Abstract
Objectives
The purpose of this study was to investigate whether pre-diagnosis exercise reduces the risk of subsequent cardiovascular events (CVEs) in women with primary breast cancer.
Background
Cardiovascular disease (CVD) is the leading nonmalignant cause of death in patients with cancer, and it is the leading cause of death in women with primary breast cancer who are older than 65 years of age.
Methods
Using a prospective design, 4,015 patients with confirmed diagnosis of primary breast cancer enrolled in the Women’s Health Initiative (WHI) completed a self-report questionnaire assessing leisure-time physical activity (i.e., exercise) in metabolic equivalent task (MET) hours per week. Age- and multivariable-adjusted Cox proportional hazards models were used to estimate associations between pre-diagnosis exercise and new-onset CVEs (i.e., heart failure [HF], myocardial infarction [MI], angina, coronary revascularization, peripheral arterial disease [PAD], carotid artery disease, transient ischemic attack [TIA], stroke, and cardiovascular death).
Results
Median follow-up was 12.7 years and 8.2 years for cardiovascular disease (CVD) mortality and CVEs, respectively, with 324 CVEs, including 89 MIs, 49 new diagnoses of HF, and 215 CVD deaths. In multivariable analysis, the incidence of composite CVEs decreased across increasing total MET h/week categories (p = 0.016). Compared with <2.5 MET-hours per week, the adjusted hazard ratio (HR) was 0.80 (95% confidence interval [CI]: 0.59 to 1.09) for 2.5 to <8.6 MET h/week; 0.9 (95% CI: 0.64 to 1.17) for 8.6 to <18 MET h/week; and 0.63 (95% CI: 0.45 to 0.88) for ≥18 MET h/week.
Conclusion
Pre-diagnosis exercise exposure is associated with a significant graded reduction in subsequent CVEs in long-term survivors of primary breast cancer.
Key Words: breast cancer, cardiovascular disease, cardiovascular events, exercise, physical activity, survivors, Women’s Health Initiative
Abbreviations and Acronyms: BMI, body mass index; CVE, cardiovascular event; CRF, cardiorespiratory fitness; CT, clinical trial; HF, heart failure; HT, hormone therapy; IQR, interquartile range; MET, metabolic equivalent task; MI, myocardial infarction; OS, observational study; PAD, peripheral arterial disease; TIA, transient ischemic attack; WHI, Women’s Health Initiative
Central Illustration
Advances in screening and adjuvant therapy have led to continual improvements in cancer-specific mortality among women with primary breast cancer. The current 5-year survival rate is 89.4% compared with 74.6% from 1975 to 1979 (1). As a result, patients with breast cancer have now sufficient longevity to be at increased risk of normal age-related pathologies: primarily, cardiovascular disease (CVD). In fact, CVD has now surpassed breast cancer as the leading cause of death in patients with primary breast cancer above the age of 65 years 2, 3.
Interestingly, efforts in cardio-oncology have focused primarily on the cardiac-centric consequences of breast-cancer therapy, with resting assessment of left ventricular ejection fraction being used to assess the incidence of asymptomatic and symptomatic myocardial injury that predisposes to heart failure (HF). However, it is now established that the adverse consequences of breast cancer therapy extend beyond the heart, with myocardial injury occurring in conjunction with (mal)adaptation of other organ systems. Many anticancer therapies cause unique and varying degrees of injury to other components of the cardiovascular system, including the pulmonary-vascular and blood-skeletal muscle axes. To this end, incremental exercise tolerance testing, which evaluates cardiorespiratory fitness (CRF), provides a robust, integrated assessment of cardiovascular reserve capacity. In previous work, we—as well as others—observed that CRF can decline between 5% and 20% in women receiving chemotherapy-containing adjuvant therapy, and this decline persists even years following the cessation of treatment. As such, intervention strategies with the capacity to not only prevent cancer-therapy–associated myocardial injury but also attenuate injury across the entire cardiovascular system may have significant clinical importance.
General physical activity, as well as planned physical activity (i.e., exercise), is a strong independent predictor of cardiovascular events (CVEs), as well as CVD and all-cause mortality in women from the general population. This reduction in risk translates into a 3-year increase in life expectancy (4). Exercise improves the reserve capacity of all organs compromising the cardiovascular system.
Much less is known regarding the impact of exercise on CVD in women with breast cancer. In one study, higher self-reported post-diagnosis exercise exposure was associated with a graded reduction in CVEs (i.e., new diagnosis of coronary artery disease [CAD], HF, valve abnormality, arrhythmia, stroke, or CVD death) in 2,973 patients with primary breast cancer (5). In another study of 55 survivors of breast cancer without cardiovascular risk factors who had received anthracyclines as part of their cancer therapy, physical activity delayed the development of diastolic dysfunction and symptoms of HF (6). Of importance, both studies evaluated the impact of exercise in the period following a diagnosis of breast cancer. A critical corollary to such work is whether exposure to exercise in the period before diagnosis affects the risk of treatment-related CVEs. In other words, are women who are regularly exercising before diagnosis better able to tolerate cancer therapy owing to lower toxicity, including cardiovascular-related toxicity? To our knowledge, whether exercise in the period before diagnosis alters subsequent risk of CVEs in the post-diagnosis period has not been investigated.
Accordingly, we determined the association between pre-diagnosis exercise and risk of CVEs in women with patients with primary breast cancer participating in the Women’s Health Initiative (WHI). We hypothesized that exercise would reduce the risk of CVEs (including HF, myocardial infarction [MI], angina, coronary revascularization, peripheral arterial disease [PAD], carotid artery disease, transient ischemic attack [TIA], stroke, or cardiovascular death) in a dose-dependent manner beyond CV risk factors and treatment exposure.
Methods
The design of the WHI clinical trial (CT) and observational study (OS) are described elsewhere (7). In brief, the WHI was initiated in 1992, recruiting postmenopausal women aged between 50 and 79 years of age at 1 of 40 clinical centers within the United States. The total study population included 161,808 women (OS, n = 93,646; CT, n = 61,132) between October 1993 and December 1998. Participants completed in-person visits and questionnaires at baseline and during follow-up. At the end of the main WHI study in 2005, participants were invited to enroll in an Extension Study, and those who consented were followed annually by mail from 2005 to 2010. The institutional review boards from all WHI-affiliated institutions approved the study protocol, and all participants provided informed consent.
Self-reports of cancer were verified by physician adjudicators after review of medical records and pathology reports. Final adjudication and coding of findings was done at the WHI Clinical Coordinating Center, according to the National Cancer Institute Surveillance, Epidemiology, and End Results (NCI-SEER) guidelines. Self-reports of cardiovascular outcomes similarly initiated a review of medical records by physician adjudicators. Reports of participant death and cause of death were also adjudicated by study physicians. The National Death Index was systematically searched for all participants including those lost to follow-up. At the time of this study, WHI data on mortality were complete through December 2014.
The participants in this cohort included patients with confirmed diagnoses of nonmetastatic (localized or regional stage) primary breast cancer during the main WHI study. Women with CVD (MI, angina, coronary artery bypass graft, percutaneous coronary intervention/percutaneous transluminal coronary angioplasty, TIA, stroke, heart failure), history of any other malignancy prior to enrollment in WHI, and/or body mass index (BMI) ≤18.5 kg/m2 were excluded from this analysis (Supplemental Figure 1). Our final analytic cohort included 4,015 patients within the WHI OS and CT studies.
Exercise exposure assessment
Exercise exposure was assessed as leisure-time physical activity and ascertained in the baseline and follow-up questionnaires (8). Specifically, patients were asked to report the frequency (i.e., never to 1 to 7 days per week); duration (<20 min, 20 to 39 min, 40 to 59 min, ≥1 h); and speed (<2 mph [strolling], 2 to 3 mph [average/normal walking], 3 to 4 mph [fairly fast walking], or >4 mph) of walking sessions per week. The frequency and duration of moderate (i.e., biking outdoors, exercise machine, calisthenics, easy swimming, popular or folk dancing) and vigorous (aerobics, jogging, tennis, and swimming laps) activity were also assessed. The midpoint value for ranges of frequency and exercise duration were imputed, and multiplied to calculate hours per week. Metabolic equivalent task (MET) values were assigned for walking (average = 3 METs, fairly fast = 4 METs, or very fast = 4.5 METs), and to classify moderate intensity recreational (4 METs) and vigorous-intensity recreational (7 METs) activities. The MET level was then multiplied by hours per week to calculate total MET hours per week (9). During WHI follow-up, leisure-time physical activity (exercise) was assessed for CT participants at years 1, 3, 6, and 9 and for OS participants in years 3 to 8. For the current study, we used the exercise data that were collected at the visit closest to breast cancer diagnosis and that were between 5 years and 1 month before diagnosis. We categorized exercise as quartiles: <2.50 MET h/week (n = 994); 2.50 to <8.625 (n = 1,008); 8.625 to < 18.00 (n = 1,011); and ≥ 18.00 (n = 1,002). Patients were also categorized and assessed as 2 groups (≥9 MET h/week [N = 1,976] and <9 MET h/week [N = 2,039]), based on physical activity levels according to the national exercise guidelines. The reliability and validity of the exercise instrument has been established (7). To ascertain the validity of the physical activity questionnaire, the questionnaire data were compared with accelerometer data in a previous study (r = 0.73, with 100% sensitivity for meeting the physical activity guidelines) (10).
Definition of cardiovascular outcomes
The primary CVD outcome was the first occurrence of newly diagnosed heart failure, MI, angina, coronary revascularization, PAD, carotid artery disease, TIA, stroke, or cardiovascular death, occurring after diagnosis of breast cancer. For this study, the combined CVD event was ascertained through the end of the Extension Study. Individual incident CVEs were also studied; these were MI, HF, coronary heart disease (CHD) death, and CVD death. CHD was defined as acute MI necessitating overnight hospitalization, death due to CHD, or silent MI identified on serial electrocardiography.
Statistical analysis
Demographic information, physical measurements, and medication inventories were collected at WHI enrollment. When available, information that had been updated closer to the breast cancer diagnosis was used. Characteristics were described by quartiles of exposure to exercise (in MET hours per week) and compared using chi-square tests for categorical variables. Age-adjusted and multivariable-adjusted Cox proportional hazards models were used to assess the associations of quartiles of METs and incident CVD events presented as hazard ratios (HRs) and 95% confidence intervals (CIs). For analysis of the combined CVD event, follow-up was the number of days from cancer diagnosis to the first incident CVE. In analyses of MI and HF as individual incident events, women who had experienced an earlier CVE (e.g., angina before MI) continued to be followed for the specific outcome being studied. Otherwise, follow-up was censored at the last documented follow-up contact, death, or September 30, 2010 (whichever came first) for analyses of the combined CVD event, MI, and HF. For death from CHD or CVD, women were followed until September 30, 2015. CHD-specific mortality and CVD-specific mortality were defined as the time from cancer diagnosis to a CHD death or CVD death, respectively. Women who experienced earlier nonfatal CVD events continued to be followed for CVD-specific mortality events. Event times were censored at the time of a death from another cause. Otherwise, women were followed until September 30, 2015.
All models were adjusted for age at WHI enrollment as a continuous variable. We also adjusted for race or ethnicity (white, black, Hispanic, other), smoking status (never, past, current), BMI (<25, 25 to <30, ≥30 kg/m2 [corresponding to categories of normal weight, overweight, and obese]), stage (localized, regional), education (less than high school, high school diploma/GED, school after high school, college degree, or higher), WHI study (HT randomized, DM randomized, not in HT, OS enrolled), hormone therapy/trial arm (nonuser, estrogen-alone, estrogen + progestin), family history of MI, and comorbidities index (count of diabetes, hypertension, history of high cholesterol treated with pharmacotherapy, history of chronic obstructive pulmonary disease (COPD), lupus/rheumatoid arthritis, history of liver disease, history of stomach ulcer). We included strata for age at diagnosis (50 to 59, 60 to 64, 65 to 69, 70 to 74, ≥75 years) and time-varying extension study participation (yes/no) in the models, allowing the baseline hazards to vary in these strata. Tests for linear trend across increasing categories of exercise were conducted by modeling the median values of each category as a single continuous variable in the models. We also estimated associations between CVD outcomes and exercise levels defined by national exercise guidelines (≥9 MET h/week [N = 1,976] referent to <9 MET h/week [N = 2,039]). As a sensitivity analysis, we analyzed a subset of women (n = 1,603) with cancer-treatment information available from Medicare data. For this group, cancer-treatment variables were included in the models as surgery (none, lumpectomy, mastectomy); chemotherapy (yes/no); radiation (yes/no). All analyses were conducted using SAS (version 9.4, SAS Institute Inc., Cary, North Carolina) or Stata (version 12, StataCorp, College Station, Texas). All statistical tests were 2-sided, and statistical significance was defined as p < 0.05.
Results
The median time from exercise assessment to breast cancer diagnosis was 12 months (interquartile range [IQR]: 6 to 23 months). The median duration of follow-up for cardiovascular events analysis was 8.2 years, IQR: 6.0 to 10.6 years. For death outcomes, median follow-up was 12.7 years (IQR: 10.4 to 14.9 years). During this period, a total of 324 CVEs, including 89 MIs, 49 new diagnoses of HF, and 215 CVD deaths (96 attributed to CHD) were observed. As expected, disease grade, race, BMI, smoking status, education, family income, and comorbidities index were associated with total METs (Table 1).
Table 1.
Participant Characteristics for Breast Cancer Cases, by Level of Exercise in the 5 Years Before Diagnosis (N = 4,015)
| MET h/week |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <2.50 (n = 994) |
2.50 to <8.625 (n = 1,008) |
8.625 to <18.00 (n = 1,011) |
≥18.00 (n = 1,002) |
p Value | |||||
| n | % | n | % | n | % | n | % | ||
| Age at cancer diagnosis, yrs | 0.647 | ||||||||
| 50 to 59 | 140 | 14.1 | 133 | 13.2 | 122 | 12.1 | 153 | 15.3 | |
| 60 to 64 | 202 | 20.3 | 217 | 21.5 | 228 | 22.6 | 223 | 22.3 | |
| 65 to 69 | 241 | 24.2 | 239 | 23.7 | 257 | 25.4 | 224 | 22.4 | |
| 70 to 74 | 217 | 21.8 | 210 | 20.8 | 206 | 20.4 | 218 | 21.8 | |
| 75+ | 194 | 19.5 | 209 | 20.7 | 198 | 19.6 | 184 | 18.4 | |
| Disease stage | 0.717 | ||||||||
| Localized | 744 | 74.8 | 760 | 75.4 | 744 | 73.6 | 758 | 75.6 | |
| Regional | 250 | 25.2 | 248 | 24.6 | 267 | 26.4 | 244 | 24.4 | |
| Disease grade | <0.001 | ||||||||
| Well differentiated | 219 | 22.0 | 266 | 26.4 | 272 | 26.9 | 259 | 25.8 | |
| Moderately differentiated | 350 | 35.2 | 389 | 38.6 | 379 | 37.5 | 396 | 39.5 | |
| Poorly differentiated/anaplastic | 294 | 29.6 | 241 | 23.9 | 276 | 27.3 | 243 | 24.3 | |
| Unknown/not done | 131 | 13.2 | 112 | 11.1 | 84 | 8.3 | 104 | 10.4 | |
| Race | <0.001 | ||||||||
| White | 840 | 84.5 | 889 | 88.2 | 897 | 88.7 | 904 | 90.2 | |
| Black | 90 | 9.1 | 49 | 4.9 | 58 | 5.7 | 45 | 4.5 | |
| Hispanic/Latino | 43 | 4.3 | 27 | 2.7 | 19 | 1.9 | 17 | 1.7 | |
| Other/unspecified | 21 | 2.1 | 43 | 4.3 | 37 | 3.7 | 36 | 3.6 | |
| BMI (kg/m2) | <0.001 | ||||||||
| <25 | 186 | 18.7 | 292 | 29.0 | 397 | 39.3 | 453 | 45.2 | |
| 25 to <30 | 342 | 34.4 | 345 | 34.2 | 354 | 35.0 | 332 | 33.1 | |
| ≥30 | 457 | 46.0 | 358 | 35.5 | 255 | 25.2 | 211 | 21.1 | |
| Unknown | 9 | 0.9 | 13 | 1.3 | 5 | 0.5 | 6 | 0.6 | |
| Smoking status | <0.001 | ||||||||
| Never | 489 | 49.2 | 532 | 52.8 | 495 | 49.0 | 465 | 46.4 | |
| Past | 427 | 43.0 | 421 | 41.8 | 470 | 46.5 | 497 | 49.6 | |
| Current | 75 | 7.5 | 55 | 5.5 | 43 | 4.3 | 40 | 4.0 | |
| Unknown | 3 | 0.3 | 0 | 0.0 | 3 | 0.3 | 0 | 0.0 | |
| Education | <0.001 | ||||||||
| Less than high school | 42 | 4.2 | 32 | 3.2 | 24 | 2.4 | 21 | 2.1 | |
| High school diploma or GED | 205 | 20.6 | 171 | 17.0 | 138 | 13.6 | 92 | 9.2 | |
| School after high school | 353 | 35.5 | 346 | 34.3 | 348 | 34.4 | 341 | 34.0 | |
| College degree or higher | 387 | 38.9 | 448 | 44.4 | 493 | 48.8 | 537 | 53.6 | |
| Unknown | 7 | 0.7 | 11 | 11.1 | 8 | 0.8 | 11 | 1.1 | |
| Family history of MI | 520 | 55.3 | 502 | 52.3 | 509 | 52.7 | 489 | 51.2 | 0.455 |
| Comorbidities Index | <0.001 | ||||||||
| 0 | 282 | 28.4 | 349 | 34.6 | 375 | 37.1 | 388 | 38.7 | |
| 1 | 405 | 40.7 | 403 | 40.0 | 422 | 41.7 | 386 | 38.5 | |
| 2 | 211 | 21.2 | 164 | 16.3 | 132 | 13.1 | 145 | 14.5 | |
| 3+ | 45 | 4.5 | 51 | 5.1 | 35 | 3.5 | 33 | 3.3 | |
| Unknown | 51 | 5.1 | 41 | 4.1 | 47 | 4.6 | 50 | 5.0 | |
| Cardiovascular conditions | |||||||||
| Treated hypercholesterolemia | 121 | 12.8 | 112 | 11.6 | 114 | 11.8 | 91 | 9.6 | 0.151 |
| Treated diabetes | 82 | 8.2 | 54 | 5.4 | 45 | 4.5 | 29 | 2.9 | <0.001 |
| Hypertension (measured or treated) | 556 | 55.9 | 520 | 51.6 | 474 | 46.9 | 446 | 44.5 | <0.001 |
| Medication use | |||||||||
| Statins | 120 | 12.1 | 111 | 11.0 | 119 | 11.8 | 83 | 8.3 | 0.026 |
| ACE inhibitors | 100 | 10.1 | 88 | 8.7 | 66 | 6.5 | 75 | 7.5 | 0.025 |
| Beta-blockers | 114 | 11.5 | 98 | 9.7 | 64 | 6.3 | 82 | 8.2 | <0.001 |
| Angiotensin II receptor antagonists | 19 | 1.9 | 27 | 2.7 | 18 | 1.8 | 21 | 2.1 | 0.514 |
| Regular aspirin use | 246 | 24.7 | 216 | 21.4 | 229 | 22.7 | 244 | 24.4 | 0.261 |
| Hormone therapy use at WHI baseline | <0.001 | ||||||||
| Nonuser | 475 | 47.8 | 459 | 45.5 | 412 | 40.8 | 408 | 40.7 | |
| Estrogen alone | 238 | 23.9 | 224 | 22.2 | 231 | 22.8 | 220 | 22.0 | |
| Estrogen + progesterone | 281 | 28.3 | 325 | 32.2 | 368 | 36.4 | 373 | 37.2 | |
| Unknown type | 1 | 0.1 | |||||||
Values are n (%). The p values were based on chi-square tests that excluded the unknown category.
ACE = angiotensin converting enzyme; BMI = body mass index; GED = general education degree; MET = metabolic equivalent task; MI = myocardial infarction; WHI = Women’s Health Initiative.
Age-adjusted analyses
There was a decreasing trend in age-adjusted HR for the primary endpoint of CVEs (HR: 0.77 [95% CI: 0.58 to 1.03], 0.75 [95% CI: 0.56 to 0.99], 0.59 [95% CI: 0.43 to 0.80]; p for trend = 0.001) across increasing quartiles of exercise, compared with the referent first quartile (Table 2). Findings were similar for secondary endpoints of cardiovascular death (HR: 0.68 [95% CI: 0.47 to 0.98], 0.56 [95% CI: 0.38 to 0.82], 0.62 [95% CI: 0.43 to 0.90] p = 0.022) across increasing quartiles of physical activity; and CHD death (HR: 0.59 [95% CI: 0.36 to 0.99], 0.45 [95% CI: 0.26 to 0.79], 0.40 [95% CI: 0.22 to 0.72]; p = 0.003). There was a similar but nonsignificant age-adjusted trend for HF (p = 0.08) and nonsignificant age-adjusted associations for MI (p = 0.26).
Table 2.
Age-Adjusted and Multivariable-Adjusted Hazard Ratios of Cardiovascular Events According to Categories of Pre-Diagnosis Exercise (MET h/week)
| Total (N = 4,015) | MET h/week |
p Value for Trend | ||||
|---|---|---|---|---|---|---|
| <2.50 (n = 994) | 2.50 to <8.625 (n = 1,008) | 8.625 to <18.00 (n = 1,011) | ≥18.00 (n = 1,002) | |||
| Median MET h/week | 8.67 | 0.0 | 5.25 | 13.00 | 26.33 | |
| Cardiovascular events∗ | ||||||
| No. of events (annualized %) | 342 (1.14) | 103 | 88 | 86 | 65 | |
| Age-adjusted HR (95% CI) | Ref. | 0.77 (0.58–1.03) | 0.75 (0.56–0.99) | 0.59 (0.43–0.80) | 0.001 | |
| Multivariable-adjusted HR (95% CI)† | Ref. | 0.80 (0.59–1.09) | 0.86 (0.64–1.17) | 0.63 (0.45–0.88) | 0.016 | |
| MI | ||||||
| No. of events (annualized %) | 89 (0.29) | 25 | 22 | 24 | 18 | |
| Age-adjusted HR (95% CI) | Ref. | 0.79 (0.45–1.40) | 0.84 (0.48–1.48) | 0.67 (0.37–1.24) | 0.262 | |
| Multivariable-adjusted HR (95% CI)† | Ref. | 0.83 (0.44–1.53) | 1.05 (0.57–1.92) | 0.68 (0.34–1.36) | 0.373 | |
| Heart failure | ||||||
| No. of events (annualized %) | 49 (0.16) | 18 | 11 | 12 | 8 | |
| Age-adjusted HR (95% CI) | Ref. | 0.58 (0.27–1.22) | 0.63 (0.30–1.31) | 0.43 (0.19–1.00) | 0.075 | |
| Multivariable-adjusted HR (95% CI)† | Ref. | 0.64 (0.29–1.43) | 0.94 (0.43–2.04) | 0.57 (0.23–1.44) | 0.366 | |
| Cardiovascular death | ||||||
| No. of events (annualized %) | 215 (0.44) | 69 | 54 | 45 | 47 | |
| Age-adjusted HR (95% CI) | Ref. | 0.68 (0.47–0.98) | 0.56 (0.38–0.82) | 0.62 (0.43–0.90) | 0.022 | |
| Multivariable-adjusted HR (95% CI)‡ | Ref. | 0.73 (0.50–1.06) | 0.60 (0.40–0.90) | 0.69 (0.46–1.04) | 0.109 | |
| CHD death (annualized %) | ||||||
| No. of events | 96 (0.22) | 36 | 25 | 19 | 16 | |
| Age-adjusted HR (95% CI) | Ref. | 0.59 (0.36–0.99) | 0.45 (0.26–0.79) | 0.40 (0.22–0.72) | 0.003 | |
| Multivariable-adjusted HR (95% CI)‡ | Ref. | 0.65 (0.38–1.10) | 0.46 (0.25–0.83) | 0.41 (0.21–0.78) | 0.006 | |
Annualized percentage is the total number with the event divided by the total person-years for all women at risk for the event.
CHD = coronary heart disease; CI = confidence interval; HR = hazard ratio; MET = metabolic equivalent task; MI = myocardial infarction; WHI = Women’s Health Initiative.
Cardiovascular events include heart failure, myocardial infarction, angina, coronary revascularization, peripheral artery disease, carotid artery disease, transient ischemic attack, stroke, and cardiovascular death.
Follow-up through September 30, 2010. Adjusted for age at WHI enrollment (continuous), race (white, black, Hispanic, other), smoking status (never, past, current), body mass index (<25, 25 to <30, ≥30 kg/m2), stage (localized, regional), education (less than high school, high school diploma/GED, school after high school, college degree or higher), study (HT randomized; DM randomized, not in HT; OS enrolled), hormone therapy/trial arm (non-user, estrogen-alone, estrogen + progestin), family history of MI, and comorbidities index (count of diabetes, hypertension, treated hypercholesterolemia, history of chronic obstructive pulmonary disease (COPD), lupus/rheumatoid arthritis, history of liver disease, history of stomach ulcer). Models are stratified by age at diagnosis (50 to 59, 60 to 64, 65 to 69, 70 to 74, ≥75 years) and extension study participation (yes/no), allowing the baseline hazards to vary in these strata.
Deaths as of September 30, 2015, including from NDI searches. Adjusted for age at WHI enrollment (continuous), race (white, black, Hispanic, other), smoking status (never, past, current), body mass index (<25, 25 to <30, ≥30 kg/m2), stage (localized, regional), education (less than high school, high school diploma/GED, school after high school, college degree or higher), study (HT randomized; DM randomized, not in HT; OS enrolled), hormone therapy/trial arm (nonuser, estrogen-alone, estrogen + progestin), family history of MI, and comorbidities index (count of diabetes, hypertension, treated hypercholesterolemia, history of COPD, lupus/rheumatoid arthritis, history of liver disease, history of stomach ulcer). Models are stratified by age at diagnosis (50 to 59, 60 to 64, 65 to 69, 70 to 74, ≥75 years).
Multivariable-adjusted analyses
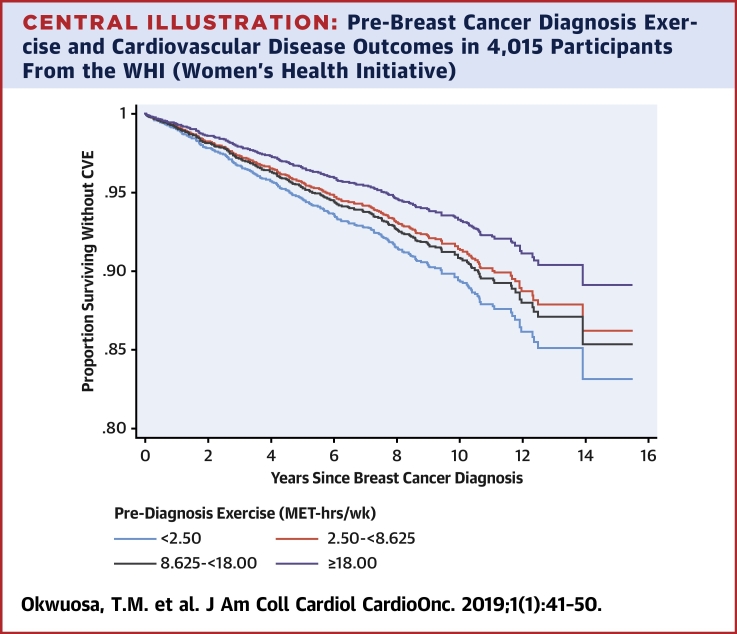
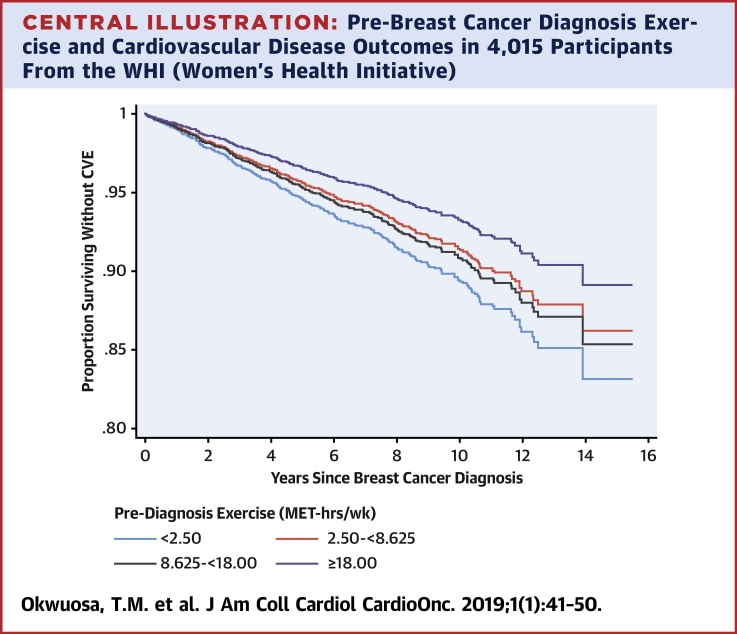
Compared with the referent first quartile, there was a decrease in HR across increasing quartiles of exercise for the primary endpoint (HR: 0.80 [95% CI: 0.59 to 1.09 for the second quartile]; 0.86 [95% CI: 0.64 to 1.17 for the third quartile); and 0.63 [95% CI: 0.45 to 0.88 for the fourth quartile]; p for trend = 0.016) (Central Illustration). A similar relationship was observed for CHD death (HR: 0.65 [95% CI: 0.38 to 1.10 for the second quartile]; 0.46 [95% CI: 0.25 to 0.83 for the third quartile]; and 0.41 [95% CI: 0.21 to 0.78 for the fourth quartile]; p = 0.006) as shown in Table 2. The p values for trend across increasing quartiles of exercise for MI, HF, or CVD death were not statistically significant.
Central Illustration.
Pre-Breast Cancer Diagnosis Exercise and Cardiovascular Disease Outcomes in 4,015 Participants From the WHI (Women’s Health Initiative)
Estimated survival probabilities from multivariable Cox models in relation to quartiles of pre-breast cancer diagnosis exercise exposure (MET h/week). Figure details the proportion surviving without a CVE (first occurrence of newly diagnosed heart failure, myocardial infarction, angina, coronary revascularization, peripheral artery disease, carotid artery disease, transient ischemic attack, stroke, or cardiovascular death occurring after diagnosis of breast cancer [n = 342 with CVD]). CVD = cardiovascular disease; CVE = cardiovascular event; IQR = interquartile range; MET = metabolic equivalent task; WHI = Women’s Health Initiative.
CVEs based on exercise guidelines
In multivariable analyses, only CHD death remained statistically significant (HR: 0.56 [95% CI: 0.35 to 0.89]; p = 0.014) when high exercise level was compared with low exercise level (Table 3). The multivariable HRs for the primary endpoint and cardiovascular death were lower (0.84 [95% CI: 0.67 to 1.06] and 0.79 [95% CI: 0.35 to 0.89], respectively), but this did not reach significance.
Table 3.
Multivariable-Adjusted Hazard Ratios of Cardiovascular Events According to National Exercise Guidelines (Pre-Diagnosis Exercise Level) for Breast Cancer for Breast Cancer Patients (N = 4,015)
| MET h/week |
p Value | ||
|---|---|---|---|
| <9 (n = 2,039) | ≥9 MET (n = 1,976) | ||
| Median MET h/week | 2 | 18 | |
| Cardiovascular events∗ | |||
| No. of events | 193 | 149 | |
| Age-adjusted HR (95% CI) | Ref | 0.77 (0.62–0.95) | 0.015 |
| Multivariable-adjusted HR (95% CI)† | Ref | 0.84 (0.67–1.06) | 0.153 |
| MI | |||
| No. of events | 47 | 42 | |
| Age-adjusted HR (95% CI) | Ref | 0.89 (0.58–1.34) | 0.574 |
| Multivariable-adjusted HR (95% CI)† | Ref | 0.99 (0.62–1.58) | 0.970 |
| Heart failure | |||
| No. of events | 29 | 20 | |
| Age-adjusted HR (95% CI) | Ref | 0.71 (0.40–1.25) | 0.237 |
| Multivariable-adjusted HR (95% CI)† | Ref | 0.95 (0.51–1.78) | 0.885 |
| Cardiovascular death | |||
| No. of events | 123 | 92 | |
| Age-adjusted HR (95% CI) | Ref | 0.74 (0.57–0.97) | 0.031 |
| Multivariable-adjusted HR (95% CI)‡ | Ref | 0.79 (0.59–1.06) | 0.117 |
| CHD death | |||
| No. of events | 61 | 35 | |
| Age-adjusted HR (95% CI) | Ref | 0.57 (0.37–0.86) | 0.007 |
| Multivariable-adjusted HR (95% CI)‡ | Ref | 0.56 (0.35–0.89) | 0.014 |
CHD = coronary heart disease; CI = confidence interval; HR = hazard ratio; MET = metabolic equivalent task; MI = myocardial infarction; WHI = Women’s Health Initiative.
Cardiovascular events include heart failure, myocardial infarction, angina, coronary revascularization, peripheral artery disease, carotid artery disease, transient ischemic attack, stroke, and cardiovascular death.
Follow-up through September 30, 2010. Adjusted for age at WHI enrollment (continuous), race (white, black, Hispanic, other), smoking status (never, past, current), body mass index (<25, 25 to <30, ≥30 kg/m2), stage (localized, regional), education (less than high school, high school diploma/GED, school after high school, college degree or higher), study (HT randomized; DM randomized, not in HT; OS enrolled), hormone therapy/trial arm (nonuser, estrogen-alone, estrogen + progestin), family history of MI, and comorbidities index (count of diabetes, hypertension, treated hypercholesterolemia, history of COPD, lupus/rheumatoid arthritis, history of liver disease, history of stomach ulcer). Models are stratified by age at diagnosis (50 to 59, 60 to 64, 65 to 69, 70 to 74, ≥75 years) and extension study participation (yes/no), allowing the baseline hazards to vary in these strata.
Deaths as of September 30, 2015, including from National Death Index (NDI) searches. Adjusted for age at WHI enrollment (continuous), race (white, black, Hispanic, other), smoking status (never, past, current), body mass index (<25, 25 to <30, ≥30 kg/m2), stage (localized, regional), education (less than high school, high school diploma/GED, school after high school, college degree or higher), study (HT randomized; DM randomized, not in HT; OS enrolled), hormone therapy/trial arm (nonuser, estrogen-alone, estrogen + progestin), family history of MI, and comorbidities index (count of diabetes, hypertension, treated hypercholesterolemia, history of COPD, lupus/rheumatoid arthritis, history of liver disease, history of stomach ulcer). Models are stratified by age at diagnosis (50 to 59, 60 to 64, 65 to 69, 70 to 74, ≥75 years).
In a sensitivity analysis of Medicare subset of 1,603 participants, in which we adjusted for breast cancer-treatment variables, including chemotherapy and/or radiation therapy, and surgery, our findings were similar (Supplemental Tables 1 to 3).
Discussion
In this large prospective observational cohort study, pre-diagnosis exercise was associated with substantial graded reductions in composite of subsequent newly diagnosed, first-incident cardiovascular events or CHD death in patients with primary breast cancer (Central Illustration). Conversely, meeting national exercise guidelines for healthy adults was associated with decreased risk of CHD death but not the primary endpoint of CVEs. This study is the first to show that exposure to exercise before a diagnosis of cancer may protect against or mitigate the established adverse cardiovascular consequences observed in patients with breast cancer, adding to the growing evidence base supporting the importance of exercise to prevent CVEs in high-risk populations.
As hypothesized, our primary analyses demonstrated that pre-diagnosis exercise was associated with a 20% to 37% reduction in the risk of CVEs, even after controlling for important clinical covariates including pre-existing cardiovascular risk factors and other chronic health conditions. However, risk of individual events—specifically, MI and HF—were not affected, suggesting that exercise may confer greater risk reduction in other events included in the composite endpoint, such as angina, coronary revascularization, PAD, or stroke. Isolating the impact of exercise on these individual conditions was not possible, however, because of a low number of events. Given that this is the first study to investigate the impact of pre-diagnosis exercise on CVEs in patients with cancer, direct comparisons with previous work is not possible. Nevertheless, 2 studies have examined the impact of post-diagnosis, post-adjuvant therapy exercise exposure on CVD-specific morbidity and mortality in adult patients with cancer 5, 11. Congruent with the current data, both studies found that exercise was associated with between a 9% to 35% and 13% to 53% reduction in a composite measure of CVEs among 2,973 patients with primary breast cancer (median age of 57 years, 8.6 years median follow-up) (5) and 1,187 adult survivors of Hodgkin’s lymphoma (median age of 31.2 years, 11.9 years median follow-up) (11), respectively. Despite similar magnitude of risk reduction, there were notable differences between the findings related to post-diagnosis exercise compared with pre-diagnosis exercise in the current study. Specifically, in this study, for the primary endpoint, only 18 MET hours per week were associated with a significant reduction in risk of composite CVEs. This is in contrast to our previous findings, in which 9 MET h/week, approximately equivalent to the Centers for Disease Control (CDC) guidelines for healthy adults, was sufficient to significantly reduce risk. The reasons for these discrepancies are not clear but may relate to methodological differences across studies pertaining to exercise assessment, categorization, as well as ascertainment and classification of CVEs. Nevertheless, although the current findings suggest that protection from breast-cancer–related cardiovascular injury requires 18 MET h/week —double the current CDC guidelines—it is noteworthy that 9 MET h/week is the minimal recommended level of exercise, and greater benefits are observed above this level. As such, the findings of our study still support current guideline recommendations.
From a mechanistic perspective, the protective effects of pre-diagnosis exercise may work in a distinct but complementary manner of exercise performed during cytotoxic therapy. First, patients engaging in higher pre-diagnosis exercise will likely have a more favorable cardiovascular risk profile, including higher cardiorespiratory fitness (or cardiovascular reserve capacity) in comparison with less active or inactive patients. It is conceivable that patients with higher cardiovascular reserve capacity tolerate the unique and varying degrees of direct cardiovascular toxic effects of locoregional and systemic therapy (12). However, data to support the notion that women with higher cardiorespiratory fitness at diagnosis experience less treatment-related toxicities are not currently available. Another explanation is that patients engaging in regular exercise before diagnosis have higher proclivity to maintain regular exercise post-diagnosis during adjuvant therapy; exercise data collected on a small subsample of patients in this study support this notion (data not shown). Randomized trials demonstrate that structured aerobic training attenuates treatment-induced declines in cardiorespiratory fitness and other markers of cardiovascular health (e.g., endothelial function, resting heart rate, body composition) compared with nonexercising, usual care in patients undergoing conventional combination chemotherapy for primary breast cancer 13, 14, 15, 16, 17. Whether exercise attenuation of cancer therapy-associated cardiovascular injury lowers long-term CVEs is not known.
There is an anticipated substantial increase in the number of patients at high risk of cardiovascular toxicity over the next 20 years (18). As such, the growing importance of late-occurring cardiovascular toxicity in breast as well as a number of long-term cancer survivors cannot be understated, as such toxicities may be starting to—or have the potential to—offset further improvements in overall survival witnessed over the past 4 decades (3). Our initial data, together with previous epidemiological findings and data from a growing number of small clinical trials creates a strong rationale to develop a research agenda to investigate comprehensively the role of pre-diagnosis cardiovascular risk profiles as well as exposure to nonpharmacological and pharmacological cardiovascular protective therapies on subsequent incidence of CVEs, together with role of such factors after diagnosis and across the treatment continuum.
Study limitations
Our study has limitations. Of these, the most important is that exercise exposure was assessed by a self-administered questionnaire with well-known limitations, and therefore some misclassification of exposure is possible. In addition, residual confounding through the impact of other lifestyle factors is likely. Although assessment of pre-diagnosis exercise is less prone to reverse causality in comparison with exercise assessed in the post-diagnosis setting, it is not possible to delineate whether higher exercise exposure reflects lower CV or other chronic disease burden as opposed to a direct exercise-induced effect. Only randomized trial data can answer this question. Our study questionnaire only assessed exercise exposure that ranged from immediately to 5 years before diagnosis of breast cancer, creating interpatient heterogeneity in timing of assessment of exposure to exercise. Our study also lacked consideration of modifiable risk factors.
Conclusions
Pre-diagnosis exercise exposure may be a strategy to lower CVD risk in patients with primary breast cancer. These findings add to the growing evidence highlighting the importance of exercise to manage cancer treatment-related acute and chronic late effects in the large and growing number of cancer survivors.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In this prospective cohort study of 4,015 women from the Women’s Health Initiative Study, pre-diagnosis exercise in breast cancer patients is associated with a significant graded reduction in subsequent cardiovascular events.
TRANSLATIONAL OUTLOOK: Randomized clinical trials are necessary to determine the cardioprotective benefits of exercise in patients with cancer and survivors.
Acknowledgments
The authors thank the WHI investigators and staff for their dedication and the study participants for making the program possible. A short list of WHI investigators follows: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller. Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, Washington) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, Massachusetts) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, California) Marcia L. Stefanick; (The Ohio State University, Columbus, Ohio) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, Arizona) Cynthia A. Thomson; (University at Buffalo, Buffalo, New York) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, Florida) Marian Limacher; (University of Iowa, Iowa City/Davenport, Iowa) Robert Wallace; (University of Pittsburgh, Pittsburgh, Pennsylvania) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker. Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, North Carolina) Sally Shumaker.
Footnotes
The Women's Health Initiative (WHI) program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, and HHSN268201100004C. Dr. Jones is supported by research grants from the National Cancer Institute, AKTIV Against Cancer, and the Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). The opinions expressed are those of the authors and do not necessarily reflect the views of the U.S. Department of Health and Human Services or the National Institutes of Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
For a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Howlader N., Noone A.M., Krapcho M. National Cancer Institute; Bethesda, MD: 2016. SEER Cancer Statistics Review, 1975–2013. [Google Scholar]
- 2.Patnaik J.L., Byers T., DiGuiseppi C., Dabelea D., Denberg T.D. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu J., Wu L., Jiang M. Real-world impact of non-breast cancer-specific death on overall survival in resectable breast cancer. Cancer. 2017 doi: 10.1002/cncr.30617. [DOI] [PubMed] [Google Scholar]
- 4.Lee D.C., Pate R.R., Lavie C.J., Sui X., Church T.S., Blair S.N. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64:472–481. doi: 10.1016/j.jacc.2014.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones L.W., Habel L.A., Weltzien E. Exercise and risk of cardiovascular events in women with nonmetastatic breast cancer. J Clin Oncol. 2016;34:2743–2749. doi: 10.1200/JCO.2015.65.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy A.C., GulAcsi B.P., CserEp Z., Hangody L., Forster T. Late cardiac effect of anthracycline therapy in physically active breast cancer survivors: a prospective study. Neoplasma. 2017;64:92–100. doi: 10.4149/neo_2017_111. [DOI] [PubMed] [Google Scholar]
- 7.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.McTiernan A., Kooperberg C., White E. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women's Health Initiative Cohort Study. JAMA. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 9.Ainsworth B.E., Haskell W.L., Leon A.S. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exercise. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Johnson-Kozlow M., Rock C.L., Gilpin E.A., Hollenbach K.A., Pierce J.P. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- 11.Jones L.W., Liu Q., Armstrong G.T. Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32:3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeely M.L., Campbell K.L., Rowe B.H., Klassen T.P., Mackey J.R., Courneya K.S. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34–41. doi: 10.1503/cmaj.051073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones L.W., Fels D.R., West M. Modulation of circulating angiogenic factors and tumor biology by aerobic training in breast cancer patients receiving neoadjuvant chemotherapy. Cancer Prev Res. 2013;6:925–937. doi: 10.1158/1940-6207.CAPR-12-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courneya K.S., Segal R.J., Mackey J.R. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25:4396–4404. doi: 10.1200/JCO.2006.08.2024. [DOI] [PubMed] [Google Scholar]
- 15.Segal R., Evans W., Johnson D. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. J Clin Oncol. 2001;19:657–665. doi: 10.1200/JCO.2001.19.3.657. [DOI] [PubMed] [Google Scholar]
- 16.Courneya K.S., McKenzie D.C., Mackey J.R. Effects of exercise dose and type during breast cancer chemotherapy: multicenter randomized trial. J Natl Cancer Inst. 2013;105:1821–1832. doi: 10.1093/jnci/djt297. [DOI] [PubMed] [Google Scholar]
- 17.van Waart H., Stuiver M.M., van Harten W.H. Effect of low-intensity physical activity and moderate- to high-intensity physical exercise during adjuvant chemotherapy on physical fitness, fatigue, and chemotherapy completion rates: results of the PACES randomized clinical trial. J Clin Oncol. 2015;33:1918–1927. doi: 10.1200/JCO.2014.59.1081. [DOI] [PubMed] [Google Scholar]
- 18.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA: Cancer J Clin 2016;66:271–89. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.