Corresponding Author
Key Words: antihypertensive drugs, blood pressure, cancer, renin angiotensin aldosterone, tyrosine kinase inhibitors, VEGF inhibitors
Vascular endothelial growth factor (VEGF) inhibitors (VEGFIs), through their antiangiogenic actions, have transformed the landscape of cancer therapy by improving clinical outcomes and patient survival. These drugs are among the most widely prescribed for solid tumors 1, 2 and retinopathy (3), and there is growing interest in their use for other diseases such as psoriasis (4). However, with the benefits of VEGFIs are unwanted effects, in particular blood pressure (BP) elevation. This class effect has been observed in all VEGFI drug trials. In the most striking example, hypertension was identified in 87% of patients after only 1 week of cediranib treatment (5). Considering the vascular target of VEGFIs, their association with cardiovascular on-target side effects is not surprising (6). BP elevation, which occurs rapidly, within hours to days, reflects effective inhibition of VEGF signaling and accordingly has been considered a biomarker of tumor responsiveness 6, 7.
Although there is unambiguous experimental and clinical evidence that VEGFIs cause hypertension, the pathophysiology and underlying molecular mechanisms remain elusive. In addition, there is a paucity of clinical information on how best to diagnose and treat VEGFI-mediated cardiovascular toxicities, with current practice relying on trial data. Oncology trials tend to report hypertension as a categorical phenomenon, relying on frequently changing definitions provided by the Common Terminology Criteria for Adverse Events. These rarely align with major hypertension guidelines, which currently recommend tight BP control with targets as low as 130/80 mm Hg 8, 9. Whether these lower BP targets are applicable to VEGFI-treated cancer patients is unknown. Also, it is unclear whether enforcing strict BP targets is appropriate in this population if this means VEGFI dose reduction, treatment interruption, or drug cessation with consequent suboptimal cancer response. On the other hand, patients receiving VEGFIs may be at increased risk of end-organ complications of hypertension. In particular, direct myocardial toxicity of VEGFIs and increased afterload as a consequence of hypertension may predispose to left ventricular systolic dysfunction and heart failure (10). Posterior reversible encephalopathy syndrome, although rare, is another potential complication, as are nephropathy and renal failure, especially in the context of renal cancer 6, 10. Hypertension is not only a problem with systemic VEGFIs but may also be a concern with local treatment, as suggested by a few studies demonstrating worsening hypertension and nephrotoxicity in patients treated with intravitreal VEGFI therapy for retinopathy 11, 12, 13. Preventing these complications, or at least intervening at an early and potentially reversible phase, should be the goal of management to allow the continuation of VEGFI therapy at optimum doses for maximum anticancer effect 6, 14 (Figure 1).
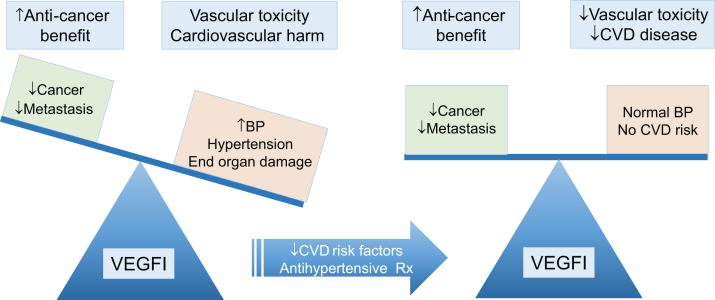
Figure 1.
VEGFI Beneficial and Harmful Effects Leading to Improved Cancer Survival With Increased Cardiovascular Toxicities, Particularly Hypertension
Ideal management of patients should balance antiangiogenic and cardiovascular toxicities so that anticancer treatment is optimized in the absence of hypertension and cardiovascular disease. BP = blood pressure; CVD = cardiovascular disease; Rx = prescription; VEGFI = vascular endothelial growth factor inhibitor.
Notwithstanding the uncertainties of applying population-based thresholds for the diagnosis and treatment of hypertension, the absolute rise in BP after starting VEGFIs may be as important, if not more so. The Cardiovascular Toxicities Panel of the National Cancer Institute recommends: 1) formal cardiovascular risk assessment before commencing VEGFI treatment; and 2) initiation of antihypertensive therapy in patients with a rise in diastolic BP of >20 mm Hg, even in those who would conventionally be considered normotensive (14). These recommendations are based on expert opinion rather than evidence, as reflected by the focus on diastolic BP rather than systolic BP, which is the element associated with cardiovascular events and the component that major hypertension guidelines emphasize 8, 9. The most effective agent for reduction of VEGFI-induced BP rise is also undefined, and other than the avoidance of nondihydropyridine calcium channel blockers (CCBs), no class is currently recommended over another (14). Nondihydropyridine CCBs inhibit cytochrome P450 3A4, the enzyme that metabolizes VEGFIs, leading to potentially high VEGFI plasma levels, which may aggravate VEGFI-induced hypertension (7).
With the upsurge of VEGFIs as part of modern chemotherapy protocols, the challenges to effectively manage cardiovascular toxicities increase (1). Unless gaps in knowledge are addressed and underlying mechanisms better understood, the clinical burden of cardiovascular toxicities in VEGFI-treated patients will continue to grow. Tackling some of these challenges are 2 studies in this issue of JACC: CardioOncology, which provide insights into VEGFI-associated BP elevation by retrospective analysis of data from real-world American patients treated at 2 large oncology centers.
Waliany et al. (15) provide a robust analysis of the Stanford Renal Cancer Database to describe the magnitude and timing of VEGFI-associated BP rise in 228 patients before and during treatment with tyrosine kinase inhibitors of VEGF. In support of previous studies, they confirmed that VEGFIs cause an increase in BP, with axitinib having the greatest potency. CCBs and potassium-sparing diuretic drugs were the most effective antihypertensive agents in these patients. An important finding in this study that deserves more attention is the lack of effect of inhibitors of the renin angiotensin aldosterone system (RAAS). Neither angiotensin-converting enzyme (ACE) inhibitors nor angiotensin receptor blockers (ARBs) lowered BP in VEGFI-induced hypertension in this study (15). This cannot be attributed to ethnicity factors because only 3.1% of patients were African Americans, who are typically less responsive to RAS inhibitors than whites.
The second paper in this issue, by Bottinor et al. (16), interrogated the database of patients in the Vanderbilt electronic health record and hypothesized that antihypertensive therapy with RAAS inhibitors (ACE inhibitors, ARBs, aldosterone antagonists, or renin inhibitors) mitigates VEGFI-mediated hypertension more effectively than other antihypertensive drugs. They studied 1,013 subjects and, similar to other studies, reported a clear relationship between VEGFI and development or aggravation of hypertension. The BP increase during VEGFI treatment in the Vanderbilt cohort was lowest in patients treated at baseline with RAAS inhibitors versus CCB and diuretic agents. Although both papers in this issue 15, 16 are retrospective studies, and both are based on robust information from large databases, conclusions about RAAS inhibition in the management of VEGFI-related hypertension are divergent. Multiple factors likely contribute to this, including the experimental design, baseline characteristics of patient cohorts, type of cancer (renal versus nonrenal), timing of antihypertensive therapy (pre- or post-commencement of VEGFI treatment), and possibly the lack of involvement of the RAAS in the pathophysiology of VEGFI-induced hypertension. This is supported by preclinical and clinical studies showing: 1) no change in plasma renin or aldosterone levels in cancer patients treated with sorafenib (17); 2) decreased plasma renin activity and reduced renin concentration with no change in aldosterone levels in cancer patients treated with sunitinib (18); and 3) decreased renin expression and reduced aldosterone urinary excretion in mice treated with a monoclonal antibody targeting the major VEGF receptor, VEGFR2 (19). These studies have clinical significance because they suggest that targeting the RAAS might not be the preferred antihypertensive treatment strategy in VEGFI-induced hypertension, a notion supported by findings in the Stanford Renal Cancer cohort (15).
Several other important points are highlighted by the 2 studies in this issue of JACC: CardioOncology 15, 16. Patients receiving VEGFIs for cancer therapy have substantial baseline cardiovascular comorbidity. Exacerbation of hypertension occurs in the majority, and there is now further real-world evidence to reinforce this point and, in particular, to intensify BP surveillance during the first 4 to 6 weeks of VEGFI therapy 1, 15, 16. This may be even more pertinent with more selective agents such as axitinib. However, even after the identification and initial treatment of the VEGFI-associated BP rise, current strategies are not good enough to return BP to baseline during VEGFI treatment. Although both studies raise questions about the most efficacious drug to prevent or treat VEGFI-associated hypertension we still do not have a robust answer, and in particular, the effectiveness of ACE inhibitors and ARBs in these patients is unclear. Optimal antihypertensive therapy has not yet been defined, and accordingly, the selection of classes of antihypertensive drugs should be based on particular comorbidities and cancer types in individual patients.
Furthermore, BP targets from the population as a whole are extrapolated to the VEGFI-treated cancer population. Whether this is appropriate or not is unclear, especially since the cancer population may be more susceptible to end-organ effects because of the sudden nature of the BP rise coupled with the direct toxic effects of VEGFIs on the vasculature, myocardium, and kidneys (6). On the other hand, evidence-based BP thresholds at which VEGFI dose reduction or interruption should occur are still required. These are needed to ensure that this is only done when there is a genuine risk of cardiovascular morbidity becoming more prescient than the index cancer. In addition to defining the optimum agent for the prevention and treatment of the VEGFI-associated BP rise, it is of particular importance to identify those drugs that might provide added value over and above the reduction of BP per se in order to provide additional cardioprotective and renoprotective effects. It is as important to identify antihypertensive drugs that are not effective and that should probably be avoided.
Unless adequately powered randomized controlled trials to assess effects of different antihypertensive drugs in the prevention or treatment of VEGFI-associated hypertension are conducted, clinical treatment will remain suboptimal, and management will rely on expert opinion rather than evidence. Trials should have clinically relevant endpoints that address the control of BP and its target organ effects (left ventricular systolic dysfunction and renal dysfunction) versus cancer outcomes, including VEGFI dose reduction and treatment interruption. The field, although advancing, is still in its infancy regarding the understanding of the underlying mechanisms of VEGFI-induced hypertension and evidence-based best clinical practice. Clinical decisions and research questions that rely so heavily on understanding the balance between cardiovascular risk and optimum cancer care will only be adequately tackled by continued and increasing cardiovascular-oncology collaboration.
Footnotes
Dr. Touyz is supported by a British Heart Foundation Chair award (CH/12/4/29762). Dr. Lang has received speaker fees from Novartis and Pfizer.
References
- 1.Pal S., Gong J., Mhatre S.K. Real-world treatment patterns and adverse events in metastatic renal cell carcinoma from a large US claims database. BMC Cancer. 2019;19:548. doi: 10.1186/s12885-019-5716-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaelson M.D., Gupta S., Agarwal N. A phase Ib study of axitinib in combination with crizotinib in patients with metastatic renal cell cancer or other advanced solid tumors. Oncologist. 2019 Jun 6 doi: 10.1634/theoncologist.2018-0749. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li J., Xu J., Chen Y., Zhang J., Cao Y., Lu P. Efficacy comparison of intravitreal anti-VEGF therapy for three subtypes of neovascular age-related macular degeneration: a systematic review and meta-analysis. J Ophthalmol. 2018;2018:1425707. doi: 10.1155/2018/1425707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malecic N., Young H.S. Novel investigational vascular endothelial growth factor (VEGF) receptor antagonists for psoriasis. Expert Opin Investig Drugs. 2016;25:455–462. doi: 10.1517/13543784.2016.1153064. [DOI] [PubMed] [Google Scholar]
- 5.Robinson E.S., Matulonis U.A., Ivy P. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol. 2010;5:477–483. doi: 10.2215/CJN.08111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Touyz R.M., Lang N.N., Herrmann J., van den Meiracker A.H., Danser A.H.J. Recent advances in hypertension and cardiovascular toxicities with vascular endothelial growth factor inhibition. Hypertension. 2017;70:220–226. doi: 10.1161/HYPERTENSIONAHA.117.08856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humphreys B.D., Atkins M.B. Rapid development of hypertension by sorafenib: toxicity or target? Clin Cancer Res. 2009;15:5947–5949. doi: 10.1158/1078-0432.CCR-09-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams B., Spiering W., Agabiti Rosei E. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 9.Whelton P.K., Carey R.M., Aronow W.S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [published correction appears in J Am Coll Cardiol 2018;71:2275–9] [DOI] [PubMed] [Google Scholar]
- 10.Dobbin S.J.H., Cameron A.C., Petrie M.C., Jones R.J., Touyz R.M., Lang N.N. Toxicity of cancer therapy: what the cardiologist needs to know about angiogenesis inhibitors. Heart. 2018;104:1995–2002. doi: 10.1136/heartjnl-2018-313726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hanna R.M., Lopez E.A., Hasnain H. Three patients with injection of intravitreal vascular endothelial growth factor inhibitors and subsequent exacerbation of chronic proteinuria and hypertension. Clin Kidney J. 2019;12:92–100. doi: 10.1093/ckj/sfy060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanna R.M., Barsoum M., Arman F., Selamet U., Hasnain H., Kurtz I. Nephrotoxicity induced by intravitreal vascular endothelial growth factor inhibitors: emerging evidence. Kidney Int. 2019;96:572–580. doi: 10.1016/j.kint.2019.02.042. [DOI] [PubMed] [Google Scholar]
- 13.Rasier R., Artunay O., Yuzbasioglu E., Sengul A., Bahcecioglu H. The effect of intravitreal bevacizumab (Avastin) administration on systemic hypertension. Eye (Lond) 2009;23:1714–1718. doi: 10.1038/eye.2008.360. [DOI] [PubMed] [Google Scholar]
- 14.Maitland M.L., Bakris G.L., Black H.R., Cardiovascular Toxicities Panel, Convened by the Angiogenesis Task Force of the National Cancer Institute Investigational Drug Steering Committee Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waliany S., Sainani K.L., Park L.S., Zhang C.A., Srinivas S., Witteles R.M. Increase in blood pressure associated with tyrosine kinase inhibitors targeting vascular endothelial growth factor. J Am Coll Cardiol CardioOnc. 2019;1:24–36. doi: 10.1016/j.jaccao.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bottinor W.J., Shuey M.M., Manouchehri A. Renin-angiotensin-aldosterone system modulates blood pressure response during vascular endothelial growth factor receptor inhibition. J Am Coll Cardiol CardioOnc. 2019;1:14–23. doi: 10.1016/j.jaccao.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belcik J.T., Qi Y., Kaufmann B.A. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol. 2012;60:618–625. doi: 10.1016/j.jacc.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kappers M.H., van Esch J.H., Sluiter W., Sleijfer S., Danser A.H., van den Meiracker A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 19.Facemire C.S., Nixon A.B., Griffiths R., Hurwitz H., Coffman T.M. Vascular endothelial growth factor receptor 2 controls blood pressure by regulating nitric oxide synthase expression. Hypertension. 2009;54:652–658. doi: 10.1161/HYPERTENSIONAHA.109.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]