Abstract
Background
Atrial fibrillation (AF) is a common cardiovascular complication affecting patients with cancer, but management strategies are not well established.
Objectives
The purpose of this retrospective cohort study was to evaluate cross-sectional patterns of anticoagulation (AC) use in patients with cancer with AF or atrial flutter (AFL) on the basis of their risk for stroke and bleeding.
Methods
Patients with cancer and electrocardiograms showing AF or AFL performed at Moffitt Cancer Center in either the inpatient or outpatient setting were included in this retrospective analysis. We described percentages of AC prescription by stroke and bleeding risk, as determined by individual CHA2DS2-VASc and HAS-BLED scores, respectively. Multivariable logistic regression evaluated clinical variables independently associated with anticoagulant prescription.
Results
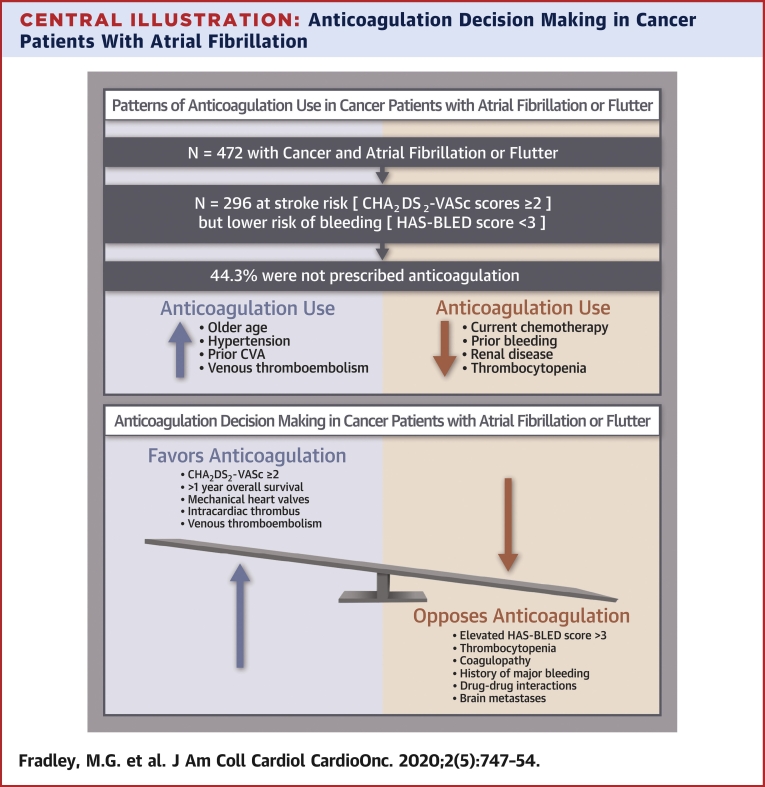
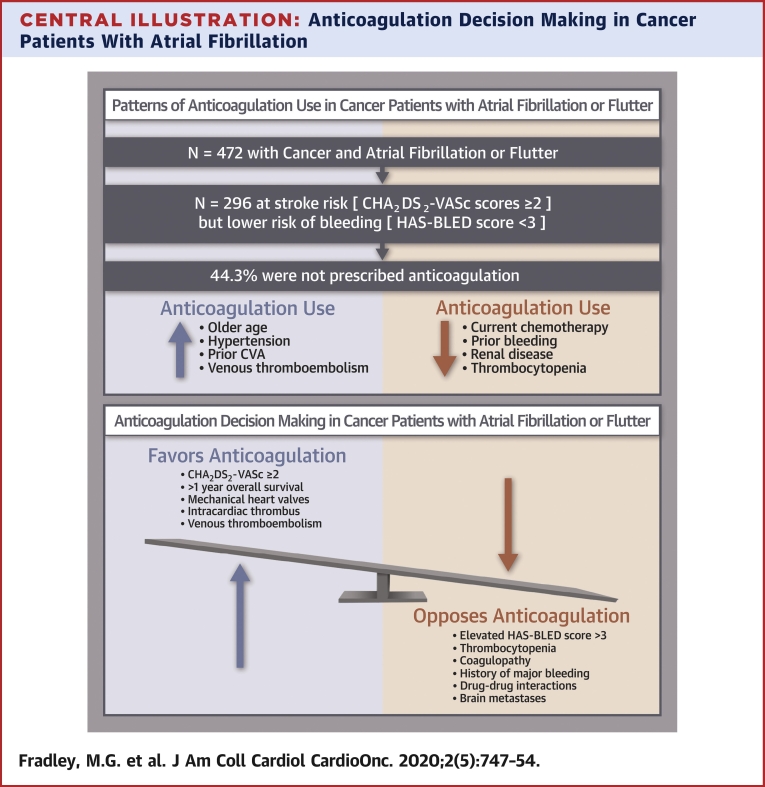
The prevalence of electrocardiography-documented AF or AFL was 4.8% (n = 472). The mean CHA2DS2-VASc score was 2.8 ± 1.4. Among patients with CHA2DS2-VASc scores ≥2 and HAS-BLED scores <3, 44.3% did not receive AC, and of these, only 18.3% had platelet values <50,000/μl. In multivariable analysis, older age, hypertension, prior stroke, and history of venous thromboembolism were each directly associated with AC use, while current chemotherapy use, prior bleeding, renal disease, and thrombocytopenia were each inversely associated with AC use.
Conclusions
Nearly one-half of patients with cancer, the majority with normal platelet counts, had an elevated risk for stroke but did not receive AC. In addition to known predictors, current chemotherapy use was independently associated with a lower odds of AC use. This study highlights the need to improve the application of AF treatment algorithms to cancer populations.
Key Words: anticoagulation, atrial fibrillation, cancer, cardio-oncology
Abbreviations and Acronyms: AC, anticoagulation; AF, atrial fibrillation; AFL, atrial flutter; CI, confidence interval; DOAC, direct oral anticoagulant agent; ECG, electrocardiogram; EMR, electronic medical record; MCC, Moffitt Cancer Center; OR, odds ratio
Central Illustration
Advances in cancer therapies over the past several decades have led to dramatic improvements in cancer outcomes (1). As a result, patients with cancer are living longer and in many cases surviving their disease. There is increasing recognition that patients with cancer and survivors are at increased risk for various cardiovascular complications including arrhythmias such as atrial fibrillation (AF) and atrial flutter (AFL) (2). The development of AF in patients with cancer is promoted by genetics, risk factors, systemic inflammation, and neurohormonal changes (3). The prevalence of AF ranges between 2% and 15% in patients with cancer, with higher rates reported for certain classes of antineoplastic drugs, such as tyrosine kinase inhibitors (4).
Stroke is one of the most serious adverse sequelae associated with AF and AFL. The rate of stroke in patients with cancer may be higher than that of the general population, as both cancer and AF are independent risk factors for ischemic stroke (5). In one study, the baseline prevalence of stroke was 27% in patients with AF and history of cancer compared with 20% in patients without cancer, although the cancer group was older and had higher CHA2DS2-VASc scores (6).
Anticoagulation (AC) is therefore the mainstay in the management of AF, with the goal of decreasing the risk for thromboembolic events (7).
Patients with cancer, particularly those undergoing therapy, and cancer survivors are not well represented in clinical trials of AC, making AC management in this patient population a challenge (8). Oral AC is prescribed to about 85% of patients with nonvalvular AF in the general population (9); however, it is unclear whether patients with cancer are prescribed currently recommended AC regimens. The aim of this retrospective cohort study was to evaluate cross-sectional patterns of AC prescription in cancer patients with AF or AFL at Moffitt Cancer Center (MCC).
Methods
This retrospective analysis was conducted in accordance with the ethical standards of institutional and national research committee and with the Declaration of Helsinki of 1964. Our study was approved by University of South Florida Institutional Review Board (Pro00031630) and by the MCC Scientific Review Committee (MCC 19292). As this was a retrospective chart review, informed consent was determined to be not necessary.
Between January 1, 2016, and December 31, 2016, patients who had at least 1 electrocardiogram obtained at MCC in either the inpatient or outpatient setting were identified. From this sample, the percentage of patients with both cancer diagnoses and documented AF or AFL on electrocardiography were included in the cohort. All electrocardiographic diagnoses of AF or AFL were manually adjudicated by the study electrophysiologist and confirmed with an associated International Classification of Diseases-Ninth Revision or International Classification of Diseases-10th Revision code in the electronic medical record (EMR).
Patient-related data are maintained in the EMR for all inpatient and outpatient evaluations at MCC. Medical records of patients who met the inclusion criteria (cancer diagnosis and at least 1 electrocardiographically documented episode of AF or AFL) were comprehensively reviewed, and baseline characteristics associated with the CHA2DS2-VASc (10) and HAS-BLED (11) scores were collected, including heart failure or cardiomyopathy, hypertension, age, diabetes, stroke or transient ischemic attack, vascular disease, sex, renal disease (dialysis, transplantation, or creatinine >2.26 mg/dl), liver disease (cirrhosis or total bilirubin >2 times normal and aspartate transaminase or alanine transaminase >3 times normal), prior major bleeding (any bleeding requiring hospitalization and/or causing a decrease in hemoglobin level of >2 g/l and/or requiring blood transfusion that was not a hemorrhagic stroke) or increased bleeding risk (transfusion dependency due to hematologic abnormalities), labile international normalized ratio (<2 or >3 for 60% of the time), excessive alcohol intake (>8 alcoholic drinks per week), and medications that increase bleeding risk (nonsteroidal anti-inflammatory drugs, aspirin, antiplatelet agents, and anticoagulant agents).
Additional variables of interest included hemoglobin and platelet counts at the time of AF or AFL diagnosis, history of venous thromboembolism, Karnofsky score, left ventricular ejection fraction derived from echocardiography, and cancer type. We also evaluated for the occurrence of perioperative AF, chemotherapy exposure within 3 months of the incident arrhythmia, and active chemotherapy use at the time of the documented AF or AFL episode. All variables were confirmed using International Classification of Diseases-9th Revision or International Classification of Diseases-10th Revision codes in the EMR. AC regimens were assessed and confirmed by the presence of a prescription in the EMR within 30 days of the electrocardiographic recording of AF or AFL.
The percentage of anticoagulant prescription among patients was categorized by stroke and bleeding risk as determined by individual CHA2DS2-VASc and HAS-BLED scores, respectively. An increased risk for stroke was defined as a CHA2DS2-VASc score ≥2, and increased bleeding risk was defined as a HAS-BLED score ≥3. Summary data are presented as mean ± SD for normally distributed numeric variables, median with interquartile range for numeric variables not normally distributed, and counts and percentages for categorical variables. Univariable comparisons of baseline characteristics between patients with and without AC used Welch’s t-test for normally distributed continuous variables, the Mann-Whitney-Wilcoxon test for continuous variables not normally distributed, and the chi-square test for categorical variables and proportions. This analysis was replicated among the subset of patients with CHA2DS2-VASc scores ≥2 and HAS-BLED scores <3. Multivariable logistic regression was performed to evaluate the independent relationship, expressed as adjusted odds ratios (ORs) and 95% confidence intervals (CIs), between clinical variables and the decision to prescribe anticoagulant agents in patients with AF. No variable selection methods were applied; all clinically relevant study variables and cancer subtypes were included in the multivariable model. Model assumptions of linearity were assessed by visually comparing continuous predictor values and logit values. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for data analysis. Statistical significance was defined as a p value <0.05 for all analyses.
Results
A total of 9,857 patients had at least 1 electrocardiogram obtained at MCC in either the inpatient or outpatient setting. Of this sample, 472 patients (4.8%) had both cancer diagnoses and documented AF or AFL on electrocardiography; this population constituted the study cohort. Baseline demographics and clinical characteristics of the cohort are presented in Table 1. The mean age was 73.0 ± 10.4 years, and 67.7% were men. The mean CHA2DS2-VASc score was 2.8 ± 1.4. Among this group, 213 patients (45.1%) were prescribed AC and 259 patients (54.9%) were not. Patients who were prescribed AC were older (76.0 years vs. 70.5 years; p < 0.001) and more likely to have hypertension (71.8% vs. 53.3%; p < 0.001). Patients with histories of AF (64.3% vs. 30.5%; p < 0.001) and those with elevated CHA2DS2-VASc scores ≥2 (92.0% vs. 74.5%; p < 0.001) were also more likely to be prescribed AC.
Table 1.
Baseline Characteristics According to Anticoagulation Status
| Total (N = 472) | No AC (n = 259) | AC (n = 213) | p Value | |
|---|---|---|---|---|
| Age (yrs) | 73.0 ± 10.4 | 70.5 ± 11.4 | 76.0 ± 9.1 | <0.001 |
| Female | 152 (32.2) | 84 (32.4) | 68 (31.9) | 0.883 |
| Hypertension | 291 (61.7) | 138 (53.3) | 153 (71.8) | <0.001 |
| Heart failure | 37 (7.8) | 14 (5.4) | 23 (10.8) | 0.031 |
| Prior atrial fibrillation | 216 (45.8) | 79 (30.5) | 137 (64.3) | <0.001 |
| Diabetes | 114 (24.2) | 58 (22.4) | 56 (26.3) | 0.286 |
| Prior stroke | 37 (7.8) | 12 (4.6) | 25 (11.7) | 0.004 |
| Vascular disease | 96 (20.3) | 47 (18.1) | 49 (23.0) | 0.192 |
| Renal disease | 23 (4.9) | 17 (6.6) | 6 (2.8) | 0.060 |
| Liver disease | 4 (0.8) | 3 (1.2) | 1 (0.5) | 0.417 |
| Prior major bleed | 80 (16.9) | 73 (28.2) | 7 (3.3) | <0.001 |
| Labile INR | 5 (1.1) | 2 (0.8) | 3 (1.4) | 0.413 |
| VTE | 33 (7.0) | 13 (5.0) | 20 (9.4) | 0.095 |
| Chemotherapy, current use | 232 (49.2) | 151 (58.3) | 81 (38.0) | <0.001 |
| Chemotherapy, not current but in prior 3 months | 53 (11.2) | 30 (11.6) | 23 (10.8) | 0.903 |
| Perioperative atrial fibrillation | 133 (28.2) | 92 (35.5) | 41 (19.2) | <0.001 |
| NSAID | 97 (20.6) | 60 (23.2) | 37 (17.4) | 0.151 |
| Ibrutinib | 4 (0.8) | 4 (1.5) | 0 (0) | 0.188 |
| Platelet count <50,000/μl | 63 (13.3) | 61 (23.6) | 2 (0.9) | <0.001 |
| Karnofsky score | 90 (70–90) | 90 (70–90) | 90 (80–90) | 0.509 |
| LVEF (%)∗ | 63 (58–63) | 63 (58–63) | 63 (58–63) | 0.301 |
| Brain metastases | 8 (1.7) | 7 (2.7) | 1 (0.5) | 0.131 |
| Cancer type | ||||
| Heme | 116 (24.6) | 92 (35.5) | 24 (11.3) | <0.001 |
| GI | 73 (15.5) | 39 (15.1) | 34 (16.0) | 0.887 |
| Cutaneous | 66 (14.0) | 22 (8.5) | 44 (20.7) | <0.001 |
| GU | 66 (14.0) | 31 (12.0) | 35 (16.4) | 0.209 |
| Lung | 63 (13.3) | 33 (12.7) | 30 (14.1) | 0.771 |
| Breast | 22 (4.7) | 11 (4.2) | 11 (5.2) | 0.802 |
| GYN | 11 (2.3) | 5 (1.9) | 6 (2.8) | 0.742 |
| Sarcoma | 11 (2.3) | 3 (1.2) | 8 (3.8) | 0.120 |
| Other | 44 (9.3) | 23 (8.9) | 21 (9.9) | 0.838 |
| CHA2DS2-VASc score | 2.8 ± 1.4 | 2.4 ± 1.4 | 3.2 ± 1.3 | <0.001 |
| CHA2DS2-VASc score ≥2 | 389 (82.4) | 193 (74.5) | 196 (92.0) | <0.001 |
| HAS-BLED score | 1.7 ± 0.9 | 1.7 ± 1.1 | 1.7 ± 0.8 | 0.788 |
| HAS-BLED score ≥3 | 95 (20.1) | 64 (24.7) | 31 (14.6) | 0.006 |
Values are mean ± SD, n (%), or median (interquartile range).
AC = anticoagulation; GI = gastrointestinal; GU = genitourinary; GYN = gynecologic; Heme = hematologic; INR = international normalized ratio; LVEF = left ventricular ejection fraction; NSAID = nonsteroidal anti-inflammatory drug; VTE = venous thromboembolism.
Echocardiographic data were available for 230 of the 472 patients.
In contrast, patients on active chemotherapy at the time of the documented AF or AFL episode (38.0% vs. 58.3%; p < 0.001) were less likely to be prescribed AC, as were patients with elevated HAS-BLED scores ≥3 (14.6% vs. 24.7%; p = 0.0062). Patients with hematologic malignancies were less likely to be prescribed AC, while patients with cutaneous malignancies were more likely. There were no differences in AC status in the other cancer groups. Among all patients who were on active chemotherapy or treated in the preceding 3 months, only 36.5% were prescribed AC. Regarding anticoagulant class, 38% received warfarin and 54% received a direct oral anticoagulant agent (DOAC). The remaining 8% were prescribed low–molecular weight heparin. Among the DOACs, apixaban was prescribed to 42%, rivaroxaban to 47%, dabigatran to 9%, and edoxaban to the remaining 2%.
Of the 296 patients deemed potentially appropriate candidates for AC (i.e., those at elevated risk for stroke [CHA2DS2-VASc score ≥2] but lower risk for bleeding [HAS-BLED score <3]), 44.3% were not prescribed AC. Comparisons were made to understand which factors were associated with AC use in patients with CHA2DS2-VASc scores ≥2 and HAS-BLED scores <3 (Table 2). AC was not prescribed in 48.9% of women and 51.1% of men. Baseline hypertension was more prevalent in those prescribed AC (72.7% vs. 56.5%; p = 0.004), as was history of AF. Interestingly, only 18.3% of those patients who were not prescribed AC had thrombocytopenia with a platelet count <50,000/μl. Among the 154 patients without histories of AF or AFL with CHA2DS2-VASc scores ≥2 and HAS-BLED scores <3, 60.4% were not prescribed AC. Patient characteristics were similar to those of the whole study cohort (Supplemental Table 1).
Table 2.
Clinical Variables According to Anticoagulation Status in Patients With CHA2DS2-VASc Scores ≥2 and HAS-BLED Scores <3: Full Cohort (N = 296)
| Total (N = 296) | No AC (n = 131) | AC (n = 165) | p Value | |
|---|---|---|---|---|
| Age (yrs) | 74.9 ± 8.3 | 73.8 ± 8.8 | 76.2 ± 7.6 | 0.003 |
| Female | 123 (41.6) | 64 (48.9) | 59 (35.8) | 0.020 |
| Hypertension | 194 (65.5) | 74 (56.5) | 120 (72.7) | 0.004 |
| Heart failure | 25 (8.4) | 7 (5.3) | 18 (10.9) | 0.091 |
| Prior atrial fibrillation | 143 (48.3) | 39 (29.8) | 104 (63.0) | <0.001 |
| Diabetes | 81 (27.4) | 34 (26.0) | 47 (28.5) | 0.634 |
| Prior stroke | 29 (9.8) | 10 (7.6) | 19 (11.5) | 0.265 |
| Vascular disease | 61 (20.6) | 25 (19.1) | 36 (21.8) | 0.564 |
| Renal disease | 8 (2.7) | 5 (3.8) | 3 (1.8) | 0.293 |
| Liver disease | 3 (1.0) | 2 (1.5) | 1 (0.6) | 0.433 |
| Prior major bleed | 27 (9.1) | 26 (19.8) | 1 (0.6) | <0.001 |
| Labile INR | 3 (1.0) | 2 (1.5) | 1 (0.6) | 0.476 |
| VTE | 21 (7.1) | 7 (5.3) | 14 (8.5) | 0.404 |
| Chemotherapy, current use | 142 (48.0) | 77 (58.8) | 65 (39.4) | <0.001 |
| Chemotherapy, noncurrent, prior 3 months | 36 (12.2) | 18 (13.7) | 18 (10.9) | 0.592 |
| Perioperative atrial fibrillation | 81 (27.4) | 47 (35.9) | 34 (20.6) | 0.006 |
| NSAID | 34 (11.5) | 20 (15.3) | 14 (8.5) | 0.108 |
| Ibrutinib | 2 (0.7) | 2 (1.5) | 0 (0) | 0.383 |
| Platelet count <50,000/μl | 26 (8.8) | 24 (18.3) | 2 (1.2) | <0.001 |
| Karnofsky score | 80 (70–90) | 80 (70–90) | 90 (70–90) | 0.682 |
| LVEF (%) | 60 (58–63) | 63 (58–63) | 58 (58–63) | 0.512 |
| Brain metastases | 5 (1.7) | 4 (3.1) | 1 (0.6) | 0.246 |
| Cancer type | ||||
| Heme | 65 (22.0) | 45 (34.4) | 20 (12.1) | <0.001 |
| GI | 49 (16.6) | 20 (15.3) | 29 (17.6) | 0.688 |
| Cutaneous | 41 (13.9) | 8 (6.1) | 33 (20.0) | <0.001 |
| GU | 41 (13.9) | 15 (11.5) | 26 (15.8) | 0.357 |
| Lung | 40 (13.5) | 19 (14.5) | 21 (12.7) | 0.805 |
| Breast | 16 (5.4) | 8 (6.1) | 8 (4.8) | 0.841 |
| GYN | 10 (3.4) | 4 (3.1) | 6 (3.6) | 0.783 |
| Sarcoma | 10 (3.4) | 3 (2.3) | 7 (4.2) | 0.541 |
| Other | 25 (8.4) | 10 (7.6) | 15 (9.1) | 0.797 |
Values are mean ± SD, n (%), or median (interquartile range).
Abbreviations as in Table 1.
Multivariable analyses were subsequently performed to identify factors independently associated with the prescription of AC in patients with cancer with AF (Table 3). Older age, hypertension (OR: 2.50; 95% CI: 1.50 to 4.22; p < 0.001), prior stroke (OR: 1.67; 95% CI: 1.03 to 2.82; p = 0.043) and history of venous thromboembolism (OR: 2.83; 95% CI: 1.04 to 8.24; p = 0.038) were each independently associated with AC prescription. Current chemotherapy administration was inversely associated with AC prescription (OR: 0.55; 95% CI: 0.32 to 0.93; p = 0.027), as was thrombocytopenia (OR: 0.10; 95% CI: 0.01 to 0.38; p = 0.004), history of major bleeding (OR: 0.09; 95% CI: 0.03 to 0.23; p < 0.001), and the presence of perioperative AF (OR: 0.38; 95% CI: 0.22 to 0.65; p < 0.001).
Table 3.
Clinical Variables Associated With Anticoagulation Prescription in Multivariable Logistic Regression Analysis: Full Cohort (N = 472)∗
| Covariate | Adjusted Odds Ratio (95% CI) |
|---|---|
| Age 65–74 yrs (vs. <65 yrs) | 2.04 (1.01–4.25) |
| Age ≥75 yrs (vs. <65 yrs) | 2.65 (1.30–5.52) |
| Gender (female vs. male) | 0.74 (0.44–1.22) |
| Hypertension | 2.50 (1.50–4.22) |
| Heart failure | 2.38 (0.95–6.29) |
| Prior atrial fibrillation | 3.02 (1.86–4.96) |
| Diabetes | 0.84 (0.47–1.48) |
| Prior stroke | 1.67 (1.03–2.82) |
| Vascular disease | 0.82 (0.44–1.51) |
| Renal disease | 0.24 (0.07–0.77) |
| Liver disease | 0.89 (0.03–12.39) |
| Prior major bleeding | 0.09 (0.03–0.23) |
| Labile INR | 1.09 (0.13–12.22) |
| VTE | 2.83 (1.04–8.24) |
| Chemotherapy, current use (vs. no use) | 0.55 (0.32–0.93) |
| Chemotherapy, noncurrent, prior 3 months (vs. no use) | 0.50 (0.24–1.05) |
| Perioperative atrial fibrillation | 0.38 (0.22–0.65) |
| NSAID | 0.31 (0.17–0.56) |
| Ibrutinib† | — |
| Platelet count <50,000/μl | 0.10 (0.01–0.38) |
| Karnofsky score | 1.00 (0.97–1.03) |
| LVEF | 0.99 (0.94–1.04) |
| Brain metastases | 0.12 (0.01–0.93) |
CI = confidence interval; other abbreviations as in Table 1.
All variables listed in the table were included in the multivariable model; all variables were categorized as binary (yes or no), with the exception of age, categorized as <65 years, 65 to 74 years, or ≥75 years; Karnofsky score, categorized as 0 to 100; and LVEF, categorized as 0 to 100. All cancer subtypes indicated in Tables 1 and 2 were also included in the model to control for confounding.
None of the patients treated with ibrutinib were prescribed anticoagulation.
Discussion
Our study showed that almost one-half of eligible patients (44.4%) were not prescribed AC despite an elevated risk for stroke and acceptable risk for bleeding (Central Illustration). Furthermore, patients undergoing current chemotherapy were less likely to be prescribed AC, as were patients with bleeding history, renal disease, and thrombocytopenia. Similarly, among those patients without a histories of AF/AFL, 60.4% did not receive AC. Patients with hematologic malignancies were also less likely to receive AC, possibly secondary to a perceived increased risk for bleeding from cytopenia.
Central Illustration.
Anticoagulation Decision Making in Cancer Patients With Atrial Fibrillation
Anticoagulation for atrial fibrillation–related thromboembolism prophylaxis can be challenging in patients with concurrent cancer. In this population, decision making must be individualized weighing factors that promote the use of anticoagulation against those unique cancer-specific circumstances that are likely to increase bleeding risk and other complications. CVA = cerebrovascular accident.
Suboptimal use of AC in patients with cancer with AF can lead to significant negative health and economic consequences. In a large retrospective study, patients with AF with histories of cancer were less likely to fill prescriptions for AC than those without cancer, regardless of cancer type (6). AC prescription was more common when patients were referred to cardiologists, and there were fewer strokes without an increase in the risk for bleeding (6). There are also various comorbid conditions, including mechanical heart valves, intracardiac thrombus, or venous thromboembolism, that are associated with increased AC use.
Management of AF is challenging in patients with cancer (Central Illustration). Traditional thromboembolic risk score calculators, such as the CHA2DS2-VASc score, may be less useful in this patient population. These models do not include certain clinical characteristics, such as presence of malignancy and life expectancy, and may not accurately predict stroke risk in patients with cancer (4). Patell et al. (12) found that CHADS2 score may be more predictive of stroke risk in patients with cancer compared with CHA2DS2-VASc score. A study by D’Souza et al. (13) also suggested the CHA2DS2-VASc score may not be an adequate assessment of thromboembolic risk in patients with recent cancer, with patients with cancer having lower rates at higher CHA2DS2-VASc scores compared with control subjects without cancer.
Assessing bleeding risk in the cancer population is also important. Traditionally, the HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding, labile international normalized ratio, elderly, drug or alcohol use) has been used for the general population. However, its use in patients with cancer has been questioned, as it does not consider the bleeding tendencies of malignancy and its associated therapies. In fact, 1 study demonstrated that cancer was the strongest predictor of bleeding when it was included as an individual covariate along with the other HAS-BLED variables (14).
Thrombocytopenia is a major safety concern in this patient population. Significant thrombocytopenia may occur in patients with cancer for a variety of reasons, including myelosuppressive chemotherapy, bone marrow tumor invasion, and immune-mediated phenomena (15). A platelet value of <50,000/μl is a generally accepted threshold to modify AC regimens. Indeed, AC is generally contraindicated for platelet counts below this range (16). In addition, patients with platelet counts <100,000/μl were excluded from clinical trials with DOACs (17). In our study, among those patients who were eligible for but did not receive AC, only 18.9% had platelet counts of <50,000/μl, emphasizing the underuse of AC in this patient population. Similarly, prior major bleeding was associated with less AC. Although this finding is not entirely surprising, it should not be considered an absolute contraindication to future AC, particularly if related to a reversible cause that has been corrected. As such, a shared decision-making approach for AC should be pursued in these unique situations.
The safety and efficacy of DOACs in the cancer population have relied mostly on post hoc analyses. Data from the ARISTOTLE (Apixaban Versus Warfarin in Patients With Atrial Fibrillation) trial showed preserved safety and efficacy of apixaban versus warfarin among patients with AF with and without active cancer (18). In this retrospective analysis, apixaban was associated with a greater benefit in the composite outcome of stroke or systemic embolism, myocardial infarction, and death in patients with active cancer compared with those without. Additionally, data from the ENGAGE-AF–TIMI 48 (Edoxaban Versus Warfarin in Patients With Atrial Fibrillation–Thrombolysis In Myocardial Infarction 48) trial showed that patients with active malignancy had higher rates of death and major bleeding compared with those without active malignancy; however, the efficacy and safety profile of edoxaban relative to warfarin was similar despite the presence of malignancy (19). In contrast, data from the ROCKET-AF (Rivaroxaban Versus Warfarin in Nonvalvular Atrial Fibrillation) and RE-LY (Dabigatran Versus Warfarin in Patients With Atrial Fibrillation) trials demonstrated that the risk for bleeding in patients with cancer was 2 to 6 times higher than in patients without cancer (20,21). Vitamin K antagonists have largely been replaced by DOACs for stroke prophylaxis in patients with cancer with AF. The potential for interactions with various cancer therapies, the need for frequent laboratory monitoring, and less than optimal time in the therapeutic range are some of the drawbacks of vitamin K antagonists (22). This is confirmed in our real-world cohort, in which the majority of patients in 2016 received DOACs. We would expect this number to be even higher given updated AF and AC guidelines (23). It should be noted that vitamin K antagonists remain first-line therapy in patients with cancer and valvular AF (24).
Interestingly, our study showed that current chemotherapy use was associated with lower initiation of AC. There are various potential reasons for this finding. For example, there is a common but unproven theory that there is little long-term risk for recurrent AF or thromboembolism once the offending chemotherapy is stopped, and therefore, AC is not needed. It is also possible that the tendency to withhold AC in the perichemotherapy period is due to associated coagulation imbalance through decreased hepatic production of coagulation factors or platelet dysfunction, leading to an increased concern for bleeding complications (25). However, endothelial injury caused by certain chemotherapy drugs often leads to platelet aggregation and increases the risk for thrombosis, as evidenced by increased rates of venous thromboembolism (25), supporting the notion that withholding AC in this setting should be done only on a case-by-case basis. Similarly, there was an inverse relationship with perioperative AF and AC use despite the fact that the risk for thromboembolism in this setting is elevated (26). The risks and benefits of AC for AF or AFL that develops in the setting of cancer therapy and/or surgery warrant additional research and investigation.
Study limitations
First, our study was retrospective in nature. Second, our study was not powered, by design, to detect a clinically meaningful difference in thromboembolic events in the group that did not receive AC. Third, the true incidence of AF could be higher than what is shown by our study, but it was believed to be beyond the scope of this analysis to extend the duration of AF detection and determine the true burden of AF. Fourth, we recognize that both the CHA2DS2-VASc and HAS-BLED scores have significant limitations in the cancer population, but there are no other superior risk scores for patients with cancer, and these remain the most widely used stroke and bleeding risk algorithms in both the general population and patients with cancer specifically. It is also worth noting that our data suggest a sex discrepancy in AC prescribing practices, with fewer women receiving blood thinners despite CHA2DS2-VASc scores ≥2 and HAS-BLED scores <3. The most current guidelines recommend AC for women with CHA2DS2-VASc scores ≥3 (23), so our application of a score ≥2 for both men and women may have biased these findings. Nevertheless, the study population was from 2016, when AC recommendations for a CHA2DS2-VASc score ≥2 were applied uniformly regardless of sex (27). Finally, we chose to evaluate 9 different cancer groups, but certain classifications had low representation, which may affect the ability to draw significant conclusions in specific subgroups.
Conclusions
Almost one-half of patients with cancer with AF who were eligible for AC were not prescribed it in our single-center study. Current chemotherapy administration significantly reduced the likelihood of receiving AC. Our findings raise awareness of this issue because of the potential devastating consequences of thromboembolic events in patients with AF or AFL not receiving AC.
Perspectives.
COMPETENCY IN PATIENT CARE: AF is a commonly encountered cardiovascular complication in patients with cancer, and AF can increase the risk for stroke and systemic thromboembolism. This study demonstrates that patients with cancer may not be receiving adequate AC despite an increased risk for stroke and low bleeding risk. It is essential for cardiologists and electrophysiologists to work with oncologists to provide appropriate treatment to patients with cancer with AF.
TRANSLATIONAL OUTLOOK: Arrhythmias are a frequent and serious complication associated with cancer and cancer therapeutics. These clinical data motivate future clinical and translational studies to: 1) determine the true thromboembolic potential of cancer-associated AF; and 2) develop cancer-specific AF thromboembolism risk stratification and management algorithms.
Author Disclosures
Dr. Fradley has received a research grant from Medtronic; and has received consulting fees from Abbott and Takeda. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Gregory Piazza, MD, MS, served as the Guest Associate Editor for this paper. Anju Nohria, MD, served as Guest Editor-in-Chief for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Miller K.D., Nogueira L., Mariotto A.B. Cancer treatment and survivorship statistics 2019. CA Cancer J Clin. 2019;69:363–385. doi: 10.3322/caac.21565. [DOI] [PubMed] [Google Scholar]
- 2.Fradley M.G., Viganego F., Kip K. Rates and risk of arrhythmias in cancer survivors with chemotherapy-induced cardiomyopathy compared with patients with other cardiomyopathies. Open Heart. 2017;4 doi: 10.1136/openhrt-2017-000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahman F., Ko D., Benjamin E.J. Association of atrial fibrillation and cancer. JAMA Cardiol. 2016;1:384–386. doi: 10.1001/jamacardio.2016.0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alomar M., Fradley M.G. Electrophysiology translational considerations in cardio- oncology: QT and beyond. J Cardiovasc Transl Res. 2020;13:390–401. doi: 10.1007/s12265-019-09924-y. [DOI] [PubMed] [Google Scholar]
- 5.Malavasi V.L., Fantecchi E., Gianolio L. Atrial fibrillation in patients with active malignancy and use of anticoagulants: under-prescription but no adverse impact on all- cause mortality. Eur J Intern Med. 2019;59:27–33. doi: 10.1016/j.ejim.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 6.O’Neal W.T., Claxton J.S., Sandesara P.B. Provider specialty, anticoagulation, and stroke risk in patients with atrial fibrillation and cancer. J Am Coll Cardiol. 2018;72:1913–1922. doi: 10.1016/j.jacc.2018.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinberg B.A., Piccini J.P. Anticoagulation in atrial fibrillation. BMJ. 2014;348:g2116. doi: 10.1136/bmj.g2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanz A.P., Gomez J.L.Z. AF in cancer patients: a different need for anticoagulation? Eur Cardiol. 2019;14:65–67. doi: 10.15420/ecr.2018.32.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boriani G., Proietti M., Laroche C. Contemporary stroke prevention strategies in 11 096 European patients with atrial fibrillation: a report from the EURObservational Research Programme on Atrial Fibrillation (EORP-AF) Long-Term General Registry. Europace. 2018;20:747–757. doi: 10.1093/europace/eux301. [DOI] [PubMed] [Google Scholar]
- 10.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor–based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 11.Pisters R., Lane D.A., Nieuwlaat R., de Vos C.B., Crijns H.J., Lip G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 12.Patell R., Gutierrez A., Rybicki L., Khorana A.A. Usefulness of CHADS2 and CHA2DS2-VASc scores for stroke prediction in patients with cancer and atrial fibrillation. Am J Cardiol. 2017;120:2182–2186. doi: 10.1016/j.amjcard.2017.08.038. [DOI] [PubMed] [Google Scholar]
- 13.D’Souza M., Carlson N., Fosbol E. CHA2DS2-VASc score and risk of thromboembolism and bleeding in patients with atrial fibrillation and recent cancer. Eur J Prev Cardiol. 2018;25:651–658. doi: 10.1177/2047487318759858. [DOI] [PubMed] [Google Scholar]
- 14.Brown J.D., Goodin A.J., Lip G.Y.H., Adams V.R. Risk stratification for bleeding complications in patients with venous thromboembolism: application of the HAS- BLED bleeding score during the first 6 months of anticoagulant treatment. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosarla R.C., Vaduganathan M., Qamar A., Moslehi J., Piazza G., Giugliano R.P. Anticoagulation strategies in patients with cancer: JACC review topic of the week. J Am Coll Cardiol. 2019;73:1336–1349. doi: 10.1016/j.jacc.2019.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez Fernandez C., Formiga F., Camafort M. Antithrombotic treatment in elderly patients with atrial fibrillation: a practical approach. BMC Cardiovasc Disord. 2015;15:143. doi: 10.1186/s12872-015-0137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pastori D., Antonucci E., Violi F. Thrombocytopenia and mortality risk in patients with atrial fibrillation: an analysis from the START registry. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melloni C., Dunning A., Granger C.B. Efficacy and safety of apixaban versus warfarin in patients with atrial fibrillation and a history of cancer: insights from the ARISTOTLE trial. Am J Med. 2017;130:1440–1448.e1. doi: 10.1016/j.amjmed.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 19.Fanola C.L., Ruff C.T., Murphy S.A. Efficacy and safety of edoxaban in patients with active malignancy and atrial fibrillation: analysis of the ENGAGE AF–TIMI 48 trial. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.008987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel M.R., Mahaffey K.W., Garg J. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 21.Connolly S.J., Ezekowitz M.D., Yusuf S. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 22.Zamorano J.L., Lancellotti P., Rodriguez Munoz D. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for Cancer Treatments and Cardiovascular Toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 23.January C.T., Wann L.S., Calkins H. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019;74:104–132. doi: 10.1016/j.jacc.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Fernandez T., Martin-Garcia A., Roldan Rabadan I. Atrial fibrillation in active cancer patients: expert position paper and recommendations. Rev Esp Cardiol (Engl Ed) 2019;72:749–759. doi: 10.1016/j.rec.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Kvolik S., Jukic M., Matijevic M., Marjanovic K., Glavas-Obrovac L. An overview of coagulation disorders in cancer patients. Surg Oncol. 2010;19:e33–e46. doi: 10.1016/j.suronc.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 26.Butt J.H., Olesen J.B., Havers-Borgersen E. Risk of thromboembolism associated with atrial fibrillation following noncardiac surgery. J Am Coll Cardiol. 2018;72:2027–2036. doi: 10.1016/j.jacc.2018.07.088. [DOI] [PubMed] [Google Scholar]
- 27.January C.T., Wann L.S., Alpert J.S. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.