Abstract
Transthyretin amyloid cardiomyopathy (ATTR-CM) has emerged as an increasingly identified etiology of heart failure. Fortunately, the disease now has an approved therapy, with many others under development. Assessment of prognosis in ATTR-CM is critical to inform patients about the disease course and guide clinical decisions. This review discusses the evidence behind clinical, biomarker, and imaging findings that inform prognosis in patients with ATTR-CM and can assist providers in the shared decision-making process during management of this disease.
Key Words: amyloidosis, biomarkers, cardiac magnetic resonance, cardiomyopathy, echocardiography, nuclear imaging
Abbreviations and Acronyms: 99mTc-PYP, 99mTc-pyrophosphate; AF, atrial fibrillation; ATTR-CM, transthyretin amyloid cardiomyopathy; CMR, cardiac magnetic resonance; eGFR, estimated glomerular filtration rate; H/CL, heart to contralateral; HF, heart failure; LGE, late gadolinium enhancement; MCF, myocardial contraction fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; SVI, stroke volume index; TTR, transthyretin; V122I, valine-122-isoleucine
Central Illustration
Highlights
-
•
Prognostic factors for ATTR-CM can guide patient expectations and inform clinical decisions.
-
•
Clinical features, blood biomarkers, and imaging obtained during workup for ATTR-CM convey prognostic information.
-
•
Further studies in determining the incremental value of prognostic factors are warranted.
Transthyretin (TTR) amyloid cardiomyopathy (ATTR-CM) is an increasingly recognized etiology of heart failure (HF) and is no longer considered an untreatable disease. Data that demonstrate survival and hospitalization benefit for the TTR stabilizer, tafamidis, and ongoing development of novel therapeutics create urgency for clinicians to recognize the disease and refer patients for therapy (1). However, determining the most appropriate management plan requires an understanding of a patient’s prognosis. In the last decade, increasing investigation of patients with ATTR-CM has led to recognition of many prognostic factors across numerous domains. We review the prognostic factors that have been identified in patients with ATTR-CM to provide the treating clinician a framework to: 1) appropriately counsel patients about the natural history of the disease; and 2) use prognostic information to guide management plans.
Prognostic Factors in ATTR-CM
Clinical features
Clinical features, often easily gleaned during an initial encounter with a patient with ATTR-CM, may have significant impact on survival and morbidity. We highlight several features here.
Functional assessments
As TTR amyloid fibrils progressively deposit in the myocardium, patients can develop worsening HF symptoms and poor functional status, which carry independent prognostic information. In a study of 93 patients with wild-type ATTR-CM, there was a stepwise worsening of survival with increasing New York Heart Association (NYHA) functional class with a steep decline at class III; median survival for NYHA functional classes I, II, III, and IV were 4.6, 4.1, 2.1, and 1.3 years, respectively (2). In addition to mortality, presence of NYHA functional class III or IV HF was found to be an independent risk factor for a composite of adverse cardiovascular outcomes, including development of atrial arrhythmias, atrioventricular block, HF hospitalization, and stroke (3,4). Data from the ATTR-ACT (Transthyretin Amyloidosis Cardiomyopathy Clinical Trial) showed that patients with NYHA functional class III had an attenuated benefit from tafamidis compared with those with better NYHA functional classes, which emphasized the need for early detection and treatment of ATTR-CM (1). Worse performance on other functional tests, such as the 6-min walk test, was also associated with worse survival (5).
Comorbidities
Conduction system involvement in ATTR-CM is frequently associated with tachyarrhythmias and bradyarrhythmias. Atrial arrhythmias, such as atrial fibrillation (AF) or atrial flutter, are found in up to 40% to 60% of patients, depending on the cohort (1,6, 7, 8). Although categorical presence of AF in patients with ATTR-CM does not appear to be independently associated with mortality, AF is associated with other high-risk features, including increased risk of HF, renal impairment, diastolic dysfunction, and impaired invasive hemodynamic findings (7,8). In patients with ATTR-CM, AF also carries an increased risk of thromboembolism; a recent study that assessed cardioversion outcomes of AF in patients with cardiac amyloidosis found a high procedure cancellation rate (28%), primarily due to the presence of atrial thrombus (9). The development of bradyarrhythmias that required a pacemaker was also associated with worse survival and likely served as a marker of advanced disease (2).
Because the diagnosis of ATTR-CM, especially wild-type TTR, often occurs in older adults, patients frequently have significant age-related comorbidities that may affect survival. For example, up to 16% of patients who underwent transcatheter aortic valve replacement for severe aortic stenosis had nuclear scintigraphy scans consistent with ATTR-CM (10). Although aortic stenosis was not specifically shown to modify prognosis in patients with cardiac amyloidosis, patients in this study had a high-risk phenotype, with most (75%) having NYHA functional class III symptoms, worse diastolic dysfunction, and a higher frequency of low-flow, low-gradient aortic stenosis compared with control subjects (10). Conversely, incidental diagnosis of cardiac amyloidosis was shown to be an incrementally poor prognostic factor in patients with severe aortic stenosis (11,12).
Renal dysfunction was also identified as a risk factor for worse prognosis. In a large cohort of 869 patients with ATTR-CM evaluated at the United Kingdom National Amyloidosis Centre, estimated glomerular filtration rate (eGFR) at a threshold of <45 ml/min/1.73 m2 was an independent predictor of mortality on multivariable analysis (13). Median survival was approximately 2.5 years for patients who had eGFR below the threshold compared with approximately 5 years for those above the threshold. Impaired renal function might be a sequela of renovascular congestion from progressive HF or may pre-exist the development of ATTR-CM and subsequently limit effective volume status modulation. The strength of this association led to the implementation of eGFR in a prognostic staging system further described in the following.
Genotype
Prognosis in hereditary TTR amyloidosis is modified by specific genotype and subsequent degree of cardiac involvement in phenotype. A common genotype present in 3% to 4% of the African-American population in the United States is the valine-122-isoleucine (V122I) variant (14). This appears to portend a different prognosis compared with non-V122I variants and wild-type TTR. In a cohort of 1,034 patients with ATTR-CM (711 with wild-type, 205 with V122I, 118 with non-V122I), patients with the V122I variant had significantly worse survival (median survival of 2.6 years) compared with those with non-V122I variants (median survival of 5.8 years) or wild-type TTR (median survival of 4.8 years) (5). Although patients with the V122I variant had evidence of more advanced cardiac disease at time of diagnosis, the association with worsened survival remained even after controlling for biomarker-based staging (see the following) (5,13). Patients with the V122I variant also had shorter survival times from onset of cardiac symptoms to diagnosis, which potentially signaled a more pathogenic genotype (5).
Blood Biomarkers
As in other etiologies of HF, routine blood biomarkers have an important role in the prognostication for patients with ATTR-CM. Whether due to direct toxic effects on myocardial cells or from myocardial stress from amyloid deposition, troponin and natriuretic peptide elevations in blood have demonstrated particular value in prognostication, both independently and in combination in staging systems (6). The Mayo Clinic group assessed a cohort of 360 patients with wild-type ATTR-CM and demonstrated that elevations above thresholds of troponin T (0.05 ng/ml) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (3,000 pg/ml) each contributed to a prognostic 3-point staging system: Stage I (elevation of neither biomarker); Stage II (elevation in 1 biomarker); and Stage III (elevation in both biomarkers). Median survival for patients classified as Stage I was 5.5 years and 1.7 years for those classified as Stage III.
Renal dysfunction and elevations in NT-proBNP were also recently found to be components of another robust prognostic staging system (13). Patients with eGFR <45 ml/min/1.73 m2 and NT-proBNP >3,000 pg/ml had significantly worse outcomes compared with those not meeting these thresholds. Using a similar staging scheme to that previously described, the median survival was 5.7, 3.9, and 2.0 years for patients classified as Stages I, II, and III, respectively. The association of higher stage with worse survival remained significant even after the cohort was separated into different types of ATTR-CM (wild-type, V122I, or non-V122I).
Because of this dramatic difference in survival based on readily available, relatively inexpensive, and easily interpretable biomarkers in the context of these validated staging systems, it is important for clinicians to consider measuring these biomarkers during the initial workup for ATTR-CM in all patients.
Imaging
Imaging studies, particularly echocardiography and nuclear scintigraphy, are routinely obtained as a part of the diagnostic workup for ATTR-CM. Several features, if found on imaging studies, have been associated with poor outcomes and allow the treating clinician to use this existing imaging information to further assist in the prognostic assessment of patients with ATTR-CM.
Echocardiography
Myocardial deposition of TTR amyloid fibrils alters the morphology of the ventricular walls, which leads to increased thickness, decreased compliance, and impaired contractile function. However, patients often have normal or preserved left ventricular ejection fractions (LVEFs) in the early stages of ATTR-CM. Furthermore, although an LVEF decline (e.g., <50%) has been associated with worse outcomes, this association has been inconsistent (6,15). An alternative to LVEF for measuring left ventricular (LV) systolic function is the myocardial contraction fraction (MCF), which is calculated as the ratio of LV stroke volume to myocardial volume (16). With progression of cardiac amyloidosis, MCF consistently declines, whereas LVEF may remain stable due to proportional decreases in stroke volume and end-diastolic volume. In a secondary analysis of the international Transthyretin Amyloidosis Outcomes Survey database, an MCF of <25% was associated with a median survival of <3 years, whereas an MCF of ≥25% was associated with a median survival of >6.8 years (17). The specific MCF threshold of 25% is an arbitrary dichotomization of a continuous variable but serves as a method to apply this imaging marker as a risk factor for worse prognosis. For example, an MCF of 15% likely identifies even greater disease progression and a worse prognosis than an MCF of 25%. This theme is repeated in many of the risk factors discussed in the following.
Global longitudinal strain is another sensitive marker of impaired systolic function early in the course of ATTR-CM. Typical longitudinal strain patterns in patients with ATTR-CM show relative LV apical sparing compared with basal and mid segments. In a cohort of 79 patients with cardiac amyloidosis (including those with light-chain, mutant TTR, and wild-type TTR), longitudinal strain was demonstrated to correlate significantly with late gadolinium enhancement (LGE), a cardiac magnetic resonance (CMR) surrogate for amyloid infiltration, as well as amyloid deposition when analyzed histologically (18). In the same study, Ternacle et al. (18) further demonstrated that impaired apical longitudinal strain (threshold of 14.5%) predicted major adverse cardiac events, which suggested that impairment of typically spared apical strain might also serve as a risk factor for worse prognosis.
However, MCF and global longitudinal strain are not routinely available or calculated in most echocardiography laboratories. Stroke volume index (SVI) is more commonplace and also has strong prognostic implications. Using a threshold of 33 ml/m2 as representative of a low cardiac output state, Milani et al. (19) not only demonstrated SVI to be an independent predictor of survival in a cohort of 754 patients with light-chain cardiac amyloidosis, but also showed that it performed similarly to MCF and LV strain in survival models. Future investigation of the prognostic potential of routinely obtained echocardiographic measures for patients with ATTR-CM is warranted.
Nuclear scintigraphy
Nuclear scintigraphy can be used to reliably detect TTR amyloid deposition and diagnose ATTR-CM, but certain patterns in 99mTc-pyrophosphate (99mTc-PYP) scans can also be prognostic. In a multicenter cohort of 121 patients, patients with a heart to contralateral (H/CL) ratio of <1.6 had a median survival of >5 years, but those with a ratio of ≥1.6 had a median survival of <3 years (15). Higher H/CL ratios above the 1.6 threshold likely also identify patients with increasingly worse prognosis.
In a different cohort of 54 patients with ATTR-CM, it was found that a pattern of decreased apical 99mTc-PYP uptake compared with mid and basal segment 99mTc-PYP uptake was present in patients with ATTR-CM, mirroring the apical-sparing pattern often seen in echocardiography (20). Reduction in the apical-sparing ratio (e.g., more diffuse 99mTc-PYP uptake) was associated with worse survival. Because 99mTc-PYP scanning is frequently used as a diagnostic strategy for patients suspected of having ATTR-CM, the clinician should also be aware that important prognostic information can be conveyed in the results.
CMR
CMR findings of LGE in a diffuse, subendocardial distribution are typical of amyloid cardiomyopathy. Anecdotally, CMR may be obtained during workup of ATTR-CM as a method to distinguish it from hypertrophic cardiomyopathy or as a screening study before catheter ablation of AF. Again, specific findings from this imaging technique can carry important prognostic information. Transmural LGE has been found to be an independent predictor of worse survival for patients with either light-chain amyloid cardiomyopathy or ATTR-CM, even after adjustment for NT-proBNP and other echocardiographic measures (21). Patients with subendocardial LGE had better survival than those with transmural LGE (3-year overall survival of approximately 80% vs. 50%, respectively). It was also demonstrated, using CMR T1 mapping, that increases in extracellular volume, a measure of amyloid burden that correlates with both the distribution of LGE and abnormal cardiac uptake on nuclear scintigraphy scans, was associated with worse survival (22).
Although significant prognostic information can be gained from CMR techniques, we do not recommend routinely obtaining this study solely for prognostic information because: 1) it is unclear how much incremental value it would add to the additional prognostic markers already discussed; 2) presence of significant renal dysfunction or implantable devices prohibits this technique; and 3) CMR would not likely be cost-effective if pursued solely for prognostication. However, if CMR data are available, treating clinicians should be aware that important prognostic information can be gained.
Putting it Together
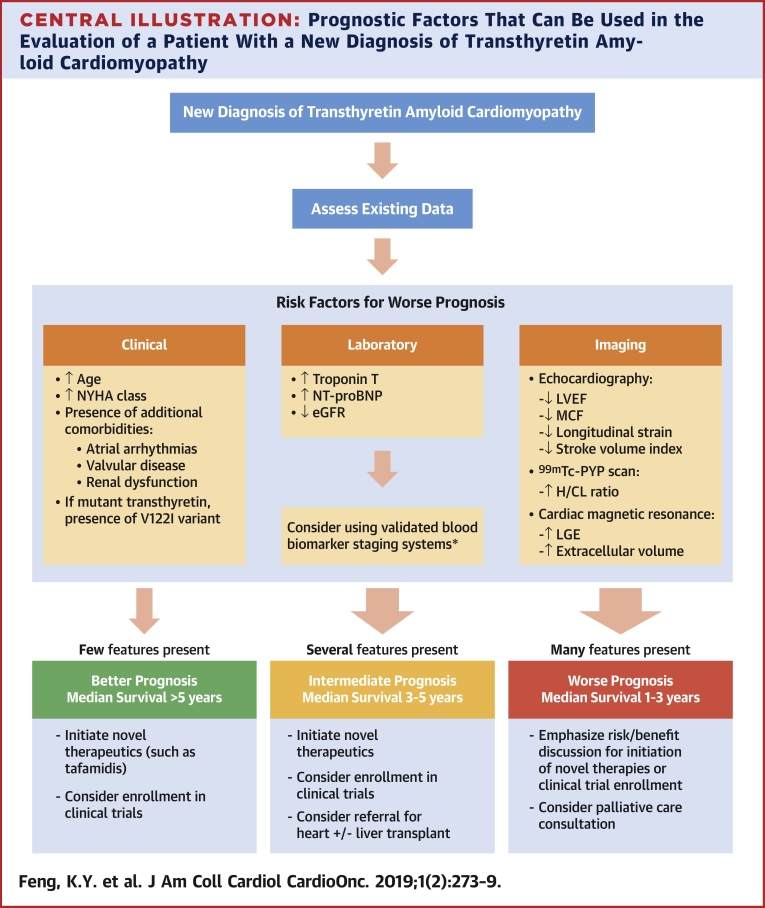
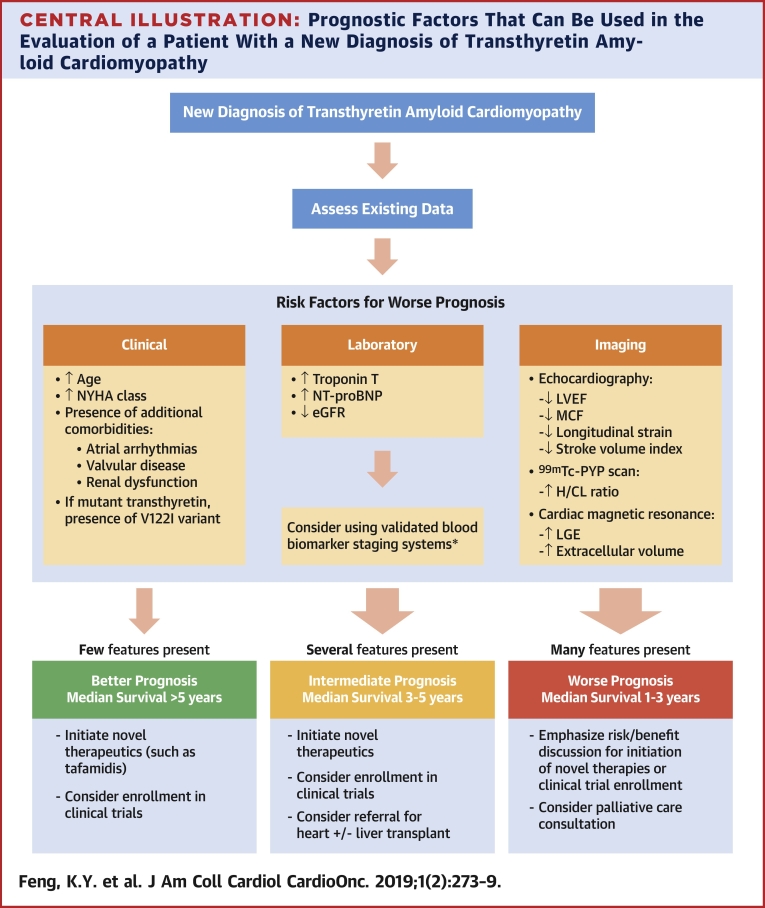
Clinicians can synthesize data from a variety of clinical assessments, biomarkers, and multimodality imaging studies to better inform patients with ATTR-CM about their prognosis (Central Illustration). Risk factors for worse outcomes are: 1) specific clinical features (worse performance on functional assessments, specific genotypes, and comorbidities, such as renal dysfunction); 2) increased myocardial stress evidenced by elevated biomarkers (troponin and NT-proBNP); 3) worsening pump function visualized via echocardiography (LVEF, MCF, SVI or global strain/strain patterns); and 4) magnitude and pattern of amyloid infiltration visualized via uptake on nuclear scans (H/CL ratio) or via CMR (LGE and extracellular volume). Although it is unclear the magnitude to which each factor adds incremental prognostic information, providers should be aware of the prognostic information inherent in even routine studies.
Central Illustration.
Prognostic Factors That Can Be Used in the Evaluation of a Patient With a New Diagnosis of Transthyretin Amyloid Cardiomyopathy
Various clinical, biomarker, and imaging parameters can be used in assessing prognosis, but this is not meant to imply that all factors be measured in all patients, but rather that risk factors for worse prognosis can be identified from available studies obtained during the diagnostic process for transthyretin amyloid cardiomyopathy. *Laboratory studies are readily available, relatively inexpensive, and easily interpretable in the context of validated staging systems and are recommended in all patients (6,13). 99mTc-PYP = 99mTc-pyrophosphate; eGFR = estimated glomerular filtration rate; H/CL = heart to contralateral; LGE = late gadolinium enhancement; LVEF = left ventricular ejection fraction; MCF = myocardial contraction fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; V122I = valine-122-isoleucine.
Determining prognosis guides discussions regarding management. Patients with few poor prognostic risk factors and early stage disease tend to have good short-term survival (median survival >5 years). The emergence of novel therapies, such as tafamidis, have the potential to change the natural history of ATTR-CM and conceivably lengthen survival significantly. Such patients should be promptly referred for consideration of novel therapies and screened for enrollment in clinical trials. Patients with several poor prognostic risk factors (e.g., abnormal biomarkers, higher H/CL ratios, and so on) have an intermediate prognosis, with median survival estimated at approximately 3 to 5 years. For these patients, similar to those with few risk factors, initiation of novel therapies and/or referral for clinical trials should be promptly considered. If patients continue to clinically worsen despite optimal management, referral to an advanced HF clinic should be made for consideration of heart and/or heart−liver transplantation before the disease progresses to irreversible multiorgan failure or worsening comorbidities precludes consideration of transplantation.
Unfortunately, because of the demographics of wild-type ATTR-CM, patients often present at an older age with numerous comorbidities (previously treated malignancy, atherosclerotic vascular disease, severe aortic stenosis, and so on) or at advanced stages of HF with biomarker and imaging evidence of significant amyloid deposition. Management decisions for this group of patients should emphasize the discussion of risks and benefits of therapy. Novel therapies in the treatment of ATTR-CM can be expensive, and the cost can be prohibitive for many patients. Data from the ATTR-ACT trial, which excluded patients with significant renal dysfunction (eGFR <25 ml/min/1.73 m2), showed that patients with NYHA functional class III had a diminished benefit from the drug compared with those with less functionally limiting disease (1). Because of the amount of time required to show benefit of tafamidis in the trial, it is currently unclear whether initiating TTR stabilizing therapy in patients with advanced disease (e.g., those in NYHA functional class IV or those with significant renal dysfunction) would offer meaningful benefit. As novel therapeutics continue to be developed, patients with advanced disease might benefit more from investigational agents aimed at disrupting and resorbing amyloid fibrils already deposited in tissue (23). In addition, at advanced stages of disease, palliative care discussions regarding symptom management are also reasonable.
Lastly, accurate prognostication also furthers the development of clinical trials. Prospective validation of prognostic factors may lead to more efficient surrogates for tracking disease progression because use of mortality and other adverse events require prolonged study time, which results in increased resources and time to conduct investigations, and delays in the development of potentially beneficial therapies. Once validated, these risk factors can also then translate to routine clinical care.
Conclusions
Clinicians can use a wide variety of clinical assessments, biomarkers, and imaging modalities to inform prognostic assessments for patients newly diagnosed with ATTR-CM. Prognostic determinations will assist in the shared decision-making process of treating this complex disease, especially in a landscape of rapidly changing therapies.
Footnotes
This paper was funded by the Duke Clinical Research Institute. Dr. Khouri has received research support from and is a member of the Speakers Bureau of Alnylam Pharmaceuticals; and has received an honorarium from and a member of the Advisory Board of Pfizer. Dr. Felker has received research support from Amgen, Merck, Novartis, Otsuka America Pharmaceutical, and Roche Diagnostics; and has served as a consultant for Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Medscape, LLC, Medtronic PLC, MyoKardia, Novartis, Trevena, Alynylam Pharmaceuticals, and Cardionomic. Dr. DeVore has received support from the American Heart Association, Amgen, the National Heart, Lung, and Blood Institute, and Novartis; and has been a consultant for Novartis. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Maurer M.S., Schwartz J.H., Gundapaneni B. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018;379:1007–1016. doi: 10.1056/NEJMoa1805689. [DOI] [PubMed] [Google Scholar]
- 2.Pinney J.H., Whelan C.J., Petrie A. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapezzi C., Merlini G., Quarta C.C. Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009;120:1203–1212. doi: 10.1161/CIRCULATIONAHA.108.843334. [DOI] [PubMed] [Google Scholar]
- 4.Galat A., Rosso J., Guellich A. Usefulness of (99m)Tc-HMDP scintigraphy for the etiologic diagnosis and prognosis of cardiac amyloidosis. Amyloid. 2015;22:210–220. doi: 10.3109/13506129.2015.1072089. [DOI] [PubMed] [Google Scholar]
- 5.Lane T., Fontana M., Martinez-Naharro A. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140:16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 6.Grogan M., Scott C.G., Kyle R.A. Natural history of wild-type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016;68:1014–1020. doi: 10.1016/j.jacc.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 7.Longhi S., Quarta C.C., Milandri A. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid. 2015;22:147–155. doi: 10.3109/13506129.2015.1028616. [DOI] [PubMed] [Google Scholar]
- 8.Mints Y.Y., Doros G., Berk J.L., Connors L.H., Ruberg F.L. Features of atrial fibrillation in wild-type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Heart Fail. 2018;5:772–779. doi: 10.1002/ehf2.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El-Am E.A., Dispenzieri A., Melduni R.M. Direct current cardioversion of atrial arrhythmias in adults with cardiac amyloidosis. J Am Coll Cardiol. 2019;73:589–597. doi: 10.1016/j.jacc.2018.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castano A., Narotsky D.L., Hamid N. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38:2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcante J.L., Rijal S., Abdelkarim I. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. J Cardiovasc Magn Reson. 2017;19:98. doi: 10.1186/s12968-017-0415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Treibel T.A., Fontana M., Gilbertson J.A. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging. 2016;9 doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 13.Gillmore J.D., Damy T., Fontana M. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018;39:2799–2806. doi: 10.1093/eurheartj/ehx589. [DOI] [PubMed] [Google Scholar]
- 14.Buxbaum J.N., Ruberg F.L. Transthyretin V122I (pV142I)* cardiac amyloidosis: an age-dependent autosomal dominant cardiomyopathy too common to be overlooked as a cause of significant heart disease in elderly African Americans. Genet Med. 2017;19:733–742. doi: 10.1038/gim.2016.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castano A., Haq M., Narotsky D.L. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol. 2016;1:880–889. doi: 10.1001/jamacardio.2016.2839. [DOI] [PubMed] [Google Scholar]
- 16.King D.L., El-Khoury Coffin L., Maurer M.S. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–329. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 17.Rubin J., Steidley D.E., Carlsson M., Ong M.-L., Maurer M.S. Myocardial contraction fraction by M-mode echocardiography is superior to ejection fraction in predicting mortality in transthyretin amyloidosis. J Card Fail. 2018;24:504–511. doi: 10.1016/j.cardfail.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ternacle J., Bodez D., Guellich A. Causes and consequences of longitudinal LV dysfunction assessed by 2D strain echocardiography in cardiac amyloidosis. J Am Coll Cardiol Img. 2016;9:126–138. doi: 10.1016/j.jcmg.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 19.Milani P., Dispenzieri A., Scott C.G. Independent prognostic value of stroke volume index in patients with immunoglobulin light chain amyloidosis. Circ Cardiovasc Imaging. 2018;11 doi: 10.1161/CIRCIMAGING.117.006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sperry B.W., Vranian M.N., Tower-Rader A. Regional variation in technetium pyrophosphate uptake in transthyretin cardiac amyloidosis and impact on mortality. J Am Coll Cardiol Img. 2018;11:234–242. doi: 10.1016/j.jcmg.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Fontana M., Pica S., Reant P. Prognostic value of late gadolinium enhancement cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2015;132:1570–1579. doi: 10.1161/CIRCULATIONAHA.115.016567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Naharro A., Treibel T.A., Abdel-Gadir A. Magnetic resonance in transthyretin cardiac amyloidosis. J Am Coll Cardiol. 2017;70:466–477. doi: 10.1016/j.jacc.2017.05.053. [DOI] [PubMed] [Google Scholar]
- 23.Alexander K.M., Evangelisti A., Witteles R.M. Emerging therapies for transthyretin cardiac amyloidosis. Curr Treat Options Cardiovasc Med. 2019;21:40. doi: 10.1007/s11936-019-0743-2. [DOI] [PubMed] [Google Scholar]