Immune checkpoint inhibitor (ICI) therapy has significantly improved the prognosis of many advanced cancers. ICIs target cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death-1 (PD-1), or programmed cell death-ligand 1 (PD-L1) and increase the antitumor immune response. Several immune-related side effects, including colitis, pneumonitis, hepatitis, and thyroiditis, have been reported since the U.S. Food and Drug Administration approval of the first ICI, ipilimumab, in 2011 (1). Cardiovascular adverse effects such as myocarditis are uncommon, but bear high mortality rates of nearly 50% (2). ICI myocarditis typically occurs early, and potential factors associated with ICI myocarditis are combination therapy (e.g., nivolumab [anti–PD-1] and ipilimumab [anti–CTLA-4] therapy), presence of a thymoma, and diabetes (3,4). Acute myocarditis after a first course of combination ICI therapy was assessed through a detailed study of 2 clinical cases (5). In both patients, myocardial lymphocytic CD8 infiltrates were identified on autopsy with no clear evidence of acute viral infection. As far as the pathophysiology, the theory of a common antigen or high frequency of T-cell receptor sequences amongst the myocardium, skeletal muscle, and tumors has been suggested.
However, the clinical presentation of ICI myocarditis is heterogeneous, with preserved left ventricular ejection fraction in one-half of the cases, and high rates of conduction abnormalities and ventricular arrhythmias (3). ICI myocarditis can also occur late, although notably, there is limited information on late-onset myocarditis with long-term ICI therapy (2). We herein describe a case of late myocarditis in a patient on long-term ICI therapy.
Case Presentation
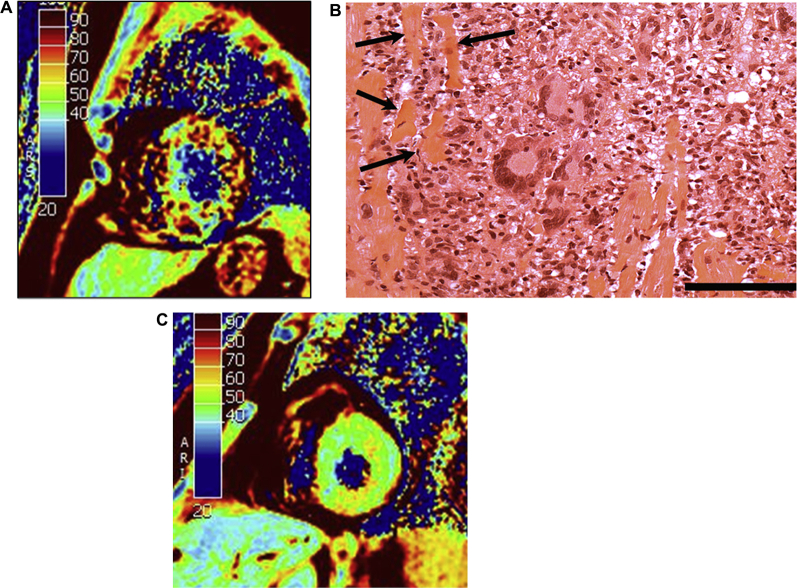
A 57-year-old male patient presented with myalgias and dyspnea after the 27th course of 2nd-line ICI monotherapy (Nivolumab [anti–PD-1] 3 mg/kg every 3 weeks) for metastatic renal cell carcinoma. The electrocardiogram showed sinus tachycardia, low QRS voltage, and T-wave inversion in the anterior leads with no ST-segment changes. Cardiac biomarkers were elevated (troponin I 4,700 ng/l, B-type natriuretic peptide 466 ng/l). Left heart catheterization revealed normal coronary arteries. Cardiac magnetic resonance demonstrated severe and diffuse myocardial edema (mean myocardial T2 = 74 ms, normal values <55 ms [Figure 1], high T1 values of 1,163 ms, high extravascular volume of 35.5%), and severe biventricular dysfunction (left ventricular ejection fraction [LVEF] 32%). A right ventricular endomyocardial biopsy (EMB) was performed and intravenous steroids (methylprednisolone 1 g/day) were started within 24 h of intensive care unit admission and continued for 3 days, then tapered to prednisone 2 mg/kg/day. Peak high-sensitivity troponin I was 5,800 ng/l on day 3 of his hospitalization.
Figure 1.
CMR Imaging at Diagnosis, Endomyocardial Biopsy at Diagnosis, and CMR at Recovery (6-Week Follow-Up)
(A) Initial cardiac magnetic resonance (CMR) at 1.5-T, short axis; T2 mapping. (B) Endomyocardial biopsy at high magnification showing extensive and complex inflammatory infiltrate made by many mononuclear cells, giant cells, and some polymorphonuclear cells. Inflammation was aggressive and associated with several necrotic cardiac myocytes (arrows). Hematoxylin and eosin stain. Bar: 25 μm. (C) 6-week follow-up CMR at 1.5-T, short-axis; T2 mapping.
Histopathological analysis of the EMB specimen showed intense myocardial inflammation comprised of numerous giant cells associated with many mononuclear cells, and prominent myocyte necrosis (Figure 1). Immunohistochemistry showed that the inflammatory cells were mainly CD68-positive giant cells and macrophages and CD8-positive T-lymphocytes, many of them exhibiting granzyme B, perforin, and TIA1 cytotoxicity markers. PD-L1 was expressed only by inflammatory cells and not by cardiomyocytes. CD4 and CD20 lymphocytes as well as NKp46-positive NK cells were poorly represented (not shown).
The patient’s hemodynamics remained stable, without cardiogenic shock, and the LVEF improved gradually over 8 days in the intensive care unit. No sustained ventricular arrhythmias or high-degree conduction abnormalities were observed. The LVEF improved to 50% before discharge, although there was right ventricular impairment. The patient was discharged on oral heart failure medications (angiotensin-converting enzyme inhibitors and beta-blockers) and prednisone 1 mg/kg/day on day 13. Rechallenge with ICI therapy was deemed too high-risk at the time and was withheld after a multidisciplinary discussion. The patient had close follow-up in the cardio-oncology clinic, heart failure medications were titrated to maximum-tolerated doses, and steroids were subsequently tapered. An ambulatory 24-h Holter monitor showed no significant rhythm or conduction disorders.
To investigate the presence of viral markers of cardiac infection, frozen EMBs were sampled and analyzed at a quaternary university hospital laboratory (EA-4684, Cardiovir, Reims, France). Molecular assays for the detection of viral genomes were performed for enteroviruses (EVs), human herpesviruses (HHV1 to HHV8), and human parvovirus B19 (HPVB19). Only enterovirus RNA genomes were detected, and the RNA genome copy number was then estimated by a standardized RT–quantitative polymerase chain reaction assay. The mean EV-B viral load was 6000 genome copies/μg of total nucleic acid extracted, indicative of a moderate viral genomic replication load in the cardiac tissue. To differentiate between acute and persistent EV-B infection, we investigated the proportions and 5ʹ uncoding region sizes of EV-B populations using referenced molecular assays. Three EV-B populations were identified with a major proportion (45.6%) of full-length viral forms associated with 2 minor viral RNA populations, characterized by deletions ranging respectively from 8 to 36 (34.1%) and 37 to 50 nucleotides (20.3%) in the 5ʹ uncoding region. Sequencing of the viral protein 1 gene was performed and resulted in the genotypic identification of an original echovirus-19 strain (GenBank accession no. MN596948); nucleotide and amino acid sequences percent identities were 78.51% and 93.42%, respectively, with the echovirus-19 strain Burke (GenBank accession no. AY302544.1). Sequence identities between the EMB virus isolate and the prototype echovirus-19 strain Burke demonstrated a homologous serotype. Immunohistochemistry for viral protein 1 detection was performed on heart biopsies and showed foci of positive cardiomyocytes surrounded by inflammatory infiltrates, indicating endomyocardial viral protein synthesis activities.
Steroids were gradually tapered and discontinued at 3 months in light of the viral analysis. Cardiac magnetic resonance imaging showed at 6 weeks: 1) resolution of myocardial edema (average myocardial T2 = 50 ms (Figure 1), T1 values of 917 ms, and extravascular volume decreased to 29%); 2) recovery of LVEF to 68%; and 3) improvement of right ventricular function with an ejection fraction of 44% at 6 weeks to 51% at 6 months follow-up. The cancer had not progressed at 6 months follow-up, and the patient reported normal functional status. ICI therapy was not restarted at the time of last follow-up.
Discussion
This case highlights the heterogeneity of pathogenesis of acute myocarditis on ICI therapy. We describe here acute myocarditis with long-term ICI therapy, secondary to giant cell myocarditis due to enterovirus.
Giant cell myocarditis is uncommon; its pathogenesis is poorly understood, and prognosis is poor. Recent data from an international registry including 220 patients with histologically proven severe myocarditis demonstrated that giant cell myocarditis bears the worst prognosis (6). The favorable outcomes in our patient, in terms of biventricular systolic recovery and the absence of further relapse, are unexpected outcomes in this case of giant cell myocarditis. To the best of our knowledge, only 1 other case of giant cell myocarditis on ICI therapy has thus far been published. The context differed from our patient’s given that the tumor was melanoma, the therapy was ipilimumab (targeting CTLA-4), and cardiovascular death was the outcome (7).
The moderate EV-B RNA load associated with major 5ʹ full-length viral populations supported the hypothesis of an opportunistic acute viral infection as the cause of the late-onset fulminant myocarditis in our patient. Endomyocardial inflammatory damage could result from EV-induced cytopathic effects and cellular autoimmune mechanisms. The presence of endomyocardial giant cells may have been related both to viral and ICI-induced autoimmune cellular dysfunctions, acting synergistically and resulting in myocarditis. As far as the pathophysiology, the PD-1/PD-L1 pathway plays a critical role in suppressing inflammation induced by viral infection, allograft transplantation, as well as autoimmune mechanism, protecting tissues from acute histopathological damage. Viruses, specifically EV-B, have developed various strategies to escape from innate immune responses including blockade of the PD-1/PD-L1 pathway. Indeed, cleavage activities of EV-B proteinases can down-regulate PD-L1 expression in cardiac cells in vivo and in vitro inducing severe inflammatory damage (8). Here, on EMB, PD-L1 was only expressed by inflammatory cells and not by cardiomyocytes. Total disruption of the PD-1/PD-L1 pathway both by EV-B infection and ICI in cardiac tissue might explain the clinical and histopathological severity of our case. We further hypothesized that ICI therapy cessation may have reestablished T-cell immunity, resulting in viral clearance and complete clinical and imaging recovery.
Although the number of patients eligible to ICI treatment is increasing dramatically, the pathogenesis of myocarditis needs to be further investigated. Based on only 2 extensively studied cases (5) and 2 retrospective cohorts limited to approximately 30 participants each (3,4), societies have released case definitions for these emerging clinical syndromes (9). The American Heart Association (AHA) has recently proposed diagnostic criteria for ICI myocarditis diagnosis based mainly on clinical characteristics, abnormal biomarkers, and/or cardiac imaging abnormalities (9). The recent publication of ICI myocarditis definitions by the AHA has the value of standardizing criteria to improve reporting. Steroids are the first-line front treatment of ICI myocarditis, despite poor evidence to support this, followed by other immune modulators, plasma exchange, or even CTLA-4 agonist (abatacept) infusions. In the present study case, steroids were introduced and then discontinued after the confirmation of active viral markers on EMB. Indeed, the prognosis of T-cell ICI myocarditis is partly driven by early initiation of steroid treatment (10), which should not be held when awaiting histological or viral results.
Endomyocardial biopsy is recommended whenever possible in fulminant myocarditis (9). It is, however, likely to be overlooked in cancer patients. This case report emphasizes the risk for opportunistic viral infection during long-term ICI therapy and utility of biopsy. The first mechanism to be considered in the setting of ICI myocarditis is T-cell mediated, prompting steroids as a first-line therapy early after hospital admission. Endomyocardial biopsy should be performed whenever possible to rule out infectious causes of myocarditis to guide therapy and further our understanding of the potential varying presentations of ICI myocarditis.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Wang D.Y., Salem J.-E., Cohen J.V. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4:1721. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salem J.-E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor–related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ammirati E., Veronese G., Brambatti M. Fulminant versus acute nonfulminant myocarditis in patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2019;74:299–311. doi: 10.1016/j.jacc.2019.04.063. [DOI] [PubMed] [Google Scholar]
- 7.Reuben A., Petaccia M. de M., McQuade J. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology. 2017;6 doi: 10.1080/2162402X.2017.1361097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T., Chen S., Wang X. Aberrant PD-1 ligand expression contributes to the myocardial inflammatory injury caused by coxsackievirus B infection. Antiviral Res. 2019;166:1–10. doi: 10.1016/j.antiviral.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Bonaca M.P., Olenchock B.A., Salem J.-E. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]