Abstract
CD19-specific chimeric antigen receptor (CAR) T cell therapies have shown remarkable early success in highly refractory and relapsing hematological malignancies. However, this potent therapy is accompanied by significant toxicity. Cytokine release syndrome and neurotoxicity are the most widely reported, but the true extent and characteristics of cardiovascular toxicity remain poorly understood. Thus far, adverse cardiovascular effects observed include sinus tachycardia and other arrhythmias, left ventricular systolic dysfunction, profound hypotension, and shock requiring inotropic support. The literature regarding cardiovascular toxicities remains sparse; prospective studies are needed to define the cardiac safety of CAR T cell therapies to optimally harness their potential. This review summarizes the current understanding of the potential cardiovascular toxicities of CD19-specific CAR T cell therapies, outlines a proposed cardiac surveillance protocol for patients receiving this new treatment, and provides a roadmap of the future direction of cardio-oncology research in this area.
Key Words: cardiomyopathy, cytokine release syndrome, immunotherapy, leukemia, lymphoma
Abbreviations and Acronyms: ALL, acute lymphoblastic leukemia; CAR, chimeric antigen receptor; CI, confidence interval; CMR, cardiac magnetic resonance imaging; CR, complete response; CRS, cytokine release syndrome; IL, interleukin; LVSD, left ventricular systolic dysfunction; TTE, transthoracic echocardiography
Central Illustration
Highlights
-
•
CD19-specific CAR T cell therapies represent a new breakthrough in the treatment of relapsing and refractory hematological malignancies.
-
•
The adverse effects observed in early trials and recent retrospective studies have suggested that CAR T cell therapies are associated with cardiovascular toxicity.
-
•
Prospective studies using multimodality cardiac imaging and novel and established biomarkers are needed to define the cardiac safety of CAR T cell therapies to fully harness their life-saving potential.
Genetically modified chimeric antigen receptor (CAR) T cells specifically targeting CD19 have shown remarkable promise in the treatment of highly refractory and relapsing hematological malignancies in pediatric and adult populations, including acute lymphoblastic leukemia (ALL) and large B-cell lymphoma. However, this potent therapy is tempered by serious, potentially life-threatening complications (1). Cytokine release syndrome (CRS) and neurotoxicity are the most common and widely appreciated, but the true extent and characteristics of cardiovascular toxicities remain poorly defined. This review appraises the current literature on the latter and seeks to outline research priorities in cardiotoxicity to maximize the cardiovascular safety of CAR T cell therapies.
CAR T Cell Therapy
A CAR is a recombinant fusion protein that is capable of activating T cells upon recognition of a specific antigen, resulting in the killing of target cells. The first CAR designs aimed at combating cancer emerged in the late 1980s and early 1990s, but only recent designs have seen significant clinical success. In particular, immense excitement has been generated by the early success of CD19-specific CAR T cells in the treatment of highly refractory and relapsing hematological malignancies (2,3). CD19 is an effective target because it is expressed throughout B-cell lineage development and has frequent and high-level expression on the surface of nearly all B-cell malignancies. In addition, it is not found on other normal tissues, including the heart, and is not shed as a soluble form (4).
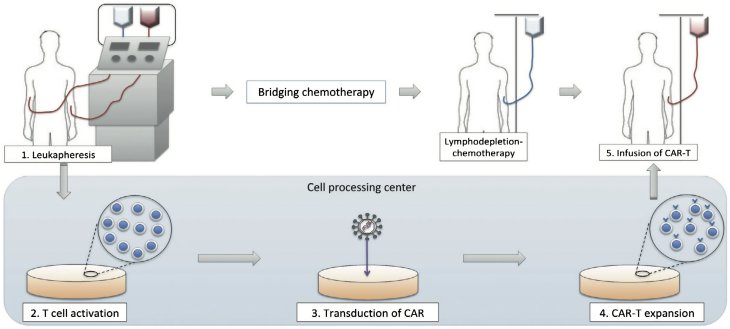
The process of manufacturing CAR T cells requires 2 to 3 weeks (Figure 1). Autologous T cells are first collected from the patient and then genetically modified ex vivo with lentiviral or retroviral vectors to re-program the T cells to recognize tumor cells expressing a tumor-associated antigen (in this context, CD19). The CAR T cells undergo rapid multiplication to generate therapeutic quantities before being administered to the patient in a single infusion (3,5).
Figure 1.
Manufacturing CAR T Cell Therapy
Autologous T cells are collected from the patient. A chimeric receptor antibody (CAR) that recognizes CD19 is inserted into the cell surface using lentiviral or retroviral vectors. The CAR T cells undergo ex vivo rapid multiplication to generate therapeutic quantities. The patient undergoes lymphodepleting chemotherapy (usually a combination of fludarabine and cyclophosphamide) before receiving an infusion of the CAR T cells to reduce the native population of T cells, which in turn promotes CAR T-cell expansion.
Reproduced from Makita et al. (45) with permission.
Before the infusion of CAR T cells, patients undergo lymphodepleting chemotherapy, most commonly with a combination of fludarabine and cyclophosphamide. This approach is to suppress the patient’s endogenous T cell compartment, which in turn promotes the in vivo expansion of the transferred CAR T cell product (6).
Two CD19-specific CAR T cell therapies have now been licensed for use with hematological cancers: axicabtagene ciloleucel (Yescarta, Gilead Sciences, Inc., Foster City, California) and tisagenlecleucel (Kymriah, Novartis Pharmaceuticals Corporation, East Hanover, New Jersey) have both received approval by the U.S. Food and Drug Administration and the European Commission and European Medicines Agency during 2017 and 2018. Axicabtagene ciloleucel has been approved for the treatment of relapsed/refractory large B-cell lymphoma in adult patients; tisagenlecleucel has been approved for the treatment of both the former and in relapsed/refractory B-cell precursor ALL in pediatric and young adult patients up to 25 years of age.
The unprecedented response to CAR T cell therapies in these studies must be appreciated in the context of a patient population in which curative options are limited or even exhausted, and for whom palliative therapy would usually be the mainstay of treatment. The early pivotal studies that established the efficacy of CAR T cell therapies in this patient cohort achieved complete remission (CR) rates of 52% to 90% with responses maintained in the range of 39% to 50% at 1 year. Retrospective analyses of adults with relapsed/refractory large B-cell lymphoma suggest, in stark contrast to those receiving CAR T cell therapies, an objective response of 26% and CR in only 7% with conventional (non-CAR) therapies. The poor response to conventional therapies was associated with a median survival of 6.3 months, and low 1- and 2-year survival rates of 28% and 20%, respectively (7); this should be compared with the 76% and 50% survival rates reported in the statistical analyses of the early pivotal CAR T cell therapy trial cohorts (8,9).
Tisagenlecleucel
Tisagenlecleucel was developed through a collaboration between the University of Pennsylvania and Novartis. It achieved early success through its pilot study within the Philadelphia Children’s Hospital with a 90% CR rate in a pediatric and young adult population with relapsed/refractory B-cell ALL (10). Thereafter, it became the first CAR T cell product to receive U.S. Food and Drug Administration approval following the single-arm, global multicenter ELIANA (Determine Efficacy and Safety of CTL019 in Pediatric Patients With Relapsed and Refractory B-cell ALL and High Risk B-cell ALL at First Relapse. Determine Feasibility and Safety of CTL019 Therapy in Pediatric Patients With High Risk B-cell ALL That Relapsed < 6 Months Post All-HSCT) study. Sixty-one (81%) of its 75 patients with follow-up out to 3 months after infusion were in ongoing CR. Relapse-free survival, in those with a response to treatment, at 6 and 12 months was 80% and 59%, respectively (8).
Tisagenlecleucel has also been tested in adult populations through the JULIET (Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients) study, a Phase II, single-arm, global multicenter study. The analysis of 93 adults with relapsed/refractory diffuse large B-cell lymphoma with follow-up out to 3 months demonstrated either complete or partial responses in 52% of the 93 patients (11,12).
Axicabtagene ciloleucel
Axicabtagene ciloleucel is another CD19-targeting CAR T cell therapy and has been explored in adult populations with large B-cell lymphomas. The initial results of the single-arm, global and multicenter ZUMA-1 (Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma) study were published in 2017 and the 2-year follow-up analysis was published in 2019. Of the 101 patients who were assessed after infusion with axicabtagene ciloleucel, 83% (n = 84) reached the primary endpoint of an objective response at 1 month (a composite of both CR [n = 59, 58%] and partial response). Median progression-free survival in the whole cohort was reported as 5.9 months with the duration of response lasting 11.1 months (9,13). Subgroup analysis at 24 months indicates that in patients who achieve CR, the progression-free survival is estimated to be 72.0% (95% confidence interval [CI]: 56.0% to 83.0%), and although the median overall survival was not reached during the study period, this was estimated to be 50.5% (95% CI: 40.2% to 59.7%). These findings illustrate the durability of responses that can be achieved after a single infusion of CAR T cells.
Adverse Effects of CAR T Cell Therapy
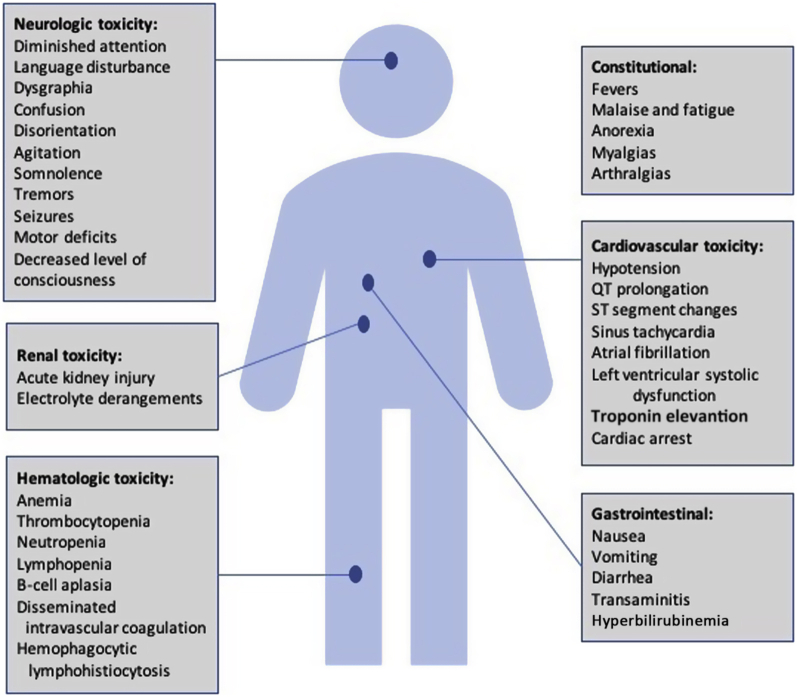
The utilization of CAR T-cell therapies has, however, been accompanied by significant toxicities (Table 1, Figure 2). The most common and well-appreciated is that of CRS. The pivotal ZUMA-1 and JULIET studies of CAR T-cell therapies in adults with relapsed/refractory B-cell lymphoma were complicated by CRS in 93% and 58% of their patient cohorts, respectively; severe grade 3 or 4 CRS was only noted in 13% and 22%, however (13,14). Similarly, the rate of CRS in the ELIANA study was 77% in its pediatric cohort with relapsed/refractory ALL (8).
Table 1.
CD19-Specific CAR T Cell Therapy Toxicities
| Maude et al. (10), 2014 (N = 30) | Neelapu et al. (ZUMA-1) (13), 2017 (N= 101) | Fitzgerald et al. (25), 2017 (N= 39) | Maude et al. (ELIANA) (8), 2018 (N = 75) | Burstein et al. (24), 2018 (N = 98) | Schuster et al. (JULIET) (11,12), 2018/2019 (N = 93) | Alvi et al. (26), 2019 (N = 137) | |
|---|---|---|---|---|---|---|---|
| Cardiovascular toxicities | |||||||
| Profound hypotension requiring inotropic support or shock | 27% | 14% | 33% | 17% | 24% | 9% | 4% (6 patients developed shock leading to cardiac deaths) |
| Left ventricular systolic dysfunction | — | — | 2% | — | 10% | — | 6% |
| Pulmonary edema | — | — | — | 6% | — | — | 4% (shortness of breath, hypoxia, signs of volume overload, and NT-proBNP >3000 pg/ml) |
| Fluid overload | — | — | 5% | ||||
| ECG and rhythm abnormalities | — | — | Sinus tachycardia (median peak HR 170 beats/min) | — | ST- segment changes (18%) | — | New-onset arrhythmias including supraventricular tachycardia, atrial fibrillation, or flutter (4%) |
| Biomarker abnormalities | — | — | — | — | NT-proBNP (92%) Lactate (79%) Mixed venous saturation (52%) |
- | Troponin elevation (54%) |
| Other toxicities | |||||||
| Cytokine release syndrome | 100% | 93% | 92% | 77% | — | 58% | 59% |
| Neurotoxicity | 43% | 64% | 33% | 40% | — | 21% | — |
| Management | |||||||
| Treatment with tocilizumab | 30% | 43% | 33% | 37% | 21% | 14% | 41% |
| Treatment with corticosteroids | 20% | 27% | 21% | — | — | 10% | — |
| Treatment with cardioprotective medications | — | — | — | — | Beta-blockers (21%) ACE inhibitors (17%) |
— | — |
CD19-specific chimeric antigen receptor (CAR) T cell therapy is associated with toxicities, including cytokine release syndrome, neurotoxicity, and a number of cardiovascular complications as shown in the early pivotal trials.
— = data have not been studied or reported within the publication; ACE = angiotensin-converting enzyme; ECG = electrocardiography; ELIANA = Determine Efficacy and Safety of CTL019 in Pediatric Patients With Relapsed and Refractory B-cell ALL and High Risk B-cell ALL at First Relapse. Determine Feasibility and Safety of CTL019 Therapy in Pediatric Patients With High Risk B-cell ALL That Relapsed <6 Months Post All-HSCT; HR = heart rate; JULIET = Study of Efficacy and Safety of CTL019 in Adult DLBCL Patients; NT-proBNP = N-terminal pro–B-type natriuretic peptide; ZUMA-1 = Long-Term Safety and Activity of Axicabtagene Ciloleucel in Refractory Large B-Cell Lymphoma.
Figure 2.
CAR T Cell Toxicities
Significant multiorgan toxicities can be associated with chimeric receptor antibody (CAR) T-cell therapy.
Cytokine release syndrome
CRS is a phenomenon that is associated with (but not limited to) CAR T cell therapy. It is also recognized in a number of different settings, including monoclonal antibody therapies such as with the anti-CD20 agent rituximab. The clinical syndrome occurs in response to widespread release of inflammatory cytokines and chemokines when large numbers of lymphocytes (B cells, T cells, or natural killer cells) or myeloid cells (macrophages, dendritic cells, or monocytes) are activated (15); in this setting, it is triggered by activation of T cells upon engagement of the CAR by CD19. This action leads to a release, among others, of interleukin (IL)-2, soluble IL-2Rα, interferon gamma, IL-6, soluble IL-6R, and granulocyte-macrophage colony-stimulating factor by the activated T cells as well as other inflammatory cytokines and chemokines by bystander immune cells (16). In particular, IL-6 has been shown to be an important mediator of toxicity in CRS (15).
Symptoms of CRS can vary significantly. Diagnosing the grade of CRS is key to appropriate management. Early CAR T cell clinical trials produced a number of different CRS grading systems, but there was a lack of consensus and significant variability among them. More recently, the American Society for Transplantation and Cellular Therapy published a consensus grading for CRS (17), the details of which are described in Table 2. CRS is characterized mainly by constitutional symptoms such as high fever, malaise and fatigue, anorexia, myalgias, and nausea. At the other end of the spectrum, CRS can involve any organ system in the body, including the cardiovascular, nervous, respiratory, gastrointestinal, hepatic, renal, and hematological systems.
Table 2.
ASTCT Consensus Grading for Cytokine Release Syndrome
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|
| Fever Temperature ≥38°C With or without constitutional symptoms (which include myalgia, arthralgia, and malaise) |
+ | + | + | + |
| With | ||||
| Hypotension | – | + No vasopressors required |
+ Requiring one vasopressor without vasopressin |
+ Requiring multiple vasopressors (excluding vasopressin) |
| And/or | ||||
| Hypoxia | – | + Requiring low-flow nasal cannula or blow-by |
+ Requiring high-flow nasal cannula, facemask, nonrebreather mask, or Venturi mask |
+ Requiring positive pressure (i.e., CPAP, BiPAP, intubation, mechanical ventilation) |
The assessment and grading of cytokine release syndrome has not been uniform, particularly early on in the experience with CAR T-cell therapy. Subsequently, this has prompted a consensus approach to grading the severity of cytokine release syndrome, which was released by the American Society for Transplantation and Cellular Therapy (ASTCT) in 2019.
BiPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure.
The symptoms typically manifest within days and occasionally weeks after CAR T cell infusion, in keeping with maximal in vivo T cell expansion in some translational studies (15). Research has focused on attempts to identify which groups of patients are at the highest risk for severe CRS to target prevention. High disease burden (and thus antigenic load) remains the strongest risk factor for severity of CRS (18,19), but the dose of cells infused, presence of other comorbidities, and early onset of CRS (within the first 3 days) are also important. Translational research continues in an attempt to identify and validate predictive biomarkers (16,19).
Management of CRS depends on the grade (17) and is outlined in detail in an excellent review by Neelapu et al. (16); it was also explored in detail in the 2019 American Society of Clinical Oncology Educational Book (20). Low-grade CRS can often be managed with supportive care alone, but in more severe cases, blockade of the IL-6 pathway is recommended either with tocilizumab, a monoclonal antibody targeting IL-6 receptors, or with siltuximab, a monoclonal antibody that binds to soluble IL-6. The experience with tocilizumab in CRS is more extensive; it has been approved by the U.S. Food and Drug Administration and has become standard of care for the treatment of CRS complicating CAR T cell therapy (5). However, not all cases of severe CRS will respond to IL-6 pathway interruption; in these cases in which CRS is refractory to tocilizumab, corticosteroid therapy is recommended. Thus far, the use of IL-6 receptor blockade and/or corticosteroid therapy does not result in a higher rate of cancer relapse (21). This has led to the administration of tocilizumab and corticosteroid earlier in the course of CRS. The usual practice in many centers is use of tocilizumab as a first-line therapy in patients who develop grade 2 or higher CRS, with the addition of steroids as a second-line therapy when tocilizumab has been ineffective (16,20). This stepwise approach to the management of CRS is represented in a treatment algorithm in Figure 3.
Figure 3.
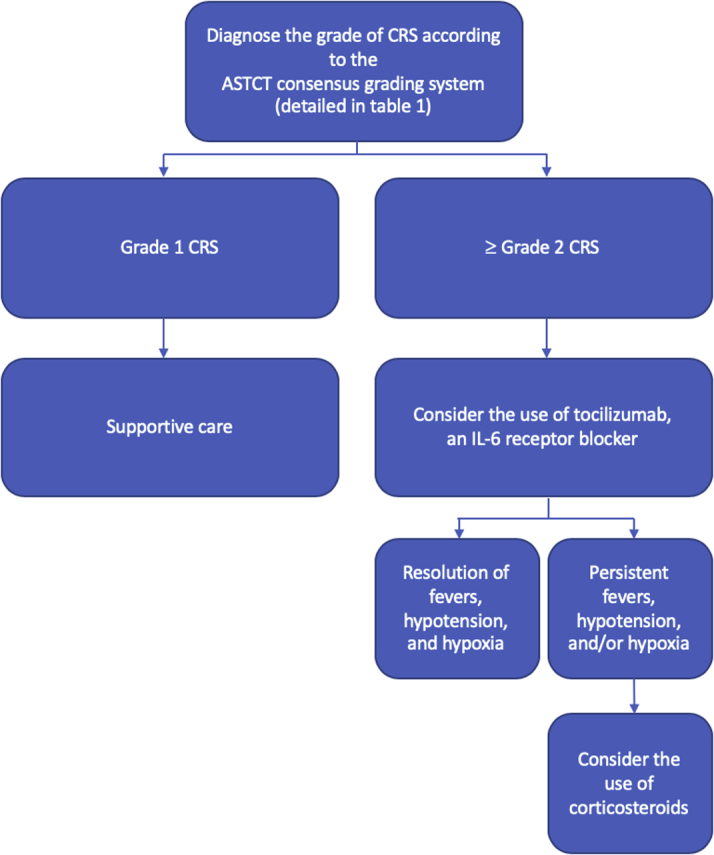
Management of CRS
Low-grade cytokine release syndrome (CRS) can be managed with supportive care alone. Tocilizumab, an interleukin (IL)-6 receptor blocker, is considered as a first line of treatment in patients with grade 2 or higher CRS. When this approach is ineffective, a dose of corticosteroids is the second-line treatment. ASTCT = American Society for Transplantation and Cellular Therapy.
Neurotoxicity
Neurotoxicity or immune effector cell–associated neurotoxicity syndrome (17) is another recognized complication of CAR T cell therapies that can occur in up to 50% of treated patients (18). It has a wide spectrum of manifestations. Early signs include diminished attention, language disturbance, and dysgraphia before progressing to confusion, disorientation, agitation, aphasia, somnolence, and tremors. At the severe end of the spectrum of this syndrome, patients may experience seizures, motor and sensory deficits, transverse myelitis, and decreased levels of consciousness accompanied by evidence of increased intracranial pressure (16,22). IL-6 has been implicated as a potential underlying mechanism for neurotoxicity, much as it has been for CRS. However, neurotoxicity does not always occur concurrently with CRS and may even be detected in the absence of CRS, suggesting additional or alternative mechanisms (18,23).
Cardiovascular adverse effects
Clinical manifestations
Cardiovascular complications and toxicity are less well defined due to the paucity of data in the literature. Two retrospective studies (24,25) reported by the investigators at the Children’s Hospital of Philadelphia assessed the cardiovascular adverse effects of CAR T cell therapies specifically in pediatric and young adult populations. Until recently, data within the adult population were lacking. However, the retrospective study by Alvi et al. (26) in December 2019 has provided significant new insights. Table 1 provides a summary of the cardiovascular complications that have been observed.
The most prominent cardiovascular complication thus far is profound hypotension requiring vasopressor support and admission to the intensive care unit (24,25). The full spectrum of clinical manifestations, however, is broad. It can include sinus tachycardia (which is often attributed to the CRS fever), elevations in serum troponin levels, left ventricular systolic function (LVSD), decompensated heart failure, new-onset arrhythmias, and cardiovascular death (26,27).
The initial analysis by Fitzgerald et al. (25) in 2017 retrospectively assessed cardiovascular toxicity within 39 pediatric and young adult patients receiving CAR T cell therapy for relapsed/refractory ALL. Tachycardia was observed in the setting of prolonged high fevers, with a median peak heart rate of 170 beats/min. Cardiovascular dysfunction was reported in 36% (n = 14) of the cohort. This was defined in 13 patients by fluid-refractory vasoplegic shock managed with α-agonists and, in 1 patient, by LVSD managed with milrinone. Hypotension always occurred in the setting of fever. It was often resistant to treatment with a single inotropic agent; in 10 of the 17 patients requiring inotropic support, more than one agent was required. Ninety-six percent of the patients who developed cardiovascular dysfunction also had CRS; 18% had CRS grade 3 and 28% had CRS grade 4 according to the Penn Grading Scale for CRS (28).
A second retrospective analysis of the potential cardiovascular toxicity associated with CD19 CAR T cell therapy was published by Burstein et al. (24) in 2018. They described a cohort of 98 patients, of whom 24% (n = 24) developed cardiovascular dysfunction; this dysfunction was defined as significant hypotension requiring inotropic support, which included the use of milrinone, dopamine, epinephrine, norepinephrine, or vasopressin. All of the patients who developed cardiovascular dysfunction had high-grade CRS; 12% had CRS grade 3 and 88% had CRS grade 4 according to the Penn Grading Scale for CRS (28). There were no cardiovascular deaths in this cohort; there was, however, a successfully resuscitated cardiac arrest in one patient 2 months after the CAR T cell infusion. As previously identified in CRS, a heavier burden of disease, defined by a pre-treatment blast percentage >25% on bone marrow biopsy specimen, was the most significant predictor of hypotension requiring inotropic support in this patient cohort (odds ratio: 15.5; 95% CI: 5.1 to 47.1; p < 0.001). Other significant predictors included a lower pre-treatment ejection fraction (p = 0.019) or diastolic dysfunction (p = 0.021) prior to infusion of CAR T cells. In both studies, most of the patients with profound hypotension (93% and 88%, respectively) received tocilizumab (24,25).
Of the patients in the analysis by Burstein et al. (24) who had hypotension requiring inotropic support, at least 41% (n = 10) had new LVSD during hospitalization but not all patients underwent echocardiography. A deterioration in left ventricular systolic function in this study was defined as a decrease in ejection fraction >10% or fractional shortening >5% compared with baseline or an ejection fraction <55% or fractional shortening <28% when previously normal at baseline. Longer term follow-up also exhibited cardiac systolic and diastolic dysfunction in 7% (n = 7) at the time of discharge, and persistent dysfunction in just 1% (n = 1) at 6 months. New electrocardiography abnormalities related to ST-segment changes were present in 18% (n = 6) of patients with hypotension, requiring inotropic support. In the small group of patients who had serum cardiac biomarkers obtained, frequent abnormalities in B-type natriuretic peptide (92% of patients; median 580 pg/mL), lactate (79% of patients; median 3.05 mmol/l), and mixed venous saturations (52% of patients; median 68.5%) were observed (24).
The retrospective review of 137 adult patients by Alvi et al. (26) in December 2019 provided the first description of cardiovascular complications associated with CAR T cell therapy in the adult population outside of case reports and isolated events within the earlier clinical trials. Thirty-eight percent of the patients (n = 53) had pre- and post-CAR T cell infusion troponin levels measured. In 54% (n = 29), the troponin result was elevated after CAR T-cell infusion. Cardiovascular events occurred in 12% (n = 17). There were 6 cardiovascular deaths due to new-onset heart failure, arrhythmias, hypotension, shock, or cardiac arrest; an additional 6 patients developed decompensated cardiac failure requiring intravenous diuretic agents; and 5 patients had new-onset arrhythmias, including supraventricular tachycardias, atrial fibrillation, and atrial flutter.
Patients at higher risk of cardiovascular complications were identified in this study. All cardiovascular events occurred in patients who also had concomitant CRS. Furthermore, troponin elevation after CAR T cell infusion (cardiovascular event rate among patients with positive troponin vs. negative troponin after CAR T cell infusion: 55% vs. 4%; p < 0.001) and the time from onset of CRS to administration of tocilizumab (adjusted odds ratio: 1.22; 95% CI: 1.01 to 1.53; p = 0.022) were both associated with an increased risk of developing a cardiovascular event.
It would seem from the currently available data that cardiovascular toxicities observed in the CD19 CAR T cell space are predominantly noted early and are associated with CRS. Potential long-term and late-onset cardiovascular and systemic adverse effects from CAR T cell therapy remain incompletely described. A retrospective review by Cordeiro et al. (29) published in 2020 examined an 86-patient cohort who had all survived beyond 1 year following CAR T cell infusion. The authors identified the following late adverse events: significant cytopenias, hypogammaglobulinemia, infections, subsequent malignancies, immune-related events, graft-versus-host disease in previous allogeneic stem cell transplant recipients, and neurologic psychiatric events. Importantly, there were no cardiovascular complications producing late morbidity or mortality in the analysis by Cordeiro et al. Therefore, although we still do not have optimal long-term follow-up data in the CAR T cell field regarding long-term toxicity and complications, current evidence suggests that most of the cardiovascular complications associated with this novel therapy are likely to be early post-infusion and transient.
Pathophysiology
The underlying mechanisms of cardiovascular complications, such as refractory hypotension and myocardial toxicity in CAR T cell therapy, remain poorly understood but are likely to be multifactorial. Vascular leak contributes to this clinical phenomenon; significantly positive fluid balances have been reported by Fitzgerald et al. (25). Hu et al. (30) observed a frequent diagnosis of capillary leak syndrome, defined as a triad of hypotension, edema, and hemoconcentration with hypoproteinemia. However, data collection exploring cardiovascular complications has thus far been retrospective and ad hoc. Not all of these patients underwent echocardiography at the time of their hypotensive episodes. It is therefore not yet possible to completely exclude a contribution from LVSD.
It has also been proposed that LVSD in this setting represents a nonspecific stress-induced or Takotsubo cardiomyopathy (15), and not direct toxicity from CAR T cells. Stress-induced or Takotsubo cardiomyopathy is described as acute and transient LVSD associated with catecholamine surges classically precipitated by emotionally or physically stressful events (31). The LVSD associated with CAR T cell therapy occurs within the context of physiological stress from the CRS and has thus far been shown to be reversible by 6-month follow-up (24).
CAR T cell therapies have also been investigated in the treatment of other cancers, including stage III/IV melanoma and high-risk or relapsed myeloma, in which melanoma-associated antigen 3 (MAGE-A3)–specified CARs are used. In a detailed case report, Linette et al. (32) closely examined the histopathology in 2 cardiac deaths in response to MAGE-A3–specified CAR T cell therapy for melanoma and myeloma in 2 separate patients. In both patients, there was robust in vivo expansion of the T cell population with significantly elevated levels of IL-6. The CAR T cell biodistribution was analyzed, and some of the highest concentrations were found in the myocardium and pericardial fluid. The histopathological analysis found extensive cardiac myonecrosis and lymphoid infiltration with CD3+ T cells. The CD3+ T cell infiltration appeared to be specific to the myocardium, as this was not noted in other organs and tissues examined (32). This finding suggests that there may be direct T cell–mediated cardiac injury and toxicity due to off-tumor, off-target cross-reactivity of the MAGE-A3–specific CAR T cells with the myocardium. However, similar histopathological analyses in CD19-specific CAR T cell therapy deaths have not been reported, and there is no evidence of direct myocardial toxicity of CD19-specific CAR T cells.
Future Directions in Cardio-Oncology Research of CAR T Cell Therapies
There is a limited body of literature that informs the field of cardio-oncology with respect to the impact of CAR T cell therapies in hematological malignancies, and additional research is needed to fully define the extent of the problem. It is important to understand whether CAR T cell–associated cardiotoxicity is simply an early phenomenon associated with CRS or whether there are more directly cardiotoxic effects from CAR T cells, both early and late, that have yet to be defined. Indeed, once the scale and chronology of the association are clearer, further exploration of the possible pathophysiology and mechanisms will then be desirable such that evidence-based interventions may be developed.
In our view, the most immediate emphasis should be to clearly define whether cardiotoxicity exists as a distinct, separate identity underpinned by direct toxicity from CAR T cells, or if observed, presumed transient hemodynamic abnormalities are epiphenomena associated with CRS and systemic shock.
Prospective studies will need to use systematic cardiac surveillance strategies to define the true incidence and extent of cardiac injury. These must include pre-treatment assessments and close monitoring while on treatment. Imaging will play a crucial role in these strategies, including detailed, protocol-driven echocardiography, using up-to-date parameters as well as cardiac magnetic resonance imaging (CMR) (33,34). The utility of biomarkers, including troponins and natriuretic peptides, as part of the cardiac surveillance strategy in CAR T cell therapy in some centers will also need to be evaluated (35). Many novel biomarkers have generated significant interest in other cancer treatment–related cardiotoxicities, such as nitric oxide metabolites and microRNAs (miRNAs), among many others; the role of these novel biomarkers within the context of cardiovascular toxicity due to CAR T cell therapy should also be considered (36, 37, 38).
In addition, the underlying mechanism for cardiac injury needs to be elucidated to mitigate off-tumor toxicities and inform the design of next-generation CAR T cells (39). Future CAR validation must incorporate in vitro studies, including peptide scanning and tissue screening to help identify CARs which have the potential to be directly myocardiotoxic (40). Experience with MAGE-A3–specific CAR T cells indicates that off-tumor, off-target toxicity poses a significant risk to patients. Conversely, toxicity may also be due to off-tumor, on-target effects; this has generated interest in multiple-antigen targeting through the use of logic-gated T cells developed within the AND, OR, and NOT concepts of Boolean logic (41). Adding AND gated circuits will require both antigens to be present for CAR activation to occur, whereas NOT gated circuits will allow CAR activation in the presence of one antigen and only if the other is not present. This strategy has the potential to increase specificity of CARs and reduce toxicity.
Beyond these initial research objectives, the relevance of cardio-oncology within CAR T cell therapy will be to prevent and/or minimize cardiovascular complications and toxicity from occurring and progressing (33,34). Subsequently, the next phase of research will also need to examine the utility of primary and secondary prevention strategies and to provide longer term follow-up data to assess the reversibility of cardiovascular toxicity and what impact CAR T cell therapies will have on late cardiovascular outcomes.
The success of CAR T cell therapy in cancer treatment has led to interest in whether engineered T cells could also be used to target noncancer cells, and preclinical studies have sought to translate this success into the treatment of myocardial disease. Excessive cardiac fibrosis, mediated by cardiac fibroblasts, is a key pathophysiological component of many cardiac diseases and heart failure. Fibroblast activation protein is highly expressed by cardiac fibroblasts in human hearts with dilated cardiomyopathy and hypertrophic cardiomyopathy; it is not expressed by cardiomyocytes, is weakly expressed in normal human hearts, and is therefore a suitable potential target for immunotherapy. Subsequently, the administration of fibroblast activation protein-specific CAR T cells into a cohort of mice, in which cardiac injury had been induced, has been shown to significantly reduce, and in some cases completely eliminate, cardiac fibrosis compared with control mice (42). In addition, there was partial rescue of both systolic and diastolic cardiac function. Future research will therefore focus not just on cardiac injury associated with CAR T cell therapy, but indeed whether this exciting preclinical study can be translated to humans for CAR T cell therapy to successfully treat and prevent myocardial disease.
How To Screen and Monitor Patients Undergoing CAR T Cell Therapy
Adults receiving CAR T cell therapy are likely to represent a particularly at-risk patient population for cardiac injury. They have often already had previous exposure to cardiotoxic cancer treatments, including anthracyclines and thoracic radiotherapy, and often may also have other cardiovascular comorbidities. Although, the hemodynamic instability and cardiovascular complications associated with CAR T cell therapy have thus far been reversible and transient, they can be exacerbated and more challenging to manage in the setting of pre-existing cardiovascular disease (43). In this young field, cardiac evaluation before receiving CAR T cell therapy varies significantly between centers due to paucity of data in the literature and CAR T cell–specific guidelines and recommendations. The American Society of Clinical Oncology has, however, provided a Clinical Practice Guideline from 2017 for the prevention and monitoring of cardiac dysfunction in the setting of either anthracycline exposure or radiotherapy, or both. It advocates for a careful history taking and physical examination, as well as further risk stratification and assessment through the appropriate use of transthoracic echocardiography (TTE), cardiac magnetic resonance imaging (CMR) or multigated acquisition scan, and serum cardiac biomarkers (39).
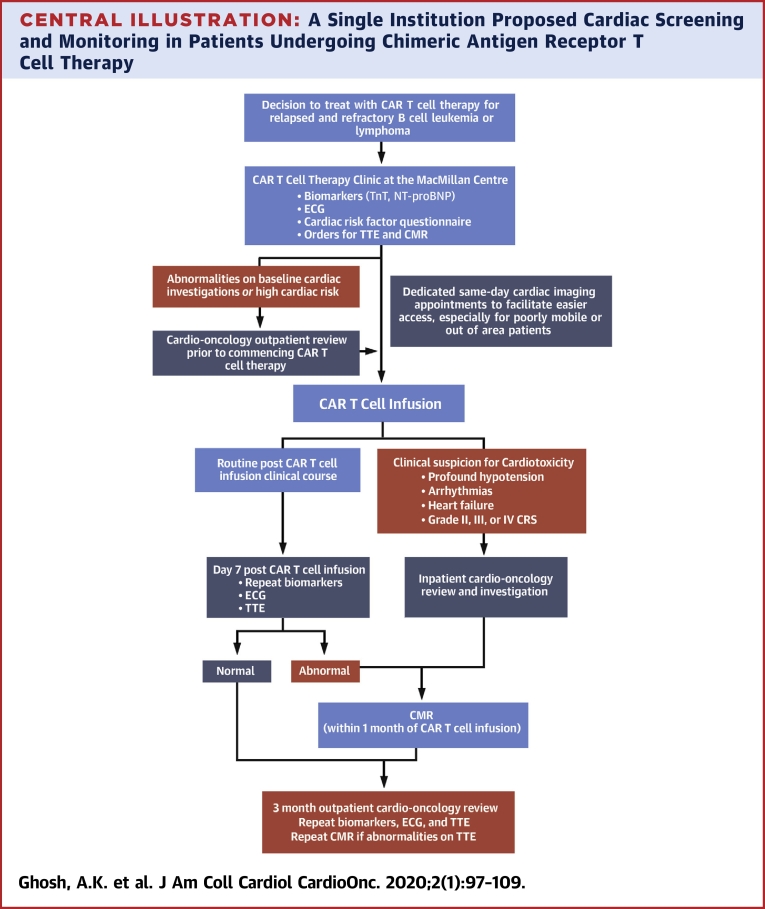
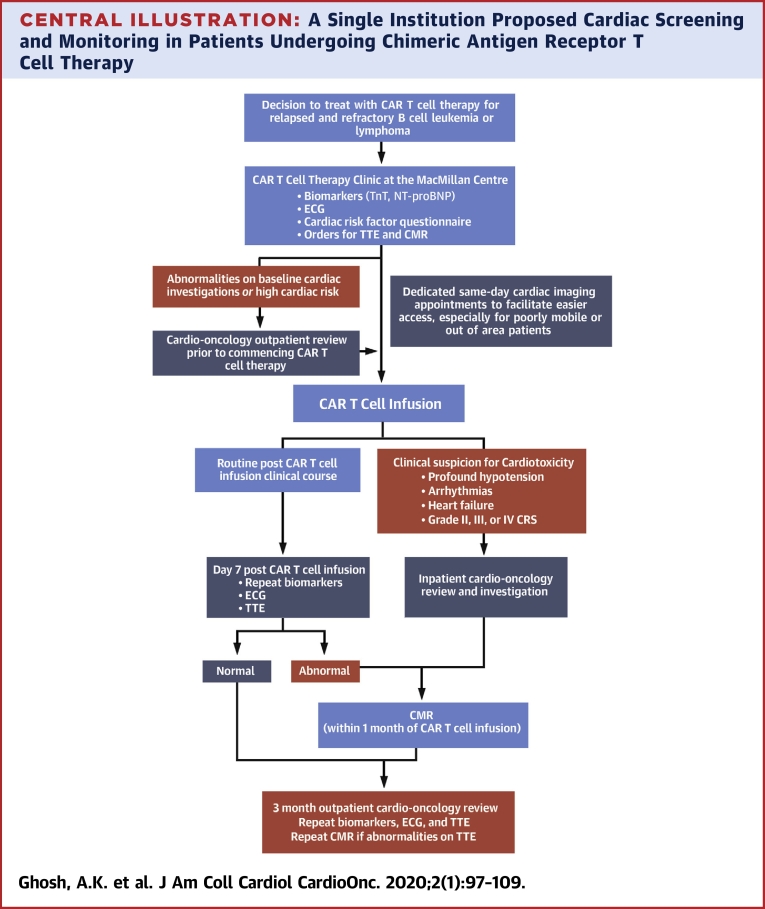
To advance our understanding of CAR T cardiotoxicity, the cardio-oncology service at the University College of London Hospital has established a cardiac screening and monitoring program in line with recommendations from the American Society of Clinical Oncology guidelines (39) and in collaboration with the CAR T cell program team (Central Illustration). Phased implementation of this program commenced in late 2019.
Central Illustration.
A Single Institution Proposed Cardiac Screening and Monitoring in Patients Undergoing Chimeric Antigen Receptor T Cell Therapy
Patient journey through cardio-oncology screening and monitoring as part of the chimeric receptor antibody (CAR) T cell therapy program at the University College of London Hospital. CMR = cardiac magnetic resonance; ECG = electrocardiography; NT-proBNP = N-terminal pro–B-type natriuretic peptide; TnT = troponin T; TTE = transthoracic echocardiography.
As part of treatment planning for CAR T cell therapy, all patients undergo risk assessment of cardiac function. In the first instance, all patients complete a questionnaire to identify those with a high cardiac risk profile. This includes patients who have had a history of chemotherapy-induced or other cardiomyopathies, pre-existing arrhythmias or coronary artery disease, or a high risk of coronary artery disease based on their calculated QRISK3-2018 score (44). All patients have blood samples drawn for troponin and N-terminal pro–B-type natriuretic peptide levels, a 12-lead electrocardiogram, cardiac imaging with a TTE (including 2-, 3-, and 4-dimensional left ventricular ejection fraction, diastolic, and global longitudinal strain assessments) and a CMR using a fast scan protocol. Significant abnormalities of the patient’s biomarkers or cardiac diagnostic tests, or a high cardiac risk profile identified by the questionnaire, will subsequently prompt cardio-oncology review before commencing CAR T cell therapy. Initiation of cardioprotective therapies with beta-blockers and either angiotensin-converting enzyme inhibitors or angiotensin receptor blockers is considered.
After administration of CAR T cell therapy, the biomarkers, 12-lead electrocardiogram, and TTE are repeated as part of cardiac surveillance. If there are abnormalities on these cardiac investigations or an elevation in the cardiac biomarkers, or in the context of clinical deterioration (including profound hypotension, arrhythmias, clinical signs and symptoms of cardiac failure, and grade III or IV CRS), these patients receive inpatient cardio-oncology review and further imaging with CMR. Initiation of a beta-blocker, angiotensin-converting enzyme inhibitor, or angiotensin receptor blocker is considered if LVSD is noted on TTE or CMR imaging.
All patients are subsequently followed up from a cardio-oncology perspective at 3 months after CAR T cell infusion. In addition to clinical assessment by the cardio-oncology team, the biomarkers, 12-lead electrocardiogram, and TTE are repeated.
There is, as yet, no evidence in the literature that examines the utility of increased cardiac surveillance in the prevention, management, or improvement of outcomes in patients receiving CAR T cell therapy. Subsequently, the data from this cardiac screening and monitoring program are being prospectively collated with the aim of providing further information to the CAR T cell and cardio-oncology community in the future.
In addition, there is at least one prospective observational study, led by the Abramson Cancer Centre of the University of Pennsylvania (NCT04026737), that is underway. Similarly, they will seek to define the incidence, natural history, and progression of cardiac dysfunction and determine the population most at risk through the use of serial echocardiography, cardiac biomarkers, clinical data, and quality of life questionnaires.
Conclusions
CAR T cell therapies have revolutionized the therapeutic approach to highly refractory and relapsing hematological malignancies in which management options had previously been limited or altogether exhausted. The potency of this treatment is accompanied by potentially significant toxicity that includes a potential risk of cardiovascular complications, with the early evidence suggesting the latter is likely to be transient. The evidence base surrounding cardiovascular complications and toxicity in particular remains limited. Multimodality cardiac imaging and established and novel biomarkers will need to drive future research to more fully define the cardiac safety profile of CAR T cell therapies, to identify the population who may be at the highest risk and enable early detection of cardiovascular complications and toxicity, and guide their management along evidence-based interventions applicable to this unique class of therapies.
Footnotes
Dr. Roddie has received speaker fees from Novartis, Gilead, and Celgen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Park J.H., Geyer M.B., Brentjens R.J. CD19-targeted CAR T-cell therapeutics for hematologic malignancies: interpreting clinical outcomes to date. Blood. 2016;127:3312. doi: 10.1182/blood-2016-02-629063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown C.E., Mackall C.L. CAR T cell therapy: inroads to response and resistance. Nature Rev Immunol. 2019;19:73–74. doi: 10.1038/s41577-018-0119-y. [DOI] [PubMed] [Google Scholar]
- 3.Salter A.I., Pont M.J., Riddell S.R. Chimeric antigen receptor–modified T cells: CD19 and the road beyond. Blood. 2018;131:2621. doi: 10.1182/blood-2018-01-785840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude S., Barrett D.M. Current status of chimeric antigen receptor therapy for haematological malignancies. Br J Haematol. 2016;172:11–22. doi: 10.1111/bjh.13792. [DOI] [PubMed] [Google Scholar]
- 5.Subklewe M., von Bergwelt-Baildon M., Humpe A. Chimeric antigen receptor T cells: a race to revolutionize cancer therapy. Transfus Med Hemother. 2019;46:15–24. doi: 10.1159/000496870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brudno J.N., Kochenderfer J.N. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol. 2017;15:31. doi: 10.1038/nrclinonc.2017.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump M., Neelapu S.S., Farooq U. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130:1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maude S.L., Laetsch T.W., Buechner J. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Locke F.L., Ghobadi A., Jacobson C.A. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maude S.L., Frey N., Shaw P.A. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schuster S.J., Bishop M.R., Tam C. Sustained disease control for adult patients with relapsed or refractory diffuse large B-cell lymphoma: an updated analysis of JULIET, a global pivotal phase 2 trial of tisagenlecleucel. Blood. 2018;132:1684. [Google Scholar]
- 12.Schuster S.J., Bishop M.R., Tam C.S. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 13.Neelapu S.S., Locke F.L., Bartlett N.L. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuster S.J., Svoboda J., Chong E.A. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med. 2017;377:2545–2554. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee D.W., Gardner R., Porter D.L. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–195. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neelapu S.S., Tummala S., Kebriaei P. Chimeric antigen receptor T-cell therapy—assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee D.W., Santomasso B.D., Locke F.L. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brudno J.N., Kochenderfer J.N. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey N. Cytokine release syndrome: who is at risk and how to treat. Best Prac Res Clin Oncol. 2017;30:336–340. doi: 10.1016/j.beha.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Santomasso B., Bachier C., Westin J., Rezvani K., Shpall E.J. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. American Society of Clinical Oncology Educational Book. 2019:433–444. doi: 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]
- 21.June C.H., Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379:64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belin C., Simard C., Dos Santos A. JS1.4 Neurological follow-up of neurotoxicity after CAR T cell therapy in lymphoma patients: a French neurological multi-center survey. J Neurooncol. 2019;21 iii5–iii5. [Google Scholar]
- 23.Gust J., Finney O.C., Li D. Glial injury in neurotoxicity after pediatric CD19-directed chimeric antigen receptor T cell therapy. Ann Neurol. 2019;86:42–54. doi: 10.1002/ana.25502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein D.S., Maude S., Grupp S., Griffis H., Rossano J., Lin K. Cardiac profile of chimeric antigen receptor T cell therapy in children: a single-institution experience. Biol Blood Marrow Transplant. 2018;24:1590–1595. doi: 10.1016/j.bbmt.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Fitzgerald J.C.M.D.P., Weiss S.L.M.D.M., Maude S.L.M.D.P. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvi R.M., Frigault M.J., Fradley M.G. Cardiovascular events among adults treated with chimeric antigen receptor T-cells (CAR-T) J Am Coll Cardiol. 2019;74:3099–3108. doi: 10.1016/j.jacc.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asnani A. Cardiotoxicity of immunotherapy: incidence, diagnosis, and management. Curr Oncol Rep. 2018;20:44. doi: 10.1007/s11912-018-0690-1. [DOI] [PubMed] [Google Scholar]
- 28.Porter D.L., Hwang W.T., Frey N.V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordeiro A., Bezerra E.D., Hirayama A.V. Late events after treatment with CD19-targeted chimeric antigen receptor modified T cells. Biol Blood Marrow Transplant. 2020;26:26–33. doi: 10.1016/j.bbmt.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu Y., Feng J., Shao M., Huang H. Profile of capillary-leak syndrome in patients received chimeric antigen receptor T cell therapy. Blood. 2018;132:5204. doi: 10.1038/s41409-022-01562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina de Chazal H., Del Buono M.G., Keyser-Marcus L. Stress cardiomyopathy diagnosis and treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1955–1971. doi: 10.1016/j.jacc.2018.07.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Linette G.P., Stadtmauer E.A., Maus M.V. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plana J.C., Thavendiranathan P., Bucciarelli-Ducci C., Lancellotti P. Multi-modality imaging in the assessment of cardiovascular toxicity in the cancer patient. J Am Coll Cardiol Img. 2018;11:1173. doi: 10.1016/j.jcmg.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 34.Seraphim A., Westwood M., Bhuva A.N. Advanced imaging modalities to monitor for cardiotoxicity. Curr Treat Options Oncol. 2019;20:73. doi: 10.1007/s11864-019-0672-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu A.F., Ky B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart. 2016;102:425. doi: 10.1136/heartjnl-2015-307894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel L., Rassaf T., Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy-related cardiotoxicity. J Thorac Dis. 2018:S4282–S4295. doi: 10.21037/jtd.2018.08.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan L.L., Lyon A.R. Role of biomarkers in prediction of cardiotoxicity during cancer treatment. Curr Treat Options Cardiovasc Med. 2018;20:55. doi: 10.1007/s11936-018-0641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leger Kasey J., Leonard D., Nielson D., de Lemos James A., Mammen Pradeep P.A., Winick Naomi J. Circulating microRNAs: potential markers of cardiotoxicity in children and young adults treated with anthracycline chemotherapy. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.004653. e004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armenian S.H., Lacchetti C., Barac A. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2017;35:893–911. doi: 10.1200/JCO.2016.70.5400. [DOI] [PubMed] [Google Scholar]
- 40.Cameron B.J., Gerry A.B., Dukes J. Identification of a titin-derived HLA-A1–presented peptide as a cross-reactive target for engineered MAGE A3–directed T cells. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Charrot S., Hallam S. CAR-T cells: future perspectives. HemaSphere. 2019;3:e188. doi: 10.1097/HS9.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aghajanian H., Kimura T., Rurik J.G. Targeting cardiac fibrosis with engineered T cells. Nature. 2019;573:430–433. doi: 10.1038/s41586-019-1546-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganatra S., Carver J.R., Hayek S.S. Chimeric antigen receptor T-cell therapy for cancer and heart: JACC Council Perspectives. J Am Coll Cardiol. 2019;74:3153–3163. doi: 10.1016/j.jacc.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hippisley-Cox J., Coupland C., Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi: 10.1136/bmj.j2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Makita S., Imaizumi K., Kurosawa S., Tobinai K. Chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma: opportunities and challenges. Drugs Context. 2019;8:212567. doi: 10.7573/dic.212567. [DOI] [PMC free article] [PubMed] [Google Scholar]