Abstract
Objectives
The goal of this study was to compare the risk of cardiotoxicity with osimertinib versus all other drugs and versus epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (TKIs) (erlotinib, afatinib, and gefitinib) in the U.S. Food and Drug Administration Adverse Events Reporting System (FAERS), a pharmacovigilance database.
Background
Osimertinib has been shown to improve outcomes in T790M-positive non–small cell lung cancer patients who progress on EGFR-TKI therapy and in the frontline setting in EGFR mutated non–small cell lung cancer. In pivotal trials, osimertinib was associated with higher rates of cardiotoxicity compared with the control arm.
Methods
FAERS was queried for “Cardiac failure,” “Electrocardiogram QT-prolonged,” “Atrial Fibrillation (AF),” “Myocardial Infarction (MI),” and “Pericardial Effusion” secondary to “Osimertinib,” “Erlotinib,” “Afatinib,” “Gefitinib,” and all other drugs from 2016 to 2018. Disproportionality signal analysis was performed by calculating the reporting odds ratio (ROR) with its 95% confidence interval (CI). The ROR was considered significant when the lower limit of the 95% CI was >1.0.
Results
The ROR (95% CI) for cardiac failure, atrial fibrillation (AF), QT prolongation, myocardial infarction, and pericardial effusion due to osimertinib versus all other drugs in FAERS was 5.4 (4.2 to 7.1), 4.0 (2.8 to 5.8), 11.2 (7.9 to 15.8), 1.6 (0.9 to 2.6), and 8.2 (4.8 to 14), respectively. The ROR (95% CI) for cardiac failure, AF, QT prolongation, myocardial infarction, and pericardial effusion in comparing osimertinib versus other EGFR-TKIs was 2.2 (1.5 to 3.2), 2.1 (1.3 to 3.5), 6.6 (3.4 to 12.8), 1.2 (0.6 to 2.3), and 1.6 (0.8 to 3.3).
Conclusions
The RORs for cardiac failure, AF, and QT prolongation were higher due to osimertinib compared with other TKIs. Electrocardiographic monitoring for QT prolongation and monitoring for signs and symptoms of heart failure should be considered in patients taking osimertinib.
Key Words: cardiotoxicity, EGFR mutation, non–small cell lung cancer, osimertinib, QT prolongation
Abbreviations and Acronyms: AF, atrial fibrillation; CI, confidence interval; FAERS, U.S. Food and Drug Administration Adverse Events Reporting System; FDA, U.S. Food and Drug Administration; EGFR, epidermal growth factor receptor; LVEF, left ventricular ejection fraction; NSCLC, non–small cell lung cancer; ROR, reporting odds ratio; TKI, tyrosine kinase inhibitor
Central Illustration
Osimertinib is an oral, third-generation, irreversible, epidermal growth factor receptor–tyrosine kinase inhibitor (EGFR-TKI) that is selective for both EGFR-TKI–sensitizing and T790M-resistant mutations in patients with non-small cell lung cancer (NSCLC) (1). NSCLC harboring EGFR mutations accounts for 10% of the patients in the United States and 35% in Asia (2, 3, 4). Traditionally, EGFR mutations were believed to be more prevalent among nonsmokers and women, but they are also found in patients with NSCLC who are smokers and men (5). Current guidelines for molecular testing in lung cancer recommend testing for EGFR mutations in all advanced lung adenocarcinoma (NSCLC) patients to guide frontline therapy (6).
Osimertinib first gained U.S. Food and Drug Administration (FDA) approval in the United States in November 2015 for T790M-positive NSCLC patients who had progression with standard EGFR-TKIs (7). More recently, osimertinib showed improved outcomes in the frontline setting in patients with EGFR-mutated NSCLC compared with standard EGFR-TKIs, leading to osimertinib being the preferred TKI for EGFR-mutated NSCLC (8). In this pivotal study, although osimertinib was associated with fewer grade 3 or higher adverse events (AEs) compared with standard EGFR-TKIs (34% vs. 45%), osimertinib was associated with an increased risk of cardiotoxicity compared with the control arm. Specifically, grade 3 or higher QT prolongation was reportedly higher in the osimertinib arm. However, a meta-analysis of pivotal studies showed that osimertinib was associated with not only an increased risk of QT prolongation but also an increased risk of cardiac failure (9). Herein, we retrospectively reviewed the U.S. Food and Drug Administration Adverse Events Reporting System (FAERS), a pharmacovigilance database, for the incidence of cardiotoxicity due to osimertinib compared with other drugs approved by the FDA and also specifically versus other EGFR-TKIs.
Methods
This study is a retrospective analysis that used data queries from the FAERS pharmacovigilance monitoring database. FAERS is a public database that contains nearly 17 million AE reports, medication error reports, and product quality complaints reported by health care professionals, manufacturers, and consumers from around the world since 1968. These reports are managed by the FDA and evaluated by clinical reviewers in the Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research. Data in each event report, where applicable, include individual case identification numbers for reference, the suspected pharmaceutical, reason for use, adverse reactions, nature of the event (i.e., serious), outcomes (e.g., hospitalized, death, other outcomes), sex (male, female, or unknown), age, weight, event date, initial FDA receipt date, latest FDA receipt date, pharmaceutical company, reporter (e.g., health care professional, consumer, pharmaceutical company, unknown), concomitant medications, latest manufacturer received date, country where the event occurred, and manufacturer control number. Individual names and date of birth are excluded from these lists.
The present study involved data queries of the FAERS database between January 1, 2016, and September 30, 2018, for AEs secondary to EGFR-TKIs, namely “Osimertinib,” “Erlotinib,” “Afatinib,” and “Gefitinib.” Only EGFR-TKIs with >100 AEs in the database were chosen for analysis (dacomitinib was excluded). We chose January 1, 2016, as the start date for all TKIs to decrease bias, given that it represents a time period post–osimertinib FDA approval. Of note, other TKIs were FDA-approved before osimertinib. We queried for AEs classified by group queries according to the Medical Dictionary for Regulatory Activities that had ≥30 events. There were 6 cardiac-related AEs due to TKIs that met that criteria, namely: “Cardiac failure,” “Atrial Fibrillation,” “Electrocardiogram QT prolonged,” “Myocardial infarction,” “Pericardial Effusion,” and “Cardiac failure congestive” (Supplemental Table 1). Cardiac failure congestive and cardiac failure AEs were grouped together for analysis. FAERS was accessed on January 28, 2019.
To compare the risk of cardiotoxicity in osimertinib-treated cases versus reported events from other drugs in the database, a disproportionality analysis was conducted by using the reporting odds ratio (ROR) (Supplemental Table 2). ROR is a measure of the magnitude of association between an exposure to a pharmaceutical and the odds of a specific outcome occurring (10). ROR was considered significant when the lower limit of the 95% confidence interval (CI) was >1.0. The risk of cardiotoxicity due to osimertinib was compared with all other drugs and with other EGFR-TKIs (gefitinib, afatinib, and erlotinib) during the same time period; disproportionality signal analysis was used to calculate the ROR. The median time and interquartile range for the AE were calculated based on the start date of the TKI. AEs were classified as serious versus nonserious per definitions according to FAERS (Supplemental Methods).
Results
The total number of AEs in FAERS from all drugs was 5,138,230. Of those, 8,450 AEs were due to osimertinib, erlotinib, afatinib, or gefitinib. Of 8,450 AEs, 2,454 were secondary to osimertinib; 5,836 were due to other EGFR-TKIs (erlotinib, afatinib, or gefitinib); and 160 were due to the combination, in which osimertinib and any of the 3 other EGFR-TKIs were listed as a possible cause. A total of 315 cardiac AEs were noted, 150 (6.1%) due to osimertinib, 8 (5%) due to osimertinib combined with other TKIs, and 157 (2.7%) due to other TKIs (Table 1).
Table 1.
Adverse Events Due to EGFR-TKIs in FAERS From 2016 to 2018
| Total (N = 8,450) | Osimertinib (n = 2,454) | Osimertinib + Other TKIs (n = 160) | Other TKIs (n = 5,836) | |
|---|---|---|---|---|
| Cardiac failure | 120 (1.4) | 57 (2.3) | 1 (0.6) | 62 (1.1) |
| Atrial fibrillation | 64 (0.8) | 30 (1.2) | 1 (0.6) | 33 (0.6) |
| QT prolongation | 49 (0.6) | 33 (1.3) | 4 (2.5) | 12 (0.2) |
| Myocardial infarction | 46 (0.5) | 16 (0.7) | 0 (0.0) | 30 (0.5) |
| Pericardial effusion | 36 (0.4) | 14 (0.6) | 2 (1.3) | 20 (0.3) |
| Total | 315 (3.7) | 150 (6.1) | 8 (5.0) | 157 (2.7) |
Values are n (% of total in each treatment category).
EGFR-TKI = epidermal growth factor receptor–tyrosine kinase inhibitor; FAERS = U.S. Food and Drug Administration Adverse Events Reporting System.
As detailed in Table 2, cardiac failure was the most common AE caused by osimertinib, followed by QT prolongation. More than 90% of the reactions due to cardiac failure and QT prolongation were serious. In 19.3% of the cardiac failure cases, osimertinib and at least 1 other non-TKI drug were considered as leading to the AE. Similarly, in the case of QT prolongation, 33% of the cases had osimertinib and at least 1 other non-TKI drug was considered as leading to the AE. For the subset of patients for whom event timing was available, the median time to event was 29 days for cardiac failure and 23 days for QT prolongation.
Table 2.
Details of Cardiac Related AEs Due to Osimertinib
| Cardiac Failure | QT Prolongation | Atrial Fibrillation | Myocardial Infarction | Pericardial Effusion | |
|---|---|---|---|---|---|
| Total number of reported AEs | 57 | 33 | 30 | 16 | 14 |
| Sex | |||||
| Female | 45/56 (80.3) | 19/28 (67.9) | 20/29 (69) | 10/15 (66.6) | 6 (54.5) |
| Male | 11/56 (19.6) | 9/28 (27.3) | 9/29 (31) | 5/15 (33.3) | 5 (45.5) |
| Age, yrs (no. of patients for whom data was available) | (n = 46) | (n = 25) | (n = 23) | (n = 2) | (n = 7) |
| Range (minimum–maximum) | 77.5 (48–92) | 66 (41–85) | 75 (59–91) | 78.5 (50–91) | 64 (42–69) |
| Type of reaction | |||||
| Serious | 55 (96.5) | 31 (93.9) | 30 (100.0) | 16 (100.0) | 12 (85.7) |
| Nonserious | 2 (3.5) | 2 (6.1) | 0 (0.0) | 0 (0.0) | 2 (14.3) |
| Outcome | |||||
| Hospitalization | 29 (50.9) | 13 (39.3) | 16 (53.3) | 5 (31.2) | 6 (42.9) |
| Death | 17 (29.8) | 10 (30.3) | 9 (30.0) | 6 (37.5) | 5 (35.7) |
| Life-threatening | 2 (3.5) | 2 (6.1) | 1 (3.3) | 2 (12.5) | 1 (7.1) |
| Other outcomes | 6 (10.5) | 6 (18.2) | 4 (13.3) | 3 (18.7) | 0 (0.0) |
| Nonserious | 2 (3.5) | 2 (6.1) | 0 (0.0) | 0 (0.0) | 2 (14.3) |
| Disabled | 1 (1.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Suspected drugs | |||||
| Osimertinib | 46 (80.7) | 22 (66.6) | 24 (80.0) | 14 (87.5) | 13 (92.9) |
| Osimertinib + ≥1∗ | 11 (19.3) | 11 (33.3) | 6 (20.0) | 2 (12.5) | 1 (7.1) |
| Outcome counts by year received | |||||
| 2016 | 11 (19.3) | 5 (15.1) | 9 (30.0) | 6 (37.5) | 5 (35.7) |
| 2017 | 23 (40.3) | 12 (36.4) | 6 (20.0) | 5 (31.2) | 6 (42.9) |
| 2018 | 23 (40.3) | 16 (48.5) | 15 (50.0) | 5 (31.2) | 3 (21.4) |
| Reporter | |||||
| Health care professional | 51 (89.5) | 33 (100.0) | 28 (93.3) | 13 (81.2) | 11 (78.6) |
| Consumer | 6 (10.5) | 0 (0.0) | 2 (6.6) | 3 (18.7) | 3 (21.4) |
| Region of AE | |||||
| Asia | 28 (49.1) | 18 (54.6) | 13 (43.3) | 6 (37.5) | 4 (28.6) |
| Americas | 16 (28.1) | 10 (30.3) | 9 (30.0) | 6 (37.5) | 7 (50.0) |
| Europe | 1 (22.8) | 5 (15.1) | 8 (26.7) | 3 (18.7) | 2 (14.3) |
| Australia | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (6.2) | 1 (7.1) |
| Time to AE, days; number of patients for whom data were available | (n = 33) | (n = 19) | (n = 16) | (n = 8) | (n = 4) |
| Median (interquartile range) | 29 (16.5–95.5) | 23 (14.0–55.0) | 60 (12.5–362.0) | 26.5 (9.0–63.5) | 65 (11.0–182.0) |
Values are n, n/N (%), or n (%).
AE = adverse event.
Drugs other than epidermal growth factor receptor–tyrosine kinase inhibitors.
According to the disproportionality signal analysis, the ROR for osimertinib versus all other drugs in FAERS for cardiac failure was elevated at 5.4 (95% CI: 4.2 to 7.1); for atrial fibrillation, 4.0 (95% CI: 2.8 to 5.8); for QT prolongation, 11.2 (95% CI: 7.9 to 15.8); for pericardial effusion, 8.2 (95% CI: 4.8 to 14.0); and for myocardial infarction, not significantly different at 1.6 (95% CI: 0.9 to 2.6) (Figure 1, Supplemental Table 3). Among reactions with elevated RORs, the reported outcome of death was highest for pericardial effusion at 35.7%.
Figure 1.
Disproportionality Signal Analysis Calculated by Using the ROR
The reporting odds ratio (ROR) was calculated for osimertinib versus all drugs and osimertinib versus other tyrosine kinase inhibitors (TKIs). Compared with all other drugs in the U.S. Food and Drug Administration Adverse Events Reporting System database, osimertinib was associated with increased cardiac failure, atrial fibrillation, QT prolongation, and pericardial effusion. RORs for osimertinib versus other TKIs were elevated for cardiac failure, atrial fibrillation, and QT prolongation. CI = confidence interval.
The ROR comparing osimertinib versus other EGFR-TKIs for cardiac failure was elevated at 2.2 (95% CI: 1.5 to 3.2); for atrial fibrillation, 2.1 (95% CI: 1.3 to 3.5); for QT prolongation, 6.6 (95% CI: 3.4 to 12.8); for myocardial infarction, not significantly different at 1.2 (95% CI: 0.6 to 2.3); and for pericardial effusion, not significantly different at 1.6 (95% CI: 0.8 to 3.3) (Figure 1).
Discussion
In FAERS, of all the AEs reported with osimertinib, 6.1% were cardiac related. Osimertinib was found to have an increased risk of cardiac failure, atrial fibrillation, QT prolongation, and pericardial effusion compared with all other drugs in the FAERS (Central Illustration). The risk of myocardial infarction was not increased compared with other drugs in FAERS. The ROR was highest for QT prolongation followed by pericardial effusion, cardiac failure, and atrial fibrillation.
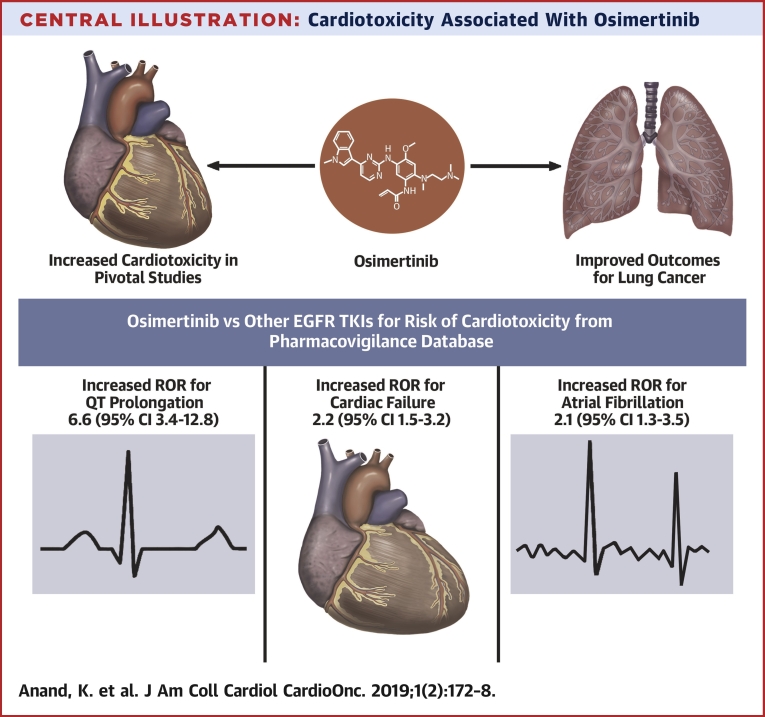
Central Illustration.
Cardiotoxicity Associated With Osimertinib
Osimertinib has improved outcomes for epidermal growth factor receptor (EGFR)-mutated lung cancer. In pivotal studies, osimertinib has been linked to an increased risk of QT prolongation and cardiac failure. We performed a retrospective study of the U.S. Food and Drug Administration Adverse Events Reporting System database and found that osimertinib increased the reporting odds ratio (ROR) for QT prolongation, cardiac failure, and atrial fibrillation compared with standard EGFR–tyrosine kinase inhibitors (TKIs) (erlotinib, gefitinib, or afatinib). CI = confidence interval.
In the comparison of osimertinib versus other EGFR-TKIs for risk of cardiotoxicity, there was an increased risk of cardiac failure, atrial fibrillation, and QT prolongation. Again, the ROR for QT prolongation versus other EGFR-TKIs was highest at 6.6. The RORs for myocardial infarction and pericardial effusion were not significantly increased.
Cardiovascular AEs are a well-known toxicity of TKIs used in chronic myeloid leukemia, such as with imatinib (first-generation BCR-ABL1 TKI), dasatinib (second-generation BCR-ABL1 TKI), and ponatinib (third-generation BCR-ABL1 TKI) (11). However, this has not been reported in clinical trials with first-generation anti–EGFR-TKIs (erlotinib or gefitinib) or second-generation anti–EGFR-TKIs (afatinib) (12, 13, 14, 15). The AURA3 trial (Randomized Phase III Study of Osimertinib vs Platinum-Pemetrexed for EGFR T790M-Positive Advanced NSCLC) compared osimertinib versus chemotherapy and determined that cardiotoxicity (defined by a decrease in left ventricular ejection fraction [LVEF] ≥10% and to an LVEF <50%) occurred in 5% of the osimertinib arm, with a median time of 5.5 months to onset of LVEF decline (7). The rate of QT prolongation was also high in the osimertinib arm in the AURA3 trial (3% vs. 0%). Similarly, in the FLAURA trial (Osimertinib in Untreated EGFR-Mutated Advanced Non–Small-Cell Lung Cancer), which compared osimertinib versus a first-generation EGFR-TKI (gefitinib or erlotinib) in the frontline setting, there was a higher rate of QT prolongation (10% vs. 4%) and LVEF decrease ≥10% to <50% with osimertinib (3% vs. 1%) (8). Osimertinib has also been reported to lead to severe cardiac dysfunction such as myocarditis. Oyakawa et al. (16) described a case of osimertinib-induced myocarditis in which even after 12 weeks of discontinuation of osimertinib, there was no improvement in LVEF.
The underlying mechanism of osimertinib-induced cardiotoxicity remains unclear. Osimertinib and its active metabolite AZ5104 not only inhibit EGFR but also inhibit HER2 (human epidermal growth factor receptor-2) in vitro (1). Given the known risk of cardiotoxicity with anti-HER2 agents such as trastuzumab (17), HER2 inhibition may be related to cardiotoxicity (18,19). EGFR signaling itself has been shown to be protective in the setting of catecholamine excess in a mouse model in which treatment with erlotinib enhanced myocardial injury induced by isoproterenol infusion (20).
Study limitations
This analysis was a retrospective study of reported events in FAERS, and as such, baseline cardiac characteristics of patients taking TKIs are not known. The time to event for all cases with AEs was not available, nor was the grade of toxicity. Moreover, the actual incidence of cardiotoxicity with osimertinib cannot be determined because it is possible that not all events are reported within FAERS. As such, there are similar limitations in the ROR estimate. AE reporting for a drug may be influenced by extent of use, publicity, and bias (21). Although the use of disproportionality analysis through pharmacovigilance databases to determine the increased risk of AEs secondary to a particular drug has been shown in various settings (21,22), it is critical that any hypotheses generated by using pharmacovigilance databases are validated through prospective studies.
The significance of the present study is that it informs the potential increased risk of cardiotoxicity with osimertinib, which is now frontline treatment for EGFR-mutated NSCLC. In pivotal studies, the increased risk of cardiac failure and QT prolongation has been reported; analysis of FAERS corroborates this finding and also indicates that osimertinib is associated with an increased risk of atrial fibrillation. NSCLC harboring EGFR mutations accounts for 10% of U.S. patients and 35% in Asia (2, 3, 4). As EGFR inhibitors continue to improve survival in NCSLC (23), there remains a need to monitor patients for signs and symptoms of treatment-related toxicity, particularly cardiotoxicity. We recommend that clinicians consider an electrocardiography (ECG) at baseline when initiating osimertinib and at periodic intervals to monitor for QT prolongation. Concomitant drugs that can increase QT prolongation should be avoided while taking osimertinib. As per the osimertinib package insert, if the QT interval is >500 ms the osimertinib should be withheld until the QT interval is <480 ms or recovers to baseline. If the baseline QT is >480 ms, then osimertinib should be resumed at one-half the dose (24). Patients should also be monitored for signs and symptoms of cardiac failure while taking osimertinib, but it is likely that more evidence is needed before recommending standardized echocardiogram monitoring for every patient.
Our study raises many questions that require additional studies; for example, what is the mechanism of osimertinib-induced cardiotoxicity? Is there a patient population that is at increased risk of cardiotoxicity compared with others? Is the cardiotoxicity caused by osimertinib reversible or irreversible?
Conclusions
Rates of QT prolongation, cardiac failure, and atrial fibrillation were found to be higher due to osimertinib compared with other EGFR-TKIs in FAERS, a pharmacovigilance database. ECG monitoring for QT prolongation and monitoring for signs and symptoms of heart failure should be considered while taking osimertinib.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The risk of cardiotoxicity, as defined by cardiac failure, QT prolongation, and atrial fibrillation, is higher with osimertinib compared with older generation EGFR-TKIs. ECG monitoring for QT prolongation should be considered while taking osimertinib, and any concomitant QT-prolonging medications should be minimized. Patients should be monitored for signs and symptoms for heart failure while taking osimertinib.
TRANSLATIONAL OUTLOOK: Further research is needed to determine the underlying mechanism of cardiotoxicity due to osimertinib. Multidisciplinary efforts are needed to identify patients at increased risk for cardiotoxicity due to osimertinib.
Acknowledgments
The authors acknowledge Matthew Landry and the Office of Strategic Research Initiatives at Houston Methodist for creation of the Central Illustration.
Footnotes
Dr. Bernicker serves on the advisory board for Guardant Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. This study was presented in part as a poster presentation at the ASCO Annual Meeting, May 31 to June 4, 2019, Chicago, Illinois.
Appendix
For supplemental tables and Methods, please see the online version of this paper.
Contributor Information
Kartik Anand, Email: kartikanand88@gmail.com.
Eric H. Bernicker, Email: bernicker@houstonmethodist.org.
Appendix
References
- 1.Cross D.A., Ashton S.E., Ghiorghiu S. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch T.J., Bell D.W., Sordella R. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non–small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 3.Paez J.G., Jänne P.A., Lee J.C. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 4.Pao W., Miller V., Zakowski M. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D'Angelo S.P., Pietanza M.C., Johnson M.L. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29:2066–2070. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lindeman N.I., Cagle P.T., Aisner D.L. Updated molecular testing guideline for the selection of lung cancer patients for treatment with targeted tyrosine kinase inhibitors: guideline from the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med. 2018;142:321–346. doi: 10.5858/arpa.2017-0388-CP. [DOI] [PubMed] [Google Scholar]
- 7.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 9.Thein K., Swarup S., Ball S. 1388P Incidence of cardiac toxicities in patients with advanced non-small cell lung cancer treated with osimertinib: a combined analysis of two phase III randomized controlled trials. Ann Oncol. 2018;29 mdy292.011. [Google Scholar]
- 10.Rothman K.J., Lanes S., Sacks S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13:519–523. doi: 10.1002/pds.1001. [DOI] [PubMed] [Google Scholar]
- 11.Moslehi J.J., Deininger M. Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol. 2015;33:4210–4218. doi: 10.1200/JCO.2015.62.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maemondo M., Inoue A., Kobayashi K. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 13.Mok T.S., Wu Y.L., Thongprasert S. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 14.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y.L., Zhou C., Hu C.P. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 16.Oyakawa T., Nakashima K., Naito T. Cardiac dysfunction caused by osimertinib. J Thoracic Oncol. 2017;12:e159–e160. doi: 10.1016/j.jtho.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Seidman A., Hudis C., Pierri M.K. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. 2002;20:1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 18.Slamon D., Eiermann W., Robert N. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Viani G.A., Afonso S.L., Stefano E.J., De Fendi L.I., Soares F.V. Adjuvant trastuzumab in the treatment of HER-2-positive early breast cancer: a meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Noma T., Lemaire A., Naga Prasad S.V. Beta-arrestin-mediated beta1-adrenergic receptor transactivation of the EGFR confers cardioprotection. J Clin Invest. 2007;117:2445–2458. doi: 10.1172/JCI31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salem J.E., Manouchehri A., Bretagne M. Cardiovascular toxicities associated with ibrutinib. J Am Coll Cardiol. 2019;74:1667–1678. doi: 10.1016/j.jacc.2019.07.056. [DOI] [PubMed] [Google Scholar]
- 22.Anand K., Burns E.A., Ensor J., Rice L., Pingali S.R. Mycobacterial infections with ruxolitinib: a retrospective pharmacovigilance review. Clin Lymphoma Myeloma Leuk. 2019 Aug 26 doi: 10.1016/j.clml.2019.08.008. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Lin J.J., Cardarella S., Lydon C.A. Five-year survival in EGFR-mutant metastatic lung adenocarcinoma treated with EGFR-TKIs. J Thoracic Oncol. 2016;11:556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Astrazeneca pharmaceuticals. Tagrisso (osimertinib) [package insert]. U.S. Food and Drug Administration website. Revised April 2018. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2018/208065s008lbl.pdf. Accessed September 15, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.