Radiation therapy (RT) to treat thoracic malignancies is associated with short- and long-term cardiovascular (CV) complications, including pericarditis and pericardial effusion, coronary artery disease (CAD), valvular heart disease (VHD), cardiomyopathy, myocardial fibrosis, conduction abnormalities, and dysautonomia. These complications increase morbidity and mortality in survivors of thoracic malignancies, particularly those with Hodgkin’s lymphoma, breast cancer, and lung cancer. The risk of radiation cardiotoxicity increases with chest radiation dose of ≥15 Gy (highest risk ≥35 Gy), mean heart dose, younger age at diagnosis, use of concomitant cardiotoxic chemotherapy, presence of CV disease or associated risk factors, and time from RT (1,2).
In this primer, we use a case to illustrate our approach to the diagnosis and management of RT-associated cardiotoxicity.
Clinical Case
A 45-year-old female Hodgkin’s lymphoma survivor presented to the survivorship clinic. She reported mild dyspnea with inclines and no previous CV disease. She was treated for Hodgkin’s lymphoma at age 19 with splenectomy, followed by doxorubicin, bleomycin, vinblastine and dacarbazine ×6 cycles (cumulative anthracycline dose 300 mg/m2) and mantle radiation (40 Gy). Her examination revealed a heart rate (HR) of 84 beats/min, blood pressure (BP) of 122/84 mm Hg, and was otherwise unremarkable. An electrocardiogram showed sinus rhythm at 87 beats/min and left ventricular (LV) hypertrophy with repolarization abnormalities. Laboratory evaluation revealed glucose of 96 mg/dl, cholesterol of 263 mg/dl, triglycerides of 152 mg/dl, and high-density lipoprotein of 60 mg/dl, respectively.
How do we manage CV risk in survivors treated with thoracic RT?
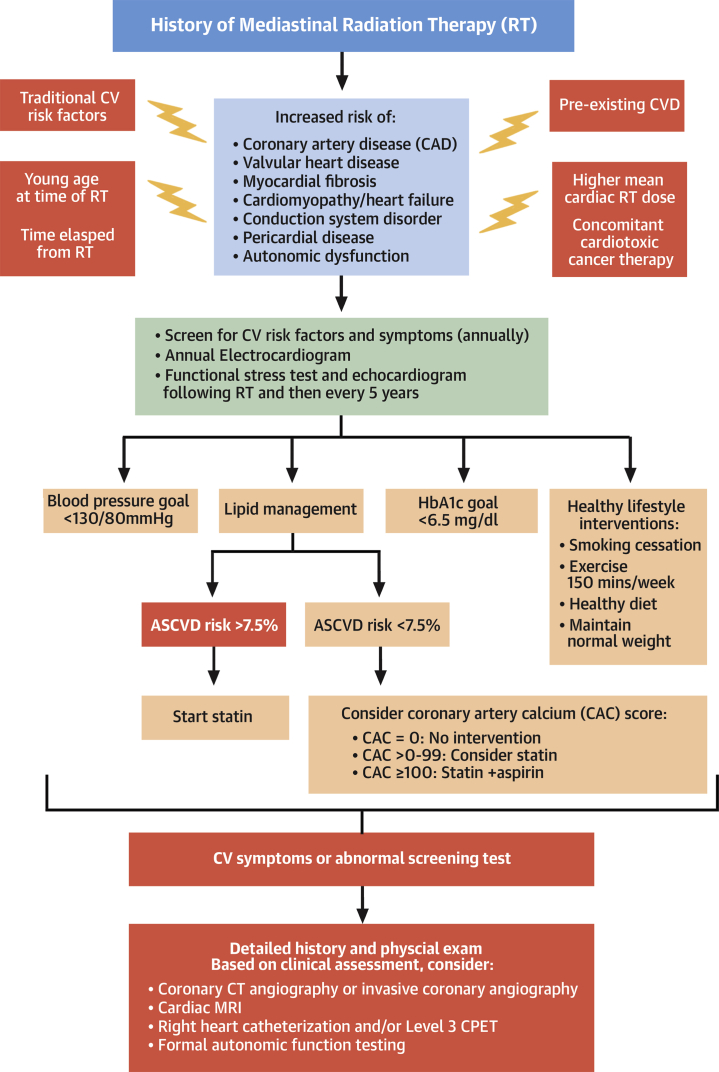
This patient had several risk factors for RT cardiotoxicity, including an RT dose >35 Gy, anthracycline exposure, was 26 years from RT, and had hyperlipidemia. The Childhood Cancer Survivor Study CV risk calculator classified her as high risk, with a 10% probability of developing heart failure or CAD by age 50 years (3). Consensus statements recommend aggressive CV risk factor modification to prevent CV events (1). However, her atherosclerotic cardiovascular disease risk score of 1% categorized her as very low risk, with the recommendation to not initiate statins (4). Current CV disease prevention guidelines do not include RT as a risk modifier. Thus, in patients age 30 years or older with high-risk features and an atherosclerotic cardiovascular disease score <7.5%, we extrapolate from the literature on CV risk prediction and often consider coronary artery calcium (CAC) scoring to refine CV risk assessment and to guide primary prevention statin and aspirin therapy (5). If CAC = 0, we recommend no statin or aspirin therapy; if CAC = 0 to 99, statins may be considered; and if CAC ≥100, we recommend statin and aspirin therapy (Figure 1). Many patients undergo routine noncardiac computed tomography for cancer staging or surveillance. The presence of vascular calcification on these scans can also be used to identify at-risk patients who may benefit from aggressive risk factor modification. In addition, BP should be controlled to a goal of <130/80 mm Hg, and survivors should be screened and treated for diabetes according to established guidelines (4). All patients should be advised to eat a heart healthy diet and to participate in 150 min/week of moderate to vigorous exercise (4) (Figure 1).
Figure 1.
How to Screen for, Diagnose, and Manage Radiation Cardiotoxicity
Risk factors for radiation-associated heart disease. An algorithm is provided for screening, risk modification, and diagnostic testing in patients who received thoracic radiation. ASCVD = atherosclerotic cardiovascular disease; CPET = cardiopulmonary exercise testing; CT = computed tomography; CV = cardiovascular; CVD = cardiovascular disease; HbA1C = glycosylated hemoglobin; MRI = magnetic resonance imaging.
Key points
-
•
Traditional CV risk factors increase the risk of radiation cardiotoxicity.
-
•
Monitoring and guideline-based modification of CV risk factors is recommended to reduce radiation cardiotoxicity.
How do we diagnose radiation cardiotoxicity?
Because of the increased risk of CV complications, consensus statements recommend screening for CV disease, even in asymptomatic survivors. A functional noninvasive stress test and echocardiogram are recommended 5 to 10 years following RT and every 5 years thereafter, unless concerning signs or symptoms occur earlier (1,2) (Figure 1). Stress echocardiography is the preferred modality at our institution to evaluate ischemia. Compared with echocardiography, radionuclide imaging, using either single-photon emission computerized tomography or positron emission tomography, has similar sensitivity and specificity for detecting CAD but carries significant radiation exposure.
Case continued
A screening echocardiogram revealed a LV ejection fraction (LVEF) of 50% (LV end-diastolic dimension 4 cm) with indeterminate diastolic function (septal e′ of 4 cm/s, E/e′ of 34.0, peak tricuspid regurgitant velocity of 2.35 m/s). The aortic and mitral leaflets were thickened with trace aortic insufficiency and mild to moderate mitral regurgitation. Exercise stress echocardiography was performed. She exercised to a workload of 7 metabolic equivalents with an appropriate HR (91 to 141 beats/min) but blunted BP (118/60 to 116/58 mm Hg) response. HR recovery was 10 beats/min (normal <12 beats/min). Oxygenation remained 100% throughout exercise. She stopped secondary to dyspnea and lightheadedness. There were no electrocardiographic changes or regional wall motion abnormalities suggestive of ischemia.
The patient’s borderline LVEF was likely related to her anthracycline and radiation exposure. Although anthracyclines usually cause dilated cardiomyopathy with reduced EF, RT can lead to heart failure, secondary to myocardial fibrosis with restrictive cardiomyopathy and preserved EF (1). These patients tend to have normal wall thickness, reduced LV dimensions, and abnormal diastolic parameters on echocardiography. Pericardial thickening and constriction might also be present. Detection of a characteristic septal bounce with respiration and significant respiratory variation in pulse-wave Doppler of mitral and tricuspid inflows suggests constriction (1). Cardiac magnetic resonance imaging, although rarely used as first-line modality, can complement echocardiography. In addition to measuring LVEF, post-contrast, T1-weighted imaging (late gadolinium enhancement) on cardiac magnetic resonance imaging can detect myocardial and pericardial fibrosis (1). Cardiac computed tomography can be particularly helpful to detect pericardial thickening and calcification (1). Invasive hemodynamic assessment using simultaneous right and left heart catheterization can also be used to evaluate restriction and/or constriction. However, distinguishing their relative hemodynamic contributions can sometimes be difficult in survivors with both complications.
As with this patient, VHD is a frequent complication seen 10 to 20 years after RT (1). RT can cause both regurgitant and stenotic lesions, with the mitral and aortic valves involved more frequently than the tricuspid valve (1). Echocardiography is the mainstay for evaluation of VHD and reveals fibrotic leaflet thickening, leaflet retraction, and late calcification. Serial transthoracic echocardiography is used to assess progression of VHD, and transesophageal echocardiography may be used for better characterization of valvular anatomy before intervention.
This patient demonstrated good functional capacity that correlated with her minimal symptoms. However, her inability to augment systolic BP with exercise raised concerns for severe left main or triple-vessel CAD, cardiomyopathy with diminished contractile reserve, severe VHD not appreciated on transthoracic echocardiography, and/or dysautonomia. Because of her high-risk status, we recommended invasive coronary angiography or coronary computed tomography angiography to rule out significant CAD. Coronary computed tomography angiography has a high negative predictive value for CAD and significantly decreased radiation exposure compared with radionuclide imaging (1). Thus, it can be considered in patients with inability to exercise, equivocal stress testing, or symptoms despite normal stress testing (possibly due to balanced ischemia).
In the absence of significant CAD, we would consider right heart catheterization with hemodynamic assessment at rest and with exercise to assess filling pressures and contractile reserve. Because thoracic RT also affects the lungs and chest wall in patients with exertional dyspnea as the predominant symptom, cardiopulmonary exercise testing, with or without right heart catheterization, can be an alternative diagnostic strategy to differentiate between cardiac and pulmonary limitations to exercise.
Autonomic dysfunction, which manifests as an elevated resting HR, abnormal HR recovery after exercise, abnormal BP response to exercise, or postural hypotension, can be evaluated by formal autonomic function testing that includes HR variability (parasympathetic), BP response to physiological stimuli (e.g., head-up tilt test and Valsalva maneuver [sympathetic]), and assessment of distal nerve (sudomotor) function (6).
Key points
-
•
Surveillance echocardiography and functional noninvasive stress testing to detect radiation cardiotoxicity should be performed every 5 years, starting 5 to 10 years after RT.
-
•
Exercise, rather than pharmacological stress testing, provides important diagnostic and prognostic information beyond ischemia and should be performed, if possible.
Case continued
Cardiac catheterization revealed a 60% proximal left anterior descending artery stenosis with LV end-diastolic pressure of 15 mmHg. Cardiopulmonary exercise testing confirmed a hypotensive response to exercise (140/80 to 120/80 mm Hg). Her peak oxygen consumption was 18.3 ml/kg/min (80% predicted by Wasserman formula) with a normal peak oxygen pulse, which suggested adequate cardiac function. The ratio of minute ventilation to maximal ventilatory volume was increased (0.84), which suggested a pulmonary limitation to exercise. Her abnormal BP response was presumed secondary to dysautonomia, and we recommended a structured exercise training program and low-dose diuretic therapy for mildly elevated filling pressures.
Over the next 8 years, she was seen annually, with serial echocardiograms performed every 2 to 3 years to assess for progression of VHD. Her dyspnea gradually worsened until she could no longer work. An echocardiogram revealed a LVEF of 45% to 50%, moderate aortic insufficiency, mild mitral stenosis, and severe mitral regurgitation. Right and left heart catheterization revealed progressive elevation of filling pressures (right atrial pressure of 13 mm Hg, pulmonary capillary wedge pressure of 25 mm Hg, and a cardiac index of 3.7 l/min/m2) and stable left anterior descending artery disease.
How do we treat radiation cardiotoxicity?
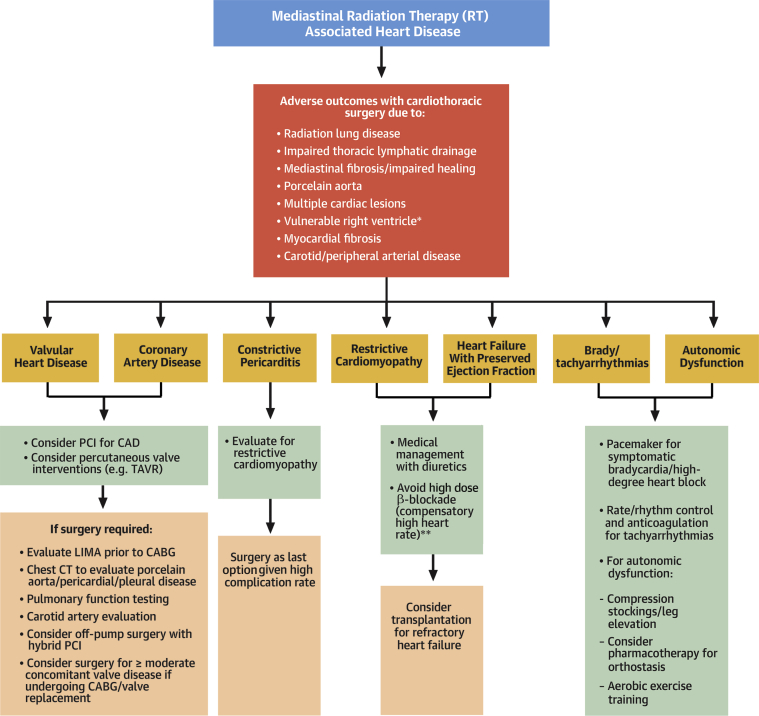
Her workup suggested symptomatic, severe mitral regurgitation. Along with adequate BP control and diuretic therapy, mitral valve replacement should be considered. Cardiac surgery, particularly re-operative surgery, is associated with increased mortality in patients who have had RT compared with patients without RT (7). This is due to a combination of factors, including comorbid pulmonary disease, impaired thoracic lymphatics, porcelain aorta, multiple cardiac lesions, vulnerable right ventricular function, and myocardial fibrosis. Therefore, when feasible, percutaneous options should be considered, especially for isolated CAD or VHD (8) (Figure 2).
Figure 2.
How to Manage Radiation Associated Heart Disease
Management strategies and their pitfalls for various radiation-associated cardiotoxicities. ∗The right ventricle is vulnerable to surgery due to underlying fibrosis, remodeling and dysfunction as a result of radiation therapy. ∗∗For restrictive cardiomyopathy. CABG = coronary artery bypass grafting; CAD = coronary artery disease; LIMA = left internal mammary artery; PCI = percutaneous coronary intervention; TAVR = transcatheter aortic valve replacement; other abbreviations as in Figure 1.
In a study of transcatheter aortic valve replacement (TAVR) compared with surgery for severe aortic stenosis in 110 patients with previous RT, TAVR was associated with reduced hospital stays, post-operative atrial fibrillation, and 30-day mortality (9). Currently, no data are available on percutaneous mitral valve interventions in patients who received thoracic RT. Two studies evaluated outcomes after percutaneous coronary intervention (PCI) for RT-associated CAD. One study showed no difference in myocardial infarction, cardiac, or all-cause mortality (10), whereas the other showed increased cardiac and all-cause mortality (11) in patients who had received RT versus controls subjects without RT. Furthermore, Liang et al. (10) reported similar restenosis rates after PCI in patients who had received RT and control subjects. Despite these conflicting results, we recommend PCI for isolated coronary lesions and for complex lesions in patients with high surgical risk.
In patients who need surgery, pre-operative evaluation should include noncontrast chest computed tomography and pulmonary function testing to assess aortic calcification and comorbid lung and/or pleural disease (8) (Figure 2). Surgery for RT-associated constriction carries a worse prognosis compared with other etiologies; careful evaluation for myocardial fibrosis and/or restriction should be performed before undertaking pericardial stripping (8) (Figure 2). In patients undergoing coronary artery bypass grafting, the left internal mammary artery should be examined and used as a conduit to improve long-term graft patency (8) (Figure 2). Because re-operation carries a high risk of adverse outcomes, concomitant moderate valvular lesions should be corrected at the time of initial surgery (8) (Figure 2). Because of the risks of re-operation, mechanical prosthetic valves may be preferable in younger patients. However, in patients with increased bleeding risk due to other comorbidities, biological prosthetic valves may carry lower morbidity (8).
Key points
-
•
Cardiac surgery, especially re-operative surgery, for radiation cardiotoxicity is associated with adverse outcomes and percutaneous options should be considered, if feasible.
-
•
In patients undergoing surgery, careful pre-operative assessment should be performed, and concomitant lesions of more than moderate severity should be corrected at the time of initial surgery to optimize outcomes.
conclusions
The patient underwent mechanical aortic and mitral valve replacement and single-vessel coronary artery bypass grafting with the left internal mammary artery to the left anterior descending artery. Her post-operative course was complicated by difficult to control atrial fibrillation, pleural effusions that required prolonged chest tube drainage, and persistent combined diastolic and systolic heart failure that required continued diuretic therapy and neurohormonal blockade.
In summary, thoracic RT is associated with significant cardiotoxicity that can manifest many years after treatment. Minimizing radiation dose, optimizing CV risk factors, and early detection of CV complications are important to improving outcomes. Management of radiation cardiotoxicity can be challenging due to the complexity of the disease, comorbid conditions, and technical limitations of surgery. Evolving radiation practices and advances in radiation technology have reduced cardiac radiation exposure over the last 2 decades. These changes in RT, combined with increased availability of percutaneous cardiac therapy options, will hopefully improve CV outcomes for patients with thoracic malignancies.
Author Disclosures
Dr. Nohria has received research support from Amgen, Inc.; and has been a consultant for Takeda Oncology and AstraZeneca. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Lancellotti P., Nkomo V.T., Badano L.P. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26:1013–1032. doi: 10.1016/j.echo.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Armenian S.H., Hudson M.M., Mulder R.L. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.St. Jude Children’s Research Hospital. CCSS Cardiovascular Risk Calculator. Available at: https://ccss.stjude.org/tools-and-documents/calculators-and-other-tools/ccss-cardiovascular-risk-calculator.html. Accessed October 2020.
- 4.Arnett D.K., Blumenthal R.S., Albert M.A. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2019;74:1376–1414. doi: 10.1016/j.jacc.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenland P., Blaha M.J., Budoff M.J., Erbel R., Watson K.E. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72:434–447. doi: 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman R., Chapleau M.W. Testing the autonomic nervous system. Handb Clin Neurol. 2013;115:115–136. doi: 10.1016/B978-0-444-52902-2.00007-2. [DOI] [PubMed] [Google Scholar]
- 7.Wu W., Masri A., Popovic Z.B. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation. 2013;127:1476–1485. doi: 10.1161/CIRCULATIONAHA.113.001435. [DOI] [PubMed] [Google Scholar]
- 8.Desai M.Y., Windecker S., Lancellotti P. Prevention, diagnosis, and management of radiation-associated cardiac disease: JACC Scientific Expert Panel. J Am Coll Cardiol. 2019;74:905–927. doi: 10.1016/j.jacc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D., Guo W., Al-Hijji M.A. Outcomes of patients with severe symptomatic aortic valve stenosis after chest radiation: transcatheter versus surgical aortic valve replacement. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang J.J., Sio T.T., Slusser J.P. Outcomes after percutaneous coronary intervention with stents in patients treated with thoracic external beam radiation for cancer. J Am Coll Cardiol Intv. 2014;7:1412–1420. doi: 10.1016/j.jcin.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 11.Reed G.W., Masri A., Griffin B.P., Kapadia S.R., Ellis S.G., Desai M.Y. Long-term mortality in patients with radiation-associated coronary artery disease treated with percutaneous coronary intervention. Circ Cardiovasc Interv. 2016;9 doi: 10.1161/CIRCINTERVENTIONS.115.003483. [DOI] [PubMed] [Google Scholar]