Abstract
Background
Many patients with cancer have a hypercoagulable state and an increased risk of developing venous thromboembolism (VTE), arterial occlusion, and pulmonary emboli. Patients with cancer may also have an increased risk of bleeding with anticoagulant treatment. Recent trials have reported that direct oral anticoagulants (DOACs) are noninferior to the low-molecular-weight heparin, dalteparin, in preventing VTE, but have a higher bleeding rate.
Objectives
This study compared the efficacy and risks of DOACs versus dalteparin in patients with cancer-related VTEs across all randomized controlled trials (RCTs).
Methods
This study performed a systematic analysis of RCTs published in PubMed, SCOPUS, and Google Scholar from September 1, 2007 through March 31, 2020 that reported clinical outcomes of treatment with DOACs versus dalteparin in patients with cancer with acute VTE. Two investigators independently performed study selection and data extraction. Extracted data were recorded and exported to statistical software for all analyses (OpenMetaAnalyst).
Results
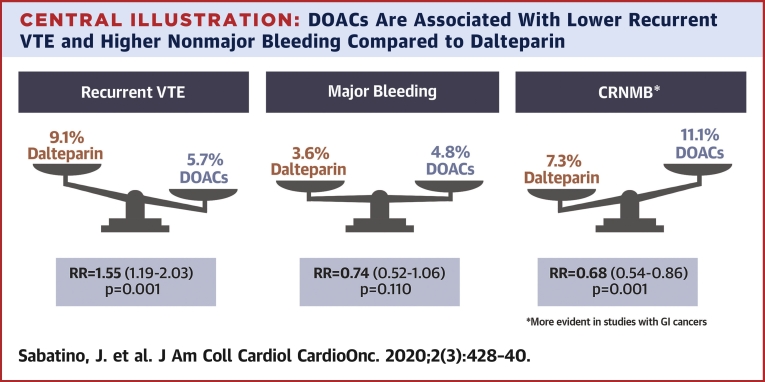
This study included 4 randomized trials (N = 2,907). Compared with DOACs, dalteparin was associated with higher VTE recurrence (risk ratio [RR]: 1.55; 95% confidence interval [CI]: 1.19 to 2.03; p = 0.001), whereas clinically relevant nonmajor bleeding (CRNMB) was significantly less frequent with dalteparin than that with DOACs (RR: 0.68; 95% CI: 0.54 to 0.86; p = 0.001). The risk of CRNMB was largely observed with patients with gastrointestinal malignancies. No significant differences were observed in major bleeding (RR: 0.74; 95% CI: 0.52 to 1.06; p = 0.11).
Conclusions
DOACs were noninferior to dalteparin in preventing VTE recurrence in patients with cancer without a significantly increased risk of major bleeding. However, DOACs were associated with higher rates of CRNMB compared with dalteparin, primarily in patients with gastrointestinal malignancies.
Key Words: cancer, direct oral anticoagulants, DOACs, hypercoagulable state, venous thromboembolism
Abbreviations and Acronyms: CI, confidence interval; CRNMB, clinically relevant nonmajor bleeding; DOAC, direct oral anticoagulant; DVT, deep vein thrombosis; GI, gastrointestinal; LMWH, low-molecular-weight heparin; PE, pulmonary embolism; RCT, randomized controlled trial; RR, risk ratio; VKA, vitamin K antagonist; VTE, venous thromboembolism
Central Illustration
Cardiovascular care of patients with cancer can be complex. Patients can have a persistent hypercoagulable state and an increased risk of recurrent venous thromboembolism (VTE). Furthermore, patients with cancer have an increased bleeding risk, which may be further exacerbated by antithrombotic treatments. Patients with cancer may also develop complications of cancer treatments that further increase the risk of thrombotic and bleeding events, including: invasive diagnostic and surgical procedures; toxicity secondary to radiotherapy, anti-angiogenic agents, hormonal therapies, immunotherapy and/or chemotherapy; hepatotoxicity; renal injury; tumor friability; or thrombocytopenia (1,2).
The CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer) Investigators trial established low-molecular-weight heparin (LMWH) as the guideline-recommended first-line therapy over vitamin K antagonists (VKAs) for patients with cancer-related acute VTEs, based on data that showed a lower risk of VTE recurrence in patients who were treated with low-molecular-weight heparin (LMWH) (3,4). However, direct oral anticoagulants (DOACs), including dabigatran, apixaban, rivaroxaban, and edoxaban, demonstrated increased safety and efficacy in comparison with VKAs for management of VTE in the general population (5, 6, 7, 8). Recently, randomized controlled trials (RCTs) compared different DOACs, including edoxaban (Hokusai VTE Cancer [Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism]) (9), rivaroxaban (SELECT-D [Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial]) (10), and apixaban (ADAM VTE [Apixaban and Dalteparin in Active Malignancy Associated Venous Thromboembolism: The ADAM VTE Trial]; CARAVAGGIO [Apixaban for the Treatment of Venous Thromboembolism]) (11,12) with dalteparin with regard to efficacy and safety in preventing recurrent VTEs in patients with cancer. A previous meta-analysis (13), which evaluated results from the Hokusai VTE Cancer, SELECT-D, and ADAM VTE trials, observed a trend for reduced VTE recurrence in DOAC-treated patients, in which DOACs were found to be noninferior to LMWH in preventing VTE recurrence in patients with cancer, but were associated with an increased risk of major bleeding and clinically relevant nonmajor bleeding (CRNMB). The results of these trials were somewhat conflicting, which might have been due to differences in trial design and enrollment criteria. The recently published results of the CARAVAGGIO trial, the largest trial to date in this specific clinical setting, reported that oral apixaban therapy in patients with cancer was associated with significantly lower VTE recurrence, low rates of bleeding, and reported enhanced quality of life outcome measures compared with dalteparin (12). Applying these newly available data, we performed a meta-analysis to evaluate the efficacy and safety of DOACs compared with dalteparin in patients with cancer-related acute VTEs.
Methods
Data sources and searches
We searched PubMed, SCOPUS, and Google Scholar electronic databases from September 1, 2007 through March 31, 2020, because the first journal article was published in 2007 regarding clinical trial testing with a DOAC for the prevention of VTEs (14). We used the following keywords and the corresponding MeSH terms: “venous thromboembolism,” “cancer,” “DOAC/NOAC.” We also reviewed the reference lists of eligible studies and screened scientific abstracts and relevant websites (i.e., www.clinicaltrialresults.org; www.escardio.org; www.tctmd.com; https://accscientificsession.acc.org; and https://exhibitatsessions.org).
Study selection
Two investigators (J.S., S.D.R.) independently screened search records to identify eligible trials. There were no disagreements. RCTs were included if they compared a DOAC versus dalteparin in patients with cancer and acute VTE. Additional inclusion criteria included the following outcomes: recurrent VTE, and major bleeding or CRNMB. Exclusion criteria consisted of duplicate publications and journal articles in which trial results were published in a language other than English and/or did not report the pre-specified endpoint measure.
Data extraction and quality assessment
Two reviewers (J.S., S.D.R.) independently extracted data concerning study characteristics and event rates from full text journal articles. Two investigators (J.S., S.D.R.) independently assessed study quality using the Cochrane Risk of Bias Tool (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials). In particular, the assessment considered: randomization method; allocation concealment; blinding of patient, investigator, and outcome adjudication committee; reporting bias; attrition bias; and any other potential sources of bias, such as those related to trial designs or the risk for contamination or crossover between the groups.
Data synthesis and analysis
We extracted data from the original primary publications (9, 10, 11, 12). Efficacy outcomes of interest consisted of recurrent VTEs that included deep vein thrombosis (DVT) and pulmonary embolism (PE). Safety outcomes consisted of major bleeding and CRNMB. In addition, death as an outcome was analyzed as a secondary endpoint. We used the risk ratio (RR) with 95% confidence interval (CI) as the summary measure (15). Heterogeneity was assessed using Cochrane’s Q test, and p values <0.10 were considered indicative for heterogeneity. I2 values were calculated for estimation of variation among studies attributable to heterogeneity (16). A fixed effect was used to compute estimates for the summary effect in case of low heterogeneity (I2 <45% and Cochrane’s Q test = NS); otherwise, a random-effects model was applied (17). Meta-analysis results were reported graphically using forest plots: the measure of effect (RR) was represented by a square, with the area being proportional to study weight, as previously described (18). A p value <0.05 was considered significant. Subgroup and sensitivity analyses were conducted using fixed effect and random effects models alternatively (15, 16, 17, 18). Publication bias was assessed using funnel plots. Analyses were performed using OpenMetaAnalyst 10 (Brown University, Providence, Rhode Island) and Review Manager 5.3 (Cochrane, London, United Kingdom).
Results
Literature search and study selection
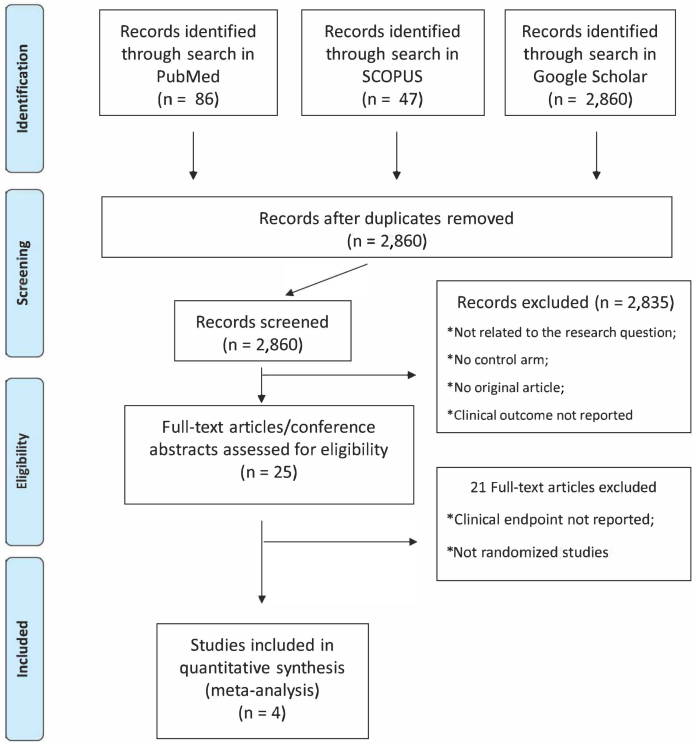
The literature search retrieved a total of 2,860 articles, after removing duplicates. By screening titles and abstracts, we identified 2,835 citations not relevant to our study aims. After a full review of the remaining manuscripts, 4 randomized controlled trials that included 2,907 patients with cancer with acute VTEs, were included in the systematic review and meta-analysis (9, 10, 11, 12). The study flowchart is described in Figure 1.
Figure 1.
Study Selection Flowchart
The flowchart describes study search, screening, and selection processes.
The 4 included studies were designed to compare the efficacy of DOACs with dalteparin for the treatment of cancer-related acute VTEs (9, 10, 11, 12). Main trial characteristics are outlined in Table 1. Treatment duration was at least 6 months for all trials (9,11,12); the Hokusai VTE trial included data from 12 months’ follow-up, and patients in the SELECT-D trial were also eligible for randomization to a further 6 months of rivaroxaban or placebo. Data from the SELECT-D trial used for calculation in this meta-analysis only refer to the 6-month follow-up with comparison to dalteparin (10). Each study reported data on VTE recurrence, major bleeding, and CRNMB. The definitions of major bleeding, CRNMB, and active cancer were homogeneous among the included studies, whereas VTE definitions were more heterogeneous. The ADAM VTE trial was the only study that included thromboembolism of the upper extremities in the inclusion criteria as a qualifying event for participating in the study. Furthermore, this study also included any arterial thromboembolism in the endpoint definition of recurrent thromboembolism (11). A detailed description of key trial definitions is included in Tables 2 and 3. The trials also represented populations with slight differences in the distribution of cancer types, as reported in Table 4.
Table 1.
Study Characteristics
| Study First Author (Ref. #); Year |
N | Mean Age (yrs) | Design | Intervention | Control | Outcome |
|---|---|---|---|---|---|---|
| CARAVAGGIO Agnelli et al. (12); 2020 |
1,155 | 67 | Open-label RCT (non-inferiority) | Apixaban | Dalteparin | Primary efficacy outcome: VTE recurrence. Primary safety outcome: major bleeding |
| SELECT-D Young et al. (10); 2018 |
406 | 67 | Open-label RCT (pilot trial) | Rivaroxaban | Dalteparin | Primary outcome: thromboembolic recurrence. Secondary outcome: major bleeding and CRNMB |
| Hokusai VTE Cancer Roskab et al. (9); 2018 |
1,046 | 64 | Open-label RCT (non-inferiority) | Edoxaban | Dalteparin | Primary outcome: composite of recurrent VTE or major bleeding |
| ADAM-VTE McBane et al. (11); 2020 |
300 | 64 | Open-label RCT (superiority) | Apixaban | Dalteparin | Primary outcome: major bleeding. Secondary outcome: VTE recurrence |
ADAM VTE = Apixaban and Dalteparin in Active Malignancy Associated Venous Thromboembolism: The ADAM VTE Trial; CARAVAGGIO = Apixaban for the Treatment of Venous Thromboembolism trial; CRNMB = clinically relevant non-major bleeding; Hokusai VTE Cancer = Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism; RCT = randomized clinical trial; SELECT-D = Comparison of an Oral Factor Xa Inhibitor With Low Molecular Weight Heparin in Patients With Cancer With Venous Thromboembolism: Results of a Randomized Trial; VTE = venous thromboembolism.
Table 2.
Definitions Used for VTE
| Study First Author (Ref. #); Year |
VTE (Qualifying Event) | VTE Recurrence |
|---|---|---|
| CARAVAGGIO Agnelli et al. (12); 2020 |
Newly diagnosed symptomatic or incidental proximal lower limb DVT or PE | Proximal DVT of the lower limbs (symptomatic or incidental), symptomatic DVT of the upper limbs, or PE (symptomatic, incidental, or fatal) occurring during the 6-month trial period |
| SELECT-D Young et al. (10); 2018 |
Symptomatic lower extremity proximal DVT, or symptomatic PE, or incidental PE | Recurrent proximal DVT, or recurrent PE (symptomatic or incidental), or fatal PE, or other sites of venous thrombosis (e.g., subclavian vein, hepatic vein, or inferior vena cava) |
| Hokusai VTE Cancer Raskob et al. (9); 2018 |
Acute, symptomatic or incidentally detected DVT involving the popliteal, femoral, or iliac vein or the inferior vena cava; acute symptomatic PE that was confirmed by means of diagnostic imaging; or incidentally detected PE involving segmental or more proximal pulmonary arteries | Symptomatic new DVT or PE, incidental (detected by means of imaging tests performed for other reasons) new DVT, or PE involving segmental or more proximal pulmonary arteries, or fatal PE or unexplained death for which PE could not be ruled out as the cause |
| ADAM-VTE McBane et al. (11); 2020 |
Acute lower or upper extremity (jugular, innominate, subclavian, axillary, brachial) DVT, PE, splanchnic (hepatic, portal, splenic, mesenteric, renal, gonadal), or cerebral vein thrombosis confirmed by appropriate cross-section imaging. | Any thromboembolic recurrence including DVT, PE, fatal PE, or arterial thromboembolism. A recurrent event was a new filling defect evident on the second study not appreciated on the original images, or when an interval study clearly showed thrombus resolution. An arterial thromboembolism could include myocardial infarction, stroke, transient ischemic attack, or peripheral arterial embolism. |
DVT = deep vein thrombosis; PE = pulmonary embolism; VTE = venous thromboembolism; other abbreviations as in Table 1.
Table 3.
Definitions Used for Bleeding Events
| Study First Author (Ref. #); Year |
Major Bleeding | CRNMB |
|---|---|---|
| CARAVAGGIO Agnelli et al. (12); 2020 |
Acute clinically overt bleeding associated with ≥1: 1) decrease in the hemoglobin level of at least 2 g/dl; 2) transfusion of ≥2 U of red cells; 3) bleeding occurring at a critical site (intracranial, intraspinal, intraocular, pericardial, intra-articular, intramuscular with compartment syndrome, or retroperitoneal); 4) bleeding resulting in surgical intervention, or fatal bleeding, all occurring during the trial drug period through 72 h after the last dose was administered |
Acute clinically overt bleeding that does not meet the criteria for major bleeding and consists of: 1) any bleeding compromising hemodynamics; 2) spontaneous hematoma >25 cm2, or 100 cm2 if there was a traumatic cause; 3) intramuscular hematoma documented by ultrasonography; 4) epistaxis or gingival bleeding requiring tamponade or other medical intervention or bleeding from venipuncture for >5 min; 5) hematuria that was macroscopic and was spontaneous or lasted for >24 h after invasive procedures; 6) hemoptysis, hematemesis, or spontaneous rectal bleeding requiring endoscopy or other medical intervention; 7) or any other bleeding considered to have clinical consequences for a patient, such as medical intervention, the need for unscheduled contact (visit or telephone call) with a physician, or temporary cessation of a study drug, or associated with pain or impairment of activities of daily life |
| SELECT-D Young et al. (10); 2018 |
Acute, clinically overt bleeding accompanied by ≥1 of the following findings: a decrease in the hemoglobin level of ≥2 g/dl over a 24-h period, transfusion of ≥2 U of packed red cells, bleeding at a critical site (including intracranial, intraspinal, intraocular, pericardial, or retroperitoneal bleeding), or fatal bleeding | Acute, clinically overt episodes, such as wound hematoma, bruising, GI bleeding, hemoptysis, hematuria, or epistaxis that did not meet the criteria for major bleeding but were associated with medical intervention, unscheduled contact with a physician, interruption or discontinuation of a study drug, or discomfort or impairment of activities of daily life |
| Hokusai VTE Cancer Raskob et al. (9); 2018 |
Overt bleeding that was associated with a decrease in the hemoglobin level of ≥2 g/dl, led to a transfusion of ≥2 U of blood, occurred in a critical site, or contributed to death | Overt bleeding that did not meet the criteria for major bleeding but was associated with the use of medical intervention, contact with a physician, interruption of the assigned treatment, discomfort, or impairment of activities of daily living. |
| ADAM-VTE McBane et al. (11); 2020 |
Overt bleeding plus a hemoglobin decrease of ≥2 g/dl or transfusion of ≥2 U of packed red blood cells, or intracranial, intraspinal/epidural, intraocular, retroperitoneal, pericardial, intra-articular, or intramuscular with compartment syndrome, or fatal bleeding | Overt bleeding not meeting the criteria for major bleeding but associated with medical intervention, an unscheduled contact with the health care team, or temporary anticoagulant cessation |
GI = gastrointestinal; other abbreviations as in Table 1.
Table 4.
Cancer Type Distribution Across Studies
| Cancer Type | CARAVAGGIO∗ Agnelli et al. (12); 2020 |
SELECT-D† Young et al. (10); 2018 |
Hokusai VTE Cancer‡ Raskob et al. (9); 2018 |
ADAM-VTE McBane et al. (11); 2019 |
||||
|---|---|---|---|---|---|---|---|---|
| Apixaban (n = 576) | Dalteparin (n = 579) | Rivaroxaban (n = 203) | Dalteparin (n = 203) | Edoxaban (n = 522) | Dalteparin (n = 524) | Apixaban (n = 150) | Dalteparin (n = 150) | |
| Colorectal | 121 (21.0) | 113 (19.5) | 55 (27.0) | 47 (23.0) | 83 (15.9) | 79 (15.1) | 18 (12.2) | 29 (19.6) |
| Upper GI | 23 (4.0) | 31 (5.4) | 15 (7.0) | 26 (12.0) | 33 (6.3) | 21 (4.0) | 7 (4.8) | 4 (2.7) |
| Lung | 105 (18.2) | 95 (16.4) | 22 (11.0) | 25 (12.0) | 77 (14.8) | 75 (14.3) | 32 (21.8) | 19 (12.8) |
| Breast | 79 (13.7) | 76 (13.1) | 20 (10.0) | 20 (10.0) | 64 (12.3) | 60 (11.5) | 16 (10.9) | 12 (8.1) |
| Genitourinary | 66 (11.5) | 73 (12.6) | 25 (13.0) | 17 (11.0) | 65 (12.5) | 71 (13.5) | 13 (8.7) | 14 (9.3) |
| Gynecological | 60 (10.4) | 59 (10.2) | 18 (9.0) | 25 (12.0) | 47 (9.0) | 63 (12.0) | 14 (9.5) | 15 (10.1) |
| Pancreatic or hepatobiliary | 44 (7.6) | 43 (7.4) | 12 (10.0) | 13 (6.0) | 49 (9.4) | 40 (7.6) | 23 (15.6) | 24 (16.2) |
| Head and neck§ | 14 (2.4) | 8 (1.4) | 0 (0.0) | 0 (0.0) | — | — | 0 (0.0) | 0 (0.0) |
| Brain tumor | 0 (0) | 0 (0) | 1 (1.0) | 2 (1.0) | || | || | 3 (2.0) | 5 (3.4) |
| Bone/soft tissue | 11 (1.9) | 7 (1.2) | 2 (1.0) | 0 (0.0) | — | — | 3 (2.0) | 1 (0.7) |
| Skin: melanoma | 4 (0.7) | 7 (1.2) | — | — | — | — | 0 (0.0) | 4 (2.7) |
| Hematological malignancy | 33 (5.7) | 52 (9.0) | 14 (7.0) | 17 (9.0) | 56 (10.7) | 55 (10.5) | 13 (8.9) | 14 (9.5) |
| Other | 16 (2.8) | 15 (2.6) | 10 (5.0) | 11 (7.0) | 48 (9.2) | 60 (11.5) | 0 (0.0) | 2 (1.4) |
Values are n (%).
Basal cell or squamous cell carcinoma of the skin, primary brain tumor or known intracerebral metastases, and acute leukemia were not included in the CARAVAGGIO trial.
Basal cell or squamous cell carcinoma of the skin were not included in the SELECT-D trial.
Basal cell or squamous cell carcinoma of the skin were not included in the Hokusai VTE Cancer trial.
Other than brain tumors.
Data not available, because brain tumors were included under “other tumors”.
Risk of bias assessments is reported in Supplemental Figure 1. Overall, the risk for selection bias, detection bias, attrition bias, and reporting bias was judged as low. All trials used appropriate randomization and allocation concealment. All studies were open label; hence, the risk for performance bias could not be completely excluded. However, endpoint adjudication committees were blinded to the treatment strategy in all trials, with the exception of the ADAM VTE study.
Measures of efficacy
Recurrence of VTE
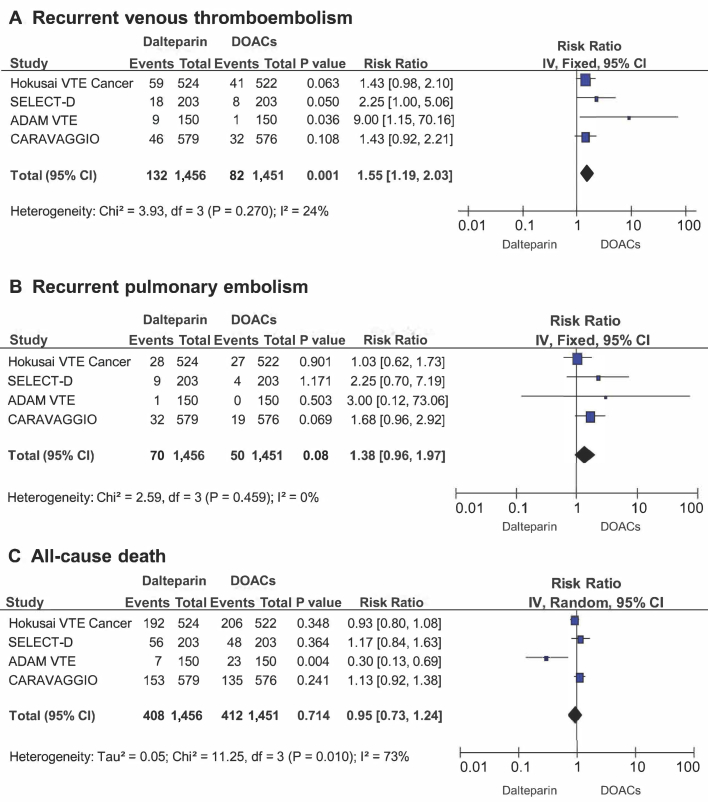
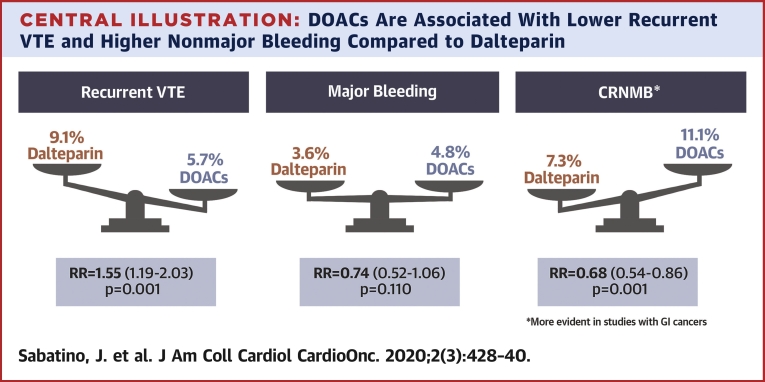
Among 2,907 patients included in the analysis, 132 (9.1%) experienced VTE recurrence in the dalteparin treatment group, and 82 patients (5.7%) had VTE recurrence in the DOAC treatment groups, which resulted in a significantly higher incidence with dalteparin compared with DOACs (RR: 1.55; 95% CI: 1.19 to 2.03; p = 0.001) (Figure 2A). Heterogeneity was low with respect to this outcome (Q = 3.93; p = 0.270; I2 = 24%). No difference was found between the subgroup of studies using a once or twice daily DOAC administration regimen. No evidence for publication bias was present at funnel plot inspection (Figure 3A).
Figure 2.
Measures of Efficacy
Forest plots illustrating results of meta-analysis on the rate of recurrent venous (A) thromboembolism, (B) pulmonary embolism, and (C) all-cause death. CI = confidence interval; DOAC = direct oral anticoagulants; I2 = inconsistency index.
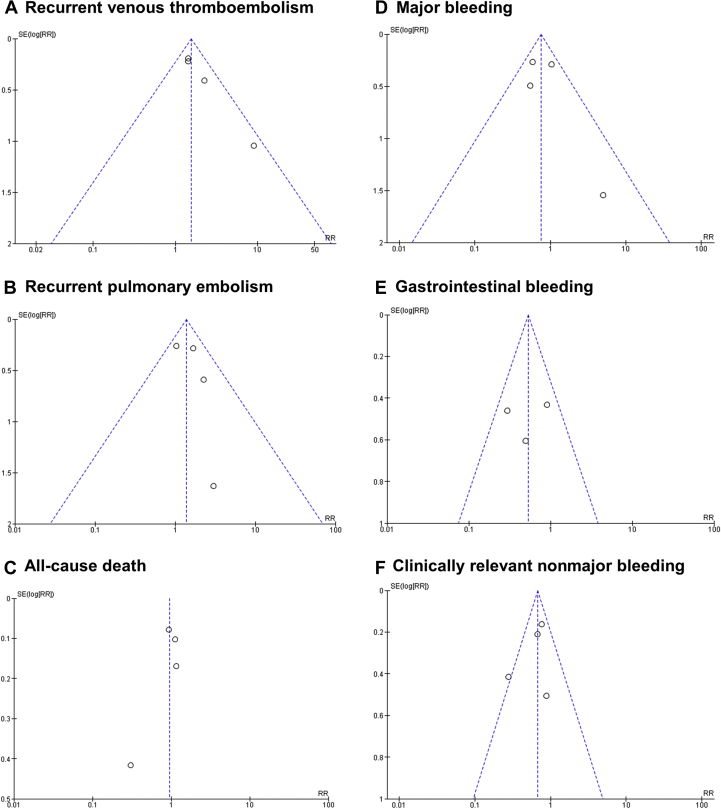
Figure 3.
Funnel Plots
Funnel plots for the assessment of publication bias are reported for all outcomes: (A) recurrent venous thromboembolism, (B) recurrent pulmonary embolism, (C) all-cause death, (D) major bleeding, (E) gastrointestinal bleeding, and (F) clinically relevant nonmajor bleeding. log[RR] = logarithm of risk ratio
Recurrence of PE
Among 2,907 patients included in the analysis, 70 (4.8%) developed PE recurrence in the dalteparin treatment group, and 50 (3.4%) experienced PE recurrence in the DOAC treatment groups, with no statistically significant difference between the treatment arms (RR: 1.38; 95% CI: 0.96 to 1.97; p = 0.080) (Figure 2B). No relevant heterogeneity was evident for this outcome (Q = 2.59; p = 0.459; I2 = 0%). No evidence for publication bias was present at funnel plot inspection (Figure 3B).
Mortality
All-cause mortality was determined in 408 patients (28.0%) who developed VTE recurrence in the dalteparin treatment groups and in 412 patients (28.4%) in the DOAC treatment groups, with no significant difference between the 2 treatment arms (RR: 0.95; 95% CI: 0.73 to 1.24; p = 0.714) (Figure 2C). Heterogeneity was determined to be high for this outcome (Q = 11.25; p = 0.010; I2 = 73%). No evidence for publication bias was found on funnel plot inspection (Figure 3C).
Measures of safety
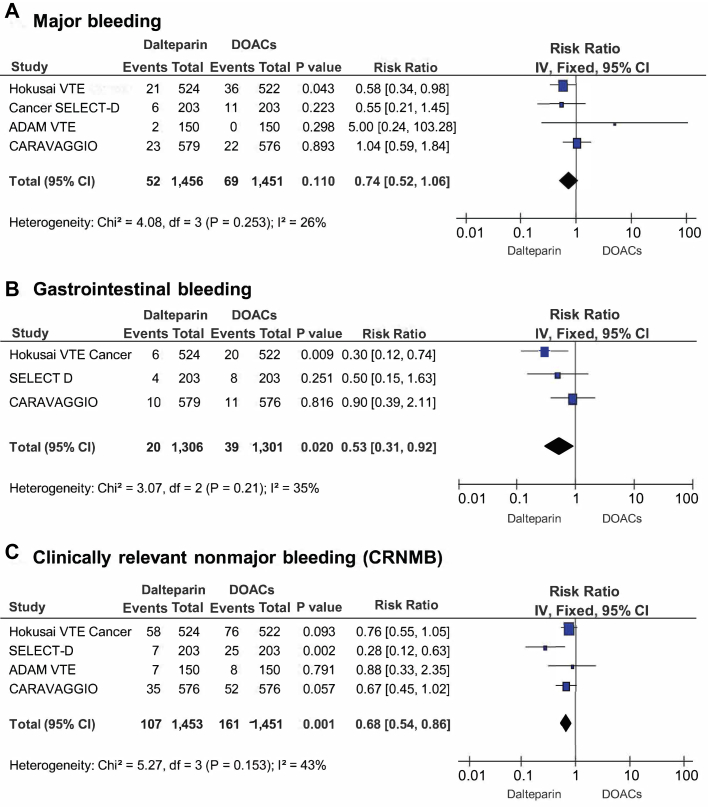
Major bleeding
Major bleeding occurred in 52 patients (3.6%) in the dalteparin treatment groups and in 69 patients (4.8%) in the DOAC treatment groups (RR: 0.74; 95% CI: 0.52 to 1.06; p = 0.110) (Figure 4A). Heterogeneity was low with respect to this outcome (Q = 4.08; p = 0.253; I2 = 26%). A significantly higher rate of major bleeding was evident with DOACs, until publication of the recent CARAVAGGIO trial findings, which changed the results of this analysis. No evidence for publication bias was determined at funnel plot inspection (Figure 3D).
Figure 4.
Measures of Safety
Forest plots illustrating results of meta-analysis on the rate of (A) major bleeding, (B) GI bleeding, and (C) clinically relevant nonmajor bleeding (CRNMB). Abbreviations as in Figures 2 and 3.
Gastrointestinal bleeding
Data on gastrointestinal (GI) bleeding were available from 3 studies (2,9,10), involving 2,607 patients. GI bleeding was reported in 20 patients (1.5%) in the dalteparin treatment groups and in 39 patients (3.0%) in the DOAC treatment groups (RR: 0.53; 95% CI: 0.31 to 0.92; p = 0.020) (Figure 4B). No evidence for publication bias was found at funnel plot inspection (Figure 3E).
CRNMB
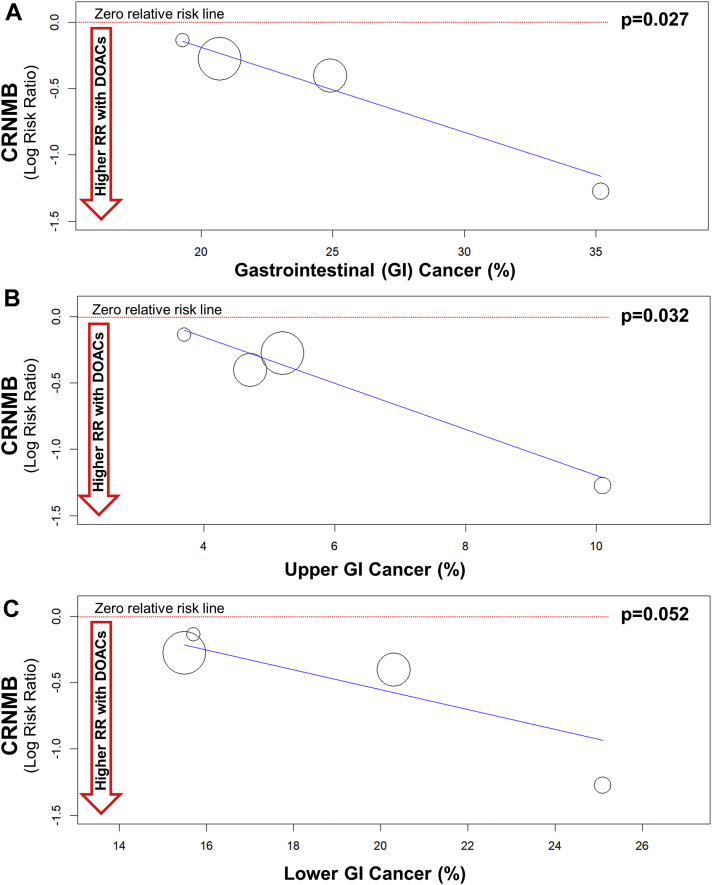
CRNMB was reported in 107 patients (7.3%) in the dalteparin treatment group and in 161 patients (11.1%) in the DOAC treatment groups (RR: 0.68; 95% CI: 0.54 to 0.86; p = 0.001) (Figure 4C). No evidence for publication bias was determined at funnel plot inspection (Figure 3F). Some heterogeneity was evident with respect to this outcome (Q = 5.27; p = 0.153; I2 = 43%). We performed a sensitivity analysis to assess the consistency of our findings. Sensitivity analysis found the most heterogeneity related to the SELECT-D study. Accordingly, removal of this trial resulted in a substantial reduction of heterogeneity (Q = 0.36; p = 0.836; I2 = 0%), with no impact on the results of the meta-analysis (RR: 0.73; 95% CI: 0.58 to 0.94; p = 0.013). Sensitivity analysis also showed that meta-analysis results were not primarily determined by the 2 largest trials, because removal of either the Hokusai VTE Cancer study (RR: 0.59; 95% CI: 0.42 to 0.84; p = 0.003) or the CARAVAGGIO trial (RR: 0.68; 95% CI: 0.51 to 0.91; p = 0.008) had a modest numerical impact on the RR, but did not significantly change the interpretation of our findings. Meta-regression analysis revealed that DOACs were associated with higher rates of CRNMB events that were primarily observed in patients with GI cancers (p = 0.027) (Figure 5A). Regarding the specific localization of GI cancer, meta-regression confirmed a significant increase of CRNMB events with upper GI cancers (p = 0.032) (Figure 5B) and a borderline increase of such events with lower GI cancers (p = 0.052) (Figure 5C).
Figure 5.
Meta-Regression Analysis
Meta-regression analysis regarding the interaction of CRNMB events with the proportion of (A) GI cancer (p = 0.027). Meta-regression confirmed a significant interaction with upper GI (B) cancer (p = 0.032) but not with (C) lower GI cancer (p = 0.052). Abbreviations as in Figures 3 and 4.
Discussion
Over the past few years, the use of DOACs has revolutionized anticoagulation treatment. Clinical trials and secondary real-world data have served to endorse DOACs as preferred therapy over VKAs for the treatment of VTEs in patients without cancer (5, 6, 7, 8).
Although patients with active cancer were excluded from most critical DOAC efficacy trials, and the CLOT trial resulted in guidelines that recommended LMWH as first-line therapy in patients with active cancer and VTE recurrence, clinical observations from real-life experience suggested that the risks of long-term treatment with LMWH might outweigh the benefits. This resulted in recent trials comparing DOACs with LMWH in preventing VTEs in patients with cancer.
Recent studies suggested that DOACs might result in an analogous or lower incidence of recurrent VTEs compared with that of dalteparin, but were also associated with an increased risk of bleeding (9, 10, 11, 12). Because the number of events observed in each study was limited, these results appeared to be conflicting and continue to be debated. A previous meta-analysis (13), that included results from the Hokusai VTE Cancer, the SELECT-D, and the ADAM VTE trials demonstrated a nonstatistically significant relative risk reduction of VTE recurrence with DOACs in patients with cancer versus patients treated with LMWH. However, DOACs were also found to be associated with an increased risk of major bleeding and CRNMB. As a consequence, most recent guidelines continue to recommend dalteparin as an initial standard treatment for the prevention of VTEs in patients with active cancer, with DOAC treatment recommended as an option only (19,20).
In our meta-analysis, DOACs were shown to be noninferior to dalteparin in preventing VTE recurrence in patients with cancer (Central Illustration). The RR of recurrent VTE was 1.55-fold greater with dalteparin compared with that of DOACs. Furthermore, although DOACs were associated with an increased risk of CRNMB compared with that of dalteparin, the risk of major bleeding was similar across the 2 treatment groups and not significantly different (p = 0.110). The additional data reported by the recently published CARAVAGGIO trial, which is the largest trial to date, provided significant evidence regarding the safety and efficacy of DOAC treatment in decreasing the risk of VTEs in patients with cancer. This evidence was welcome information following the reported results of the 3 previous smaller clinical trials, which had notable differences in trial design and enrollment criteria.
Central Illustration.
DOACs Are Associated With Lower Recurrent VTE and Higher Nonmajor Bleeding Compared to Dalteparin
Direct oral anticoagulants (DOACs) are noninferior to dalteparin to prevent venous thromboembolism (VTE) recurrence in cancer patients, with similar rates of major bleeding but higher clinically relevant nonmajor bleeding (CRNMB) events, particularly in studies in which a larger proportion of patients with gastrointestinal (GI) cancer was enrolled.
The data from the CARAVAGGIO trial concerning the risk of major bleeding were particularly noteworthy. It appears that this finding was not related to any differences in the definition of major bleeding, because this was relatively consistent across trials (Table 3). In contrast, the differences in reported major bleeding risk might have resulted from the heterogeneity in enrolled populations. Patients with primary brain tumors, brain metastases, and acute leukemia were excluded from the CARAVAGGIO trial. This might have affected the occurrence of major bleeding in patients in the treatment groups, although the proportion of patients with brain tumors or acute leukemia was also low in the remaining studies (Table 4).
Meta-regression analysis showed that the increased risk of CRNMB events observed with DOAC treatment in patients with cancer was observed with a higher proportion of GI bleeding in single studies, and the risk of CRNMB was greater in those studies with a larger proportion of patients with GI cancers (Figure 4). Although these results were not consistently statistically significant, they concurred with the 2-fold increase in GI bleeding with DOACs compared with that of dalteparin and with previous evidence. These data suggested that patients with GI cancers represented a slightly different clinical category, in which oral anticoagulation might result in a higher bleeding risk. Again, it should be noted that the CARAVAGGIO trial included fewer patients with upper GI cancer than the other studies. The observation that the risk of CRNMB events was higher with upper GI cancers compared with lower GI cancers suggested that the known effect of DOACs on upper GI bleeding might have played a role (21). It is reassuring that this phenomenon was apparently limited to CRNMB and was not associated with major bleeding. However, particular caution should be used in managing anticoagulation, because absorption of oral anticoagulants might be affected in patients with GI cancer or there might be toxicity. Caution should also be used in those who have undergone surgery of the upper GI tract (22). In addition, it is crucial to note that uncertainty remains regarding drug−drug interaction with oral anticoagulants and cancer therapies. Patients treated with powerful inducers and/or inhibitors of CYP3A4 or P-glycoprotein were primarily excluded from trial participation, and few patients treated with checkpoint inhibitors or other newly approved therapies were included in these trials.
It was reassuring that the data reported from the CARAVAGGIO trial served to confirm that DOACs were noninferior to dalteparin in preventing VTE recurrence. Despite the many differences among the 4 trials, their results concerning the prevention of recurrent VTEs in patients with cancer were consistent, with the estimated risk for recurrent VTE being increased with dalteparin. Furthermore, although the CARAVAGGIO trial excluded patients with brain tumors and included few patients with upper GI cancer, the ADAM VTE trial adopted a broader definition of the qualifying VTE event, encompassing upper extremity thrombosis. This difference, along with the smaller sample size and the slightly different distribution of cancer types compared with the other trials, might explain the lower mortality rate reported in the ADAM VTE trial.
Study limitations
As with all meta-analyses, the present analysis had limitations. Although all included studies were high quality, there was some heterogeneity among the trials. Because we were unable to define the extent to which this heterogeneity might be related to differences in trial designs and the enrolled populations, we could not completely exclude bias. Nevertheless, the meta-analysis results were consistent using both fixed-effect and random-effects calculation models, as well as across study subgroups at sensitivity analysis. The included studies encompassed all DOACs available on the market, with the exception of dabigatran, because no study evaluated the use of dabigatran against LMWHs in this clinical context. Therefore, we do not know whether these results would also apply to dabigatran. Finally, our analysis was limited to the use of dalteparin as the comparator drug, because none of the other LMWHs was selected as the control treatment arm in any of the included studies. This might be because dalteparin was tested in the CLOT study (3).
Conclusions
We demonstrated that DOACs are noninferior to LMWH in preventing VTE recurrence in patients with active cancer, although such treatment leads to an increased risk of CRNMB, which was primarily observed in patients with GI malignancy. Results of this meta-analysis of 4 randomized trials provided compelling evidence for prescribing DOACs with more confidence and perhaps to an increased number of patients with cancer who are at risk for recurrent VTEs. Initial caution was exercised with DOAC use in this setting, which was primarily related to uncertainty regarding the clinical relevance of drug interactions with anticancer treatments and concerns regarding bleeding. Nevertheless, the new evidence of noninferiority in preventing recurrent VTEs and the findings that increased bleeding risk does not involve major bleeding and is primarily related to the enrollment of patients with GI cancer should affect current clinical practice in this setting.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: DOACs are noninferior to dalteparin in preventing VTE recurrence in patients with active cancer. There is no significantly increased risk of major bleeding, but there is an elevated risk of CRNMB events, primarily related to GI cancers.
TRANSLATIONAL OUTLOOK: The new evidence of noninferiority of DOACs in protection from recurrent VTEs, along with the lack of increased major bleeding, should affect current clinical management for patients with active cancer and VTEs. Careful selection of anticoagulants and continued development of evidence-based management strategies to include DOACs are needed to help ensure the continued careful care of the growing cancer population.
Footnotes
Drs. De Rosa and Indolfi have participated in clinical studies testing all 4 drugs DOACs object of the present work; and have participated in expert consensus meetings/panels for Bayer, Pfizer–Bristol-Myers Squibb, Boehringer Ingelheim, and Daiichi-Sankyo. Dr. Indolfi has received research grants from Boehringer Ingelheim and Daiichi-Sankyo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For a supplemental figure, please see the online version of this paper.
Contributor Information
Salvatore De Rosa, Email: saderosa@unicz.it.
Ciro Indolfi, Email: indolfi@unicz.it.
Appendix
References
- 1.Prandoni P., Lensing A.W., Piccioli A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P., Trujillo-Santos J., Surico T., RIETE Investigators Recurrent thromboembolism and major bleeding during oral anticoagulant therapy in patients with solid cancer: findings from the RIETE registry. Haematologica. 2008;93:1432–1434. doi: 10.3324/haematol.13055. [DOI] [PubMed] [Google Scholar]
- 3.Lee A.Y., Levine M.N., Baker R.I. Randomized comparison of low-molecular-weight heparin versus oral anticoagulant therapy for the prevention of recurrent venous thromboembolism in patients with cancer (CLOT) investigators. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 4.Farge D., Bounameaux H., Brenner B. International clinical practice guidelines including guidance for direct oral anticoagulants in the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2016;17:e452–e466. doi: 10.1016/S1470-2045(16)30369-2. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G., Buller H.R., Cohen A., AMPLIFY Investigators Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 6.Hokusai-VTE Investigators Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. erratum: N Engl J Med 2014;370:390. [DOI] [PubMed] [Google Scholar]
- 7.Prins M.H., Lensing A.W., Bauersachs R., EINSTEIN Investigators Oral rivaroxaban versus standard therapy for the treatment of symptomatic venous thromboembolism: a pooled analysis of the EINSTEIN-DVT and PE randomized studies. Thromb J. 2013;11:21. doi: 10.1186/1477-9560-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulman S., Kakkar A.K., Goldhaber S.Z., RE-COVER II trial investigators Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation. 2014;129:764–772. doi: 10.1161/CIRCULATIONAHA.113.004450. [DOI] [PubMed] [Google Scholar]
- 9.Raskob G.E., van Es N., Verhamme P., Hokusai VTE Cancer Investigators Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 10.Young A.M., Marshall A., Thirlwall J. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 11.McBane R., II, Wysokinski W.E., Le-Rademacher J.G. Apixaban and dalteparin in active malignancy associated venous thromboembolism: the ADAM VTE Trial. J Thromb Haemost. 2020;18:411–442. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 12.Agnelli G., Becattini C., Meyer G., for the Caravaggio Investigators Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 13.Mai V., Tanguay V.F., Guay C.A. DOAC compared to LMWH in the treatment of cancer related-venous thromboembolism: a systematic review and meta-analysis. J Thromb Thrombolysis. 2020 Feb 12 doi: 10.1007/s11239-020-02055-1. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Eriksson B.I., Dahl O.E., Rosencher N. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomized, double-blind, non-inferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 15.Sorrentino S., Nguyen P., Salerno N. Standard versus ultrasound-guided cannulation of the femoral artery in patients undergoing invasive procedures: a meta-analysis of randomized controlled trials. J Clin Med. 2020;9:677. doi: 10.3390/jcm9030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Rosa S., Polimeni A., Sabatino J., Indolfi C. Long-term outcomes of coronary artery bypass grafting versus stent-PCI for unprotected left main disease: a meta-analysis. BMC Cardiovasc Disord. 2017;17:240. doi: 10.1186/s12872-017-0664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Rosa S., Sievert H., Sabatino J., Polimeni A., Sorrentino S., Indolfi C. Percutaneous closure versus medical treatment in stroke patients with patent foramen ovale: a systematic review and meta-analysis. Ann Intern Med. 2018;168:343–350. doi: 10.7326/M17-3033. [DOI] [PubMed] [Google Scholar]
- 18.Santarpia G., De Rosa S., Sabatino J., Curcio A., Indolfi C. Should we maintain anticoagulation after successful radiofrequency catheter ablation of atrial fibrillation? The need for a randomized study. Front Cardiovasc Med. 2017;4:85. doi: 10.3389/fcvm.2017.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Key N.S., Khorana A.A., Kuderer N.M. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2019;38:496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 20.Konstantinides S.V., Meyer G. The 2019 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2019;40:3453–3455. doi: 10.1093/eurheartj/ehz726. [DOI] [PubMed] [Google Scholar]
- 21.Brodie M.M., Newman J.C., Smith T., Rockey D.C. Severity of gastrointestinal bleeding in patients treated with direct-acting oral anticoagulants. Am J Med. 2018;131 doi: 10.1016/j.amjmed.2017.11.007. 573.e9−15. [DOI] [PubMed] [Google Scholar]
- 22.Suryanarayan D., Lee A.Y.Y., Wu C. Direct oral anticoagulants in cancer patients. Semin Thromb Hemost. 2019;45:638–647. doi: 10.1055/s-0039-1693479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.