Abstract
Background
An increased risk of malignancy was reported with simvastatin/ezetimibe in 1,873 patients in the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) trial.
Objectives
The purpose of this study was to clarify this unexpected finding in a larger sample size of patients stabilized after acute coronary syndrome, we conducted a prospective systematic analysis of malignancy events in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial).
Methods
Within IMPROVE-IT, 17,708 patients post–acute coronary syndrome were randomized to either ezetimibe 10 mg or matching placebo on a background of simvastatin 40 mg who took ≥1 dose of the study drug. Suspected tumors (benign and malignant) reported by investigators or identified from a review of adverse events were adjudicated by oncologists without knowledge of drug assignment. The primary malignancy endpoint included new, relapsing, or progressive malignancies (excluding nonmelanotic skin malignancies). The secondary endpoint was death due to malignancy.
Results
In this trial, 1,470 patients developed the primary malignancy endpoint during a median 6 years of follow-up. The most common malignancy locations were prostate (18.9%), lung (16.8%), and bladder (8.8%) with no differences by treatment group (p > 0.05 for each location). Kaplan-Meier 7-year rates of malignancies were similar with ezetimibe and placebo (10.2% vs. 10.3%; hazard ratio: 1.03; 95% confidence interval: 0.93 to 1.14; p = 0.56), as were the rates for malignancy death (3.8% vs. 3.6%; hazard ratio: 1.04; 95% confidence interval: 0.88 to 1.23; p = 0.68).
Conclusions
Among 17,708 patients receiving simvastatin 40 mg daily, those randomized to ezetimibe 10 mg daily had a similar incidence of malignancy and deaths due to malignancy compared with those receiving placebo during a median follow-up of 6 years (96,377 patient-years). (IMPROVE-IT: Examining Outcomes in Subjects With Acute Coronary Syndrome: Vytorin [Ezetimibe/Simvastatin] vs Simvastatin [P04103]; NCT00202878)
Key Words: acute coronary syndromes, cancer, ezetimibe, lipid-lowering therapy, lipids, malignancy, statin
Abbreviations and Acronyms: ACS, acute coronary syndrome; BMI, body mass index; CI, confidence interval; hs-CRP, high-sensitive C-reactive protein; HR, hazard ratio; KM, Kaplan-Meier; LDL-C, low-density lipoprotein cholesterol; PCSK9, proprotein convertase subtilisin kexin 9; PH, proportional hazard; RR, risk ratio
Central Illustration
Patients with established atherosclerotic cardiovascular disease are at higher risk of death attributed both to cardiovascular and noncardiovascular origin (1). Although malignancy is characterized by different biological pathways and pharmacological targets, it shares similar causal risk factors (e.g., age, smoking, inflammation, and diabetes) with cardiovascular disease (2). Therefore, both diseases can occur during a long-term clinical trial, resulting in competing risks for morbidity and mortality (3). A striking example came from the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study), in which a monoclonal antibody targeting inflammation studied primarily to reduce major adverse cardiovascular events was associated with a significant reduction of lung malignancy (4).
Meta-analyses of therapies lowering low-density lipoprotein cholesterol (LDL-C) demonstrated that prevention of cardiovascular events was proportional to the absolute reduction in LDL-C levels with no major safety concerns, specifically no increase in malignancies (5,6). However, available data from previous LDL-C–lowering clinical trials have not systematically adjudicated data for malignancy, because these were not considered disease-related events (7). An increased rate of malignancy associated with the use of the combination of ezetimibe and simvastatin was unexpectedly reported in the SEAS (Simvastatin and Ezetimibe in Aortic Stenosis) trial (8), which compared simvastatin-ezetimibe to placebo in 1,873 adults with mild-to-moderate aortic stenosis over a median follow-up of 4.4 years (8).
Ezetimibe is a nonstatin drug that inhibits the intestinal absorption of cholesterol by targeting the transmembrane protein, Nieman-Pick C1-Like 1 (9). It is recommended for further LDL-C reduction in combination with a statin (10). In IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial), the daily addition of 10 mg ezetimibe to 40 mg simvastatin in patients with acute coronary syndrome (ACS) with LDL-C levels between 50 and 125 mg/dl resulted in an incremental reduction of LDL-C to median achieved LDL-C value of 53 mg/dl versus 70 mg/dl (p < 0.001) and a significant reduction in the primary cardiovascular composite outcome compared with simvastatin alone during a median follow-up of 6 years (32.7% vs. 34.7%; hazard ratio [HR]: 0.94; 95% confidence interval [CI]: 0.89 to 0.99; p = 0.016) (11). In this present analysis, we report the results on malignancy in patients post-ACS participating in IMPROVE-IT treated with simvastatin who were randomized to ezetimibe versus placebo and followed up for a median of 6 years.
Methods
Study population
The design and results of IMPROVE-IT have been published previously (11,12). IMPROVE-IT was a multinational, double-blind, randomized, placebo-controlled trial that enrolled 18,144 patients from October 26, 2005 to July 8, 2010 after a period of stabilization following a hospitalization for ACS (11). Patients were randomized in a 1:1 manner to once daily treatment with either 40 mg simvastatin plus 10 ezetimibe or 40 mg simvastatin plus matching placebo. The study population included men and women age ≥50 years who had been hospitalized within the previous 10 days for ACS, comprising myocardial infarction with or without ST-segment elevation or high-risk unstable angina. To be eligible, the LDL-C level measured within 24 h after hospital admission had to be 50 to 125 mg/dl for patients not receiving prior lipid-lowering therapy, or 50 to 100 mg/dl for patients on prior long-term prescription of lipid-lowering therapy. Major exclusion criteria most relevant to the current analysis included active malignancy or any clinically significant condition other than atherosclerotic vascular disease. Excluded were patients with malignancy diagnosed within 5 years or who were receiving treatment for malignancy, with the exception of adequately treated in situ tumors and nonmelanotic skin malignancy. Other exclusion criteria were hemodynamic instability, acute ischemic or arrhythmic events within 24 h before enrollment, creatinine clearance <30 ml/min, active liver disease, or prior use of statin therapy with a potency higher than 40 mg of simvastatin.
Study endpoints
The primary endpoint of IMPROVE-IT was a composite of cardiovascular death, major coronary event (nonfatal myocardial infarction, rehospitalization for unstable angina, or coronary revascularization occurring ≥30 days after randomization), or nonfatal stroke. After the publications of the SEAS trial in 2008, the investigators of IMPROVE-IT designed and implemented a protocol to review all tumors occurring during the trial in previously and future-enrolled patients through the end of follow-up. The primary malignancy endpoint consisted of all first new, worsening, or relapsing malignancies, excluding nonmelanotic (i.e., basal and squamous) skin malignancies. The investigators were trained to report all suspected tumors (benign or malignant) using a prespecified data collection tool designed specifically for this study by the Executive Committee in consultation with the independent Oncology Clinical Endpoint Committee (CEC) (Supplemental Appendix). The completeness of data reported for tumors was closely monitored on site and supplemented by a review of all adverse events reported. All suspected tumors were adjudicated by a pair of independent oncologists unaware of assigned treatment and lipid levels. Pathology reports were used as a primary source of information when available. The tumors were initially classified as malignant or benign, and then subclassified as new versus present prior to randomization. If present prior to randomization, malignancies were additionally characterized as worsening versus relapsing versus stable. Further classifications were performed by organ system disease and malignancy extent. The malignancy extent for solid tumor was graded as: 1) local disease only; 2) spread to contiguous structures; 3) metastatic; and 4) unknown. The extent for leukemia, lymphoma, and other blood malignancies was graded as: 1) acute; 2) chronic; and 3) unknown. The relationship between the malignancy and the vital status was adjudicated by the Oncology CEC. Nonmalignancy deaths were reviewed by a central CEC consisting of neurologists (who reviewed stroke and intracranial hemorrhage) or cardiologists (all other nontumor cases). Disagreements between members were resolved by consensus.
Statistical analysis
Baseline characteristics stratified by the development of post-randomization malignancy are reported as frequencies and percentages for categorical variable and compared using the chi-square test. Continuous variables were reported as medians with 25th and 75th percentiles (quartile 1 to quartile 3) for continuous variables and compared using the Wilcoxon test. All analyses were performed first on a modified intention-to-treat basis defined as patients who received at least a single dose of the study drug. If a patient developed several primary malignancy endpoints, the first event was used for the primary analysis. Malignancies are presented as Kaplan-Meier (KM) rates at 7 years stratified by the treatment group and compared using the log-rank test. The cumulative incidence plots for the primary malignancy endpoint and the secondary endpoint of death due to malignancy were presented graphically using KM product-limit method. In addition, the frequencies and percentages of malignancy by location are presented by treatment group. The Cox proportional hazard (PH) regression model was used for the analysis and data presented with HR and 95% CI comparing the ezetimibe/simvastatin arm with the placebo/simvastatin arm. We tested for effect modification by evaluating the interaction terms for high-risk subgroups, including gender, age (≥75 years vs. <75 years), smoking status (current vs. past vs. never), total cholesterol quartiles, body mass index categories, high-sensitive C-reactive protein, TIMI Risk Score for Secondary Prevention (13), and randomized treatment on the risk of the primary malignancy endpoint. The PH assumption was satisfied using visual inspection of Schoenfeld residual plots and performing Supremum test for PH assumption. Sensitivity analyses were performed considering different definitions of malignancy events: 1) new, worsening, and relapsing malignancies; 2) new malignancies only; or 3) worsening or relapsing malignancies only. For each malignancy endpoint, we performed additional analyses including and excluding basal cell and squamous cell malignancies of the skin, as well as limiting malignancy events to only those with available pathology reports. We also performed a competing risk analysis integrating all-cause death as a competing outcome in the model using Fine and Gray’s method. Finally, we also evaluated the HRs in both arms with increasing duration of follow-up as done in previous publications (14,15). All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, North Carolina). A 2-sided p < 0.05 was considered as significant. The institutional review board/ethics committee at each participating center approved the protocol and amendments, and all patients gave written informed consent.
Results
There were 436 (2.4%) of the 18,144 randomized patients who did not take any study drug, thus 17,708 patients who took at least one dose of the study drug were included in this analysis (Supplemental Figure 1). Of these, 15,895 patients had no tumor event and 231 had a benign tumor only (115 in the simvastatin/ezetimibe arm and 116 in the simvastatin/placebo arm). At the end of the trial (median [interquartile range] of follow-up: 6.0 [4.3 to 7.2] years), 1,582 patients had a malignancy of which 1,470 met the primary malignancy endpoint (726 in the placebo arm and 744 in the ezetimibe arm) and 112 had nonmelanotic skin malignancy. Among the 1,470 patients who met the primary malignancy endpoint, 1,370 patients had new malignancy, 53 had progressive malignancy, and 47 had relapsing malignancy (Supplemental Figure 1).
Patients who met the primary malignancy endpoint (N = 1,470) compared with those who did not (N = 16,238) were significantly older (median age: 66.8 vs. 62.8 years; p < 0.001), had lower baseline creatinine clearance (80.7 vs. 85.0 ml/min; p < 0.001), and were more likely to be male (80.5% vs. 75.3%; p < 0.001), white (90.1% vs. 83.4%; p < 0.001), a current smoker (37.2% vs. 32.6%; p < 0.001), and to have pre-existing peripheral artery disease (7.8% vs. 5.4%; p < 0.001) or prior coronary artery bypass graft (10.9% vs. 9.1%; p = 0.025) (Table 1). No significant baseline differences between patients who did versus did not experience the primary malignancy endpoint were found for lipid values or high-sensitive C-reactive protein levels, or in the prior use of statin (35.9% vs. 34.3%; p = 0.21).
Table 1.
Baseline Characteristics by Status of Primary Malignancy Endpoint
| No Malignancy (n = 16,238)∗ | Malignancy (n = 1,470) | p Value | |
|---|---|---|---|
| Age, yrs | 62.8 (56.5, 70.7) | 66.8 (60.3, 74.0) | <0.001 |
| Body mass index, kg/m2 | 27.6 (24.9, 30.9) | 27.3 (24.7, 30.7) | 0.051 |
| Weight, kg | 81.2 (71.0, 92.7) | 81.2 (72.0, 92.5) | 0.37 |
| Male | 12,231 (75.3) | 1,183 (80.5) | <0.001 |
| Caucasian | 13,543 (83.4) | 1,324 (90.1) | <0.001 |
| Region of enrollment | |||
| North America | 6,164 (38.0) | 613 (41.7) | 0.005 |
| Western Europe | 6,428 (39.6) | 654 (44.5) | <0.001 |
| Eastern Europe | 1,330 (8.2) | 77 (5.2) | <0.001 |
| Asia Pacific | 822 (5.1) | 53 (3.6) | 0.014 |
| South America | 1,494 (9.2) | 73 (5.0) | <0.001 |
| Coexisting conditions | |||
| Diabetes | 4,412 (27.2) | 383 (26.1) | 0.36 |
| Hypertension | 9,973 (61.4) | 872 (59.3) | 0.11 |
| Heart failure | 700 (4.3) | 66 (4.5) | 0.75 |
| Peripheral arterial disease | 869 (5.4) | 115 (7.8) | <0.001 |
| Current smoking | 5,290 (32.6) | 547 (37.2) | <0.001 |
| Previous myocardial infarction | 3,375 (20.8) | 327 (22.2) | 0.19 |
| Previous percutaneous coronary intervention | 3,159 (19.5) | 306 (20.8) | 0.21 |
| Previous coronary artery bypass graft | 1,480 (9.1) | 160 (10.9) | 0.025 |
| Medications at index acute coronary syndromes | |||
| Lipid-lowering therapy | 5,730 (35.3) | 543 (37.0) | 0.20 |
| Statin | 5,564 (34.3) | 527 (35.9) | 0.21 |
| Aspirin | 6,830 (42.1) | 632 (43.1) | 0.48 |
| Creatinine clearance, ml/min | 85.0 (66.1, 107.6) | 80.7 (63.5, 98.8) | <0.001 |
| TRS2P score >3 | 1,354 (8.5) | 152 (10.5) | 0.011 |
| Type of index event | |||
| MI with ST-segment elevation | 4,634 (28.5) | 451 (30.7) | 0.083 |
| MI without ST-segment elevation | 7,676 (47.3) | 704 (47.9) | 0.66 |
| Unstable angina | 3,924 (24.2) | 315 (21.4) | 0.018 |
| Laboratory values at index event | |||
| LDL-C, mg/dl | 95.0 (79.0, 110.0) | 93.8 (78.0, 109.7) | 0.23 |
| HDL-C, mg/dl | 40.0 (33.0, 49.0) | 39.1 (33.0, 48.7) | 0.38 |
| Triglycerides, mg/dl | 120.0 (85.0, 171.8) | 121.2 (85.9, 176.2) | 0.50 |
| Hs-CRP, mg/l | 5.1 (2.0, 17.7) | 5.3 (2.5, 16.1) | 0.46 |
| Hemoglobin A1c, % | 6.1 (5.6, 7.3) | 6.1 (5.7, 7.0) | 0.81 |
| Medications at time of randomization | |||
| Aspirin | 15,770 (97.1) | 1,421 (96.7) | 0.39 |
| Beta-blocker | 14,171 (87.3) | 1,277 (86.9) | 0.69 |
| ACEI/ARB inhibitor | 12,293 (75.7) | 1,082 (73.7) | 0.08 |
| Thienopyridine | 14,067 (86.6) | 1,291 (87.9) | 0.18 |
Values are median (25th, 75th percentiles) or n (%). Wilcoxon rank-sum test of differences between with and without primary malignancies for continuous variables. Chi-square test of frequencies between with and without malignancies for categorical variables.
ACEI = angiotensin-converting enzyme inhibitor; ACS = acute coronary syndromes; ARB = angiotensin receptor blocker; HDL-C = high-density lipoprotein cholesterol; Hs-CRP = high-sensitive C-reactive protein; IQR = interquartile range; LDL-C = low-density lipoprotein cholesterol; MI = myocardial infarction; TRS2P = TIMI Risk Score for Secondary Prevention (13).
The no-malignancy group summary statistics are based on patients without malignancy diagnosis prior to death, loss to follow-up or end of the study.
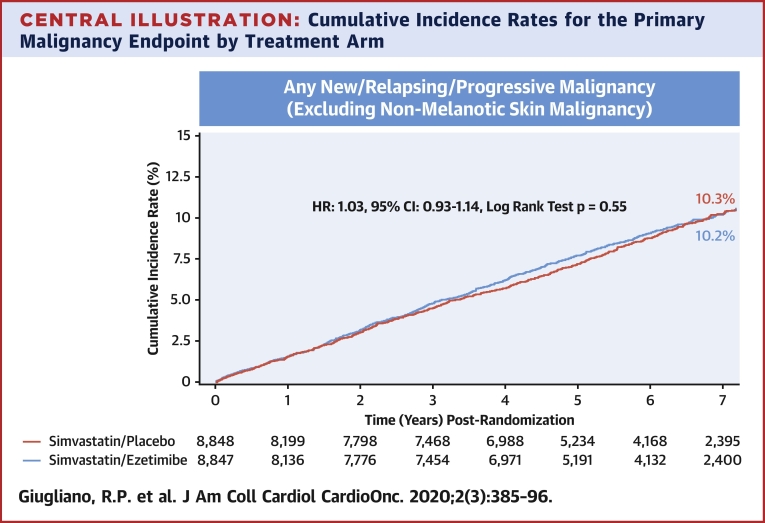
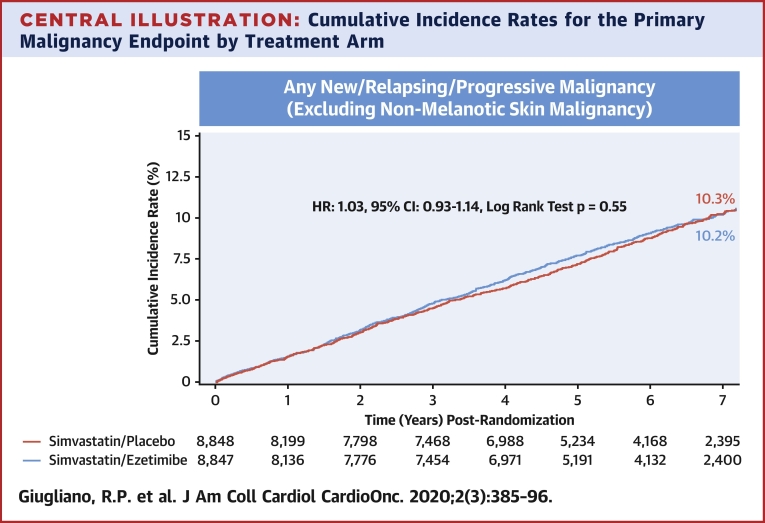
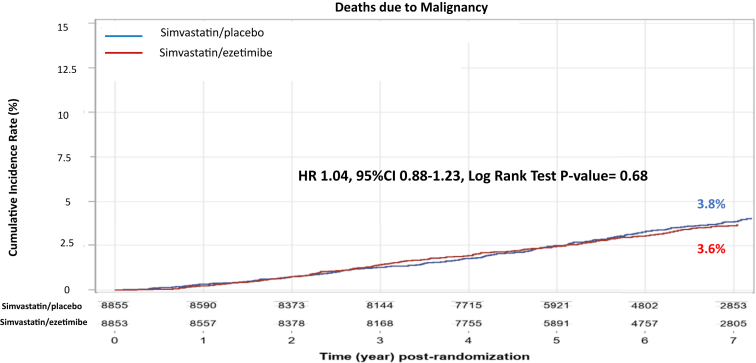
The 7-year KM rate for the primary malignancy endpoint was similar between the ezetimibe and placebo groups (7-year event rates of 10.2% vs. 10.3%; log-rank test p = 0.56; HR: 1.03; 95% CI: 0.93 to 1.14) (Central Illustration). There were 277 (3.8%) deaths due to malignancy in the ezetimibe versus 268 (3.6%) in the placebo arm (log-rank test p = 0.68; HR: 1.04; 95% CI: 0.88 to 1.23) (Figure 1).
Central Illustration.
Cumulative Incidence Rates for the Primary Malignancy Endpoint by Treatment Arm
Simvastatin/ezetimibe (red) and placebo/ezetimibe (blue). The primary malignancy endpoint was defined as new, relapsing, or progressive malignancy (excluding nonmelanotic skin malignancy). The cumulative incidence plots were presented graphically using Kaplan-Meier product-limit method. CI = confidence interval; HR = hazard ratio.
Figure 1.
Cumulative Incidence Rates for the Secondary Endpoint of Deaths Due to Malignancy by Treatment Arm
Simvastatin/ezetimibe (red) and placebo/ezetimibe (blue). The cumulative incidence plots were presented graphically using Kaplan-Meier product-limit method. CI = confidence interval; HR = hazard ratio.
In sensitivity analyses, the estimates were similar when including basal and squamous cell skin malignancy (905 vs. 908 cases; 12.5% vs. 12.8%; HR: 1.00; 95% CI: 0.91 to 1.10). The restriction of the primary endpoint to new malignancy did not significantly alter the results (690 vs. 674 cases; 9.6% vs. 9.7%; HR: 1.03; 95% CI: 0.92 to 1.14) (Table 2) nor did an analysis of all new malignancies that included basal and squamous cell skin malignancies (851 vs. 857 cases; 11.9% vs. 12.2%; HR: 0.99; 95% CI: 0.90 to 1.09). The results were consistent when limiting the primary endpoint definition to cases confirmed with pathology reports (640 vs. 629 cases; 8.8% vs. 8.9%; HR: 1.02; 95% CI: 0.92 to 1.14) (Supplemental Table 1). In the competing risk analysis with consideration of all-cause death, we found similar results (7-year event rates of 9.8% vs. 9.8%; HR: 1.03; 95% CI: 0.93 to 1.15; p = 0.53) (Supplemental Table 2).
Table 2.
Risks of Primary and Secondary Malignancy Endpoints by Treatment Arm
| Simvastatin Monotherapy (n = 8,855) |
Simvastatin/Ezetimibe (n = 8,853) |
|||||
|---|---|---|---|---|---|---|
| n | Kaplan-Meier Event Rate at 7 Years (%) | n | Kaplan-Meier Event Rate at 7 Years (%) | HR (95% CI) | p Value | |
| Primary endpoint | ||||||
| New, relapsing, or progressive malignancy (excluding nonmelanotic skin malignancy) | 726 | 10.3 | 744 | 10.2 | 1.03 (0.93–1.14) | 0.56 |
| Secondary endpoints | ||||||
| New, relapsing, or progressive malignancy (including nonmelanotic skin malignancy) | 908 | 12.8 | 905 | 12.5 | 1.00 (0.91–1.10) | 0.99 |
| New malignancy (excluding nonmelanotic skin, relapsing, or progressive malignancies) | 674 | 9.7 | 690 | 9.6 | 1.03 (0.92–1.14) | 0.63 |
| New malignancy (including nonmelanotic skin malignancy and excluding relapsing or progressive malignancy) | 857 | 12.2 | 851 | 11.9 | 0.99 (0.90–1.09) | 0.90 |
| New, relapsing, or progressive malignancy (excluding nonmelanotic skin malignancy) | 629 | 8.9 | 640 | 8.8 | 1.02 (0.92–1.14) | 0.67 |
| Deaths due to malignancy | 268 | 3.6 | 277 | 3.8 | 1.04 (0.88–1.23) | 0.68 |
CI = confidence interval; HR = hazard ratio.
In the overall population, the most common organ locations of the primary malignancy endpoint were prostate (18.9%), lung (16.8%), and bladder (8.8%). By treatment arm, the most common malignancy locations were lung (16.0% in the ezetimibe arm vs. 17.6% in the simvastatin monotherapy arm; p = 0.40), prostate in men (19.3% in the simvastatin-ezetimibe arm vs. 18.5% in the placebo arm; p = 0.72), and bladder (8.1% in the ezetimibe arm vs. 9.5% in the placebo arm; p = 0.33) (Supplemental Table 3). Breast malignancy occurred in 26.4% of female patients in the ezetimibe arm versus 18.9% patients in the placebo arm (p = 0.13) (and only 1 case in men). The rates of malignancy events did not differ by treatment group for any location (p > 0.05 for all comparisons) (Table 3). Results were similar between treatment groups for deaths due to malignancy by location (p > 0.05 for each comparison) (Supplemental Table 4). Further analyses by gender showed no significant differences between treatment groups for both the primary malignancy endpoint (p > 0.05 for each comparison) (Supplemental Tables 5 and 6) and deaths due to malignancy (p > 0.05 for each comparison) (Supplemental Tables 7 and 8). Finally, we did not observe any differences in the extent of malignancy by treatment arm (Table 4).
Table 3.
Risks of Primary Malignancy Endpoint Location by Treatment Arm
| Organ Location | Simvastatin Monotherapy (n = 8,855) |
Simvastatin/Ezetimibe (n = 8,853) |
HR (95% CI) | p Value | ||
|---|---|---|---|---|---|---|
| n | Kaplan-Meier Event Rate at 7 Years (%) | n | Kaplan-Meier Event Rate at 7 Years (%) | |||
| Lung (bronchus) | 128 | 1.92 | 119 | 1.64 | 0.93 (0.73–1.20) | 0.59 |
| Prostate (men only) | 108/6,727 | 2.07 | 116/6,687 | 2.07 | 1.06 (0.82-1.38) | 0.64 |
| Bladder | 69 | 1.07 | 60 | 0.87 | 0.87 (0.62–1.23) | 0.44 |
| Colon | 48 | 0.67 | 60 | 0.81 | 1.25 (0.86–1.83) | 0.24 |
| Melanoma | 34 | 0.52 | 35 | 0.52 | 1.03 (0.64–1.65) | 0.90 |
| Breast | 28 | 0.43 | 38 | 0.54 | 1.36 (0.83–2.21) | 0.22 |
| In women | 27/2,128 | 1.83 | 38/2,166 | 2.33 | 1.41 (0.86–2.31) | 0.17 |
| In men | 1/6,727 | 0.02 | 0/6,687 | 0.00 | NA | NA |
| Kidney or ureter | 34 | 0.51 | 31 | 0.38 | 0.91 (0.56–1.49) | 0.72 |
| Lymphoma | 27 | 0.35 | 31 | 0.43 | 1.15 (0.69–1.93) | 0.60 |
| Pancreas | 30 | 0.43 | 20 | 0.34 | 0.67 (0.38–1.17) | 0.16 |
| Leukemia | 18 | 0.24 | 21 | 0.28 | 1.17 (0.62–2.19) | 0.63 |
| Head or neck | 18 | 0.25 | 17 | 0.24 | 0.95 (0.49–1.84) | 0.88 |
| Stomach | 12 | 0.17 | 15 | 0.24 | 1.25 (0.59–2.68) | 0.56 |
| Nervous system | 4 | 0.04 | 11 | 0.19 | 2.75 (0.88–8.63) | 0.08 |
| Ovarian or fallopian tube | 2/2,128 | 0.11 | 3/2,166 | 0.19 | NA | NA |
Malignancy locations are shown in order of frequency.
NA = not available; other abbreviations as in Table 2.
Table 4.
Frequencies of Malignancy Extension by the Treatment Arm
| Malignancy Population |
Type of Malignancy | Severity | Simvastatin Monotherapy | Simvastatin/Ezetimibe | p Value |
|---|---|---|---|---|---|
| Excluding skin malignancy | Solid tumor | Local (no spread beyond the primary organ) | 328 (49.6) | 304 (45.1) | 0.15 |
| Metastatic | 212 (32.1) | 230 (34.1) | |||
| Known to spread to contiguous structures | 84 (12.7) | 109 (16.2) | |||
| Unknown | 37 (5.6) | 31 (4.6) | |||
| Leukemia, lymphoma and other blood malignancy | Acute | 12 (18.5) | 13 (18.6) | 0.98 | |
| Chronic | 11 (16.9) | 11 (15.7) | |||
| Unknown | 42 (64.6) | 46 (65.7) | |||
| Including skin malignancy | Solid tumor | Local (no spread beyond the primary organ) | 507 (60.1) | 464 (55.6) | 0.12 |
| Metastatic | 212 (25.1) | 230 (27.5) | |||
| Known to spread to contiguous structures | 87 (10.3) | 110 (13.2) | |||
| Unknown | 37 (4.4) | 31 (3.7) |
Values are n (%).
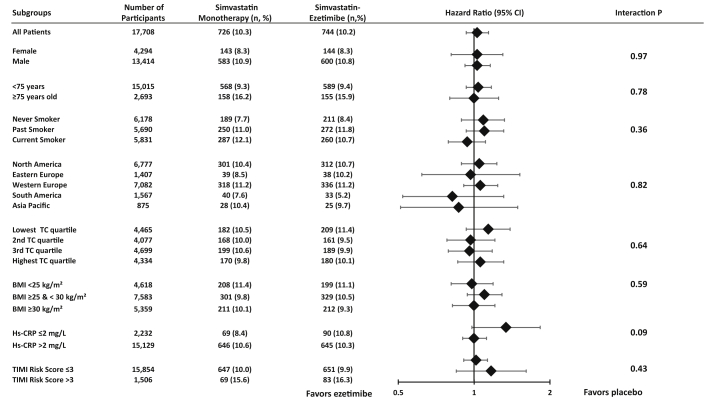
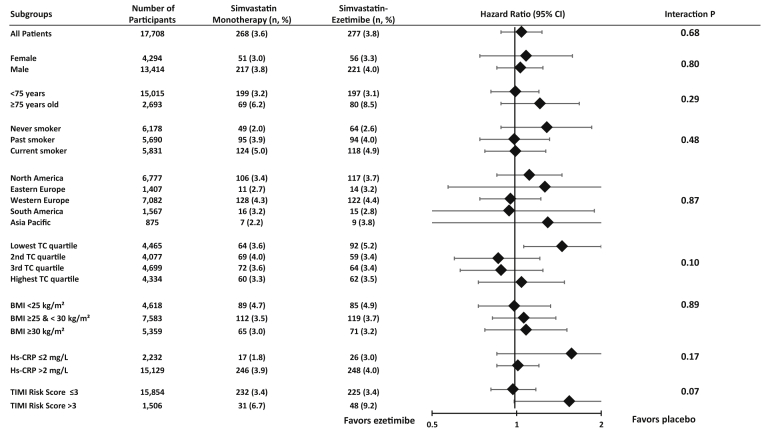
In subgroup analyses, the rates of the primary malignancy endpoint were similar between treatment groups for each of the 8 pre-specified high-risk subgroups (each p for interaction >0.05) (Figure 2). The risks for the primary malignancy endpoint with ezetimibe versus placebo were similar in men (7-year KM event rates 10.8% vs. 10.9%; HR: 1.03; 95% CI: 0.92 to 1.16; p = 0.59) and in women (8.3% vs. 8.3%; HR: 1.03; 95% CI: 0.81 to 1.30; p = 0.82; p for interaction = 0.96). The risks of death due to malignancy between treatment groups were consistent across the pre-specified high-risk subgroups (each p for interaction >0.05) (Figure 3).
Figure 2.
Primary Malignancy Endpoint by Pre-Specified High-Risk Subgroups
The number of events and the 7-year Kaplan-Meier rates are shown. BMI = body mass index; Hs-CRP = high-sensitive C-reactive protein; TC = total cholesterol. Total cholesterol: 25th percentile = 144.0 mg/dl, 50th percentile = 162.4 mg/dl and 75th percentile = 181.0 mg/dl.
Figure 3.
Deaths Due to Malignancy Endpoint by Pre-Specified High-Risk Subgroups
The number of events and the 7-year Kaplan-Meier rates are shown. Total cholesterol: 25th percentile =144.0 mg/dl, 50th percentile = 162.4 mg/dl and 75th percentile = 181.0 mg/dl. Abbreviations as in Figure 2.
During the 7 years of follow-up, there was no divergence in the KM curves over time (Figure 1); the year-by-year HRs for the primary malignancy endpoint comparing the two treatment arms did not demonstrate a progressive trend over time (Table 5).
Table 5.
Risks of Malignancy Events by Treatment Arm and Year of Follow-Up
| Endpoints | Years of Follow-Up | Simvastatin Monotherapy |
Simvastatin/Ezetimibe |
HR (95% CI) | p Value | ||
|---|---|---|---|---|---|---|---|
| N | n (%) | N | n (%) | ||||
| Primary malignancy endpoint | |||||||
| ≤1 | 8,848 | 129 | 8,847 | 133 | 1.04 (0.81–1.32) | 0.77 | |
| >1 and ≤2 | 8,199 | 125 | 8,136 | 131 | 1.05 (0.82–1.34) | 0.68 | |
| >2 and ≤3 | 7,798 | 117 | 7,776 | 130 | 1.11 (0.87–1.43) | 0.40 | |
| >3 and ≤4 | 7,468 | 95 | 7,454 | 109 | 1.15 (0.87–1.51) | 0.33 | |
| >4 and ≤5 | 6,988 | 95 | 6,971 | 96 | 1.01 (0.76–1.35) | 0.92 | |
| >5 and ≤6 | 5,234 | 80 | 5,191 | 70 | 0.88 (0.64–1.22) | 0.44 | |
| >6 and ≤7 | 4,168 | 57 | 4,132 | 44 | 0.78 (0.52–1.15) | 0.21 | |
| Deaths due to malignancy | |||||||
| ≤1 | 8,855 | 20 | 8,853 | 28 | 1.40 (0.79–2.49) | 0.25 | |
| >1 and ≤2 | 8,590 | 44 | 8,557 | 36 | 0.82 (0.53–1.27) | 0.37 | |
| >2 and ≤3 | 8,373 | 55 | 8,378 | 43 | 0.78 (0.52–1.16) | 0.22 | |
| >3 and ≤4 | 8,144 | 42 | 8,168 | 41 | 0.97 (0.63–1.49) | 0.89 | |
| >4 and ≤5 | 7,715 | 36 | 7,755 | 49 | 1.36 (0.89–2.09) | 0.16 | |
| >5 and ≤6 | 5,921 | 34 | 5,891 | 44 | 1.30 (0.83–2.04) | 0.25 | |
| >6 and ≤7 | 4,802 | 25 | 4,757 | 23 | 0.93 (0.53–1.64) | 0.80 | |
Abbreviations as in Table 2 and3.
Discussion
The data presented in this analysis of IMPROVE-IT are robust. A total of 17,708 patients with a median follow-up of 6 years (96,377 patient-years) after acute coronary syndromes were studied. The addition of ezetimibe to simvastatin did not affect the risk of malignancy. No differences were found in new, worsening, or relapsing malignancy, or after: 1) including or excluding nonmelanoma skin malignancies; 2) limiting the malignancy definition to new malignancies only or when including relapsing/worsening malignancies; or 3) restricting the endpoint to patients with available pathology reports. In addition, we did not find differences between treatment groups in deaths due to malignancy, malignancy location, or among high-risk subgroups. Finally, we did not observe any increased relative risk with ezetimibe versus placebo with longer follow-up duration to 7 years.
In the SEAS trial (approximately one-tenth the size of IMPROVE-IT) of patients with aortic stenosis, incident malignancy was diagnosed in 105 patients (11.1%) randomized to the ezetimibe-simvastatin combination, as compared with 70 patients (7.5%) in the placebo group (p = 0.01). Fatal malignancies diagnosed after discontinuation of the study drug or placebo occurred in 39 patients in the ezetimibe-simvastatin group versus 23 in the placebo group (p = 0.05). The excess malignancy risk in the ezetimibe-simvastatin group was not restricted to a single organ and the risk did not increase over time. Because simvastatin had been studied in more than 41,541 patients in large double-blind randomized trials without an increase in malignancy as previously reported, attention focused on whether the excess in malignancy in SEAS may have been related to ezetimibe (16).
Because ezetimibe inhibits the absorption, not only of cholesterol, but also of phytosterols and other nutrients that have possible protective roles against malignancy (17,18), a potential mechanism for increased risk of malignancy with ezetimibe has been proposed (19). To clarify whether an excess in malignancy existed in other large randomized studies with ezetimibe, in 2011 a combined analysis from the SHARP (Study of Heart and Renal Protection) trial (20) and an interim analysis from the ongoing IMPROVE-IT (analysis limited to 11,354 patients with a follow-up of 1 year) was performed in a total of 20,617 patients randomized to ezetimibe versus placebo (15). In contrast to the SEAS trial (mean follow-up: 4.1 years), the combined data from the SHARP trial (mean follow-up: 2.7 years) and the interim IMPROVE-IT data (mean follow-up: 1.0 year) did not show an increased risk of malignancy with ezetimibe (risk ratio: 0.96; 95% CI: 0.82 to 1.12; p = 0.61) (15). When combining data from all three datasets (SEAS, SHARP, and IMPROVE-IT), the risk ratio (RR) was 1.06 (95% CI: 0.92 to 1.22; p = 0.46). There was no evidence of an increased RR over time (p for trend = 0.54), suggesting the absence of a temporal association or causal inference between ezetimibe exposure and risk of malignancy over a follow-up period of 18,604 person-years.
Major strengths of our analysis are the systematic evaluation of all tumors (malignant or benign), a thorough classification of all possible malignancies with multiple sensitivity analyses, and an independent assessment by a panel of oncologists who were unaware of study treatment and lipid levels. In contrast, previous studies have usually reported malignancy as a safety outcome, without a broad review of all tumors or use of an independent blinded adjudication process as is typically performed for the primary and secondary efficacy endpoints related to the disease. Therefore, we agree with Peto et al. (14) that the malignancy findings reported from SEAS were likely due to chance. The present study reinforces the importance of testing previously unanticipated findings in a large and independent database with prospectively validated methods for malignancy events adjudication (7).
Some concern has been expressed that statin and other LDL-C–lowering drugs might be carcinogenic (21). Because simvastatin was a mandatory background therapy in both arms of IMPROVE-IT, our analysis cannot address any potential hypothesis regarding the association between simvastatin use and cancer. However, the cholesterol treatment trialist meta-analysis in 90,056 patients treated with statins and 14 trials showed no association between LDL-C reduction and an increased risk of malignancy with statin (statin: 2,810 events, 1.4% per year; controls: 2,804 events, 1.4% per year; RR: 1.00; 95% CI: 0.95 to 1.04) (5). In the updated analysis from the cholesterol treatment trialist cycle 2, including 174,149 patients and 27 trials, the reduction of LDL-C with statin (or high-intensity statin) had no effect on the incidence of newly diagnosed malignancy (RR: 1.00; 95% CI: 0.96 to 1.05) or on deaths to malignancy (RR: 1.00; 95% CI: 0.93 to 1.07) compared with control (placebo or low-intensity statin) over a median treatment duration of 4.8 years (22). Furthermore, there was no increased malignancy rate despite very low achieved LDL-C lowering with the proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors in the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects With Elevated Risk) and ODYSSEY-OUTCOMES (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) trials (23,24). Finally, achievement of very low LDL-C levels with ezetimibe in IMPROVE-IT (25) (<30 mg/dl) and with evolocumab in FOURIER (<20 mg/dl) at 1 month after randomization was not associated with an increase in major safety events, including new malignancies.
We acknowledge limitations to this analysis. First, although IMPROVE-IT was a large multicenter, multinational trial that enrolled a broad population of patients after an ACS hospitalization, patients were selected for enrollment who fulfilled appropriate inclusion criteria. Frail patients at higher risk of malignancy may have been less likely to qualify and/or willing to participate in this clinical trial. Therefore, the applicability of the observations described to patients excluded from IMPROVE-IT is unknown. Second, as previously reported, the discontinuation of study medication was highest early and stabilized to 8% per year (26). However, study drug discontinuation was not related to the addition of ezetimibe and data continued to be collected and analyzed regardless of whether patients continued study treatment or not (26). Third, the average follow-up of 6 years might be not long enough to detect a carcinogenic signal, particularly for slowly growing malignancies with a long latency period. However, there is no indication that the risks by treatment arm would have changed over a longer follow-up nor that the KM curves would have started to diverge beyond the available follow-up of 6 years. Fourth, the systematic prospective collection for malignancies started in 2008, roughly 3 years after the start of the trial, and consequently part of the malignancy data was collected retrospectively. Fifth, although the analyses of outcomes stratified by subgroups were pre-specified, the power was low in these subgroups. Finally, we did not measure phytosterols or other potential protective or tumor-promoting factors in blood to evaluate the effect of ezetimibe on the potential mechanistic pathways related to malignancy.
Conclusions
We found that ezetimibe did not increase the rates of malignancy nor deaths due to malignancy in 17,708 patients with recent ACS treated with simvastatin and followed up for a median of 6 years totaling 96,377 patient-years of follow-up.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: The addition of ezetimibe to simvastatin did not affect the risk of malignancy. No differences were found in new, worsening, or relapsing malignancy. Findings remained consistent after: 1) including or excluding nonmelanoma skin malignancies; 2) limiting malignancy outcome definitions to either new malignancy or including relapsing/worsening malignancy; or 3) restricting the endpoint to patients with available pathology reports. In addition, we did not find differences between treatment groups in deaths due to malignancy, malignancy location, extent of malignancy, or among high-risk subgroups. Finally, we did not observe any increased relative risk with ezetimibe versus placebo with longer follow-up duration.
TRANSLATIONAL OUTLOOK: Our findings support the 2018 Cholesterol Guidelines recommendations to intensify lipid-lowering treatment to lower LDL-C levels in patients with ACS. The use of ezetimibe in addition to simvastatin can be safely considered for the long-term lipid-lowering management of patients with ACS. Our data highlight the importance of considering malignancy outcomes in the design of long-term cardiovascular trials because the incidence of cancer endpoints approaches the incidence of traditional cardiovascular endpoints over time.
Acknowledgment
We thank Andrew J. Wagner, MD, PhD (Dana Farber Cancer Institute, Harvard Medical School, Boston, Massachusetts) for his contribution as a member of the Oncology CEC.
Footnotes
IMPROVE-IT was supported by research grants from Merck to the Brigham and Women’s Hospital and the Duke Clinical Research Group. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the article, and its final contents. Dr. Giugliano has received a research grant to his institution from Merck for the conduct of the IMPROVE-IT; has received a research grant to his institution from Amgen for other lipid-lowering trials; has received honoraria for continuing medical education activities from Amgen, Daiichi-Sankyo, and Merck; and has received consulting/advisory board fees from Amarin, Bristol-Myers Squibb, CVS Caremark, Daiichi-Sankyo, GlaxoSmithKline, Lexicon, Merck, Portola, Pfizer, and Servier. Dr. Gencer's activities in the TIMI Group, Harvard Medical Schools, are supported by grants from the Geneva University Hospitals, Eugenio Litta, and Athemis Foundations. Dr. Wiviott has received grants from Amgen, Arena, AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly, Janssen, Merck, and Sanofi; has received consulting fees from ARENA, AstraZeneca, Aegerion, Allergan, Angelmed, Boehringer Ingelheim, Boston Clinical Research Institute, Bristol-Myers Squibb, Daiichi-Sankyo, Eisai, Eli Lilly, Icon Clinical, Janssen, Lexicon, Merck, Servier, St. Jude Medical, and Xoma; and his spouse is an employee of Merck. Dr. Park is a member of the TIMI Study Group, which has received institutional research grant support through Brigham and Women’s Hospital from: Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. Dr. Fuchs has a consulting role for Agios, Amylin Pharmaceuticals, Bain Capital, CytomX Therapeutics, Daiichi-Sankyo, Eli Lilly, Entrinsic Health, Evolveimmune Therapeutics, Genentech, Merck, Taiho, and Unum Therapeutics; serves as a Director for CytomX Therapeutics; owns unexercised stock options for CytomX and Entrinsic Health; and is a co-Founder of Evolveimmune Therapeutics and has equity in this private company. Dr. Goessling has received consulting fees from and owns stock in Camp4 Therapeutics; and has received patent royalties from Fate Therapeutics. Drs. Musliner and Tershakovec were employed by Merch at the time IMPROVE-IT was conducted. Dr. Blazing has received research support from Merck for data analysis; and has received consultant/advisory board fees from Merck and Espirion. Dr. Califf is employed by Verily Life Sciences and Google Health; and is on the board of Cytokinetics. Dr. Cannon has received a research grant from Merck to his institution for his role as principal investigator of IMPROVE-IT; and has received consultant or advisory board fees from Merck. Dr. Braunwald has received a research grant to his institution from Merck for his role as Co-Chair of IMPROVE-IT and other support from Merck during the conduct of the study; research grants through his institution from AstraZeneca, Daiichi-Sankyo, and Novartis; and consultancy fees from Amgen, Cardurion, MyoKardia, NovoNordisk, and Verve.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For supplemental Methods and tables, please see the online version of this paper.
Appendix
References
- 1.Malmborg M., Christiansen C.B., Schmiegelow M.D., Torp-Pedersen C., Gislason G., Schou M. Incidence of new onset cancer in patients with a myocardial infarction - a nationwide cohort study. BMC Cardiovasc Disord. 2018;18:198. doi: 10.1186/s12872-018-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfeffer M.A. Cancer in cardiovascular drug trials and vice versa: a personal perspective. Eur Heart J. 2013;34:1089–1094. doi: 10.1093/eurheartj/ehs014. [DOI] [PubMed] [Google Scholar]
- 3.Messerli F.H., Bangalore S., Torp-Pedersen C., Staessen J.A., Kostis J.B. Cardiovascular drugs and cancer: of competing risk, smallpox, Bernoulli, and d'Alembert. Eur Heart J. 2013;34:1095–1098. doi: 10.1093/eurheartj/ehs158. [DOI] [PubMed] [Google Scholar]
- 4.Ridker P.M., MacFadyen J.G., Thuren T. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- 5.Baigent C., Keech A., Kearney P.M. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 6.Sabatine M.S., Wiviott S.D., Im K., Murphy S.A., Giugliano R.P. Efficacy and safety of further lowering of low-density lipoprotein cholesterol in patients starting with very low levels: a meta-analysis. JAMA Cardiol. 2018;3:823–828. doi: 10.1001/jamacardio.2018.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor A.J., Nissen S.E. Preliminary observations from preliminary trial results: have we finally had enough? Circ Cardiovasc Qual Outcomes. 2008;1:54–57. doi: 10.1161/CIRCOUTCOMES.108.811901. [DOI] [PubMed] [Google Scholar]
- 8.Rossebo A.B., Pedersen T.R., Boman K. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. doi: 10.1056/NEJMoa0804602. [DOI] [PubMed] [Google Scholar]
- 9.Sudhop T., Lutjohann D., Kodal A. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 10.Grundy S.M., Stone N.J., Bailey A.L. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. J Am Coll Cardiol. 2019;73:e285–e350. doi: 10.1016/j.jacc.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Cannon C.P., Blazing M.A., Giugliano R.P. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 12.Cannon C.P., Giugliano R.P., Blazing M.A. Rationale and design of IMPROVE-IT (IMProved Reduction of Outcomes: Vytorin Efficacy International Trial): comparison of ezetimbe/simvastatin versus simvastatin monotherapy on cardiovascular outcomes in patients with acute coronary syndromes. Am Heart J. 2008;156:826–832. doi: 10.1016/j.ahj.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Bohula E.A., Morrow D.A., Giugliano R.P. Atherothrombotic risk stratification and ezetimibe for secondary prevention. J Am Coll Cardiol. 2017;69:911–921. doi: 10.1016/j.jacc.2016.11.070. [DOI] [PubMed] [Google Scholar]
- 14.Peto R., Gray R., Brantom P., Grasso P. Dose and time relationships for tumor induction in the liver and esophagus of 4080 inbred rats by chronic ingestion of N-nitrosodiethylamine or N-nitrosodimethylamine. Cancer Res. 1991;51:6452–6469. [PubMed] [Google Scholar]
- 15.Peto R., Emberson J., Landray M. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–1366. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 16.Cholesterol Treatment Trialists C. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bradford P.G., Awad A.B. Phytosterols as anticancer compounds. Mol Nutr Food Res. 2007;51:161–170. doi: 10.1002/mnfr.200600164. [DOI] [PubMed] [Google Scholar]
- 18.Blanco-Vaca F., Cedo L., Julve J. Phytosterols in cancer: from molecular mechanisms to preventive and therapeutic potentials. Curr Med Chem. 2019;26:6735–6749. doi: 10.2174/0929867325666180607093111. [DOI] [PubMed] [Google Scholar]
- 19.Salen G., von Bergmann K., Lutjohann D. Ezetimibe effectively reduces plasma plant sterols in patients with sitosterolemia. Circulation. 2004;109:966–971. doi: 10.1161/01.CIR.0000116766.31036.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale K.M., Coleman C.I., Henyan N.N., Kluger J., White C.M. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 22.Cholesterol Treatment Trialists C., Emberson J.R., Kearney P.M. Lack of effect of lowering LDL cholesterol on cancer: meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS One. 2012;7 doi: 10.1371/journal.pone.0029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giugliano R.P., Pedersen T.R., Park J.G. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390:1962–1971. doi: 10.1016/S0140-6736(17)32290-0. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz G.G., Steg P.G., Szarek M. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 25.Giugliano R.P., Wiviott S.D., Blazing M.A. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT trial. JAMA Cardiol. 2017;2:547–555. doi: 10.1001/jamacardio.2017.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navar A.M., Roe M.T., White J.A. Medication discontinuation in the IMPROVE-IT trial. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.