Abstract
Objectives
The purpose of this study was to evaluate ischemic and bleeding outcomes of unselected cancer patients undergoing percutaneous coronary intervention (PCI).
Background
The number of cancer patients undergoing PCI is increasing despite concerns regarding ischemic and bleeding risks.
Methods
Between 2009 and 2017, consecutive patients undergoing PCI were prospectively included in the Bern PCI Registry. Cancer-specific data including type, date of initial diagnosis, and health status at index PCI were collected. We performed propensity score matching to adjust for baseline differences between patients with and without cancer. The primary ischemic endpoint was the device-oriented composite endpoint (cardiac death, target vessel myocardial infarction, target lesion revascularization) at 1 year, and the primary bleeding endpoint was Bleeding Academic Research Consortium (BARC) 2 to 5 at 1 year.
Results
Among 13,647 patients, 1,368 (10.0%) had an established diagnosis of cancer. The 3 leading cancer types were prostate (n = 294), gastrointestinal tract (n = 188), and hematopoietic (n = 177). At index PCI, 179 (13.1%) patients were receiving active cancer treatment. In matched analysis, there was no significant difference in device-oriented composite endpoint (11.5% vs. 10.2%; p = 0.251), whereas cardiac death and BARC 2 to 5 bleeding occurred more frequently among patients with cancer compared with those without cancer (6.8% vs. 4.5%; p = 0.010 and 8.0% vs. 6.0%; p = 0.026, respectively). Cancer diagnosis within 1 year before PCI emerged as an independent predictor for cardiac death and BARC 2 to 5 bleeding at 1 year.
Conclusions
Cancer patients carry an increased risk of cardiac mortality that was not associated with stent-related ischemic events among patients undergoing PCI in routine clinical practice. Higher risk of bleeding in cancer patients undergoing PCI deserves particular attention. (CARDIOBASE Bern PCI Registry; NCT02241291)
Key Words: bleeding, cancer, coronary artery disease, ischemia, percutaneous coronary intervention
Abbreviations and Acronyms: BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; CI, confidence interval; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; DOCE, device-oriented composite endpoint; HR, hazard ratio; IPTW, inverse probability of treatment weighting; MI, myocardial infarction; PCI, percutaneous coronary interventions; PS, propensity score
Central Illustration
Cardiovascular disease and cancer are the most common causes of death in developed countries. Improved longevity resulting from advances in early diagnosis, risk factor control, and treatment of both disease manifestations has resulted in an increasing prevalence of coronary artery disease (CAD) and cancer. Cancer is known to be associated with an increased risk of ischemic events including myocardial infarction (MI) and stroke by several mechanisms (1). The coagulable state is activated from triggering of the coagulation system by tumor cells (2). Some chemotherapeutic agents may cause premature atherosclerosis and trigger acute coronary syndrome by endothelial injury, vasospasm, and changes in lipid metabolism (3, 4, 5, 6). Finally, CAD and cancer share several common risk factors, such as smoking, sedentary lifestyle, diet, obesity, and chronic inflammation (7). In addition to an increased risk of ischemic events, cancer may predispose to bleeding because of direct and indirect effects on coagulation and hematologic parameters and the often-required surgical procedures.
Despite an increasing number of cancer patients undergoing percutaneous coronary intervention (PCI), only limited data regarding ischemic and bleeding events are available in this population, and with inconsistent results (8, 9, 10, 11). We sought to compare ischemic and bleeding outcomes between patients undergoing PCI with versus without a cancer diagnosis and to determine whether there may be cancer-specific predictors of these outcomes.
Methods
Patient population
All patients undergoing PCI at Bern University Hospital, Switzerland, have been prospectively enrolled to the Bern PCI Registry (NCT02241291) since January 2009 with no formal exclusion criteria. For the current analysis, all consecutive patients enrolled between January 2009 and January 2017 were included. Patients with missing data regarding the history of cancer were excluded. Study patient characteristics, procedure characteristics, in-hospital outcomes, and 1-year outcomes were systematically and prospectively collected. A health questionnaire was sent to all living patients with questions on rehospitalization and adverse events, followed by telephone contact in case of missing responses. General practitioners and referring cardiologists were contacted as necessary for additional information. To ascertain outcomes in patients treated for adverse events at other medical institutions, external medical records, discharge letters, and coronary angiography documentation were systematically collected and reviewed. For patients with cancer, detailed cancer characteristics were collected from medical records, patient contact, or general practitioner contact as a part of data collection for the PCI registry. PCI was performed according to current practice guideline (12). The routinely recommended dual antiplatelet therapy (DAPT) duration was 12 months (13). Informed consent was obtained from each patient. The registry was approved by the institutional ethics committee.
Cancer
If patients had multiple primary cancers before index PCI, the most recent type of cancer diagnosed before index PCI was used for the analysis. Ongoing treatment of cancer was defined as planning for surgery or currently undergoing systemic therapy (i.e., chemotherapy, hormone, and biological therapy) and/or radiation at index PCI.
Clinical endpoints and definitions
A clinical event committee consisting of 2 cardiologists (and a third referee in case of disagreement) adjudicated all events based on original source documents. The primary ischemic endpoint was the device-oriented composite endpoint (DOCE) (cardiac death, target vessel MI, and target lesion revascularization), and the primary bleeding endpoint was the Bleeding Academic Research Consortium (BARC) composite type 2 to 5. Bleeding was defined according to the BARC criteria, which is a standardized, hierarchically graded classification for bleeding in patients receiving antithrombotic therapy and consists of 6 categories (types 0 to 5), with greater category indicating more severe bleeding (14). The details of the BARC criteria are available in the Supplemental Appendix. PRECISE-DAPT score is a 5-item (age, creatinine clearance, hemoglobin, white blood cell count, and previous spontaneous bleeding) risk score and ranges from 0 to 100, with higher scores indicating a higher risk of out-of-hospital bleeding during DAPT.
Statistical analysis
Continuous variables were summarized as mean ± SD or median (1st quartile, 3rd quartile) and compared with analysis of variance or Kruskal-Wallis test based on data distribution. Binary and categorical variables were calculated as frequencies (proportions), and were compared with the chi-square test or Fisher exact test if expected cell counts were <5. Survival curves were constructed for time-to-event variables with Kaplan-Meier estimates and compared using the log-rank test. Propensity score (PS) matching and inverse probability of treatment weighting (IPTW) were performed to determine the impact of cancer on study endpoints. PS was calculated for each patient with a probit regression model predicting cancer by the following baseline variables: age, sex, body mass index, hypertension, diabetes mellitus, dyslipidemia, current smoker, family history of CAD, previous MI, previous PCI, previous coronary artery bypass graft, previous cerebrovascular accident, peripheral artery disease, renal failure, anemia, previous bleeding, MI at presentation, Killip IV, left ventricular ejection fraction, number of diseased vessels, number of lesions, left main disease, chronic total occlusion, new-generation drug-eluting stent (DES) use, potent P2Y12 (prasugrel or ticagrelor), any DAPT, anticoagulation, and statin. Matching was performed with a 1:1 nearest neighbor matching without replacement, using a caliper width equal to 0.09. Stratified Cox models were used in the analyses of matched pairs. The balance between cancer and no cancer groups was evaluated using standardized differences. Subdistribution hazard ratios with a competing risk approach were obtained using Fine and Gray’s methods (15). Multivariable Cox regression analyses were performed to determine independent predictors for cardiac death and BARC 2 to 5 and model results were presented with hazard ratio (HR), 95% confidence interval (CI), and p values. Schoenfeld residuals were used to assess the proportionality assumption. Variables pre-determined to be of clinical importance were used for adjustment (for cardiac death: age, female, cardiogenic shock, left ventricular ejection fraction, MI at presentation, renal failure, and peripheral artery disease; for BARC 2 to 5: age, female, body mass index, renal failure, prior bleeding history, and anemia) (16, 17, 18, 19, 20). The p values were 2-sided and < 0.05 was considered to be statistically significant in all analyses. Statistical analyses were performed with Stata version 15.1 (StataCorp, College Station, Texas) and R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Patients
Of 13,753 patients enrolled in the Bern PCI Registry between January 2009 and January 2017, those who had the history of cancer was unclear (n = 79) or detailed cancer information was not available (n = 27) were excluded. As a result, 13,647 were analyzed in the present study; there was complete 1-year follow-up for any event type in 93.5% of patients and for mortality in 96.5% of patients. Clinical and procedural characteristics and medication status are shown in Tables 1, 2, and 3 and Supplemental Table 1. A total of 1,368 patients (10.0%) had an established diagnosis of cancer. Cancer patients were older and had a higher prevalence of hypertension, smoking, stable CAD, and comorbidities including previous stroke, renal failure, chronic obstructive pulmonary disease, and peripheral artery disease. Cancer patients had higher PRECISE-DAPT scores compared with no cancer patients (26.4 ± 12.4 vs. 19.6 ± 12.4; p < 0.001). At discharge, DAPT and potent P2Y12 inhibitors were less frequently administered (93.9% vs. 95.3%; p = 0.024, 30.6% vs. 41.6%; p < 0.001, respectively), although anticoagulation and triple therapy were more frequently prescribed in cancer patients (11.4% vs. 7.9%; p < 0.001, 12.3% vs. 8.2%; p < 0.001, respectively).
Table 1.
Patient Characteristics
| Overall (N = 13,647) | Cancer (n = 1,368) | No Cancer (n = 12,279) | p Value | |
|---|---|---|---|---|
| Age, yrs | 67.7 ± 12.0 | 72.9 ± 9.8 | 67.1 ± 12.1 | <0.001 |
| Female | 3,549 (26.0) | 391 (28.6) | 3,158 (25.7) | 0.023 |
| Body mass index, kg/m2 | 27.4 ± 4.7 | 26.9 ± 4.6 | 27.5 ± 4.7 | <0.001 |
| Current smoker | 3,628 (26.9) | 251 (18.3) | 3,377 (27.8) | <0.001 |
| Hypertension | 9,407 (69.2) | 1,057 (77.3) | 8,350 (68.3) | <0.001 |
| Diabetes mellitus | 3,135 (23.0) | 342 (25.0) | 2,793 (22.8) | 0.062 |
| Dyslipidemia | 8,784 (64.7) | 915 (66.9) | 7,869 (64.5) | 0.078 |
| Previous myocardial infarction | 2,318 (17.0) | 268 (19.6) | 2,050 (16.7) | 0.008 |
| Previous PCI | 3,025 (22.2) | 347 (25.4) | 2,678 (21.9) | 0.004 |
| Previous CABG | 1,370 (10.0) | 169 (12.4) | 1,201 (9.8) | 0.003 |
| Family history of CAD | 3,564 (26.2) | 272 (19.9) | 3,292 (26.9) | <0.001 |
| Peripheral arterial disease | 1,112 (8.2) | 173 (12.6) | 939 (7.7) | <0.001 |
| History of cerebrovascular accident (stroke/TIA) | 990 (7.3) | 140 (10.2) | 850 (6.9) | <0.001 |
| Prior bleeding | 990 (7.3) | 140 (10.2) | 850 (6.9) | <0.001 |
| Chronic kidney disease | 3,146 (25.4) | 516 (37.9) | 2,630 (23.8) | <0.001 |
| History of atrial fibrillation or atrial flutter | 1,021 (12.0) | 173 (17.8) | 848 (11.2) | <0.001 |
| Left ventricular ejection fraction, % | 58.0 (45.0, 65.0) | 60.0 (45.0, 65.0) | 55.0 (45.0, 65.0) | 0.074 |
| Anemia | 2,885 (24.3) | 538 (39.6) | 2,347 (22.3) | <0.001 |
| Clinical indication for PCI | ||||
| Stable CAD | 6,011 (44.0) | 714 (52.2) | 5,297 (43.1) | <0.001 |
| Acute coronary syndrome | ||||
| Unstable angina | 677 (5.0) | 71 (5.2) | 606 (4.9) | 0.694 |
| Non-ST-segment elevation MI | 3,387 (24.8) | 367 (26.8) | 3,020 (24.6) | 0.075 |
| ST-segment elevation MI | 3,572 (26.2) | 216 (15.8) | 3,356 (27.3) | <0.001 |
| Cardiogenic shock at presentation | 515 (3.8) | 54 (3.9) | 461 (3.8) | 0.709 |
| PRECISE-DAPT score | 20.3 ± 12.7 | 26.4 ± 13.5 | 19.6 ± 12.4 | <0.001 |
Values are mean ± SD, n (%), or median (1st quartile, 3rd quartile).
CABG = coronary artery bypass graft; CAD = coronary artery disease; MI = myocardial infarction; PCI = percutaneous coronary intervention; TIA = transient ischemic attack.
Table 2.
Procedural Characteristics
| Overall (N = 13,647) | Cancer (n = 1,368) | No Cancer (n = 12,279) | p Value | |
|---|---|---|---|---|
| Target lesion coronary artery | ||||
| Left main artery | 594 (4.4) | 80 (5.8) | 514 (4.2) | 0.006 |
| Left anterior descending artery | 7,150 (52.4) | 664 (48.5) | 6,486 (52.8) | 0.003 |
| Left circumflex artery | 4,409 (32.3) | 461 (33.7) | 3,948 (32.2) | 0.247 |
| Right coronary artery | 5,014 (36.7) | 536 (39.2) | 4,478 (36.5) | 0.051 |
| Bypass graft | 450 (3.3) | 55 (4.0) | 395 (3.2) | 0.129 |
| Number of lesions | ||||
| 1 | 7,654 (56.1) | 760 (55.6) | 6,894 (56.1) | 0.688 |
| 2 | 3,924 (28.8) | 398 (29.1) | 3,526 (28.7) | 0.777 |
| ≥3 | 2,069 (15.1) | 210 (15.4) | 1,859 (15.1) | 0.842 |
| Lesion type | ||||
| Restenotic lesion | 926 (6.8) | 96 (7.0) | 830 (6.8) | 0.734 |
| Chronic total occlusion | 597 (4.4) | 50 (3.7) | 47 (4.5) | 0.186 |
| Access site | ||||
| Radial | 1,971 (23.1) | 233 (24.0) | 1,738 (23.0) | 0.492 |
| Femoral | 6,567 (76.9) | 739 (76.0) | 5,828 (77.0) | 0.492 |
| Stent type | ||||
| New-generation DES | 12,185 (89.3) | 1,203 (87.9) | 10,982 (89.4) | 0.097 |
| Bare metal stent | 842 (6.2) | 100 (7.3) | 742 (6.0) | 0.066 |
Values are n (%).
DES = drug-eluting stent.
Table 3.
Medication at Discharge
| Overall (N = 13,647) | Cancer (n = 1,368) | No Cancer (n = 12,279) | p Value | |
|---|---|---|---|---|
| Aspirin | 13,219 (96.9) | 1,318 (96.3) | 11,901 (97.0) | 0.217 |
| Clopidogrel | 7,851 (57.6) | 908 (66.4) | 6,943 (56.6) | <0.001 |
| Potent P2Y12 (prasugrel or ticagrelor) | 5,517 (40.5) | 419 (30.6) | 5,098 (41.6) | <0.001 |
| Any DAPT | 12,968 (95.2) | 1,284 (93.9) | 11,684 (95.3) | 0.024 |
| Oral anticoagulation | 1,116 (8.2) | 156 (11.4) | 960 (7.9) | <0.001 |
| Direct oral anticoagulants | 318 (3.4) | 48 (4.8) | 270 (3.2) | 0.012 |
| Any DAPT and OAC/DOAC | 1,149 (8.6) | 163 (12.3) | 986 (8.2) | <0.001 |
| Statin | 12,232 (90.3) | 1,170 (85.8) | 11,062 (90.8) | <0.001 |
Values are n (%).
DAPT = dual antiplatelet therapy; DOAC = direct oral anticoagulant; OAC = oral anticoagulant.
Cancer characteristics
Cancer characteristics are summarized in Tables 4 and 5, respectively. Major cancer types were prostate (n = 294, 21.5%), gastrointestinal tract (n = 188, 13.7%), hematopoietic (n = 177, 12.9%), breast (n = 172, 12.6%), bladder (n = 119, 8.7%), skin (n = 105, 7.7%), lung (n = 76, 5.6%), and head/neck (n = 75, 5.5%). A total of 203 (14.8%) patients were diagnosed within 1 year before PCI. At index PCI, 179 (13.1%) patients were being actively treated for cancer and 121 (9.1%) had metastatic cancer. Surgery was performed in 70.1% of patients; chemotherapy, hormone therapy, or biological therapy in 38.5%; and radiation in 33.4%.
Table 4.
Type of Cancer (N = 1,368)
| Head/neck | 75 (5.5) |
| Head/neck (except for thyroid) | 53 (3.9) |
| Thyroid | 22 (1.6) |
| Gastrointestinal tract | 188 (13.7) |
| Esophageal | 15 (1.1) |
| Gastric | 18 (1.3) |
| Small intestine | 6 (0.4) |
| GIST | 4 (0.3) |
| Colon/rectal | 145 (10.6) |
| Hepatic, biliary, pancreatic | 21 (1.5) |
| Liver | 11 (0.8) |
| Gallbladder | 1 (0.1) |
| Pancreatic | 9 (0.7) |
| Lung | 76 (5.6) |
| Skin | 105 (7.7) |
| Melanoma | 46 (3.4) |
| Nonmelanoma | 59 (4.3) |
| Breast | 172 (12.6) |
| Uterine, ovarian | 25 (1.8) |
| Uterine | 21 (1.5) |
| Ovarian | 4 (0.3) |
| Prostate | 294 (21.5) |
| Bladder | 119 (8.7) |
| Renal | 51 (3.7) |
| Hematopoietic | 177 (12.9) |
| Others | 65 (4.8) |
Values are n (%).
GIST = gastrointestinal stromal tumor.
Table 5.
Cancer Characteristics (N = 1,368)
| Years between cancer diagnosis and PCI (n = 1,305) | |
| ≤1 yr | 203 (14.8) |
| 1–5 yrs | 356 (26.0) |
| ≥5 yrs | 746 (54.5) |
| 5–10 yrs | 328 (24.0) |
| ≥10 yrs | 418 (30.6) |
| Stage of cancer at diagnosis (n = 621) | |
| 0 | 36 (2.6) |
| I | 170 (12.4) |
| II | 196 (14.3) |
| III | 133 (9.7) |
| IV | 86 (6.3) |
| On-going treatment at index PCI | 179 (13.1) |
| Metastasis (n = 1,107)∗ | 121 (9.1) |
| Treatment† | |
| Surgery | 959 (70.1) |
| Chemotherapy, hormone therapy, or biological therapy | 526 (38.5) |
| Radiation | 457 (33.4) |
Values are n (%).
PCI = percutaneous coronary intervention.
Patients with hematopoietic cancer were excluded.
Performed up to 1 yr after PCI.
Clinical outcomes
The PS distribution among patients with and without cancer is shown in Supplemental Figure 1. After excluding patients with missing covariates for PS calculation and considering a caliper width of 0.09, PS matching generated 1,343 pairs. The groups in the matched cohort had similar baseline characteristics, which were confirmed by the absolute values of standardized differences below 10% for all variables used in the calculation of the PS (Supplemental Tables 2 to 4).
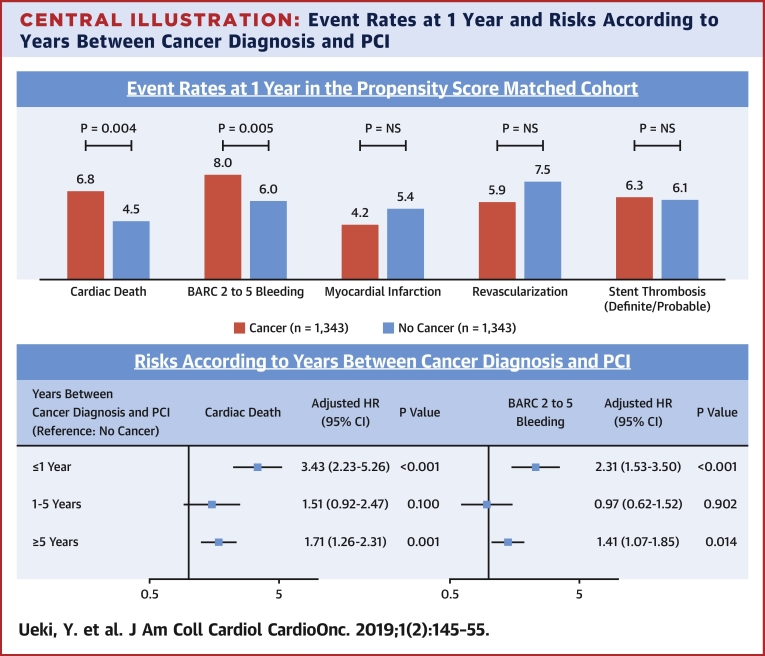
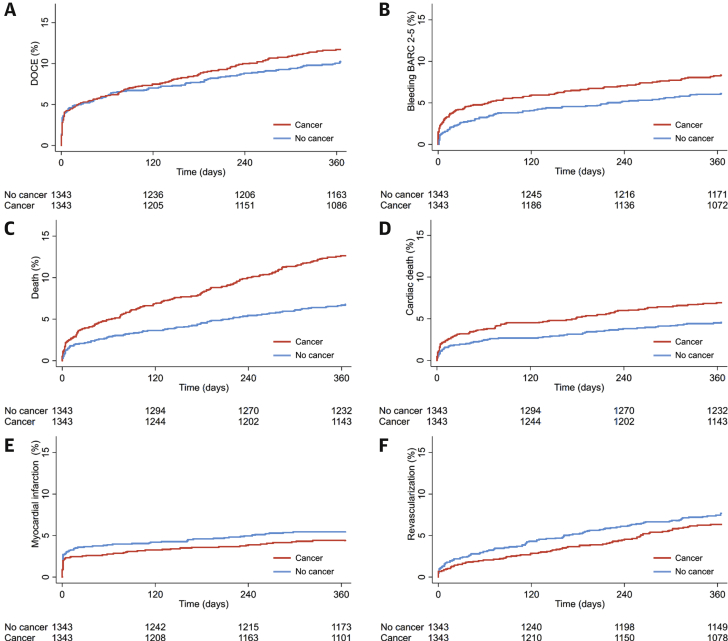
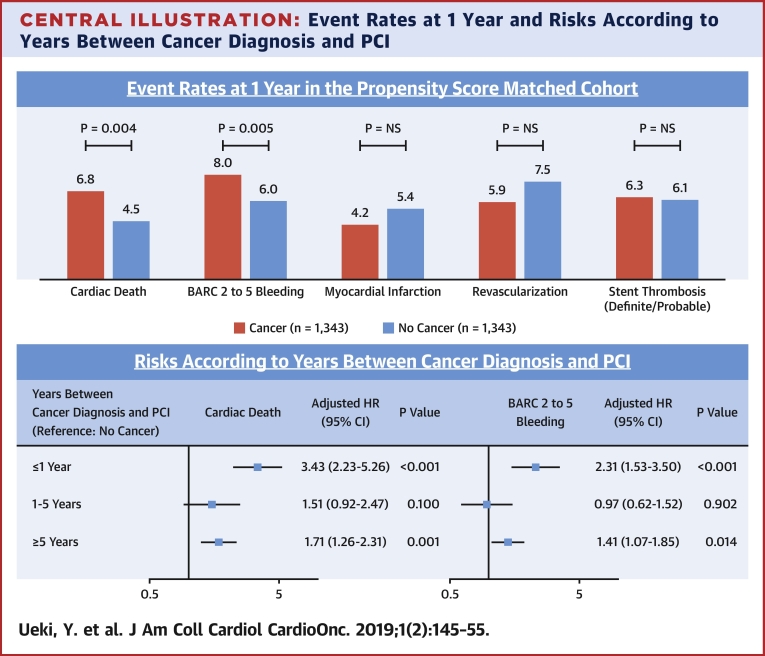
Clinical outcomes before and after PS matching and results of IPTW methods are summarized in Table 6 and Supplemental Table 5. In the PS-matched cohort, there was no significant difference in DOCE (11.5% vs. 10.2%, HR: 1.18; 95% CI: 0.93 to 1.50; p = 0.181). However, cancer patients had a higher risk of BARC 2 to 5 (8.0% vs. 6.0%; HR: 1.55; 95% CI: 1.14 to 2.11; p = 0.005), all-cause death (12.6% vs. 6.8%; HR: 2.03; 95% CI: 1.55 to 2.65; p < 0.001), cardiac death (6.8% vs. 4.5%; HR: 1.64; 95% CI: 1.17 to 2.31; p = 0.004), unclear death (2.7% vs. 1.5%; HR: 2.06; 95% CI: 1.15 to 3.67; p = 0.015), and non-CV death (5.2% vs. 1.7%; HR: 3.10; 95% CI: 1.89 to 5.06; p < 0.001). There were no significant differences in MI, revascularization, and definite/probable ST, similar to the results in the overall cohort (Supplemental Table 5). Kaplan-Meier curves for study endpoints after PS matching are shown in Figure 1.
Table 6.
Event Rates at 1 Year in the Propensity Score Matched-Cohort
| Cancer (n = 1,343) | Days to Events | No Cancer (n = 1,343) | Days to Events | HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| Primary ischemic endpoint | ||||||
| DOCE | 154 (11.5) | 48 (2, 180) | 137 (10.2) | 28 (1, 169) | 1.18 (0.93–1.50) | 0.181 |
| Primary bleeding endpoint | ||||||
| Bleeding BARC (2 to 5) | 107 (8.0) | 22 (2, 152) | 80 (6.0) | 47 (8, 175) | 1.55 (1.14–2.11) | 0.005 |
| Secondary endpoints | ||||||
| All-cause death | 169 (12.6) | 98 (19, 225) | 91 (6.8) | 99 (10, 220) | 2.03 (1.55–2.65) | <0.001 |
| Cardiac death | 91 (6.8) | 42 (4, 180) | 61 (4.5) | 54 (6, 192) | 1.64 (1.17–2.31) | 0.004 |
| Definite cardiac death | 55 (4.1) | 6 (2, 82) | 41 (3.1) | 10 (3, 130) | 1.44 (0.94–2.21) | 0.090 |
| Unclear death | 36 (2.7) | 157 (55, 263) | 20 (1.5) | 188 (72, 282) | 2.06 (1.15–3.67) | 0.015 |
| Cardiovascular death | 99 (7.4) | 42 (4, 179) | 68 (5.1) | 66 (7, 218) | 1.64 (1.18–2.27) | 0.003 |
| Noncardiovascular death | 70 (5.2) | 182 (98, 275) | 23 (1.7) | 104 (57, 220) | 3.10 (1.89–5.06) | <0.001 |
| Myocardial infarction | 57 (4.2) | 2 (1, 107) | 72 (5.4) | 2 (1, 101) | 0.77 (0.54–1.10) | 0.152 |
| Spontaneous myocardial infarction | 28 (2.1) | 118 (55, 238) | 36 (2.7) | 101 (15, 212) | 0.76 (0.46–1.27) | 0.303 |
| TV myocardial infarction | 45 (3.4) | 1 (0, 79) | 59 (4.4) | 1 (1, 53) | 0.78 (0.53–1.15) | 0.201 |
| Any revascularization | 79 (5.9) | 140 (25, 256) | 100 (7.5) | 108 (15, 210) | 0.79 (0.58–1.08) | 0.140 |
| Target lesion revascularization | 45 (3.4) | 147 (36, 250) | 49 (3.7) | 73 (8, 208) | 0.96 (0.63–1.45) | 0.831 |
| Target vessel revascularization | 60 (4.5) | 146 (35, 253) | 72 (5.4) | 96 (13, 210) | 0.87 (0.61–1.23) | 0.421 |
| Stent thrombosis (definite/probable) | 84 (6.3) | 2 (1, 18) | 82 (6.1) | 1 (1, 15) | 1.04 (0.76–1.41) | 0.814 |
| Acute | 38 (2.8) | 1 (0, 1) | 42 (3.1) | 1 (0, 1) | 0.90 (0.58–1.40) | 0.655 |
| Subacute | 33 (2.5) | 5 (3, 15) | 25 (1.9) | 9 (4, 15) | 1.33 (0.79–2.26) | 0.287 |
| Late | 14 (1.0) | 146 (84, 256) | 16 (1.2) | 162 (63, 222) | 0.93 (0.45–1.93) | 0.853 |
| Stroke | 28 (2.1) | 17 (4, 154) | 18 (1.3) | 116 (9, 215) | 1.69 (0.91–3.13) | 0.097 |
| Bleeding BARC (2) | 40 (3.0) | 33 (6, 203) | 30 (2.2) | 123 (53, 238) | 1.62 (0.98–2.70) | 0.061 |
| Bleeding BARC (3) | 71 (5.3) | 22 (1, 150) | 54 (4.0) | 35 (4, 134) | 1.47 (1.01–2.13) | 0.042 |
| Bleeding BARC (4) | 3 (0.2) | 1 (0, 34) | 4 (0.3) | 20 (11, 27) | 0.75 (0.17–3.35) | 0.706 |
| Bleeding BARC (5) | 4 (0.3) | 29 (9, 178) | 2 (0.2) | 149 (109, 190) | 4.00 (0.45–35.79) | 0.215 |
Values are n (%) or median (1st quartile, 3rd quartile). HR and 95% CI are computed from Cox models.
BARC = bleeding academic research consortium; CI = confidence interval; DOCE = device oriented composite endpoint, HR = hazard ratio; TV = target vessel.
Figure 1.
Kaplan-Meier Curves
(A) DOCE, (B) bleeding (BARC 2 to 5), (C) all-cause death, (D) cardiac death, (E) myocardial infarction, and (F) and revascularization in the propensity score matched-cohort. BARC = bleeding academic research consortium; DOCE = device oriented composite endpoint.
Results in the PS-matched cohort were similar with those derived from the IPTW methods. Results of IPTW methods with imputation of missing data remained similar (data not shown). Cancer patients had an increased risk of cardiac death after considering noncardiac death as a competing risk in the overall population and the PS-matched cohort (overall, HR: 1.66; 95% CI: 1.34 to 2.05; p < 0.001; PS-matched, HR: 1.52; 95% CI: 1.10 to 2.10; p = 0.012). Similarly, an increased risk of BARC 2 to 5 in cancer patients was still observed after considering all-cause death as a competing risk (overall, HR: 1.58; 95% CI: 1.29 to 1.94; p < 0.001; PS-matched, HR: 1.36; 95% CI: 1.02 to 1.86; p = 0.036). Causes of definite cardiac death are shown in Supplemental Table 6. Types of bleeding and medication status at discharge according to bleeding are shown in Supplemental Tables 7 and 8. There were no significant differences in potent P2Y12 use and triple therapy at discharge between patients who had bleeding events with cancer versus no cancer.
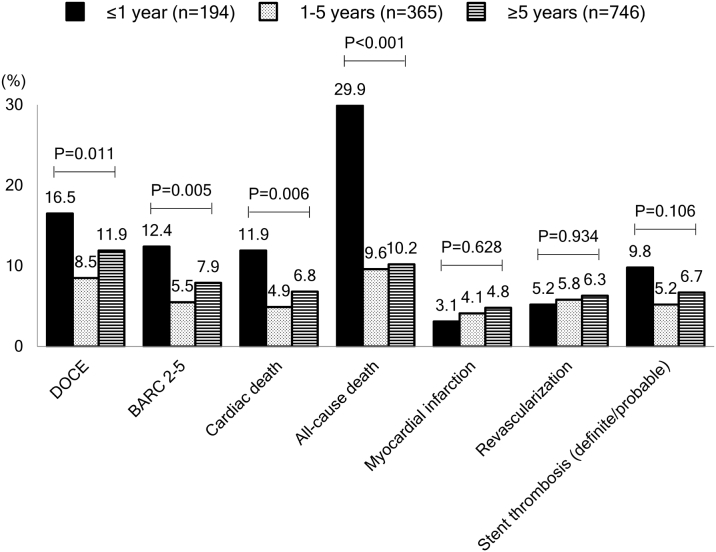
Clinical outcomes according to years between cancer diagnosis and PCI and type of cancer were also evaluated. Patients with a cancer diagnosis within 1 year before index PCI more frequently presented with anemia or acute coronary syndrome, had a higher PRECISE-DAPT score, and had a higher prevalence of metastasis compared with late diagnosis >1 year (Supplemental Tables 9 to 12). A higher incidence of cardiac death, all-cause death, and BARC 2 to 5 bleeding was observed in patients with a recent cancer diagnosis (≤1 year) (Figure 2). Among major types of cancer, lung and bladder cancer had a relatively higher incidence of cardiac death (13.2% and 10.9%, respectively) and all-cause death (36.8% and 15.1%, respectively) (Supplemental Figure 2).
Figure 2.
Event Rates According to Time Between Cancer Diagnosis and Index PCI Among Cancer Patients
The p values were based on log-rank test. Abbreviations as in Figure 1.
Predictors
Table 7 shows the results of multivariable Cox regression analyses for cardiac death and BARC 2 to 5 bleeding among patients with cancer. Cancer diagnosis within 1 year was an independent predictor for both cardiac death (adjusted HR: 1.92, 95% CI: 1.10 to 3.36; p = 0.022) and BARC 2 to 5 (adjusted HR: 1.75; 95% CI: 1.03 to 2.98; p = 0.040). A further explanatory Cox regression analysis to assess the time-dependent relation between cancer diagnosis and adverse outcomes was performed in the overall cohort. Cancer diagnosis ≤1 year and to a lower degree, cancer diagnosis ≥5 years before PCI, emerged as independent predictors for cardiac death and BARC 2 to 5 bleeding (Table 8). Results of multivariable Cox models with multiple imputation of missing data remained similar (data not shown).
Table 7.
Cox Regression Analysis For Cardiac Death And BARC 2 to 5 Bleeding Among Cancer Patients
| Cardiac Death |
BARC 2 to 5 Bleeding |
|||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
| Diagnosis within 1 yr of prior PCI | 1.92 (1.10–3.36) | 0.022 | 1.75 (1.03–2.98) | 0.040 |
| Ongoing treatment at index PCI | 0.91 (0.46–1.79) | 0.787 | 1.00 (0.55–1.80) | 0.988 |
| Type of cancer | ||||
| Bladder | 3.75 (1.50–9.39) | 0.005 | 1.16 (0.53–2.56) | 0.705 |
| Breast | 4.27 (1.39–13.13) | 0.011 | 0.92 (0.37–2.29) | 0.856 |
| Gastrointestinal tract | 4.10 (1.67–10.06) | 0.002 | 0.72 (0.33–1.57) | 0.411 |
| Head/neck | 2.75 (0.85–8.85) | 0.090 | 1.38 (0.56–3.41) | 0.483 |
| Hematopoietic | 3.72 (1.42–9.77) | 0.008 | 1.21 (0.57–2.53) | 0.621 |
| Hepatic, biliary, pancreatic | 0.81 (0.10–6.81) | 0.844 | 0.96 (0.22–4.30) | 0.963 |
| Lung | 3.64 (1.30–10.22) | 0.014 | 0.85 (0.31–2.38) | 0.762 |
| Others | 2.16 (0.60–7.79) | 0.241 | 1.70 (0.65–4.46) | 0.282 |
| Prostate | Ref. | Ref. | ||
| Renal | 2.47 (0.63–9.75) | 0.197 | 1.26 (0.45–3.51) | 0.656 |
| Skin | 3.26 (1.06–9.99) | 0.039 | 1.16 (0.49–2.72) | 0.735 |
| Uterine, ovarian | 3.45 (0.63–18.96) | 0.154 | 1.51 (0.39–5.88) | 0.551 |
Of the study patients, 93.7% (1,282 of 1,368) and 93.1% (1,274 of 1,368) were entered into the multivariable model for cardiac death and BARC 2 to 5 bleeding, respectively. Variables entered into multivariable models were as follows: for cardiac death: age, female, cardiogenic shock, left ventricular ejection fraction, myocardial infarction at presentation, chronic kidney disease, peripheral artery disease; for BARC 2 to 5 bleeding: age, female, body mass index, chronic kidney disease, prior bleeding, anemia, any dual antiplatelet therapy, and oral anticoagulant/direct oral anticoagulant at discharge.
Abbreviations as in Table 6.
Table 8.
Multivariable Cox Analysis for Cardiac Death and BARC 2 to 5 Bleeding in Overall Cohort
| Cardiac Death |
BARC 2 to 5 Bleeding |
|||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p Value | Adjusted HR (95% CI) | p Value | |
| No cancer | Ref. | Ref. | ||
| Years between cancer diagnosis and PCI | ||||
| ≤1 yr | 3.43 (2.23–5.26) | <0.001 | 2.31 (1.53–3.50) | <0.001 |
| 1–5 yrs | 1.51 (0.92–2.47) | 0.100 | 0.97 (0.62–1.52) | 0.902 |
| ≥5 yrs | 1.71 (1.26–2.31) | 0.001 | 1.41 (1.07–1.85) | 0.014 |
Of the study patients, 83.1% (11,339 of 13,647) and 82.2% (11,220 of 13,647) were entered into the multivariable model for cardiac death and BARC 2 to 5 bleeding, respectively. Variables entered into multivariable models were as follows: for cardiac death: age, female sex, current smoker, hypertension, chronic kidney disease, peripheral artery disease, myocardial infarction at presentation, cardiogenic shock, previous revascularization (PCI and/or coronary artery bypass graft), left ventricular ejection fraction, stent type (bare metal stent, first-generation DES, new-generation DES), and potent P2Y12 use at discharge; for BARC 2 to 5 bleeding: age, female sex, body mass index, prior bleeding, anemia, chronic kidney disease, potent P2Y12 use at discharge, and DAPT and oral anticoagulant use at discharge.
Discussion
The major findings of the present study are: 1) patients with cancer had an increased risk of cardiac death and bleeding, but not ischemic events such as MI, stent thrombosis, or recurrent revascularization; and 2) among patients with cancer, those with a recent cancer diagnosis (≤1 year) had an increased risk of cardiac death and bleeding (Central Illustration).
Central Illustration.
Event Rates at 1 Year and Risks According to Years Between Cancer Diagnosis and PCI
(Upper panel) Population is the propensity score matched-cohort (cancer vs. no cancer). The p values were based on Cox models. (Lower panel) Population is the overall cohort. The p values were based on Cox models. Of the study patients, 83.1% (11,339 of 13,647) and 82.2% (11,220 of 13,647) were entered into the multivariable model for cardiac death and BARC 2 to 5 bleeding, respectively. BARC = bleeding academic research consortium; CI = confidence interval; HR = hazard ratio; NS = not significant; PCI = percutaneous coronary intervention.
Previous studies evaluating outcomes of cancer patients after PCI showed inconsistent results likely attributable to the inclusion of small cohorts, lack of detailed information on cancer characteristics, and a limited number of endpoints not allowing for the estimation of the ischemic and bleeding risk (i.e., the most relevant concern in these patients) (8, 9, 10, 11,21). Potts et al. (21) reported the prevalence of cancer and in-hospital outcomes among more than 6 million patients undergoing PCI using the National Inpatient Sample database in the United States; however, scarcity of details regarding cancer characteristics, causes of death, number of endpoints, and follow-up duration substantially limit the interpretation of the results. The current study provides robust and detailed data including cancer characteristics and clinical outcomes through the 1 year following PCI derived from a large-scale, consecutively enrolled cohort (>1,000 cancer patients) that is reflective of a real-world clinical setting.
Navi et al. (1) reported that patients with newly diagnosed solid or hematologic cancer had an increased risk of arterial thromboembolism compared with patients without cancer. We did not observe significant differences in ischemic outcomes after index PCI when focusing on patient-level events (i.e., MI, revascularization, and stroke) and lesion/stent-level events (i.e., target vessel related MI, target lesion revascularization, and ST) despite concerns regarding hypercoagulability in cancer patients. Our findings are consistent with previous studies that also included cancer patients undergoing PCI (8,10). Several reasons might be considered for the absence of differences in ischemic events. First, DAPT initiated after PCI may mitigate ischemic risk in exchange for an increased risk of bleeding as observed in patients without cancer. Second, patients with very advanced cancer stage (i.e., those at higher risk for thromboembolic events) might have been managed conservatively without referral for PCI. Third, use of current devices (new-generation DES use in ∼90% of cancer patients) and contemporary PCI techniques may have a potential to achieve equivalent stent-related results regardless of the presence of cancer. Fourth, the relative lack of power (i.e., type II error) to detect differences in thrombotic events should also be considered.
Explanations for an increased risk of cardiac death without increased hazards for other ischemic endpoints are likely multifactorial. First, several adverse effects of cancer treatment might increase the risk of cardiac death (i.e., surgery: bleeding and interruption of DAPT, chemotherapy and radiation, anemia, heart failure, cardiovascular toxicity, spasm, and plaque rupture). This hypothesis is supported by our finding that patients diagnosed with cancer within 1 year before PCI had an increased risk of cardiac death because cancer treatments usually start immediately after cancer diagnosis, and the risk was attenuated over time. Second, a higher incidence of bleeding in patients with cancer may also contribute to an increased risk of cardiac death. Post-PCI bleeding (periprocedural and post-discharge) is known to be associated with increased mortality, to a greater degree than post-discharge MI (22, 23, 24, 25, 26). Third, patients with cancer had a higher risk of unclear death compared with those without. According to universal definition of cardiac death, unclear death belongs to the category of cardiac death, although by far not every unclear death is of cardiac origin (27).
In the current study, patients with cancer had a higher incidence of BARC 2 to 5 bleeding despite only marginally higher PRECISE-DAPT score. Despite the higher incidence of bleeding in cancer patients, the C-statistics of the PRECISE-DAPT score among cancer patients were numerically lower compared with no cancer patients (0.63 vs. 0.67 in overall cohort and 0.63 vs. 0.64 in PS-matched cohort), suggesting that existing bleeding risk scores might be less predictive in cancer patients (Supplemental Table 13). Several risk scores including the PRECISE-DAPT, PARIS (Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients), and DAPT scores, established for the evaluation of the risk and benefit of short versus prolonged DAPT duration among patients undergoing PCI (17,18,25) did not include cancer as a component of the risk score. The derivation dataset of these risk scores excluded cancer patients in randomized controlled trials or did not capture cancer history. Based on our findings, cancer and especially cancer diagnosed within 1 year before PCI should be acknowledged as an independent bleeding risk factor and considered when deciding on duration and intensity of DAPT. Furthermore, the effort to reduce modifiable risks (e.g., minimize duration of triple therapy and shorten DAPT duration in view of recent data suggesting this to be safe [26]) should be made to improve outcomes among cancer patients. In light of the increasing number of patients with both CAD and cancer, further studies are warranted to test the added value of cancer in the prediction of bleeding in patients undergoing PCI.
Among cancer characteristics, a recent cancer diagnosis (i.e., within 1 year before PCI) was an independent predictor for cardiac death and bleeding. Concordant with our findings, Velders et al. (9) reported that cancer diagnosis within 6 months before PCI emerged as a strong predictor for early (<7 days) cardiac death among patients with ST-segment elevation MI. An explanation may be the worse baseline characteristics in patients with a recent cancer diagnosis compared with those with late (>1 year) cancer diagnosis. It remains a question how these patients should be optimally managed in terms of timing of PCI for stable CAD, PCI procedure (devices), and antiplatelet regimen. A close collaboration between interventional cardiologists and oncologists is required to further improve outcomes, as noted in the European Society of Cardiology position paper on cancer treatment (28). Interestingly, patients with a remote cancer history (i.e., cancer diagnosis ≥5 years before PCI) also had an increased risk of cardiac death and BARC 2 to 5 bleeding (although lower as compared with the group with cancer <1 year) despite the potential survivorship bias. This finding might be explained by the differences in patient characteristics including higher PRECISE-DAPT score and the more frequent radiation therapy exposure. Of note, 18% of patients with a recent cancer diagnosis in our study were treated with bare metal stents, probably because of bleeding concerns. Current guidelines suggest the use of newer generation DES or drug-coated stents, and our data support this, as the majority of the patients were treated with newer generation DES, without excess in stent thrombosis (29).
Study limitations
First, the single-center design of this study may limit the generalizability of our findings. Second, there may be several potential unmeasured confounding factors confounding factors inherent to observational data. Third, cancer populations undergoing PCI appear to be highly heterogeneous; a stratified analysis according to each type of cancer cannot be performed in view of the limited sample size in each subgroup. Fourth, the lack of risk with ischemic events might be at least partly attributable to the relative lack of power (i.e., type II error). Fifth, the platelet count at the time point of bleeding, which is a major determinant of bleeding and is often depressed from cancer or the treatment, was not available in the current study. Finally, the primary bleeding endpoint in the current study included BARC 2, which is less associated with mortality compared with major bleeding (i.e., BARC 3 to 5).
Conclusions
Cancer patients carry an increased risk of cardiac mortality that was not associated with ischemic events among patients undergoing PCI in routine clinical practice. Bleeding occurred more frequently in cancer patients and deserves particular attention. Modifying duration and intensity of dual antiplatelet therapy could be considered.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with cancer had an increased risk of cardiac death and bleeding, but not ischemic events. Specifically, a recent cancer diagnosis (≤1 year) was an independent predictor for cardiac death and bleeding.
COMPETENCY IN PATIENT CARE: Cardiologists and oncologists should be aware that cancer patients undergoing PCI are exposed to an increased risk of cardiac death and bleeding and collaboratively make efforts to mitigate these risks especially among patients with recent cancer diagnosis (≤1 year).
TRANSLATIONAL OUTLOOK: Further studies are needed to develop algorithms to predict bleeding among cancer patients undergoing PCI, and determine the optimal DAPT intensity and regimen in this population.
Footnotes
Dr. Pilgrim has received research grants to the institution from Biotronik, Symetis/Boston Scientific, and Edwards Lifesciences; and speaker fees from Biotronik and Boston Scientific. Dr. Valgimigli has received research grants to the institution from Abbott, Terumo, Medicure, and Astrazeneca; and personal fees from Abbott, Chiesi, Bayer, Daiichi Sankyo, Amgen, Terumo, Alvimedica, Astrazeneca, Biosensors, Idorsia, Coreflow, Vifor, and Bristol-Myers Squibb SA. Dr. Windecker has received research grants to the institution from Abbott, Amgen, Bayer, Bristol-Myers Squibb, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Sinomed, and Polares. Dr. Räber has received research grants to the institution from Abbott Vascular, Biotronik, Boston Scientific, HeartFlow, Sanofi, and Regeneron; and speaker fees from Abbott, Amgen, AstraZeneca, Bayer, CSL Behring, Occlutech, and Sanofi. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
For supplemental tables and figures, please see the online version of this paper.
Appendix
References
- 1.Navi B.B., Reiner A.S., Kamel H. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rickles F.R. Mechanisms of cancer-induced thrombosis in cancer. Pathophysiol Haemost Thromb. 2006;35:103–110. doi: 10.1159/000093551. [DOI] [PubMed] [Google Scholar]
- 3.Darby S.C., Ewertz M., McGale P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 4.Frickhofen N., Beck F.J., Jung B., Fuhr H.G., Andrasch H., Sigmund M. Capecitabine can induce acute coronary syndrome similar to 5-fluorouracil. Ann Oncol. 2002;13:797–801. doi: 10.1093/annonc/mdf035. [DOI] [PubMed] [Google Scholar]
- 5.Moore R.A., Adel N., Riedel E. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri T.K., Schutz F.A., Je Y., Rosenberg J.E., Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 7.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hess C.N., Roe M.T., Clare R.M. Relationship between cancer and cardiovascular outcomes following percutaneous coronary intervention. J Am Heart Assoc. 2015;4:e001779. doi: 10.1161/JAHA.115.001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Velders M.A., Boden H., Hofma S.H. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1867–1872. doi: 10.1016/j.amjcard.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 10.Nakatsuma K., Shiomi H., Morimoto T. Influence of a history of cancer on long-term cardiovascular outcomes after coronary stent implantation (an observation from coronary revascularization demonstrating outcome study-Kyoto Registry Cohort-2) Eur Heart J Qual Care Clin Outcomes. 2018;4:200–207. doi: 10.1093/ehjqcco/qcy014. [DOI] [PubMed] [Google Scholar]
- 11.Tabata N., Sueta D., Yamamoto E. Impact of current and past cancer history on the risk of cardiovascular events following percutaneous coronary intervention: a Kumamoto University Malignancy and Atherosclerosis (KUMA) study. Eur Heart J Qual Care Clin Outcomes. 2018;4:290–300. doi: 10.1093/ehjqcco/qcx047. [DOI] [PubMed] [Google Scholar]
- 12.Windecker S., Kolh P., Alfonso F. 2014 ESC/EACTS Guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) Eur Heart J. 2014;35:2541–2619. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 13.Wijns W., Kolh P., Danchin N. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehq277. [DOI] [PubMed] [Google Scholar]
- 14.Mehran R., Rao S.V., Bhatt D.L. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 15.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16.Costa F., van Klaveren D., James S. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
- 17.Yeh R.W., Secemsky E.A., Kereiakes D.J. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. 2016;315:1735–1749. doi: 10.1001/jama.2016.3775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baber U., Mehran R., Giustino G. Coronary thrombosis and major bleeding after PCI with drug-eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. doi: 10.1016/j.jacc.2016.02.064. [DOI] [PubMed] [Google Scholar]
- 19.Farooq V., van Klaveren D., Steyerberg E.W. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet. 2013;381:639–650. doi: 10.1016/S0140-6736(13)60108-7. [DOI] [PubMed] [Google Scholar]
- 20.Fox K.A., Dabbous O.H., Goldberg R.J. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE) BMJ. 2006;333:1091. doi: 10.1136/bmj.38985.646481.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potts J.E., Iliescu C.A., Lopez Mattei J.C. Percutaneous coronary intervention in cancer patients: a report of the prevalence and outcomes in the United States. Eur Heart J. 2019;40:1790–1800. doi: 10.1093/eurheartj/ehy769. [DOI] [PubMed] [Google Scholar]
- 22.Ndrepepa G., Berger P.B., Mehilli J. Periprocedural bleeding and 1-year outcome after percutaneous coronary interventions: appropriateness of including bleeding as a component of a quadruple end point. J Am Coll Cardiol. 2008;51:690–697. doi: 10.1016/j.jacc.2007.10.040. [DOI] [PubMed] [Google Scholar]
- 23.Genereux P., Giustino G., Witzenbichler B. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. doi: 10.1016/j.jacc.2015.06.1323. [DOI] [PubMed] [Google Scholar]
- 24.Palmerini T., Bacchi Reggiani L., Della Riva D. Bleeding-related deaths in relation to the duration of dual-antiplatelet therapy after coronary stenting. J Am Coll Cardiol. 2017;69:2011–2022. doi: 10.1016/j.jacc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 25.Valgimigli M., Bueno H., Byrne R.A. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39:213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H., Domei T., Morimoto T. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 Randomized Clinical Trial. JAMA. 2019;24:2414–2427. doi: 10.1001/jama.2019.8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutlip D.E., Windecker S., Mehran R. Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. doi: 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 28.Zamorano J.L., Lancellotti P., Rodriguez Munoz D. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 29.Neumann F.-J., Sousa-Uva M., Ahlsson A. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.