amyloidosis&rank=4 Available at:
- 67.Cole D.C., Frishman W.H. Cardiovascular complications of proteasome inhibitors used in multiple myeloma. Cardiol Rev. 2018;26:122–129. doi: 10.1097/CRD.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 68.Dimopoulos M.A., Moreau P., Palumbo A. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 69.Siegel D., Martin T., Nooka A. Integrated safety profile of single-agent carfilzomib: experience from 526 patients enrolled in 4 phase II clinical studies. Haematologica. 2013;98:1753–1761. doi: 10.3324/haematol.2013.089334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stewart A.K., Rajkumar S.V., Dimopoulos M.A. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 71.Cornell R.F., Ky B., Weiss B.M. Prospective study of cardiac events during proteasome inhibitor therapy for relapsed multiple myeloma. J Clin Oncol. 2019;37:1946–1955. doi: 10.1200/JCO.19.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xiao Y., Yin J., Wei J., Shang Z. Incidence and risk of cardiotoxicity associated with bortezomib in the treatment of cancer: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0087671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carrier M., Le Gal G., Tay J., Wu C., Lee A.Y. Rates of venous thromboembolism in multiple myeloma patients undergoing immunomodulatory therapy with thalidomide or lenalidomide: a systematic review and meta-analysis. J Thromb Haemost. 2011;9:653–663. doi: 10.1111/j.1538-7836.2011.04215.x. [DOI] [PubMed] [Google Scholar]
- 74.Knight R., DeLap R.J., Zeldis J.B. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079–2080. doi: 10.1056/NEJMc053530. [DOI] [PubMed] [Google Scholar]
- 75.Moreau P., Dimopoulos M.A., Richardson P.G. Adverse event management in patients with relapsed and refractory multiple myeloma taking pomalidomide plus low-dose dexamethasone: a pooled analysis. Eur J Haematol. 2017;99:199–206. doi: 10.1111/ejh.12903. [DOI] [PubMed] [Google Scholar]
- 76.Dispenzieri A., Dingli D., Kumar S.K. Discordance between serum cardiac biomarker and immunoglobulin-free light-chain response in patients with immunoglobulin light-chain amyloidosis treated with immune modulatory drugs. Am J Hematol. 2010;85:757–759. doi: 10.1002/ajh.21822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tapan U., Seldin D.C., Finn K.T. Increases in B-type natriuretic peptide (BNP) during treatment with lenalidomide in AL amyloidosis. Blood. 2010;116:5071–5072. doi: 10.1182/blood-2010-09-305136. [DOI] [PubMed] [Google Scholar]
- 78.Gillmore J.D., Tennent G.A., Hutchinson W.L. Sustained pharmacological depletion of serum amyloid P component in patients with systemic amyloidosis. Br J Haematol. 2010;148:760–767. doi: 10.1111/j.1365-2141.2009.08036.x. [DOI] [PubMed] [Google Scholar]
- 79.Pepys M.B., Herbert J., Hutchinson W.L. Targeted pharmacological depletion of serum amyloid P component for treatment of human amyloidosis. Nature. 2002;417:254–259. doi: 10.1038/417254a. [DOI] [PubMed] [Google Scholar]
- 80.Richards D.B., Cookson L.M., Berges A.C. Therapeutic clearance of amyloid by antibodies to serum amyloid P component. N Engl J Med. 2015;373:1106–1114. doi: 10.1056/NEJMoa1504942. [DOI] [PubMed] [Google Scholar]
- 81.Richards D.B., Cookson L.M., Barton S.V. Repeat doses of antibody to serum amyloid P component clear amyloid deposits in patients with systemic amyloidosis. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aan3128. [DOI] [PubMed] [Google Scholar]
- 82.Multiple treatment session study to assess GSK2398852 administered following and along with GSK2315698. ClinicalTrials.gov identifier: NCT03044353. https://clinicaltrials.gov/ct2/show/NCT03044353 Available at:
- 83.Wall J.S., Kennel S.J., Williams A. AL amyloid imaging and therapy with a monoclonal antibody to a cryptic epitope on amyloid fibrils. PLoS One. 2012;7 doi: 10.1371/journal.pone.0052686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gertz M.A., Landau H., Comenzo R.L. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J Clin Oncol. 2016;34:1097–1103. doi: 10.1200/JCO.2015.63.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varga C., Lentzsch S., Comenzo R.L. Beyond NEOD001 for systemic light-chain amyloidosis. Blood. 2018;132:1992–1993. doi: 10.1182/blood-2018-07-865857. [DOI] [PubMed] [Google Scholar]
- 86.Wechalekar A.D., Whelan C. Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 2017;7:e546. doi: 10.1038/bcj.2017.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Witteles R.M. Doxycycline and ursodiol for ATTR amyloidosis: not ready for prime time. J Card Fail. 2019;25:154–155. doi: 10.1016/j.cardfail.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 88.Muchtar E., Gertz M.A., Kumar S.K. Digoxin use in systemic light-chain (AL) amyloidosis: contra-indicated or cautious use? Amyloid. 2018;25:86–92. doi: 10.1080/13506129.2018.1449744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Goldsmith Y.B., Liu J., Chou J., Hoffman J., Comenzo R.L., Steingart R.M. Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009;104:990–994. doi: 10.1016/j.amjcard.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 90.Murtagh B., Hammill S.C., Gertz M.A., Kyle R.A., Tajik A.J., Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95:535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 91.Sayed R.H., Rogers D., Khan F. A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015;36:1098–1105. doi: 10.1093/eurheartj/ehu506. [DOI] [PubMed] [Google Scholar]
- 92.El-Am E.A., Dispenzieri A., Melduni R.M. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. 2019;73:589–597. doi: 10.1016/j.jacc.2018.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kristen A.V., Dengler T.J., Hegenbart U. Prophylactic implantation of cardioverter-defibrillator in patients with severe cardiac amyloidosis and high risk for sudden cardiac death. Heart Rhythm. 2008;5:235–240. doi: 10.1016/j.hrthm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 94.Lin G., Dispenzieri A., Kyle R., Grogan M., Brady P.A. Implantable cardioverter defibrillators in patients with cardiac amyloidosis. J Cardiovasc Electrophysiol. 2013;24:793–798. doi: 10.1111/jce.12123. [DOI] [PubMed] [Google Scholar]
- 95.Hamon D., Algalarrondo V., Gandjbakhch E. Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016;222:562–568. doi: 10.1016/j.ijcard.2016.07.254. [DOI] [PubMed] [Google Scholar]
- 96.Varr B.C., Zarafshar S., Coakley T. Implantable cardioverter-defibrillator placement in patients with cardiac amyloidosis. Heart Rhythm. 2014;11:158–162. doi: 10.1016/j.hrthm.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 97.Russo A.M., Stainback R.F., Bailey S.R. CCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2013;61:1318–1368. doi: 10.1016/j.jacc.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 98.Dubrey S.W., Burke M.M., Hawkins P.N., Banner N.R. Cardiac transplantation for amyloid heart disease: the United Kingdom experience. J Heart Lung Transplant. 2004;23:1142–1153. doi: 10.1016/j.healun.2003.08.027. [DOI] [PubMed] [Google Scholar]
- 99.Kpodonu J., Massad M.G., Caines A., Geha A.S. Outcome of heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant. 2005;24:1763–1765. doi: 10.1016/j.healun.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 100.Lacy M.Q., Dispenzieri A., Hayman S.R. Autologous stem cell transplant after heart transplant for light chain (Al) amyloid cardiomyopathy. J Heart Lung Transplant. 2008;27:823–829. doi: 10.1016/j.healun.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 101.Grogan M., Gertz M., McCurdy A. Long term outcomes of cardiac transplant for immunoglobulin light chain amyloidosis: the Mayo Clinic experience. World J Transplant. 2016;6:380–388. doi: 10.5500/wjt.v6.i2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Davis M.K., Kale P., Liedtke M. Outcomes after heart transplantation for amyloid cardiomyopathy in the modern era. Am J Transplant. 2015;15:650–658. doi: 10.1111/ajt.13025. [DOI] [PubMed] [Google Scholar]
- 103.Davis M.K., Lee P.H., Witteles R.M. Changing outcomes after heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant. 2015;34:658–666. doi: 10.1016/j.healun.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Gray Gilstrap L., Niehaus E., Malhotra R. Predictors of survival to orthotopic heart transplant in patients with light chain amyloidosis. J Heart Lung Transplant. 2014;33:149–156. doi: 10.1016/j.healun.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Criteria requirements in adult heart allocation policy. https://optn.transplant.hrsa.gov/media/2413/adult_heart_criteria.pdf Available at: