Abstract
Background
In cancer, platelets may facilitate metastatic spread by a number of mechanisms as well as contribute to thrombotic complications. Ticagrelor, a platelet antagonist- that blocks adenosine diphosphate activation of platelet P2Y12 receptors, is widely used in the treatment of cardiovascular disease, but its efficacy in cancer remains unknown.
Objectives
This study sought to evaluate the effect of aspirin and ticagrelor monotherapy, as well as dual antiplatelet therapy, on platelet activation in cancer.
Methods
This study consisted of 2 phases: first, an in vitro study of human platelet−tumor cell interaction; and second, a randomized crossover clinical trial of 22 healthy donors and 16 patients with metastatic breast or colorectal cancer. Platelet activation and inhibition were measured by aggregometry and flow cytometry.
Results
In vitro, tumor cells induced cellular clusters that were predominantly platelet−platelet aggregates. Ticagrelor significantly inhibited formation of large tumor cell-induced platelet−platelet aggregates: 65.4 ± 4.8% to 50.9 ± 5.9% (p = 0.002) and 62.3 ± 3.1% to 48.3 ± 7.3% (p = 0.014) for MCF-7 and HT-29-induced aggregation, respectively. Supporting this finding, cancer patients on ticagrelor had significantly reduced levels of spontaneous platelet aggregation and activation compared with baseline; 14.8 ± 2.7% at baseline to 7.8 ± 2.3% with ticagrelor (p = 0.012).
Conclusions
Our findings suggested that P2Y12 inhibition with ticagrelor might reduce spontaneous platelet aggregation and activation in patients with metastatic cancer and merits further investigation in patients at high risk of cancer-associated thrombosis. (Ticagrelor-Oncology [TICONC] Study; EudraCT: 2014-004049-29)
Key Words: aspirin, breast cancer, cancer-associated thrombotic risk, colorectal cancer, metastasis, platelets, ticagrelor
Abbreviations and Acronyms: ADP, adenosine diphosphate; DAPT, dual antiplatelet therapy; TCIPA, tumor cell-induced platelet activation
Central Illustration
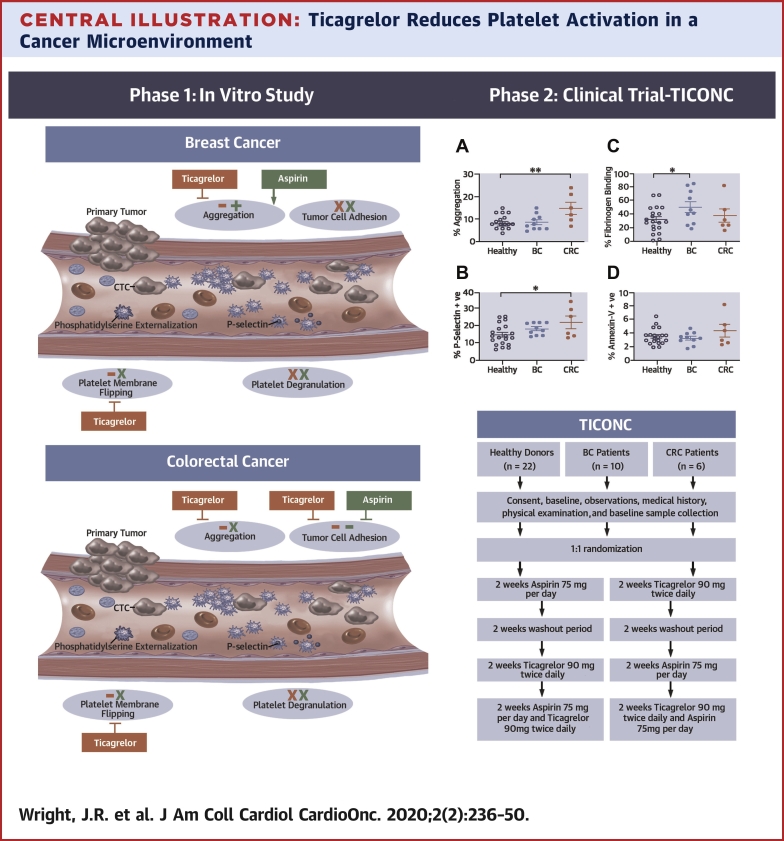
Cancer-associated thrombosis and the development of distant metastases are the prime drivers of mortality in cancer (1). Tumor cells that migrate from the primary tumor into the circulation can interact with peripheral blood cells, including platelets (2). Tumor cell−induced platelet activation (TCIPA) has been reported to increase circulating platelet activity (3) and to facilitate the metastatic process via several mechanisms (4). Bound platelets, or platelet aggregates, may also confer survival of the circulating tumor cell by shielding it from immune destruction (5) or from physical injury arising from shear stress in the microcirculation (6). Therefore, inhibiting or limiting platelet activation in patients with cancer may reduce metastasis and be beneficial in patients at risk of cancer-associated thrombosis.
Although there is growing evidence for a beneficial effect of low-dose aspirin in preventing metastatic tumor spread, research is still required to further assess its effectiveness in different types of cancer and to balance the potential benefits against risk of bleeding (7, 8, 9, 10, 11). Inhibition of platelet activation by aspirin monotherapy only affects one of the secondary platelet activation pathways, and it may be that inhibitors of alternative pathways have potential for greater impact on TCIPA. One such partial agonist, adenosine diphosphate (ADP), has been implicated in tumor cell activation of platelets (12); therefore, targeting ADP-activated pathways may have the potential to inhibit ADP-induced platelet activation facilitated by circulating tumor cells. P2Y12 antagonists, including ticagrelor, are routinely used in the treatment and prevention of arterial thrombosis (13). As a result of the PLATO (PLATelet inhibition and patient Outcomes) study (14), ticagrelor has been widely adopted clinically in the treatment of patients with cardiovascular disease, due to its superior efficacy over the earlier pro-drug, clopidogrel. Therefore, ticagrelor may be a putative antiplatelet agent for inhibition of platelet activation in cancer, potentially reducing metastatic potential and thrombotic risk. Ticagrelor has not previously been investigated for this indication in a cancer population.
This study first used an in vitro model of platelet−tumor cell interaction within the circulation to compare the effects of mono- and dual antiplatelet therapy (DAPT) on platelet activation and interaction with human breast (MCF-7) and colorectal cancer (HT-29) cells. The second part of the investigation was an interventional trial that consisted of a randomized, crossover study of the effects of ticagrelor or aspirin monotherapy and DAPT on platelets of patients with metastatic breast and colorectal cancer compared with healthy control subjects.
Methods
Complete experimental details are outlined in the Materials and Methods section of the Supplemental Appendix. The human study was approved by a regional ethics review committee and the UK Health Research Authority (15/EM/0048). All procedures performed in the study involving human participants were conducted in accordance with the principles of the Declaration of Helsinki.
Statistical analysis
Data and statistical analysis for aggregation assays and flow cytometry were performed using GraphPad Prism software v7 (GraphPad Software Incorporated, La Jolla, California). Continuous variables are presented as mean ± SEM. Within group comparisons were performed using a paired Student's t-test. Comparison of platelet activity in platelets from healthy donors and patients with metastatic cancer was analyzed using a standard t-test for 2 independent groups. TICONC study patient characteristics in Table 1 are presented as mean ± SD or number (percentage). Sample size was determined based on obtaining a 10% reduction in platelet-cancer cell interaction with 80% power. Microscopy images were analyzed using Image-J (version 1.37, National Institutes of Health Image, Bethesda, Maryland). Significance is indicated as p < 0.05, p < 0.01, and p < 0.001 or is given as not significant (NS) for p ≥ 0.05.
Table 1.
The TICONC Participant Characteristics
| Healthy Donors (n = 22) | Breast Cancer Patients (n = 10) | p Value | Colorectal Cancer Patients (n = 6) | p Value | |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 6 (27.3) | 0 (0) | 3 (50) | ||
| Female | 16 (72.7) | 10 (100) | 3 (50) | ||
| Age (yrs) | 43.5 ± 2.5 | 63.4 ± 4.8 | <0.001 | 64.0 ± 3.5 | <0.001 |
| BMI (kg/m2) | 28.9 ± 1.0 | 29.2 ± 5.7 | 0.881 | 23.6 ± 1.3 | 0.018 |
| Hb (g/l) | 125.6 ± 9.0 | 133.1 ± 8.9 | 0.592 | 128.0 ± 9.1 | 0.815 |
| Plt count (109/l) | 259 ± 21.3 | 292 ± 70.6 | 0.357 | 263 ± 135 | 0.935 |
| WBC count (109/l) | 5.60 ± 1.0 | 7.03 ± 1.1 | 0.579 | 6.08 ± 0.6 | 0.852 |
| Systolic BP (mm Hg) | 129.3 ±16.2 | 131.8 ± 17.5 | 0.699 | 120.8 ± 14.1 | 0.251 |
| Diastolic BP (mm Hg) | 80.3 ± 10.3 | 83.1 ± 15.7 | 0.542 | 79.7 ± 14.1 | 0.908 |
| Heart rate (beats/min) | 72.9 ± 11.1 | 80.2 ± 12.1 | 0.099 | 82.5 ± 18.8 | 0.113 |
Values are n (%) or mean ± SD. The p values denote t-test comparison between cancer groups and healthy control subjects.
BMI = body mass index; BP = blood pressure; Hb = hemoglobin; Plt = platelet; TICONC = Ticagrelor-Oncology study; WBC = white blood cell.
Results
In vitro study
Ticagrelor inhibits tumor cell-induced cell−cell aggregation
Initial experiments established the optimal concentrations of antiplatelet agents to be used in the in vitro study and testing the platelet response to physiological agonists in the presence and absence of aspirin and ticagrelor. ADP (10 μM) and arachidonic acid (1 mM) induced a maximal platelet aggregatory response that was significantly inhibited by 10 μM of ticagrelor (from 93.0 ± 3.5% to 2.0 ± 2.0% aggregation) or 50 μM aspirin (from 99.3 ±0.7% to 6.7 ± 2.3% aggregation), respectively. These concentrations of inhibitors were used throughout the in vitro study (Figures 1A and 1B).
Figure 1.
Cellular Aggregation in the Platelet-Tumor Cell Microenvironment
(A) Aggregation trace depicts typical agonist-induced platelet aggregation (10 μM adenosine diphosphate (ADP), and 1 mM arachidonic acid [AA]) and inhibition by antiplatelet agents (A and B) ticagrelor (Ticag) (10 μM) and aspirin (ASA) (50 μM), respectively. Platelets from normal healthy donors were pre-incubated with Ticag (10 μM), ASA (50 μM), or dual inhibitors, then stirred in the presence of (C) MCF-7 breast cancer cells or (D) HT-29 colorectal cancer cells. Values are mean percent aggregation ± SEM (n = 8). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. DAPT = dual antiplatelet therapy (ticagrelor and aspirin); Plts = platelets; TC = tumor cells.
As shown in Figure 1C (reproduced in Supplemental Figure 1A), platelets from healthy subjects, stirred without addition of tumor cells, did not spontaneously aggregate; however, the presence of MCF-7 breast cancer cells resulted in significant levels of aggregation within 10 min (platelets + MCF-7 65.4 ± 4.8%; p < 0.001; n = 8). MCF-7-induced platelet aggregation was significantly reduced by pre-treatment of platelets with ticagrelor (50.9 ± 5.9%; p = 0.002) (Figure 1C). Aspirin alone had no significant effect on MCF-7-induced aggregation (mean 63.1 ± 3.8%; p = 0.139), but as dual therapy, aspirin with ticagrelor significantly reduced aggregation in the presence of MCF-7 breast tumor cells (mean 46.1 ± 5.4%; p < 0.001) (Figure 1C). A similar pattern was seen for platelets incubated with another human breast cancer cell line, SKBR3 tumor cells (Supplemental Figure 1B).
TCIPA and inhibition of tumor cell−induced cellular aggregation followed a similar pattern when using the colorectal cancer cell line, HT-29. Aggregation in the presence of HT-29 cells was significantly reduced by ticagrelor (from 62.3 ± 3.1% to 48.3 ± 7.3%; p = 0.014; n = 8) (Figure 1D, and reproduced in Supplemental Figure 1C). Aspirin alone did not alter HT-29−induced aggregation (61.1 ± 2.7%; p = 0.344), nor did it further enhance the inhibition of ticagrelor (50.6 ± 6.5%; p = 0.013) (Figure 1D). Ticagrelor also had a significant inhibitory effect on platelets incubated with another colon cancer cell line, HCT116 (Supplemental Figure 1D).
Ticagrelor reduces tumor cell-induced, platelet−platelet interaction
In the microenvironment of the circulating tumor cell, cellular aggregates could potentially form as platelet−platelet aggregates, or between platelets and tumor cells through direct ligand-receptor binding. Analysis of samples by microscopy demonstrated that changes in cellular aggregation appeared to be predominantly due to platelet−platelet interaction rather than the interaction of platelets and tumor cells. Consistent with the aggregometry data, samples containing washed platelets alone showed an even dispersal after stirring (Figure 2A). The addition of MCF-7 cells (Figure 2, left-sided column) resulted in clumps of platelet−platelet aggregates, with some but not all, associated with a tumor cell. Pre-incubation of platelets with ticagrelor (10 μM) resulted in fewer large clumps of platelet−platelet aggregates, and more platelets remained dispersed throughout the sample. As seen in the previous aggregation experiments, aspirin (50 μM) appeared to have little effect on the inhibition of platelet−platelet aggregates. Presence of both antiplatelet agents (ticagrelor and aspirin) appeared to have a mixed effect, with platelet−platelet aggregates still being present, but also with observable platelet dispersal. Analysis of changes in platelet fluorescence demonstrated a variable change in the size of platelet aggregates, with the mean perimeter (Figure 2B) and diameter (Figure 2D) of platelet aggregates in the presence of MCF-7 cells significantly reduced in the presence of both ticagrelor and aspirin.
Figure 2.
Effect of Antiplatelet Agents on Breast and Colorectal Cancer Cell-Induced Aggregation
Platelets, fluorescently labeled in red, were pre-incubated for 10 min at 37 oC with Ticag (10 μM), ASA (50 μM), or dual inhibitors, then stirred for 10 min in the presence of MCF-7 breast cancer cells or HT-29 CRC cells. (A) Samples were stained with platelet-specific CD42a-PE conjugated antibody and images acquired using an EVOS FL cell imaging system (Thermo Fisher Scientific, Glasgow, United Kingdom). (B and C) The perimeter of platelet aggregates and (D and E) diameter of platelet aggregates were analyzed using Image-J (National Institutes of Health, Bethesda, Maryland). Data show mean ± SEM (n = 5). ∗p < 0.05; ∗∗p < 0.01 for MCF-7 (n = 3); and ∗p < 0.05; ∗∗p < 0.01 for HT29. Con = control; CRC = colorectal cancer; other abbreviations as in Figure 1.
There was less variability in the effect of the single antagonist ticagrelor observed in platelet samples that were stirred in the presence of the colorectal HT-29 cell line (Figure 2, right-sided column). Quantification of the size of platelet aggregates in the presence or absence of the 2 inhibitors demonstrated a significant decrease in perimeter (Figure 2C) and diameter (Figure 2E) of fluorescently labeled platelets in the presence of ticagrelor or DAPT.
Tumor−cell interaction induces platelet secretion and phosphatidylserine exposure
We also investigated the influence of tumor cells on other markers of platelet activation, using flow cytometric assessment of P-selectin expression (a marker of platelet degranulation) and Annexin-V binding (which is an indicator of platelet membrane externalization of phosphatidylserine, or “platelet membrane flipping”). As a positive control, and to confirm the efficacy of the antiplatelet drugs, we initially assessed platelet activation in the absence of tumor cells, in response to ADP (10 μM) or arachidonic acid (1 mM) and in the presence or absence of ticagrelor (10 μM) and aspirin (50 μM).
Unstimulated platelets expressed basal levels of P-selectin of 2.5 ± 1.2%. In contrast, significant degranulation was induced by the platelet agonist ADP (10 μM) and was significantly inhibited by pre-treatment with 10 μM ticagrelor (61.5 ± 3.2% and 4.8 ± 1.6% respectively; p < 0.001; n = 4) (Figure 3A). Arachidonic acid (1 mM) induced a similar level of degranulation as a result of thromboxane generation, and pre-treatment of platelets with aspirin (50 μM) had a much smaller, but statistically significant inhibitory effect on P-selectin expression in response to arachidonic acid (1 mM) (47.0 ± 1.1% and 39.6 ± 0.6%, respectively; p = 0.007; n = 3) (Figure 3A). In contrast to induction of secretion, ADP and arachidonic acid had only a minimal effect on externalization of phosphatidylserine, as demonstrated by <10% Annexin-V binding in response to either agonist (Figure 3B).
Figure 3.
Tumor Cell Interaction Induces Platelet Activation
The amplitude of normal physiological platelet activation leading to expression of (A) P-selectin and (B) Annexin-V binding in response to ADP (10 μM) and AA (1 mM), and subsequent inhibition in the presence of ticagrelor (10 μM) and aspirin (50 μM), were first tested in the absence of TCs. Platelet interaction with TCs induced platelet degranulation (C, D, F) and externalization of phosphatidylserine (C, E, G). Washed platelets were pre-treated with Ticag (10 μM), ASA (50 μM), or DAPT, stirred at 37 oC in the presence of MCF-7 or HT-29 cells and analyzed by flow cytometry for P-selectin and Annexin-V binding. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001 (n = 6 for both cell lines). FITC = fluorescein isothiocyanate; other abbreviations as in Figure 1.
Tumor cell interaction with platelets induced significant platelet degranulation and externalization of phosphatidylserine, as depicted in the flow cytometry (Figure 3C, left panel, Figure 3C, right panel). More specifically, incubation of platelets with MCF-7 breast cancer cells resulted in 75.2 ± 5.6% of the platelet population expressing P-selectin compared with platelets that had been stirred in the same conditions in the absence of MCF-7 (16.7 ± 3.4%; p < 0.001; n = 6) (Figure 3D). Pre-incubation of platelets with single or dual antiplatelet agents had no significant effect on degranulation, as measured by P-selectin expression (67.6 ± 10.6%, 81.5 ± 2.0%, or 68.6 ± 8.6%; p > 0.05 for all, for treatment with ticagrelor, aspirin, or dual inhibitors, respectively) (Figure 3D).
These findings were also consistent in the colorectal cancer cell line, HT-29. Incubation with the colorectal cancer cells induced most of the platelet population to express P-selectin (85.4 ± 1.8%; p = 0.013; n = 6 compared with platelets alone); however, pre-incubation of platelets with single or dual inhibitors had only a small effect on P-selectin expression that did not reach levels of significance (67.3 ± 9.1%, 80.9 ± 4.8%, and 71.2 ± 7.0% for ticagrelor, aspirin, and both inhibitors, respectively) (Figure 3F).
Surface exposure of phosphatidylserine on platelets stirred in the absence of tumor cells was <10%. However, incubation of platelets with MCF-7 cells, in the absence of any inhibitors, resulted in a significant increase of the platelet population expressing Annexin-V (22.7 ± 5.0%; p = 0.010; n = 6) (Figure 3E). Pre-incubation of platelets with ticagrelor reduced this significantly (11.7 ± 3.4%; p = 0.031). Again, aspirin alone did not have an inhibitory effect (25.9 ± 6.4%; p = 0.383), nor any additive effect when combined with ticagrelor (11.2 ± 2.6%; p = 0.014) (Figure 3E). Tumor cell-induced platelet induction of phosphatidylserine exposure was even greater with HT-29 cells (mean: 37.7 ± 9.5%; p = 0.036) (Figure 3G). This was again significantly inhibited by ticagrelor (15.2 ± 3.1%; p = 0.041) and to some extent by aspirin, either alone (27.3 ± 3.3%; p = 0.186) or combined with ticagrelor (11.8 ± 2.2%; p = 0.029) (Figure 3G).
Effect of antiplatelet agents on platelet-facilitated tumor cell-endothelial cell adhesion
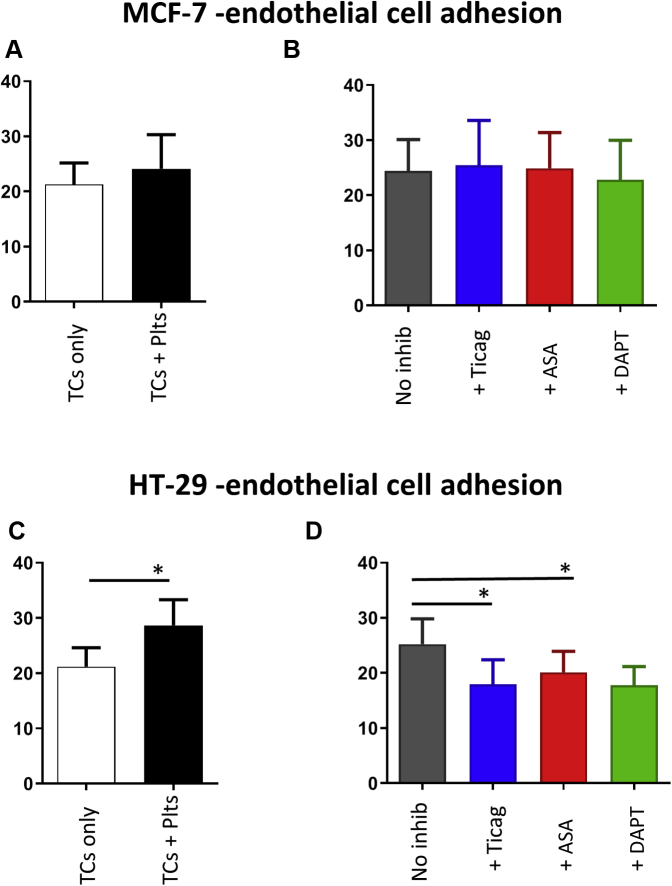
Cellular arrest and adhesion to the endothelial wall is key to metastatic disease progression. Platelets may play a role in this process, providing mechanisms of cellular interaction that facilitate the tumor cell attachment and adhesion to the endothelial wall, and therefore, may optimize the opportunity for tumor cell migration into the underlying tissues. Under static conditions, the addition of platelets increased the level of MCF-7 breast cancer cell adhesion to cultured human umbilical vein endothelial cells from 21.3 ± 3.9% to 24.1 ± 6.2%, albeit nonsignificantly (p = 0.501; n = 3) (Figure 4A). A similar but significant effect on endothelial adhesion was observed for HT-29 cells, rising from 21.2 ± 3.4% without platelets to 28.7 ± 4.7% in the presence of platelets (p = 0.015; n = 5) (Figure 4C).
Figure 4.
Platelet-Enhanced Tumor Cell−Endothelial Cell Adhesion
Platelet-rich plasma was prepared from citrated blood collected from normal healthy donors, and was pre-incubated with Ticag (10 μM), ASA (50 μM), a combination of ASA (50 μM) and Ticag (10 μM), or dimethyl sulphoxide vehicle control, then incubated for 15 min at 37oC with MCF-7 breast cancer cells and HT-29 colorectal cancer cells. Graphs show the (A and B) percentage of TC adhesion for MCF-7 and (C and D) HT-29 following 1-h incubation of each platelet−tumor cell sample with a human umbilical vein endothelial cell monolayer. Values are expressed as mean percentage of adhesion ± SEM. ∗p < 0.05 (n = 3) and (n = 5) for MCF-7 and HT-29, respectively. Abbreviations as in Figure 1.
To investigate whether antiplatelet agents altered the magnitude of platelet-facilitated tumor cell adhesion to human umbilical vein endothelial cells, platelets were pre-treated for 10 min with aspirin (50 μM), ticagrelor (10 μM), or DAPT. MCF-7 breast cancer cell adhesion appeared to be unaffected by these antiplatelet agents (Figure 4B). In contrast, HT-29 colorectal cancer cell−endothelial cell adhesion, which was more sensitive to the presence of platelets, was significantly reduced when platelets had been pre-treated with single inhibitors. Ticagrelor reduced adhesion from 25.2 ± 4.6% to 17.9 ± 4.5% (p = 0.020; n = 5), and aspirin reduced adhesion to 20.1 ± 3.8% (p = 0.030). Treatment with both therapies did not appear to further enhance the inhibitory effect (17.8 ± 3.4%) (Figure 4D).
The TICONC study
Study population
Healthy donors, patients with metastatic breast cancer, and patients with metastatic colorectal cancer were enrolled in a randomized, open-label crossover design study (Ticagrelor-Oncology [TICONC] Study; EudraCT: 2014-004049-29). Thirty-eight eligible donors (22 healthy donors, 10 patients with breast cancer, and 6 patients with colorectal cancer with advanced cancer) were recruited. The completion rate was 81.6% due to the following: rapid disease progression (n = 3), serious adverse effect (hematuria) (n = 1), and patient choice to withdraw from trial (n = 3). The inclusion criteria (Supplemental Table 1) and baseline characteristics of the populations included in the study are presented in Table 1. The mean age of the 3 subgroups ranged from 43.5 to 63.4 years, with the healthy control subjects being significantly younger than both cancer subgroups. The mean body mass index of patients with colorectal cancer was significantly lower than that of healthy donors (p = 0.018), and male patients with colorectal cancer also had lower levels of hemoglobin than healthy male donors. There was no significant difference in platelet or white blood cell count between healthy donors and cancer cohorts.
Circulating platelet activity in patients with metastatic breast or colorectal cancer
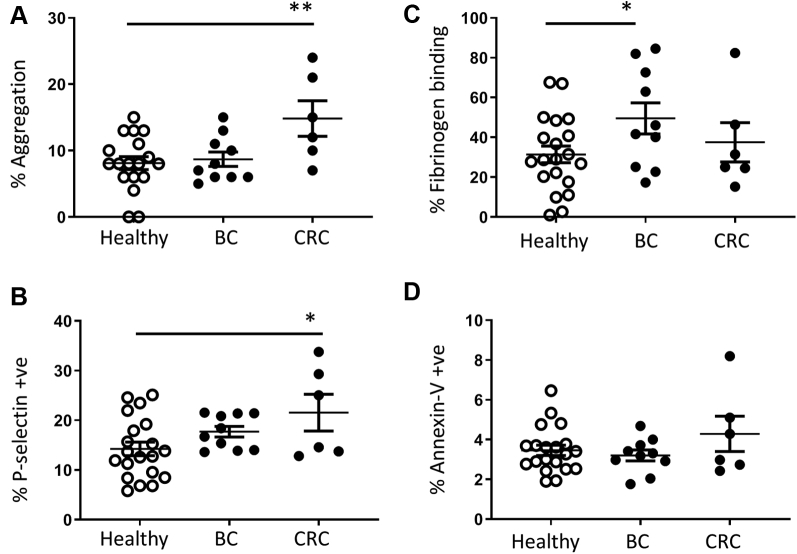
Following randomization of the trial participants (Figure 5), samples obtained at the initial trial visit (before drug treatment commenced) (Figure 6) were used for an ex vivo study of the level of platelet activation in unstimulated platelets isolated from the circulation of patients with metastatic cancer compared with healthy donors. Platelet aggregation assays showed that in the absence of additional exogenous platelet agonists, patients with metastatic colorectal cancer had significantly higher levels of spontaneous platelet aggregation (p = 0.007) than platelets from healthy donors or patients with breast cancer (Figure 6A). In support of this, platelets from patients with colorectal cancer also had significantly higher levels of P-selectin expression (p = 0.030) (Figure 6B). In contrast, platelets from participants with breast cancer bound significantly higher levels of fibrinogen (p = 0.034) (Figure 6C). There was no significant difference in Annexin-V binding across the groups (Figure 6D).
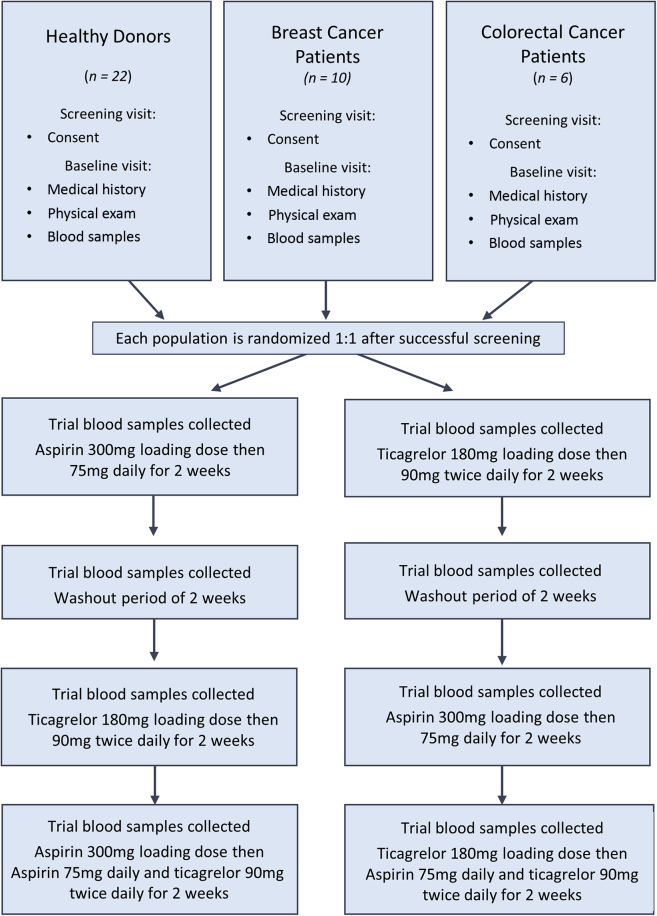
Figure 5.
The TICONC Study Design
A total of 22 healthy donors, 10 patients with breast cancer, and 6 patients with colorectal cancer were recruited to the TICONC (Ticagrelor-Oncology) study, a randomized, open-label crossover study design. All donors were aged between 18 and 85 years. Following initial screening and baseline sample collection, participants were randomized and received 2 weeks of ASA (75 mg daily) or Ticag (90 mg twice daily) followed by a 2-week washout period. This was followed by 2 weeks of remaining monotherapy, and finally, 2 weeks of DAPT. Blood samples were collected at each visit and assayed for platelet activation.
Figure 6.
Platelet Activity in Circulating Resting Platelets of Healthy Donors and Patients With Metastatic Breast or CRC
Platelet activation markers were measured in the absence of additional exogenous platelet agonists for (A) spontaneous aggregation, (B) P-selectin expression, (C) fibrinogen-binding, and (D) Annexin-V binding. Data show mean ± SEM for healthy donors (n = 22), patients with breast cancer (BC) (n = 10), and patients with CRC (n = 6). ∗p < 0.05; ∗∗p < 0.01. Abbreviations as in Figure 2.
Effect of platelet antagonists on spontaneous platelet activation in patients with metastatic cancer
Patients with colorectal cancer who took ticagrelor had significantly lower levels of spontaneous platelet aggregation compared with baseline (14.8 ± 2.7% to 7.8 ± 3.3% with ticagrelor; p = 0.012) (Figure 7A). Although aspirin did not significantly inhibit aggregation of platelets from patients with colorectal cancer, it significantly increased spontaneous platelet aggregation in participants with breast cancer (8.7 ± 1.1% at baseline to 12.6 ± 1.6% with aspirin; p = 0.026) and in healthy donors (8.1 ± 1.0% to 10.5 ± 1.4% with aspirin; p = 0.035) (Figure 7A). Although none of the antiplatelet drugs had any effect on inhibition of Annexin-V binding (Figure 7B) or P-selectin expression (Figure 7C), ticagrelor significantly reduced levels of fibrinogen binding in unstimulated platelets isolated from healthy donors (31.4 ± 4.2% at baseline to 17.9 ± 3.6% with ticagrelor; p = 0.008) (Figure 7D) and patients with metastatic breast cancer (49.5 ± 7.8% to 26.7 ± 5.2% with ticagrelor; p = 0.048) (Figure 7D). The absolute inhibitory effect of the antiplatelet drugs was tested in the presence of exogenously added platelet agonists for ADP (10 μM) (Supplemental Figure 2) and arachidonic acid (1 mM) (Supplemental Figure 3), which confirmed that platelet activation in each subgroup was significantly increased and was subsequently significantly inhibited in the presence of ticagrelor (10 μM) and aspirin (50 μM), respectively, as expected.
Figure 7.
The TICONC Trial Results: Effect of Antiplatelet Therapy on Activation Status of Circulating Resting Platelets in Healthy Donors and Patients With Metastatic BC and CRC
(A) Mean percentage of spontaneous aggregation, (B) mean percentage of Annexin-V binding, (C) mean percentage of P-selectin positive, and (D) mean percentage of fibrinogen-binding at baseline before drug treatment and during single or DAPT. Blood collected for the washout period (w/o) was drawn 2 weeks after the end of initial antiplatelet therapy. Values are mean ± SEM (healthy donors: n = 22; BC: n = 10, and CRC: n = 6). ∗p < 0.05; ∗∗p < 0.01. Abbreviations as in Figures 1, 2, and 6.
Discussion
This study investigated the potential benefit of repurposing ticagrelor, which is already widely used for the treatment of patients with cardiovascular disease, to treat cancer. Our main findings were as follows. First, in an in vitro model of platelet−tumor cell interaction, ticagrelor monotherapy, but not aspirin, primarily reduced tumor cell-induced platelet aggregation and activation (phosphatidylserine externalization) in response to human breast cancer cells (MCF-7) and colorectal cancer cells (HT-29). Second, in addition to cellular aggregation, both ticagrelor and aspirin monotherapy significantly reduced colorectal cancer cell adhesion in the presence of platelets but not the adhesion of breast cancer cells. Third, the data from the crossover study showed that in patients with metastatic cancer, ticagrelor could significantly reduce spontaneous platelet aggregation (Central Illustration). These findings support further investigation of the potential role for ticagrelor monotherapy as prophylaxis in cancer patient populations at risk for thrombotic complications (e.g., venous thromboembolism).
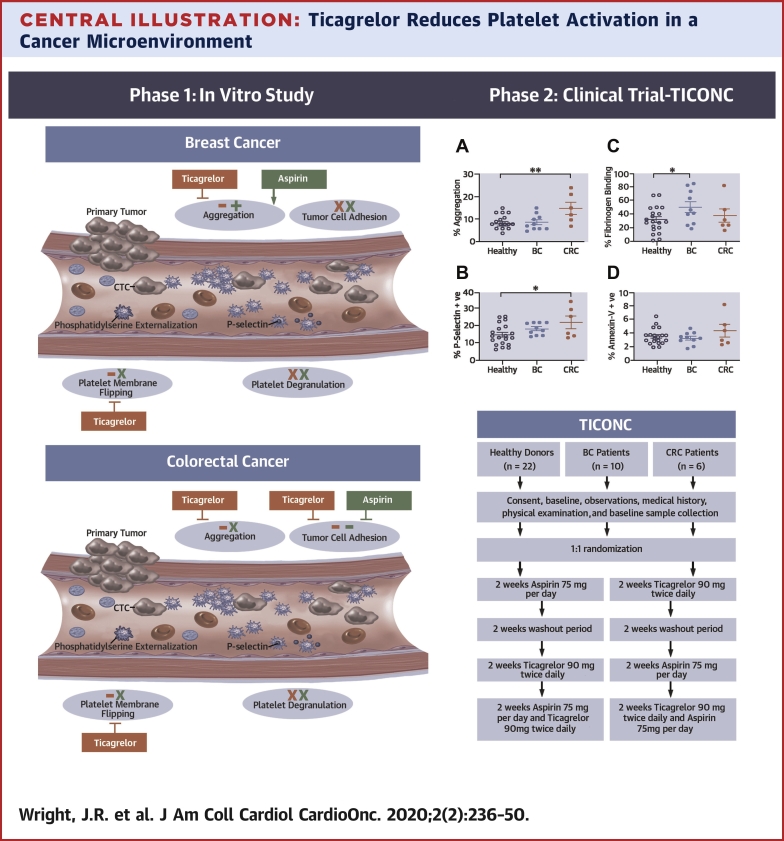
Central Illustration.
Ticagrelor Reduces Platelet Activation in a Cancer Microenvironment
(Left) Summary of the effect of antiplatelets in an in vitro model of platelet−tumor cell interaction within the circulation. Although ticagrelor inhibited aggregation and platelet membrane flipping in both cancer models, there was a lack of effect of ticagrelor or aspirin on breast tumor cell adhesion. In contrast, both drugs were effective in inhibition of colorectal cancer cell adhesion. Neither drug inhibited platelet degranulation. Ticagrelor effects shown in red, aspirin effects in green, − represents drug inhibition, + indicates enhanced activity, x represents no drug effect. (Right) Flowchart of the TICONC (Ticagrelor-Oncology) clinical trial, and levels of activation in circulating platelets isolated from patients with metastatic cancer before commencing antiplatelet therapy. BC = breast cancer; CRC = colorectal cancer; CTC = circulating tumor cells.
For continued improvement of overall cancer survival, there is a clinical need for rapidly translatable medications that are effective at reducing cancer incidence, metastasis, and cancer-associated thrombosis. Evidence that platelets contribute to cancer is mixed. Epidemiological studies that investigated the protective effect of the antiplatelet drug aspirin suggested a possible role, at least in colorectal cancer (10), but clinical trials in older adults (ASPREE [Aspirin in Reducing Events in the Elderly]) (15) or patients with diabetes (ASCEND [A Study of Cardiovascular Events iN Diabetes]) (16) were disappointing. However, platelets were reported to play an important role in cancer metastasis and cancer-associated thrombosis (4,17,18); therefore, limiting platelet activation in patients with cancer might be important in these key areas.
Tumor metastasis involves many mechanistic steps and is driven by multiple pathways, making therapeutic targeting of the different phases problematic. Platelet activation results in release of soluble mediators, such as vascular endothelial growth factor and platelet-derived growth factor, which facilitate angiogenesis (19), and sP-selectin, which is involved in cell adhesion and aggregation (20). In addition to physiological functions, these platelet-derived mediators can contribute to disease (21,22). Therefore, targeting the circulating platelet may be beneficial in reducing metastatic spread and may limit the risk of cancer-associated thrombosis. Although aspirin has been associated with reduced metastatic progression in some colorectal cancers, these findings have not been replicated in all studies or in other cancers (11). The much anticipated AddAspirin trial will further inform these key questions. However, aspirin only affects 1 of the secondary pathways for platelet activation. Therefore, more potent platelet inhibitors have the potential to have a greater effect on these processes.
To explore the effect of the antiplatelet agents on the interaction between platelets and tumor cells in the circulation, we first used an in vitro model with tumor cells and human platelets that were pre-treated with monotherapy or DAPTs. Using aspirin at a concentration we confirmed to completely inhibit platelet activation in response to 1 mM arachidonic acid, platelet aggregation in the presence of tumor cells was not inhibited by aspirin monotherapy. This was also reported in a previous in vitro study of breast cancer cell−induced platelet activation (23) and suggested that aggregation driven by tumor cells did not occur via activation of the platelet thromboxane pathway, which aspirin is known to inhibit. In contrast to cellular aggregation, aspirin significantly reduced platelet-facilitated colorectal cancer cell adhesion to endothelial cells, which suggested it might be an inhibitor of the platelet contribution to this part of the metastatic process.
An alternative secondary pathway of platelet activation is via ADP stimulation of the P2Y1 and P2Y12 receptors. In vitro and in vivo data suggested that blocking ADP was also beneficial in reducing cancer progression (24,25), and this was particularly relevant because tumor cells can release ADP into the tumor microenvironment. An in vivo study of P2Y12−/− and P2Y1−/− mice demonstrated that it was the P2Y12 receptor that contributed to ovarian tumor growth (26) and that mice treated with the P2Y12, ticagrelor, had improved survival (27). In our in vitro study, in contrast to platelet pre-treatment with aspirin, cellular aggregation in the presence of MCF-7- breast cancer cells and HT-29 colorectal cancer cells was significantly reduced by ticagrelor monotherapy, with no additive effect of dual therapy.
Platelets were reported to aggregate with tumor cells, forming a “cloak” that shields the tumor cell from immune detection (28). Therefore, we expected the aggregation recorded to consist of a heterogeneous cell population. However, analysis by microscopy revealed that in the presence of tumor cells, in the timeframe studied, cellular clusters were predominantly platelet−platelet aggregates. Ticagrelor significantly inhibited formation of tumor cell−induced large platelet−platelet aggregate complexes, which resulted in a platelet population that was more dispersed and suggested that blockade of platelet P2Y12 might potentially reduce the risk of thrombotic complications (e.g., venous thromboembolism) if, in vivo, it had the same effect.
Ticagrelor has not been previously tested in vivo for its antiplatelet effects in a cancer population. In this crossover trial (TICONC), we compared the efficacy of ticagrelor and aspirin monotherapy with DAPT on the activation of unstimulated platelets isolated from patients with advanced metastatic breast or colorectal cancer. Supporting the results of the in vitro study, in the absence of exogenously added ADP, ticagrelor significantly reduced the aggregatory response of platelets isolated from patients with higher levels of spontaneous platelet aggregation.
The mechanisms behind platelet hyperreactivity, the elevated activity level of circulating platelets, is still poorly understood, but increased spontaneous aggregation has historically been shown to correlate with poor prognosis in patients with myocardial infarction (29,30). Platelet hyperreactivity in a cancer population has been previously reported; however, the possible effects of antiplatelet agents on this population were not considered in this previous study of a small patient cohort of a mixed cancer population (3). We considered only 2 major cancers to understand any differential response to treatment between groups. In contrast to patients with colorectal cancer, platelets from normal healthy donors and patients with breast cancer had similar lower levels of spontaneous aggregation. Although in vitro monotherapy treatment with aspirin was ineffective at blocking cellular aggregation, it significantly increased the level of platelet aggregation of platelets from donors whose level of spontaneous platelet aggregation was initially low. Further study is necessary to elucidate the mechanisms behind these effects.
Ticagrelor has been widely adopted in populations of patients with high-risk coronary syndromes due to its increased efficacy and reduced mortality rates compared with other P2Y12 antagonists (e.g., clopidogrel) (14), with the most common side effects being dyspnea and minor bleeding (14,31). However, the use of this antiplatelet agent has not previously been tested in a cancer population. In the present study, 1 colorectal cancer patient presented with hematuria during the combined aspirin and ticagrelor period, which resolved once DAPT was stopped.
Study limitations
This is the first study to explore the potential benefits of ticagrelor in a cancer population; therefore, direct comparisons are not possible. Limitations of our study were predominantly related to small sample size. A statistically significant difference was considered at p < 0.05, which did not account for multiple testing because these data were exploratory and aimed to provide evidence to justify a larger, sufficiently powered study. Although we limited our study to just 2 main types of cancer, breast and colorectal, there still is a molecular subtype variation, and consequently, a difference in response to anticancer treatments. In addition, it was likely that other endogenous factors released by tumor cells that might activate platelets (e.g., thrombin) might also contribute to platelet activation in the tumor microenvironment. We observed that spontaneous aggregation of platelets was inhibited in some patients who received other antithrombotic drugs (e.g., warfarin and rivaroxaban). Therefore, to further explore and validate the findings of this exploratory study, larger scale studies need to be developed with stratification according to cancer subtypes to analyze the effect of antiplatelet and anticoagulant treatment on different patients. In addition, long-term studies are needed to determine the effectiveness of ticagrelor in reducing metastatic spread in different cancer populations.
Conclusions
The primary finding of this study demonstrated that some populations of patients with cancer have circulating platelets that are hyperreactive in comparison to platelets from healthy donors. Ticagrelor, as monotherapy but not dual therapy, was more effective than aspirin in reducing both in vitro and ex vivo tumor cell-induced platelet aggregation. This finding warrants further investigation in larger cohorts of patients with cancer to explore the potential role for ticagrelor monotherapy as prophylaxis in patients at high risk of cancer-associated venous thromboembolism, as well as long-term assessment of its ability to reduce metastatic spread and improve progression-free survival.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In cancer, platelets may facilitate metastatic spread by a number of mechanisms as well as contribute to thrombotic complications. Our in vitro studies and a small, randomized crossover clinical trial suggested significant inhibition of tumor-cell induced platelet activation during treatment with ticagrelor.
TRANSLATIONAL OUTLOOK: Large-scale randomized clinical trials are needed to assess the effects of ticagrelor in different cancer populations, evaluating its efficacy in reducing metastatic spread, and its potential for reducing the risk of cancer-associated venous thromboembolism.
Acknowledgments
The authors are grateful to all the patients with cancer and the healthy volunteers who participated in this study and to our clinical colleagues for supporting patient recruitment. The authors acknowledge statistical advice from Dr. Christopher Nelson at the Leicester National Institutes of Health Biomedical Research Unit in Cardiovascular Disease, Glenfield Hospital, Leicester, United Kingdom, and the support of the Leicester Experimental Cancer Medicine Centre, Clinical Sciences Building, Leicester Royal Infirmary, Leicester, United Kingdom.
Footnotes
This study was supported by an investigator-sponsored study grant from AstraZeneca (No. ISSBRIL0264). Dr. Thomas has received conference attendance support from Bristol-Myers Squibb; and has been a member of the Speakers Bureau for Amgen. Dr. Adlam has received funding from Abbott Vascular to support a clinical research fellow conducting unrelated research; has received in-kind support from AstraZeneca for unrelated research in addition to the funding specified for this study; and has undertaken consultancy with General Electric to support general research funds. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. This study was presented in part as a poster at the ESMO International Congress, September 27 to October 2, 2019, Barcelona, Spain.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For an expanded Methods section as well as supplemental figures and a table, please see the online version of this paper.
Appendix
References
- 1.Steeg P.S. Targeting metastasis. Nat Rev Cancer. 2016;16:201–218. doi: 10.1038/nrc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joyce J.A., Pollard J.W. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke N.M., Egan K., McFadden S. Increased platelet reactivity in patients with late-stage metastatic cancer. Cancer Med. 2013;2:564–570. doi: 10.1002/cam4.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gay L.J., Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer. 2011;11:123–134. doi: 10.1038/nrc3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philippe C., Philippe B., Fouqueray B., Perez J., Lebret M., Baud L. Protection from tumor necrosis factor-mediated cytolysis by platelets. Am J Pathol. 1993;143:1713–1723. [PMC free article] [PubMed] [Google Scholar]
- 6.Egan K., Cooke N., Kenny D. Living in shear: platelets protect cancer cells from shear induced damage. Clin Exp Metastasis. 2014;31:697–704. doi: 10.1007/s10585-014-9660-7. [DOI] [PubMed] [Google Scholar]
- 7.Chan A.T., Ogino S., Fuchs C.S. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holmes M.D., Chen W.Y., Li L., Hertzmark E., Spiegelman D., Hankinson S.E. Aspirin intake and survival after breast cancer. J Clin Oncol. 2010;28:1467–1472. doi: 10.1200/JCO.2009.22.7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bastiaannet E., Sampieri K., Dekkers O.M. Use of aspirin postdiagnosis improves survival for colon cancer patients. Br J Cancer. 2012;106:1564–1570. doi: 10.1038/bjc.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothwell P.M., Price J.F., Fowkes F.G. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 11.Rothwell P.M., Wilson M., Price J.F., Belch J.F., Meade T.W., Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 12.Heinmoller E., Schropp T., Kisker O., Simon B., Seitz R., Weinel R.J. Tumor cell-induced platelet aggregation in vitro by human pancreatic cancer cell lines. Scand J Gastroenterol. 1995;30:1008–1016. doi: 10.3109/00365529509096346. [DOI] [PubMed] [Google Scholar]
- 13.Husted S., van Giezen J.J. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallentin L., Becker R.C., Budaj A. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 15.McNeil J.J., Nelson M.R., Woods R.L. Effect of aspirin on all-cause mortality in the healthy elderly. N Engl J Med. 2018;379:1519–1528. doi: 10.1056/NEJMoa1803955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowman L., Mafham M., Stevens W. ASCEND: a study of cardiovascular events in diabetes: characteristics of a randomized trial of aspirin and of omega-3 fatty acid supplementation in 15,480 people with diabetes. Am Heart J. 2018;198:135–144. doi: 10.1016/j.ahj.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone R.L., Nick A.M., McNeish I.A. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khorana A.A., Kuderer N.M., Culakova E., Lyman G.H., Francis C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brill A., Elinav H., Varon D. Differential role of platelet granular mediators in angiogenesis. Cardiovasc Res. 2004;63:226–235. doi: 10.1016/j.cardiores.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 20.Clemetson K.J. Platelets and primary haemostasis. Thromb Res. 2012;129:220–224. doi: 10.1016/j.thromres.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 21.Blann A.D., Nadar S.K., Lip G.Y. The adhesion molecule P-selectin and cardiovascular disease. Eur Heart J. 2003;24:2166–2179. doi: 10.1016/j.ehj.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Falati S., Liu Q., Gross P. Accumulation of tissue factor into developing thrombi in vivo is dependent upon microparticle P-selectin glycoprotein ligand 1 and platelet P-selectin. J Exp Med. 2003;197:1585–1598. doi: 10.1084/jem.20021868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zara M., Canobbio I., Visconte C., Canino J., Torti M., Guidetti G.F. Molecular mechanisms of platelet activation and aggregation induced by breast cancer cells. Cell Signal. 2018;48:45–53. doi: 10.1016/j.cellsig.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Sitia G., Aiolfi R., Di Lucia P. Antiplatelet therapy prevents hepatocellular carcinoma and improves survival in a mouse model of chronic hepatitis B. Proc Natl Acad Sci U S A. 2012;109:E2165−72. doi: 10.1073/pnas.1209182109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gareau A.J., Brien C., Gebremeskel S., Liwski R.S., Johnston B., Bezuhly M. Ticagrelor inhibits platelet-tumor cell interactions and metastasis in human and murine breast cancer. Clin Exp Metastasis. 2018;35:25–35. doi: 10.1007/s10585-018-9874-1. [DOI] [PubMed] [Google Scholar]
- 26.Cho M.S., Noh K., Haemmerle M. Role of ADP receptors on platelets in the growth of ovarian cancer. Blood. 2017;130:1235–1242. doi: 10.1182/blood-2017-02-769893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gebremeskel S., LeVatte T., Liwski R.S., Johnston B., Bezuhly M. The reversible P2Y12 inhibitor ticagrelor inhibits metastasis and improves survival in mouse models of cancer. Int J Cancer. 2015;136:234–240. doi: 10.1002/ijc.28947. [DOI] [PubMed] [Google Scholar]
- 28.Nieswandt B., Hafner M., Echtenacher B., Mannel D.N. Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res. 1999;59:1295–1300. [PubMed] [Google Scholar]
- 29.Trip M.D., Cats V.M., van Capelle F.J., Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 30.Marcucci R., Valente S., Gori A.M. Global platelet hyperreactivity and elevated C-reactive protein levels predict long term mortality in STEMI patients. Thromb Res. 2014;134:884–888. doi: 10.1016/j.thromres.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 31.Storey R.F., Becker R.C., Harrington R.A. Characterization of dyspnoea in PLATO study patients treated with ticagrelor or clopidogrel and its association with clinical outcomes. Eur Heart J. 2011;32:2945–2953. doi: 10.1093/eurheartj/ehr231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.