A 53-year-old female patient, an active smoker, received a diagnosis in early 2019 of poor-risk, metastatic renal cell carcinoma with invasion of the inferior vena cava and hepatic veins. Pre-treatment transthoracic echocardiography (TTE) revealed thrombotic involvement of the right atrium with normal biventricular function (left ventricular [LV] ejection fraction [LVEF], 60%). The patient underwent unilateral total nephrectomy, tumor extraction from the inferior vena cava, and right atrial thrombectomy. Post-surgical computed tomography revealed a mass extending from the stomach to the liver and further invasion of the tumor to the liver and lungs. Adjuvant combination immunotherapy with the immune-checkpoint inhibitors (ICIs) ipilimumab (cytotoxic T-lymphocyte-associated protein 4 [CTLA-4]–targeted monoclonal antibody) and nivolumab (programmed cell death protein [PD]-1–targeted monoclonal antibody), was administered every 3 weeks for 3 cycles (1).
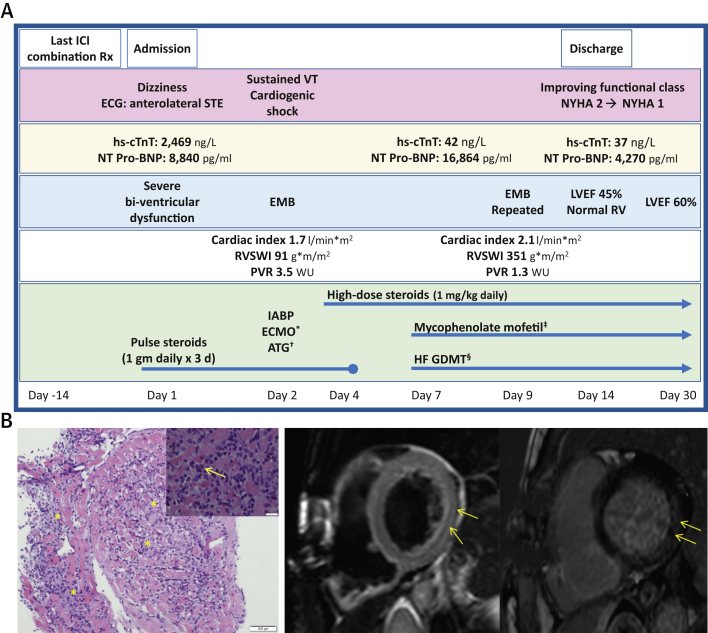
Two weeks after her last immunotherapy cycle (day 1) (Figure 1A), the patient presented to the Oncology Ambulatory Care Unit in the Davidoff Cancer Center, Rabin Medical Center (Israel) with a report of dizziness. She denied any chest pain, dyspnea, palpitations, or syncope. An electrocardiogram (ECG) revealed anterolateral ST-segment elevation. Laboratory evaluation revealed an elevated high-sensitivity cardiac troponin T (hs-cTnT) level of 2,469 ng/l (normal 0 to 14 ng/l) and an N-terminal pro–B-type natriuretic peptide (NT-proBNP) level of 8,840 pg/ml (normal adjusted for age and sex, <249 pg/ml). She was taken on an emergency basis to the cardiac catheterization laboratory and was found to have nonobstructive coronary artery disease. Left ventriculography revealed severe global LV dysfunction, and TTE confirmed severe biventricular systolic dysfunction, with an LVEF of 30%. The patient remained asymptomatic but was noted to have frequent premature ventricular beats on telemetry monitoring. Given her clinical presentation and recent treatment with ICIs, the leading diagnosis was ICI-induced myocarditis, and the decision was made to treat her with empirical pulse dose steroids (1 g methylprednisolone daily for 3 days). Despite this treatment, her condition deteriorated significantly over the next few hours as she developed refractory ventricular tachycardia and cardiogenic shock. She was treated according to the advanced cardiac life support protocol, sedated, intubated, and taken back to the catheterization laboratory for intra-aortic balloon pump insertion and transjugular endomyocardial biopsy to confirm the diagnosis. During the procedure, she continued to have recurrent episodes of sustained ventricular tachycardia. Over the next few hours, she became anuric with increasing lactic acid levels, despite intra-aortic balloon pump support. “Fast track” evaluation of the endomyocardial biopsy revealed fulminant myocarditis with abundant lymphocytes, eosinophils, and histiocytes (Figure 1B). Therefore, after a multidisciplinary meeting with her oncologists, cardiologists, and the cardiac surgery team, she began extracorporeal membrane oxygenation (ECMO), and antithymocyte globulin was added to her steroid regimen (total dose of 330 mg divided over 5 daily doses).
Figure 1.
Diagnosis and Management of a Patient With ICI-Induced Myocarditis and Cardiogenic Shock
(A) Timeline describing the patient’s clinical presentation, diagnostic findings, and treatment approach. *The patient was dependent on extracorporeal membrane oxygenation (ECMO) for 3 days, after which she was weaned from extracorporeal membrane oxygenation on the basis of hemodynamic improvement. †A total dose of 330 mg of antithymocyte globulin (ATG) was administered as divided doses over 5 days. ‡Mycophenolate mofetil (1,000 mg twice daily) was added on day 7 and continued for 1 month. §Guideline-directed medical therapy (GDMT) for heart failure (HF) was initiated and gradually titrated as the patient improved (Valsartan 80 mg twice daily which was switched on discharge to sacubitril-valsartan 49 to 51 mg twice daily, spironolactone 12.5m g increased to 25 mg once daily, and bisoprolol 1.25 mg daily, which was initiated upon discharge). (B) Endomyocardial biopsy and cardiac magnetic resonance in immune-checkpoint inhibitor (ICI)–induced myocarditis. (Left) Endomyocardial histological findings (hematoxylin and eosin stain) showing lymphocytic infiltration of the myocardium (asterisks) with surrounding eosinophils (arrow). (Right) Cardiac magnetic resonance, short-axis views showing midwall late gadolinium enhancement (arrows) in the basal and midsegmental inferolateral walls (left side) and myocardial edema on a T2 qualitative image (arrows) (right side). ECG = electrocardiogram; EMB = endomyocardial biopsy; hs-cTnT = high-sensitivity cardiac troponin; IABP = intra-aortic balloon pump; ICI = immune-checkpoint inhibitor; LVEF = left ventricular ejection fraction; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association (functional class); PVR = pulmonary vascular resistance; RV = right ventricle; RVSWI = right ventricular stroke work index; Rx = treatment; STE = ST-segment elevation; VT = ventricular tachycardia; WU = wood units.
During treatment with ECMO, she demonstrated gradual hemodynamic and clinical improvement, and ECMO support was withdrawn after 3 days. However, because of persistent LV dysfunction and increasing NT-proBNP levels (16,864 pg/ml), mycophenolate mofetil was added to her immunosuppressive regimen (day 7), which by then included prednisone 1 mg/kg daily. Both hs-cTnT and NT-proBNP levels, which were monitored during her entire hospital stay, started to decline gradually, and she was started on guideline-directed medical therapy for heart failure (Figure 1A). A hemodynamic evaluation by right-sided heart catheterization (day 9) showed normal filling pressures and a cardiac index of 2.1 l/min/m2. A follow-up endomyocardial biopsy continued to demonstrate lymphocytic infiltration, but to a lesser extent.
The patient was discharged home after a 2-week hospitalization with a favorable functional status (New York Heart Association functional class II). Her hs-cTnT and NT-proBNP levels at discharge were 34 ng/l and 4,270 pg/ml, respectively. Pre-discharge TTE showed mild LV dysfunction (LVEF, 45%) and normal right ventricular function. Cardiac magnetic resonance, performed for prognostication, showed mildly reduced LV function, increased and diffuse T2 signaling, and inferolateral late gadolinium enhancement with minimal fibrosis (Figure 1B). One month after her initial presentation with cardiogenic shock, while still receiving high-dose prednisone and mycophenolate mofetil, she continued to improve clinically (New York Heart Association functional class I to II), with declining NT-proBNP levels (1,031 pg/ml) and an LVEF of 60% on TTE.
Discussion
ICIs are specific monoclonal antibodies directed against inhibitory proteins of the cellular immune system, thus enabling an amplified T-cell–mediated immune response against cancer cells. These drugs have shown remarkable results in treating advanced metastatic cancers including melanoma, non–small cell lung cancer, and renal cell carcinoma (1). However, unchecked activation of antitumor T cells can lead to off-target autoimmune damage involving host tissues (2), including acute myocarditis. Until recently, the incidence of ICI-induced myocarditis was underestimated and poorly characterized 1, 2. Data regarding the diagnosis and management of ICI-induced myocarditis are mostly based on expert-opinion, yet this case of resolved ICI-induced myocarditis and cardiogenic shock highlights several important clinical aspects.
Given that myocarditis has a wide spectrum of clinical presentations and because the prognosis of patients with ICI-induced myocarditis in particular is very poor (3), we believe that both close surveillance and a high index of suspicion are needed to make a rapid, and possibly lifesaving, diagnosis of ICI-induced myocarditis.
Whom to follow? Combination therapy with different ICI agents is the best-established risk factor for ICI-induced myocarditis 3, 4, 5, and indeed our patient received both ipilimumab and nivolumab, which have been shown to confer a 5-fold increased risk for developing myocarditis compared with nivolumab alone (5). Moreover, although not evident in our patient, myositis is a common concomitant finding in patients with ICI-induced myocarditis (29% of cases) 5, 6, and therefore it should be regarded as a “red flag” to look for myocarditis in patients treated with ICIs. Several diagnostic algorithms have been published on how to screen these high-risk patients 5, 7, 8, but additional studies are needed to identify other patient- or cancer-related risk factors.
When to screen? In a large case series, the median time from the first dose of ICI to the onset of myocarditis was 34 days (interquartile range: 21 to 75 days) (4), and only 1 or 2 ICI doses have preceded the onset of myocarditis in the majority (93%) of fatal cases (6). However, other investigators have shown an increased risk of ICI-induced myocarditis within the first 3 months of treatment 3, 4, as was evident in our case.
How to screen? ECG findings of ICI myocarditis range from sinus tachycardia to bundle branch block, complete heart block, and ST-segment elevation (6). However, despite low diagnostic specificity, performing ECGs at baseline and before each subsequent ICI dose (doses 1 to 3) is recommended because this test is easy to perform and cost-effective (6). Similarly, cardiac biomarkers, including troponin and NT-proBNP, have shown good correlation with the diagnosis of ICI-induced myocarditis 3, 4, 5, 6, 8. Although TTE may be of limited use in predicting cardiovascular outcomes when assessing for ICI-induced myocarditis (6), pre-treatment baseline TTE is a valuable tool to detect a subsequent change in cardiac function 4, 6.
Confirming the diagnosis of acute myocarditis by endomyocardial biopsy is important (5) and may have a significant impact on patient management, as presented in this case. First, a speculative yet incorrect diagnosis of ICI-induced myocarditis may prevent patients from receiving further lifesaving immunotherapy. Notably, late gadolinium enhancement on cardiac magnetic resonance, a classic finding in patients with non–ICI-induced acute myocarditis, may be absent in 20% to 30% of patients with ICI-induced myocarditis 3, 4. Second, although data guiding treatment of ICI-myocarditis are not evidence based, a high-grade inflammatory response on endomyocardial biopsy should prompt intensification of immunosuppression to limit adverse cardiac outcomes. Depending on the severity of hemodynamic compromise, possible immunosuppressive regimens may include antithymocyte globulin, mycophenolate mofetil, and, as recently reported, abatacept or alemtuzumab 5, 9, 10, in addition to high-dose steroids. The shared mechanism of action of these drugs is the inactivation or destruction of T cells, which play a dominant role in ICI-induced myocarditis as depicted in our patient’s myocardial histological findings. Finally, because the pathology and pathophysiology of ICI-induced myocarditis resemble the dynamic process of acute cellular rejection in cardiac transplant recipients 4, 5, follow-up biopsies, as performed for acute graft rejection, may be useful to assess treatment response and to determine the duration of immunosuppression.
In conclusion, this case outlines the diagnostic work-up and management of a rapidly deteriorating patient who presented with ICI-induced myocarditis and cardiogenic shock. It demonstrates that ECG and cardiac biomarker levels can be helpful in influencing therapeutic interventions in the early stages of disease. Cardiac biomarkers can also play an important role during treatment to assess patient response and at decision crossroads. Rapid initiation of immunosuppressive therapy, according to treatment algorithms adopted from cardiac transplant recipients with acute rejection, can be beneficial in reversing the underlying pathological process and may lead to improved cardiovascular outcomes in patients with ICI-induced myocarditis. A better understanding of the triggers of myocarditis and of systematic management approaches are needed to effectively treat the growing group of patients at risk for ICI-induced myocarditis. Meanwhile, there is a critical need to educate patients, as well as their treating oncologists and cardiologists, about this life-threatening complication.
Footnotes
Dr. Nohria has received research funding from Amgen; and has served as a consultant for Takeda Oncology. Dr. Neiman has served as a consultant for Bristol-Myers Squibb. Dr. Goldvaser has received honoraria from Roche. All other authors have reported that they have that they have no relationships relevant to the contents of this paper nothing to disclose.
References
- 1.Motzer R.J., Tannir N.M., McDermott D.F. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman C.F., Proverbs-Singh T.A., Postow M.A. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016;2:1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 3.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 4.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu J.R., Florido R., Lipson E.J. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res. 2019;115:854–868. doi: 10.1093/cvr/cvz026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atallah-Yunes S.A., Kadado A.J., Kaufman G.P., Hernandez-Montfort J. Immune checkpoint inhibitor therapy and myocarditis: a systematic review of reported cases. J Cancer Res Clin Oncol. 2019;145:1527–1557. doi: 10.1007/s00432-019-02927-x. [DOI] [PubMed] [Google Scholar]
- 7.Wang D.Y., Okoye G.D., Neilan T.G., Johnson D.B., Moslehi J.J. Cardiovascular toxicities associated with cancer immunotherapies. Curr Cardiol Rep. 2017;19:21. doi: 10.1007/s11886-017-0835-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lyon A.R., Yousaf N., Battisti N.M.L., Moslehi J., Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e448. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 9.Salem J.E., Allenbach Y., Vozy A. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 10.Esfahani K., Buhlaiga N., Thebault P., Lapointe R., Johnson N.A., Miller W.H., Jr. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]