Abstract
Venous thromboembolism (VTE), including deep vein thrombosis and pulmonary embolism, represents a major cause of morbidity and mortality in patients with cancer. Arterial thromboembolism, including myocardial infarction and stroke, is also prevalent. Risk differs in subgroups, with higher rates observed in specific cancers including pancreas, stomach, and multiple myeloma. Thromboprophylaxis is recommended for most patients with active cancer hospitalized for medical illnesses and after major cancer surgery. Outpatient thromboprophylaxis is not routinely recommended, but emerging data suggest that a high-risk population that benefits from pharmacological thromboprophylaxis can be identified using a validated risk tool. Direct oral anticoagulants are emerging as the preferred new option for the treatment of cancer-associated VTE, although low-molecular-weight heparin remains a standard for patients at high bleeding risk. Management of VTE beyond the first 6 months and challenging clinical situations including intracranial metastases and thrombocytopenia require careful management in balancing the benefits and risks of anticoagulation and remain major knowledge gaps in evidence.
Key Words: arterial thromboembolism, cancer-associated thrombosis, prophylaxis, risk assessment models, treatment, venous thromboembolism
Abbreviations and Acronyms: ASCO, American Society of Clinical Oncology; ASH, American Society of Hematology; AT, antithrombin; ATE, arterial thromboembolism; CAT, cancer-associated thrombosis; CI, confidence interval; CRNMB, clinically relevant nonmajor bleeding; CVA, cerebrovascular event; DOAC, direct oral anticoagulant; DVT, deep venous thrombosis; ESMO, European Society of Medical Oncology; GI, gastrointestinal; HR, hazard ratio; ICH, intracranial hemorrhage; ISTH, International Society on Thrombosis and Haemostasis; KS, Khorana score; LMWH, low-molecular-weight heparin; MI, myocardial infarction; MM, multiple myeloma; NNT, number needed to treat; PE, pulmonary embolism; PPV, positive predictive value; RAM, risk assessment model; SPE, segmental pulmonary embolism; SSC, Scientific and Standardization Committee; SSPE, subsegmental pulmonary embolism; UHF, unfractionated heparin; VKA, vitamin K antagonist; VTE, venous thromboembolism; VVT, visceral vein thrombosis
Central Illustration
Highlights
-
•
Patients with cancer are at increased risk of VTE and ATE, with significant consequences including mortality.
-
•
RAMs combining clinical and biochemical parameters can identify high-risk patients.
-
•
Thromboprophylaxis should be considered for patients identified as high-risk for VTE.
-
•
DOACs are an emerging option for acute VTE treatment, although LMWH remains an acceptable standard.
-
•
There are limited data that address the management of ATE in patients with cancer.
-
•
A multidisciplinary approach with the oncologist and cardiologist is currently recommended.
Background and Epidemiology
Since first highlighted by Professor Armand Trousseau in the early 19th century (1), the relationship between malignancy and a clinical hypercoagulable state has been extensively studied and remains an important public health issue for patients with cancer. Even today, patients with active malignancy remain at high risk of thromboembolic events, including both venous thromboembolism (VTE) and arterial thromboembolism (ATE). VTE, including deep venous thrombosis (DVT) and pulmonary embolism (PE), is more common and can occur at any time during the history of cancer and may even be the first presenting sign of the disease (2). VTE can complicate surgery, hospitalizations, and systemic therapies and is associated with a major increase in health care resource utilization compared to patients with cancer without VTE (3). Arterial thrombotic events (ATEs), including myocardial infarction (MI), cerebrovascular event (CVA), and peripheral artery disease are leading causes of death and disability worldwide (4). Despite the well-known alterations in clotting function, malignancies are not an established independent risk factor for ATE (5). However, in patients with cancer, thromboembolism, including both VTE and ATE, is the second-leading cause of death after cancer itself, and the occurrence of thromboembolism can interrupt or delay essential cancer treatments (6,7).
VTE rates in patients with cancer are about 4- to 7-fold higher compared to healthy individuals (8) and appear to be increasing over recent years because of improved patient survival, more thrombogenic cancer treatments, extensive use of central catheters, and a better awareness of cancer-associated thrombosis (CAT) (9). Several clinical series have suggested that ATEs may be common in patients with cancer (10,11), and arterial thrombosis accounted for 5.6% of deaths in a prospective study of patients with cancer receiving outpatient chemotherapy (6).
In an United Kingdom analysis evaluating patients with cancer versus matched noncancer control individuals from the general population, the hazard ratio (HR) for VTE was 4.7 (95% confidence interval [CI]: 4.5 to 4.9), and the incidence rate was 13.9/1,000 per year (95% CI: 13.4 to 14.4) (12). A recent review estimates that approximately 15% of patients with cancer will experience VTE, and conversely, 20% of unprovoked VTEs are the first sign of an underlying malignancy (13). The incidence of ATE according to cancer subtypes and settings was investigated by Navi et al. (14), who analyzed 279,719 patients with cancer (breast, lung, prostate, colorectal, bladder, pancreatic, gastric, and non-Hodgkin lymphoma) and matched them with control individuals between 2002 and 2011 using the Surveillance, Epidemiology, and End Results (SEER) database. The incidence of ATE at 6 months was 4.7% in all patients with cancer compared to 2.2% in the matched control cohort, but the study population was mostly represented by older patients with cancer in the United States. As such, the results should be extrapolated to other populations with caution.
VTE in cancer is not limited to DVT and PE, with increasing reports of unusual site thrombosis, including the upper extremities, cerebral veins, and splanchnic veins (15). Upper extremity thrombosis is 18 times more common with active cancer, typically because of the presence of a central venous catheter (13). Splanchnic or visceral vein thrombosis (VVT) is frequently associated with cancer, especially certain gastrointestinal (GI) malignancies (16). Most of those findings are incidentally discovered on routine surveillance or restaging scans, and their potential impact on prognosis and outcomes is still uncertain (17). In contrast, ATE predominantly manifests as MI and CVA, diseases that are hardly incidental because of their significant clinical impact.
The last few years have led to a paradigm shift in both the prevention and treatment of CAT. Emerging data demonstrate benefit to patients with direct oral anticoagulants (DOACs). Large studies focused on cancer populations have been completed for both primary and secondary prevention of thromboembolism. Guidelines from different societies, including the American Society of Clinical Oncology (ASCO), International Society on Thrombosis and Haemostasis (ISTH), International Initiative on Thrombosis and Cancer, European Society of Medical Oncology (ESMO), National Comprehensive Cancer Network, and American Society of Hematology (ASH), have recently modified the recommended approach for both primary prevention and treatment (18, 19, 20, 21, 22).
In this review, we comprehensively evaluate these emerging data in the context of the risk assessment, prevention, and treatment of CAT, both venous and arterial. We searched for current data with the strongest level of evidence throughout; if unavailable, we identify that the data were derived from lower levels of evidence.
Risk Factors and Risk Assessment Models
VTE and ATE are multicausal diseases, and several risk factors have been identified. Many patient-related risk factors, including age, smoking, hypertension, and diabetes, are common to both venous and arterial events (23). In this population, risk factors can be categorized as patient-related, cancer-related, and treatment-related (Table 1) (24).
Table 1.
Clinical Risk Factors and Candidate Biomarkers for Cancer-Associated Venous Thromboembolism
| Cancer-related factors |
| Primary cancer: brain, pancreas, kidney, stomach, lung, gynecologic, lymphoma, myeloma |
| Advanced cancer stage |
| Initial period after cancer diagnosis |
| Histology (worse with adenocarcinoma) |
| Treatment-related factors |
| Major surgery |
| Hospitalization |
| Cancer therapy |
| Chemotherapy |
| Hormonal therapy |
| Antiangiogenic agents: thalidomide, lenalidomide, bevacizumab Immune checkpoint inhibitors |
| Erythropoiesis-stimulating agents |
| Transfusions |
| Central venous catheters |
| Patient-related factors |
| Older age |
| Female |
| Race (higher in Black Americans, lower in Asians/Pacific Islanders) |
| Comorbidities: infection, renal disease, pulmonary disease, obesity, arterial thromboembolism |
| Inherited prothrombotic mutations: factor V Leiden, prothrombin gene mutation |
| Prior history of venous thromboembolism |
| Poor performance status |
| Candidate biomarkers |
| Blood counts |
| Pre-chemotherapy platelet count of ≥350,000/l |
| Pre-chemotherapy leukocyte count of >11,000/l |
| Tissue factor |
| High grade of tissue factor expression by tumor cells |
| Elevated systemic tissue factor (antigen or activity) |
| D-dimer |
| Soluble P-selectin |
| C-reactive protein |
Patient-related risk factors
Data on patient-related risk factors are generally derived from large retrospective cohort studies, which include several types of cancer, and a few prospective cohort studies. Increasing age is a risk factor for VTE in patients with cancer (25), similar to the general population. In a retrospective cohort study, patients age 70 years or older undergoing chemotherapy had a 2-fold risk of developing VTE compared to younger patients (11% vs. 6%) (26). Regarding ethnicity, some studies have suggested higher rates in Black patients and lower rates in Asian patients, although data are conflicting (27). Patient functional status is important, and a poor performance status can increase the risk of VTE (28). Moreover, VTE risk is further increased in patients harboring a genetic risk factor, such as antithrombin (AT), protein C, or protein S deficiency or factor V Leiden or factor II G20210A, even though these conditions are uncommon and usually are associated with VTE at a younger age (29). Inherited prothrombotic alterations play a lower role for ATE, with the exceptions of lupus anticoagulant and hyperhomocysteinemia. Specifically, for patients with cancer, medical comorbidities including anemia, infection, obesity, pulmonary, and renal diseases are associated with up to 1.5-fold higher rates of VTE (25). Finally, patients with cancer with a prior history of VTE have a 6- to 7-fold increased absolute risk for subsequent VTE compared with patients without a prior thromboembolic event (28).
Cancer-related risk factors
The absolute risk of VTE and ATE in patients with cancer varies widely depending on the site, stage, and time after diagnosis and is based on evidence from large cohort studies; systematic assessments are missing. Primary brain tumors and pancreatic cancers are associated with the highest risk of thromboembolism (30). Stomach, esophageal, ovarian, and lung cancers also confer high risk, as well as hematologic malignancies, particularly aggressive non-Hodgkin lymphomas and multiple myeloma (MM) (24). However, it should be noted that from a public health perspective, a high proportion of VTE burden is attributable to highly prevalent cancers (breast, colorectal, prostate) despite a lower relative risk for VTE. Regional or metastatic spread is associated with a higher risk of VTE compared to localized disease (31). Approximately 50% of patients presenting with VTE at the time of diagnosis have synchronous metastasis. Timing also appears to be important; patients are at the highest risk in the first 3 months after cancer diagnosis, followed by a declining incidence. However, the risk remains higher than the general population for up to 15 years from the initial presentation (32).
According to a large retrospective cohort study, the incidence of ATE at 6 months was higher in patients with lung, gastric, and pancreatic cancers (8.3%, 6.5%, and 5.9%, respectively) (14), with MI and ischemic stroke being 2.0% and 3.0%, respectively. Moreover, advanced stage of cancer was associated with a significant increase in ATE (incidence at 6 months: 2.3% for stage 0 compared to 7.7% for stage 4). Some malignancies, such as polycythemia vera and MM, are commonly associated with arterial thrombosis (33). In a large population study in Sweden, patients with MM were found to have an increased risk of ATE at 1, 5, and 10 years after the initial diagnosis, with HRs as follows: 1.9 (95% CI: 1.8 to 2.1), 1.5 (95% CI: 1.4 to 1.6), and 1.5 (95% CI: 1.4 to 1.5), respectively (34).
Recent studies show that the anaplastic lymphoma kinase (ALK) rearrangement in lung cancer confers a higher thrombogenic risk (35). Larger validation studies are required to integrate these molecular data into clinical practice.
Treatment-related risk factors
VTE rates can increase with surgery, anticancer therapies, and supportive care treatments. Some chemotherapeutic agents have also been associated with a high burden of ATE. Surgery (especially pelvic and abdominal) in patients with cancer carries an increased risk of post-operative DVT and PE by 2- and 3-fold, respectively, when compared to patients without cancer undergoing the same procedures (36). Chemotherapy and new anticancer drugs are strong risk factors for VTE, and their growing use may partially explain its increase over the last decades. The use of systemic chemotherapy increases the risk for VTE 2- to 6-fold (37). In this class, the cisplatin thrombogenic effect is well identified: cisplatin-based regimens have a 2-fold increased risk of thromboembolic complications compared to oxaliplatin-based in patients with gastroesophageal cancer (38). Immunomodulatory drugs used in MM (thalidomide, lenalidomide, and pomalidomide) increase the risk for VTE and ATE (MI: 1.98%; CVA: 3.4%) (39), while direct-acting antiviral drugs (also potentially used in patients with cancer) are safe for prothrombotic risk (40). Antiangiogenetic drugs, such as bevacizumab, a monoclonal antibody against vascular endothelial growth factor receptor (VEGFR), increase the risk for ATE (41), as do the multitargeted agents sorafenib and sunitinib, although their precise impact on VTE is not clear (42). Recently, studies on immune checkpoint inhibitors suggest an elevated risk of both VTE and ATE, potentially due to cellular immune responses, inflammatory cytokines, and complement-mediated inflammation (43). Supportive therapies, including erythropoiesis-stimulating agents and red blood cell and platelet transfusions, contribute to the VTE burden in patients with cancer (44).
Biomarkers and CAT
Several biomarkers have been associated with CAT. High leukocyte and platelet counts and low hemoglobin levels before chemotherapy have been strongly associated with the risk of subsequent VTE (45). These parameters, derived from routinely conducted blood count studies in patients with cancer, are easily available in clinical practice and can be considered cost-effective prognostic and predictive biomarkers (46). D-dimer, a small protein fragment derived by fibrin degradation, has been studied as a predictive biomarker for VTE in cancer. High D-dimer levels are associated with an increased risk of VTE (47). However, D-dimer levels are frequently elevated in patients with cancer and vary between laboratories, and there is a lack of consensus regarding the appropriate cutoff value to be considered as high risk. Further studies are focused on other molecules, including P-selectin and tissue factor–bearing microparticles, and their potential role in VTE prediction. P-selectin has been integrated in risk assessment models (RAMs) together with clinical factors (48). To date, studies assessing the predictive utility of tissue factor-bearing microparticles show conflicting results with the best available data in pancreatic cancer; its utility beyond this disease is unclear (49).
Risk assessment models
RAMs have been developed and validated to determine which patients with cancer are at greater risk for VTE. Published RAMs are reported in Table 2 (50).
Table 2.
Comparison of Risk Assessment Models
| Item | Khorana Score∗ | Vienna CATS Score | PROTECHT Score | CONKO Score |
|---|---|---|---|---|
| Pancreatic or gastric cancer (very-high-risk tumors) | +2 | +2 | +2 | +2 |
| Lung, gynecologic, lymphoma, bladder, or testicular (high-risk tumors) | +1 | +1 | +1 | +1 |
| Pre-chemotherapy Hb of <10 g/dl or erythropoietin-stimulating agents | +1 | +1 | +1 | +1 |
| Pre-chemotherapy white blood cell count of >1 × 109/l | +1 | +1 | +1 | +1 |
| Pre-chemotherapy platelet count of ≥350 × 109/l | +1 | +1 | +1 | +1 |
| Body mass index of >35 kg/m2 | +1 | +1 | +1 | — |
| D-dimer of >1.44 mg/l | — | +1 | — | — |
| Soluble P-selectin of >53.1 ng/l | — | +1 | — | — |
| Platinum-based or gemcitabine chemotherapy | — | — | +1 | — |
| WHO performance status ≥2 | — | — | — | +1 |
CATS = cancer-associated thrombosis score; CONKO = Charité Onkologie; Hb = hemoglobin; PROTECHT = Prophylaxis Thromboembolic Events Chemotherapy; WHO = World Health Organization.
Total score: 0 = low risk; 1 to 2 = intermediate risk; ≥3 = high risk. See https://www.mdcalc.com/khorana-risk-score-venous-thromboembolism-cancer-patients.
The Khorana score (KS) was the first risk prediction model for VTE in ambulatory cancer patients (51). This score relies on 5 variables (type of cancer, components of the complete blood count [hemoglobin, platelet, and white blood cells], and body mass index) to be assessed before the initiation of chemotherapy. Each variable is assigned 1 point, except for the subclass of very high-risk tumors, which counts for 2. The score was derived from a development cohort of 2,701 patients and subsequently internally and externally validated in retrospective and prospective cohorts including more than 35,000 patients (52), and it remains the only risk assessment tool recommended by multiple guidelines (Table 2).
The Vienna CAT score adds D-dimer and soluble P-selectin measurements to the aforementioned 5 variables, improving the positive predictive value (PPV), but this has not yet been validated externally (48). The PROTECHT (Prophylaxis Thromboembolic Events Chemotherapy) study includes platinum-based or gemcitabine-based chemotherapy as additional variables (53); however, the PPV is comparable to the original score. These RAMs, as well as Onkotev and Compass, are not yet validated for use in clinical practice (54,55).
Recently, Pabinger et al. (56) from the Vienna group have proposed a new model that relies on only 2 variables: tumor site (low or intermediate, high, and very high risk) and D-dimer levels as a continuous variable, with varying cutoffs for D-dimer using a nomogram for different sites of cancer (56). This score has been validated using MICA (Multinational Cohort Study to Identify Cancer Patients at High Risk of Venous Thromboembolism), and the cross-validated C-indices of the final model were 0.68 (95% CI: 0.62 to 0.74), improving the PPV for VTE compared to the KS. This tool, however, has not yet been tested in hospitalized patients with cancer nor prospectively in studies of thromboprophylaxis.
Additionally, 2 RAMs have been specifically developed for MM: IMPEDE VTE (Immunomodulatory agent; Body Mass Index ≥25 kg/m2; Pelvic, hip or femur fracture; Erythropoietin stimulating agent; Dexamethasone/Doxorubicin; Asian Ethnicity/ Race; VTE history; Tunneled line/central venous catheter; Existing thromboprophylaxis) and SAVED (Surgery within 90 days, Asian race, VTE history, agE ≥80 years and Dexamethasone dose) (57,58). These have outperformed the current models available for MM and will potentially become new reliable options for risk stratification in this disease.
The most clear use of risk assessment tools is for the identification of high-risk patients for thromboprophylaxis, which we address in a later section. In addition to thromboprophylaxis, risk prediction scores can be used to increase awareness of the risk of VTE in both patients with cancer and providers and to provide targeted education (59). In addition, emerging studies suggest that using the KS can be helpful for the early detection of VTE using screening ultrasonography. Even though international guidelines currently do not address this question, in a multi-institutional trial, undetected VTE was observed in approximately 9% of high-risk patients as identified by a KS of ≥3 (60). A pilot study has shown that an electronic alert can help identify patients for early detection and may potentially prevent emergency department visits and hospital admissions (61). This appears to be a relevant future application of RAMs.
There are currently no validated risk tools to predict ATE in cancer. This remains a critical knowledge gap.
Prevention
Thromboprophylaxis in surgical patients with cancer
Surgery is a well-known risk factor for VTE. All patients with active malignancy undergoing major surgical procedures should be considered for pharmacological thromboprophylaxis, because they are at 2- to 3-fold times the perioperative risk for VTE compared with patients without cancer (62). In-hospital post-operative prophylaxis has long been the standard. More recently, studies have evaluated longer duration of therapy (up to 4 weeks) with in-hospital prophylaxis (7 to 10 days). These randomized trials suggest significantly lower rates of VTE with extended thromboprophylaxis (60% reduction in VTE rates, from 12% to 4.8%) with no differences in outcomes such as major bleeding or death (63).
In summary, current ASCO guidelines for prophylaxis during the perioperative period recommend the following:
-
•
All patients with malignant disease undergoing major surgical intervention should be offered pharmacological thromboprophylaxis with either unfractionated heparin (UFH) 5,000 U 2 to 4 h pre-operatively and every 8 h thereafter or low-molecular-weight heparin (LMWH) 40 mg 2 to 4 h pre-operatively or 10 to 12 h pre-operatively and 40 mg once daily thereafter, unless contraindicated because of active bleeding, high bleeding risk, or other conditions.
-
•
Thromboprophylaxis should be continued for 7 to 10 days, except for those patients who have high-risk features such as restricted mobility, obesity, history of VTE, or other additional risk factors, in whom VTE prophylaxis should be continued for up to 4 weeks. In lower-risk surgical settings, the decision on appropriate duration of thromboprophylaxis should be made on a case-by-case basis (18).
On the other hand, ESMO and ASH guidelines endorse a post-discharge duration of prophylaxis for up to 4 weeks for patients with cancer who undergo a major abdominal/pelvic surgical procedure rather than discontinuation at the time of hospital discharge (20,22).
Unfortunately, there are no validated scores to assess thrombotic or hemorrhagic risk in the oncologic surgery setting specifically.
Thromboprophylaxis in hospitalized patients with cancer
Despite the known high incidence of VTE in the cancer population, thromboprophylaxis in hospitalized patients with malignancy represents a major knowledge gap. Data from the United States DVT Registry found that hospitalized patients with malignancy are actually less likely to receive VTE prophylaxis than their noncancer counterparts (28% vs. 35%) because of the relative contraindications to pharmacological thromboprophylaxis (e.g., thrombocytopenia, active hemorrhage, or high risk for hemorrhage) (64).
Moreover, there are limited data to support the use of antithrombotic prophylaxis and limited data regarding the optimal regimen in hospitalized patients with cancer. Recently, a phase 2 trial conducted by Zwicker et al. (65) confirmed a high incidence of VTE in patients treated with fixed-dose enoxaparin (22% cumulative incidence of DVT) and showed that a weight-adjusted LMWH thromboprophylaxis approach was feasible and safe.
Multiple scoring systems have been proposed to improve VTE prevention, and in this particular setting, the Padua Prediction Score is broadly used. It considers several comorbidities/conditions assigning 3 points to active cancer, previous VTE (with the exclusion of superficial vein thrombosis), reduced mobility, and known thrombophilic condition; 2 points to recent (≤1 month) trauma and/or surgery and 1 point to older age (≥70 years), cardiac and/or respiratory failure, acute MI or ischemic stroke, acute infection and/or rheumatologic disorder, obesity (body mass index >30 kg/m2), and ongoing hormone therapy. The cutoff for high risk was identified as ≥4 points (66).
Unfortunately, even though these scoring systems include cancer diagnosis as a variable, they have been tested primarily in medical hospitalized patients and have not been validated in any specific cancer populations. In addition, evidence from the literature shows that the current prophylactic doses (enoxaparin 40 mg, dalteparin 5,000 IU, fondaparinux 2.5 mg), may not reduce the overall rate of VTE compared with placebo and may be suboptimal for high-risk populations (67). In recent retrospective studies, the ability of the KS to predict VTE in hospitalized patients was demonstrated in a post hoc analysis. Moreover, there was a greater benefit of thromboprophylaxis observed in patients with a high KS (68). Further investigations are needed to incorporate the KS or other RAMs in clinical practice for hospitalized patients with cancer. Two DOACs have recently been approved for inpatient prophylaxis, but data in patients with cancer are lacking, even though newly approved betrixaban showed similar effectiveness in patients with cancer (69, 70, 71). Finally, the duration of prophylaxis is uncertain as well. Patients with active cancer remain at higher VTE risk after discharge, but results from the EXCLAIM (Extended Prophylaxis for Venous ThromboEmbolism in Acutely Ill Medical Patients With Prolonged Immobilization) study did show a statistically significant increase in bleeding risk when antithrombotic prophylaxis was extended up to 28 days (compared to the standard 10 days), without clear benefit in VTE reduction (72).
In summary, despite the lack of specific data in patients with cancer and acknowledging the known high risk of VTE in hospitalized patients with cancer, current ASCO and ASH guidelines extrapolate based on trials of prophylaxis in medically ill patients and recommend the following:
-
•
Hospitalized patients with active malignancy and acute medical illness (heart failure, acute respiratory illness in the presence of chronic lung disease, acute infection, acute rheumatic disorder, and inflammatory bowel disease) or reduced mobility should receive pharmacological thromboprophylaxis in the absence of contraindications.
-
•
Routine pharmacological thromboprophylaxis should not be offered to patients admitted for the sole purpose of minor procedures or chemotherapy infusion, nor to patients undergoing stem cell/bone marrow transplantation (18,22).
Thromboprophylaxis in ambulatory patients with cancer
Ambulatory is defined as the period of time during which a patient is not hospitalized for surgery or medical illness or receiving end-of-life care but is in the community receiving anticancer therapy as an outpatient. Up to 74% of all cancer-associated thrombotic events occur in this setting (73). A retrospective analysis from Lyman et al. (74) from the United States IMPACT health care insurance reports that the cumulative incidence of VTE 3.5 months after starting chemotherapy was 7.3% (range: 4.6% to 11.6%) and was 13.5% by 12 months (range: 9.8% to 21.3%), varying widely depending on cancer site (74).
Starting in the 1990s, a study from Levine et al. (75) first investigated thromboprophylaxis in cancer outpatients. They showed that low-dose warfarin in women with metastatic breast cancer was associated with an 85% reduction in relative risk for VTE, with no increase in bleeding rate, compared to the control arm. More recently, several studies addressed the question of thromboprophylaxis in the outpatient setting, enrolling broad populations with different types of malignancies, with a focus on specific cancers carrying a high risk for VTE such as pancreatic cancer or MM.
The PROTECHT (Prophylaxis Thromboembolic Event Chemotherapy) study included patients with lung, breast, GI, head and neck, and ovarian cancers randomly assigned to receive daily subcutaneous nadroparin (3,800 U) or placebo. Rates of VTE in high-risk patients were 11.1% with placebo and 4.5% with nadroparin (number needed to treat [NNT] 15 vs. 77 in low- and intermediate-risk patients) without increasing the risk of major or clinically relevant nonmajor bleeding (CRNMB) (53). Similar results were observed in the SAVE-ONCO (Semuloparin for Thromboprophylaxis in Patients Receiving Chemotherapy for Cancer) trial, in which patients with any metastatic or locally advanced solid tumors starting chemotherapy were randomly divided to receive the ultra-low-molecular-weight heparin semuloparin or placebo. Despite the low rate of events in the control arm (3.4%), the study demonstrated a significant reduction in the incidence of VTE in patients receiving semuloparin (1.2%), with no increase in the incidence of major bleeding (76). A subgroup analysis of this trial showed NNTs of 25 for high-risk patients (defined as KS of ≥3) and 333 for low-risk patients. A recently updated Cochrane review stated that primary thromboprophylaxis with LMWH significantly reduced the rate of symptomatic VTE in ambulatory cancer patients receiving chemotherapy compared to no prophylaxis (risk reduction [RR]: 0.54; 95% CI: 0.38 to 0.75) and demonstrated that assuming a risk of 7.1 symptomatic VTE events per 100 patients, 30 (95% CI: 23 to 56) patients would need to be treated to prevent a single event (77). Those results confirm, once again, the need to stratify the thromboembolic risk in these patients to obtain the greatest benefit/risk ratio.
Strong evidence on the benefits of anticoagulation in high-risk populations has been gathered by studies focused on high thromboembolic risk tumors. For instance, the PROSPECT-CONKO-004 (Prospective, Randomized Trial of Simultaneous Pancreatic Cancer Treatment With Enoxaparin and Chemotherapy) trial was designed to analyze the efficacy of enoxaparin in patients with locally advanced or metastatic pancreatic cancer undergoing systemic chemotherapy. It demonstrated a reduction in the VTE rate from 9.87% to 1.25% at 3 months and from 15.13% to 5% at 12 months (78). Another study of patients with pancreatic cancer, FRAGEM (A Phase II Randomized Study of Chemo-Anticoagulation [Gemcitabine–Dalteparin] Versus Chemotherapy Alone [Gemcitabine] for Locally Advanced and Metastatic Pancreatic Adenocarcinoma), showed a reduction in the rate of VTE from 23% to 3.4% in the intervention, with an NNT of only 6 (79). Similar evidence has been observed in MM; an Italian study compared the efficacy and safety of thromboprophylaxis with low-dose aspirin or LMWH in patients with newly diagnosed MM treated with lenalidomide and showed a reduction in VTE rate, without major hemorrhagic complications, both for LMWH and aspirin (80). MM is the only malignancy in which aspirin thromboprophylaxis is recommended.
DOACs, particularly the factor Xa inhibitors apixaban, rivaroxaban, and edoxaban, are being widely investigated for use in patients with cancer. Currently, all 3 factor Xa inhibitors are approved by regulatory agencies for treatment of CAT, but they are not broadly licensed for primary prophylaxis of VTE, except after elective major orthopedic surgery or in specific scenarios, as described below. Dosing regimens for prophylaxis and treatment of VTE of approved DOACS are summarized in Table 3. Data on their efficacy and safety for primary prevention of VTE in patients with cancer emerged in early 2019, when results from 2 large randomized controlled trials became available.
Table 3.
Direct Oral Anticoagulants Dosing Regimens for Prophylaxis and Treatment of Venous Thromboembolism
| Drug | Prophylaxis | Treatment |
|---|---|---|
| Apixaban | 2.5 mg orally twice daily | 10 mg twice daily for the first 7 days, followed by 5 mg twice daily |
| Rivaroxaban | 10 mg orally once daily | 15 mg orally every 12 h for 21 days, followed by 20 mg once daily |
| Edoxaban | Not applicable | 60 mg daily after at least 5 days of low-molecular-weight heparin |
The CASSINI (Rivaroxaban for Thromboprophylaxis in High-Risk Ambulatory Patients with Cancer) trial enrolled 841 high-risk ambulatory cancer patients (defined as KS of ≥2) negative for DVT at baseline screening, to randomly receive rivaroxaban 10 mg daily or placebo for up to 6 months (81). Patients with primary or metastatic brain cancer and those at high risk of bleeding were excluded. More than 50% of the study population had a GI malignancy (pancreas: 32.8%; gastric or gastroesophageal: 20.7%). Over the entire 6-month follow-up, the composite primary endpoint of objectively confirmed DVT-, PE-, and VTE-related death occurred in 6.0% of patients in the rivaroxaban arm and 8.8% in the placebo group (HR: 0.66; 95% CI: 0.40 to 1.09; p = 0.10). Even though not unexpected in this population, 47% of enrolled patients prematurely discontinued the study drug (either rivaroxaban or placebo); however, during the on-treatment period, patients on rivaroxaban experienced a lower number of primary endpoint events compared to patients on placebo (HR: 0.40; 95% CI: 0.20 to 0.80; p = 0.007; NNT: 26).
Major bleeding and CRNMB did not differ in the 2 arms (rivaroxaban/placebo) (HR: 1.96; 95% CI: 0.59 to 6.49; p = 0.26 and HR: 1.34; 95% CI: 0.54 to 3.32; p = 0.53, respectively). Estimates suggested a potential benefit on mortality in the rivaroxaban arm, although these were not statistically significant (20.0% in patients on rivaroxaban vs. 23.8% in patients on placebo; HR: 0.83; 95% CI: 0.62 to 1.11; p = 0.21).
The AVERT (Apixaban to Prevent Venous Thromboembolism in Patients with Cancer) trial assessed the efficacy and safety of apixaban 2.5 mg twice daily for thromboprophylaxis in ambulatory patients with cancer who were at high risk for VTE (defined similarly to CASSINI trial as a KS of ≥2) (82). A total of 574 patients were enrolled in the 2 arms, without baseline screening for prevalent VTE. Unlike in CASSINI, patients with primary brain tumors and myeloma were not excluded. Approximately one-half of the population had a diagnosis of lymphoma (26.1%) or gynecologic malignancies (25.4%). At the 6-month follow up, the rate of objectively confirmed VTE (primary efficacy outcome) was significantly lower in the apixaban arm compared to placebo (4.2% vs. 10.2%; HR: 0.41; 95% CI: 0.26 to 0.65; p < 0.001). The rate of major bleeding (primary safety outcome) was significantly higher in patients randomized to receive apixaban compared to placebo (3.5% vs. 1.8%; HR: 2.00; 95% CI: 1.01 to 3.95; p = 0.046), whereas CRNMB events were similar between the 2 arms (7.3% in the apixaban arm vs. 5.5% in the placebo arm; HR: 1.28; 95% CI: 0.89 to 1.84). Rates of death from any cause were similar between the 2 treatment arms, but a higher rate was observed in the intervention arm (12.2% for apixaban vs. 9.8% for placebo arm; HR: 1.29; 95% CI: 0.98 to 1.71). Further evidence will follow these randomized controlled trials for including the prophylactic use of DOACs in clinical practice in cancer outpatients. The characteristics and results of both studies are synthesized in Table 4.
Table 4.
Study Characteristics and Results for the CASSINI and AVERT Trials for VTE Prophylaxis
| Study | CASSINI | AVERT |
|---|---|---|
| Patients | 841 patients with cancer and a KS of ≥2 Patients with primary or metastatic brain cancer and those at risk for bleeding were excluded |
574 patients with cancer and a KS of ≥2 Nonmelanoma skin cancers, acute leukemia, myeloproliferative neoplasms, and those at high risk for bleeding were excluded |
| Type of cancers | Solid tumors and lymphomas | Solid and primary brain tumors, lymphomas, and myeloma |
| Baseline screening | Yes | No |
| Duration, days | 180 | 180 |
| Treatment Arms | Rivaroxaban 10 mg Daily |
Placebo | Apixaban 2.5 mg Twice Daily |
Placebo |
|---|---|---|---|---|
| VTE, % | 6.0 | 8.8 | 4.2 | 10.2 |
| HR (95% CI); p value | 0.66 (0.40–1.09); p = 0.10 | 0.41 (0.26–0.65); p < 0.001 | ||
| Major bleeding, % | 2.0 | 1.0 | 3.5 | 1.8 |
| HR (95% CI); p value | 1.96 (0.59–6.49); p = 0.26 | 2.00 (1.01–3.95); p = 0.046 | ||
| CRNMB, % | 2.7 | 2.0 | 7.3 | 5.5 |
| HR (95% CI); p value | 1.34 (0.54–3.32); p = 0.53 | 1.28 (0.89–1.84); p = NR | ||
| Mortality, % | 20.0 | 23.3 | 12.2 | 9.8 |
| HR (95% CI); p value | 0.83 (0.62–1.11); p = 0.21 | 1.29 (0.98–1.71); p = NR | ||
| Mortality benefit | Potential | No | ||
AVERT = Apixaban to Prevent Venous Thromboembolism in Patients with Cancer; CI = confidence interval; CRNMB = clinically relevant nonmajor bleeding; HR = hazard ratio; KS = Khorana score; NR = not reported; VTE = venous thromboembolism.
In summary, for ambulatory cancer patients, currently available international guidelines, including the most recent guidelines published by ASH in 2021, suggest the following:
-
•
Routine pharmacological thromboprophylaxis should not be offered to all outpatients with cancer.
-
•
High-risk outpatients with cancer (KS of 2 or higher before starting a new systemic chemotherapy regimen) may be offered thromboprophylaxis with apixaban, rivaroxaban, or LMWH, provided there are no significant risk factors for bleeding and no drug-drug interactions. The decision should be accompanied by a discussion with the patient about the relative benefits and harms, drug cost, and duration of prophylaxis.
-
•
Patients with MM receiving thalidomide or lenalidomide (in combination with dexamethasone) should receive thromboprophylaxis with aspirin or LMWH in low-risk situations and LMWH in high-risk ones (18,22).
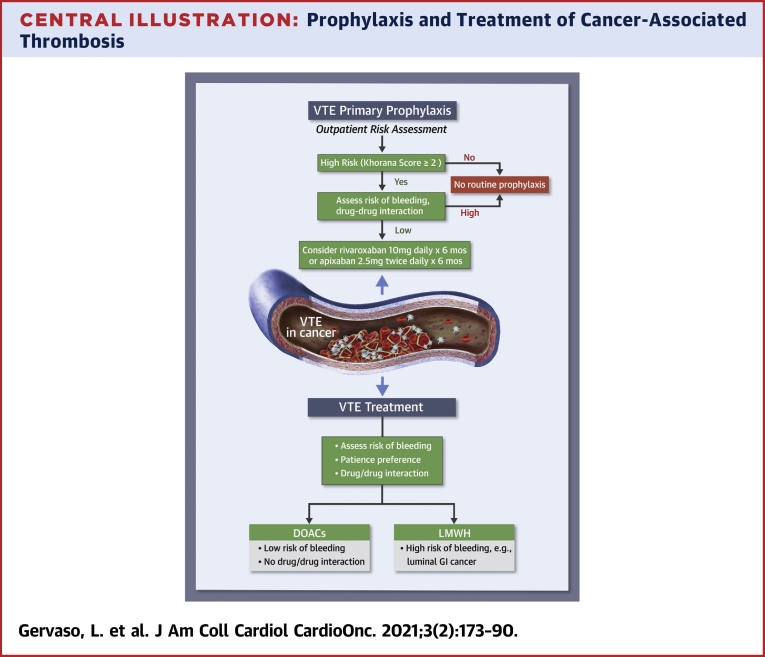
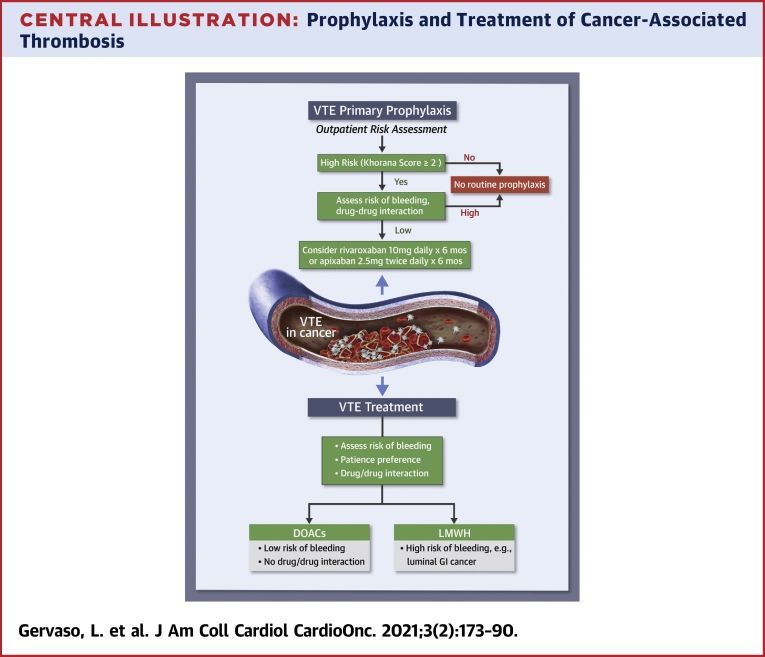
The Central Illustration outlines key points to evaluate for suggesting thromboprophylaxis in ambulatory patients with cancer.
Central Illustration.
Prophylaxis and Treatment of Cancer-Associated Thrombosis
The choice of pharmacological anticoagulation in patients with cancer should be based on several aspects, which include type of cancer, risk of bleeding, drug-drug interactions, and patient preferences. DOAC = direct oral anticoagulant; LMWH = low molecular weight heparin; VTE = venous thromboembolism.
Treatment and Secondary Prevention
Choice and duration of treatment
Appropriate treatment of VTE in patients with cancer is crucial, because of the negative impact on survival and the high rate of complications, including recurrent VTE and bleeding (up to 12% and 21% yearly, respectively) (7). Different therapeutic options are currently available for patients. Traditionally, cancer-associated VTE has been treated with vitamin K antagonists (VKAs), even though a growing body of evidence suggests a possible resistance to warfarin. LMWHs have shown to be superior to VKAs, and they are the cornerstone for treatment of thromboembolic events in cancer over the last 2 decades. The seminal CLOT (Randomized Comparison of Low-Molecular-Weight Heparin Versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients With Cancer) study randomly assigned 672 patients with cancer and acute symptomatic VTE to receive initial treatment with dalteparin at a dose of 200 IU/kg subcutaneous once daily for 5 to 7 days, followed by a coumarin derivative with a target international normalized ratio of 2.5 or therapeutic dalteparin (200 IU/kg once daily for the first month, then 150 IU/kg) for 6 months (83). During the 6-month study period, 8.0% of patients in the dalteparin group had recurrent VTE compared to 15.8% of patients in the VKA group (HR: 0.48; 95% CI: 0.30 to 0.77, p = 0.002). No significant difference was observed between the 2 groups in the rates of major and any bleeding. A decade later, in 2015, the CATCH (Comparison of Acute Treatments in Cancer Haemostasis) trial compared the LMWH tinzaparin at a dose of 175 IU/kg once daily for 6 months with tinzaparin initially for 5 to 10 days, followed by transition to warfarin with a target international normalized ratio of 2 to 3. Similar to the prior study, the rate of VTE decreased from 10% in the warfarin group to 6.9% in the tinzaparin group, although this was not statistically significant (HR: 0.65; 95% CI: 0.41 to 1.03; p = 0.07). Major bleeding rates were similar in the 2 arms, while CRNMB events were significantly lower in the tinzaparin group (11% and 16%; p = 0.03) (84).
On the basis of the CLOT trial, and as confirmed by a Cochrane review (85), LMWH is recommended as the first-line therapy for the short- and long-term management of CAT by different international guidelines (18,20). However, subcutaneous administration is often an obstacle for patient compliance, and moreover, renal insufficiency and cost are limitations for their use. Indeed, although not recommended as the preferred treatment in cancer VTE, VKAs are still widely used, given the oral route of administration and the relatively low cost. A retrospective analysis from Khorana et al. (86) including more than 100,000 medical prescriptions for VTE in patients with cancer showed that oral agents, and in particularly warfarin, are the most commonly used anticoagulants, accounting for approximately 50% of the total, with LMWH in 40% and DOACs in approximately 10% (86).
DOACs are currently recommended as a the first-line treatment for acute DVT and PE in patients without cancer, but for a long time, because of a lack of data on efficacy and safety, their use was not recommended in patients with cancer. However, with the publication of 3 dedicated cancer trials, head-to-head comparisons between DOACs and standard antithrombotic therapy are now available (87). The HOKUSAI-VTE (Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism) Cancer trial was a noninferiority trial that randomized 1,050 patients with cancer and with acute symptomatic or incidental VTE to receive edoxaban (60 mg daily after at least 5 days of LMWH therapy) or dalteparin (200 IU/kg daily for 1 month, followed by 150 IU/kg daily) for up to 6 to 12 months with a minimum duration of follow-up of 9 months (88). The primary endpoint (composite endpoint of the first recurrent VTE or major bleeding within 12 months) occurred in 12.8% of patients in the edoxaban arm compared to 13.5% in the dalteparin arm (HR with edoxaban: 0.97; p = 0.006 for noninferiority). Edoxaban was noninferior to dalteparin regardless of treatment duration (HR: 0.97; 95% CI: 0.70 to 1.36; p = 0.006 for noninferiority). The rates of recurrent VTE did not differ between the edoxaban and dalteparin groups (7.9% vs. 11.3%; HR: 0.71; 95% CI: 0.48 to 1.06; p = 0.09), whereas the rate of major bleeding was significantly higher with edoxaban compared to dalteparin (6.9% vs. 4.0%, respectively; HR: 1.77; 95% CI: 1.03 to 3.04; p = 0.04), with a predominant occurrence in patients with GI cancer, both resected and unresected (12.5% vs. 3.6%; HR: 4.0; 95% CI: 1.5 to 10.6; p = 0.005).
Further evidence has been derived from the SELECT-D (Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism) randomized trial (89). A total of 406 patients with symptomatic or incidental VTE were randomized to receive rivaroxaban (15 mg twice daily for 3 weeks followed by 20 mg once daily) or dalteparin (200 IU/kg daily for 1 month followed by 150 IU/kg daily) for 6 months. At the 6-month follow-up, the cumulative rate of recurrent VTE was significantly lower in the rivaroxaban arm compared to dalteparin (4% vs. 11%; HR: 0.43; 95% CI: 0.19 to 0.99). The cumulative rate of major bleeding was not different between the 2 treatment groups (6% vs. 4%, respectively; HR: 1.83; 95% CI: 0.68 to 4.96), while the rate of CRNMB was significantly higher in patients treated with rivaroxaban (13% vs. 4%, respectively; HR: 3.76; 95% CI: 1.63 to 8.69). Similar to the HOKUSAI trial, most major bleeding events in the rivaroxaban group (7 of 11) tended to occur in GI malignancies, and CRNMB also occurred in GI (9 of 25) or genitourinary (11 of 25) sites. Specifically, esophageal and gastroesophageal cancers seemed to be at high risk, with a 3-fold increased rate of major bleeding with rivaroxaban compared to dalteparin (36% vs. 11%). Data from those studies reflect real-world clinical practice; more than half of the patients, indeed, had metastatic disease (53% and 58%, respectively), and approximately 70% of them were receiving active anticancer treatment while in the trial. Moreover, the rates of VTE in the LMWH arms of the HOKUSAI-VTE and SELECT-D trials (11.3% and 11%, respectively) were consistent with those reported in the landmark CLOT and CATCH trials (9% and 7.2%, respectively), as was major bleeding events (4% for both HOKUSAI-VTE and SELECT-D vs. 6% and 3% for CLOT and CATCH, respectively).
Most recently, the Caravaggio trial randomized 1,155 patients with cancer with a diagnosis of symptomatic or incidental acute proximal DVT or PE to receive either apixaban (at a dose of 10 mg twice daily for the first 7 days, followed by 5 mg twice daily) or subcutaneous dalteparin (at a dose of 200 IU/kg of body weight once daily for the first month, followed by 150 IU/kg once daily). Population characteristics were similar to those of prior studies, with approximately 60% undergoing concurrent active anticancer treatments; the most common malignancies were colorectal and lung, accounting for approximately 40% of the total in both arms. The primary outcome of recurrent VTE occurred in 5.6% in the apixaban group and in 7.9% in the dalteparin group (HR: 0.63; 95% CI: 0.37 to 1.07; p < 0.001 for noninferiority). Major bleeding, the primary safety outcome, occurred in 3.8% in the apixaban group and in 4.0% in the dalteparin group (HR: 0.82; 95% CI: 0.40 to 1.69; p = 0.60); these results are in contrast to previous studies, especially for GI bleeding, even though this was not a prespecified trial outcome. Studies characteristics and results are summarized in Table 5.
Table 5.
Study Characteristics and Results for Hokusai Cancer VTE, SELECT-D, and Caravaggio Studies for VTE Treatment
| Hokusai Cancer VTE | SELECT-D | Caravaggio | |
|---|---|---|---|
| Population | 1,046 patients with cancer and acute symptomatic or incidentally detected VTE | 406 patients with cancer and acute symptomatic or incidentally detected VTE | 1,155 patients with cancer and a diagnosis of symptomatic or incidental acute proximal DVT or PE |
| Duration, months | 12 | 6 | 6 |
| Treatment Arms | Edoxaban | Dalteparin | Rivaroxaban | Dalteparin | Apixaban | Dalteparin |
|---|---|---|---|---|---|---|
| Metastatic disease, % | 52.5 | 53.4 | 58 | 58 | 67.5 | 68.4 |
| Concurrent anticancer treatment, % | 71.6 | 73.1 | 69 | 70 | 60.8. | 63.4 |
| Recurrent VTE, % | 7.9 | 11.3 | 4 | 11 | 5.6 | 7.9 |
| HR (95% CI); p value | 0.71 (0.48–1.06); p = 0.09 | 0.43 (0.19–0.99); p = NR | 0.63 (0.37–1.07); p < 0.001 | |||
| Major bleeding, % | 6.9 | 4.0 | 6 | 4 | 3.8 | 4.0 |
| HR (95% CI); p value | 1.77 (1.03–3.04); p = 0.04 | 1.83 (0.68–4.96); p = NR | 0.82 (0.40–1.69); p = 0.60 | |||
| CRNMB, % | 14.6 | 11.1 | 13 | 4 | 9.0 | 6.0 |
| HR (95% CI); p value | 1.38 (0.98–1.94); p = NR | 3.76 (1.63–8.69); p = NR | 1.42 (0.88–2.30); p = NR | |||
| Mortality, % | 39.5 | 36.6 | 25 | 30 | 23.4 | 26.4 |
| HR (95% CI); p value | 1.12 (0.92–1.37); p = NR | NR | 0.82 (0.62–1.09); p = NR | |||
SELECT-D = Anticoagulation Therapy in Selected Cancer Patients at Risk of Recurrence of Venous Thromboembolism; other abbreviations as in Table 4.
Despite the small sample size, the results from the pilot ADAM-VTE (Apixaban and dalteparin in active malignancy-associated venous thromboembolism) trial had a similarly favorable risk–benefit ratio for apixaban in the therapeutic setting, with a major bleeding rate (the primary endpoint) that was no different between the 2 groups (0% in the apixaban arm vs. 1.4% in the dalteparin arm; p = 0.138) and a VTE recurrence rate fairly lower for apixaban (0.7% vs. 6.3%; HR: 0.099; 95% CI: 0.013 to 0.780; p = 0.0281) (89).
Based on these data, ASCO guidelines state that for long-term anticoagulation, LMWH, edoxaban, or rivaroxaban for at least 6 months is preferred because of improved efficacy over VKAs. VKAs are inferior but may be used if LMWH or DOACs are not accessible. There is an increase in major bleeding risk with DOACs, particularly observed in GI and potentially genitourinary malignancies (except in the Caravaggio trial, although the GI cancer subgroup data have not yet been published). Caution with DOACs is also warranted in other settings with high risk for mucosal bleeding. Drug-drug interactions should be evaluated before using a DOAC, considering that rivaroxaban and apixaban should not be used concomitantly with potent inhibitors or inducers of P-glycoprotein or cytochrome P450 3A4 (18).
The ideal duration of anticoagulation has not been assessed, but based on available evidence, current guidelines recommend LMWH use (over VKAs) for a minimum of 6 months to treat established VTE in patients with cancer. An extended duration of anticoagulant therapy has been proposed for patients with active cancer, because the risk of recurrent VTE remains high as long as the cancer is active, and stopping anticoagulation for reasons other than major bleeding leads to a higher rate of recurrence in the active cancer patient cohort (90). Only 2 prospective multicenter studies (DALTECAN [Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months], TiCAT [Tinzaparin in cancer associated thrombosis beyond 6 months]) have assessed the safety and efficacy of extended therapy with LMWH up to 12 months in patients with cancer and acute VTE (91,92). Safety was appropriate in both studies, and the rate of recurrent VTE decreased from 4.5% to 5.7% to approximately 1% during months 7 to 12. Overall, these results show a possible favorable risk-benefit ratio for extended treatment. On the other hand, whatever drug is used, treatment for cancer-associated VTE is also burdensome for patients, and the indication to continue antithrombotic therapy until the cancer is active often translates into lifelong anticoagulation. The need for prolonged anticoagulation should be periodically re-evaluated by assessing additional risk factors, such as metastasis or progressive disease, prior history of VTE, ongoing systemic chemotherapy or prothrombotic regimens, and risk of bleeding.
In summary, for the choice and the duration of treatment, current ASCO guidelines suggest the following:
-
•
Initial anticoagulation may include LMWH (preferred over UFH if renal function is normal), fondaparinux, or rivaroxaban.
-
•
LMWH, edoxaban, or rivaroxaban for at least 6 months is preferred for long-term anticoagulation over VKAs. DOACs are associated with an increased risk of major bleeding, particularly for GI malignancies.
-
•
Anticoagulation beyond the initial 6 months should be considered for patients with metastatic cancer and/or on active cancer treatment, with periodic reassessment of the risk/benefit ratio.
The main aspects to consider in the decision-making process for CAT treatment are summarized in the Central Illustration.
Incidental VTE
Incidental VTE, defined as VTE discovered on scans ordered for other reasons (typically cancer staging or restaging) without any clinical suspicion at the time of diagnosis, contribute to up half of all VTE events in patients with cancer (93). In addition to PE and DVT, incidental findings also include VVT. In a specific cohort of patients with GI malignancies, DVT was incidentally discovered in one-half of the patients and PE in 35% of the total, while the rest were asymptomatic central catheter thrombosis (94). Management of these events remains controversial. Many retrospective studies and registries suggest similar rates of mortality and recurrence between asymptomatic and symptomatic VTE (95).
International guidelines recommend the same initial and long-term anticoagulation for incidental PE as for patients with symptomatic PE. According to a recent review published by the ASH (96), management of incidental VTE should differ according to the location of the thrombotic event. Anticoagulation is clearly recommended for proximal DVT, segmental PE (SPE), and multiple subsegmental PE (SSPE) because of their negative impact on prognosis. However, for isolated SSPE without an ultrasound-detected lower limb DVT, clinical and radiographic monitoring alone can be considered on a case-by-case evaluation. Management of isolated distal DVT is also uncertain; 2 studies evaluated the clinical course of symptomatic distal DVT in patients with cancer (97,98) and showed a similar risk of death, recurrence, and major bleeding compared to proximal DVT. Even though incidental distal DVT was not specifically evaluated, these findings suggest that distal DVT may worsen prognosis in patients with cancer, and a course of anticoagulation could be preferable over a watchful approach. More evidence is required to understand the complete benefit, treatment dose, and duration. Finally, VVT may benefit from anticoagulant treatment in patients without high risk of bleeding, but there are no data. Guidelines support a case-by-case decision (96).
In summary, guidelines recommend the following:
-
•
Incidental VTE events should be treated in the same manner as symptomatic events given their similar clinical outcomes, with the exception of isolated SSPE.
Recurrent VTE during anticoagulation
Recurrent VTE despite appropriate anticoagulation is, unfortunately, not rare among patients with cancer. Lack of compliance, temporary cessation of therapy because of bleeding or procedures, inadequate dosing, cancer progression, or heparin-induced thrombocytopenia are possible reasons for VTE recurrence. Very limited evidence is available, and an empirical approach has been proposed by the ISTH (99).
LMWH is considered the preferred approach. Patients who experience recurrent VTE should be transitioned to therapeutic LMWH if on treatment with UFH, VKA (in range), or DOACs. patients with cancer and symptomatic recurrent VTE despite optimal anticoagulation with LMWH should continue with LMWH at a higher dose, starting with an increase of 25% of the current dose or resuming the therapeutic weight-adjusted dose if the patient has been receiving a nontherapeutic dose. If there is an observed improvement, the same dose of LMWH should be used. Further escalation in case of no clinical improvement could be done based on anti-Xa peak levels (99). The utilization of a vena cava filter is also suggested for certain situations (18).
In summary, specific recommendations for these clinical scenarios are not evidence-based, and the strength is weak; however, the ISTH recommends the following:
-
•
Patients with recurrent VTE despite therapeutic anticoagulation should be treated with LMWH if they are being managed on other anticoagulants, or they should continue LMWH at a higher dose, starting with a 25% increase of the current dose.
Special Situations With High Bleeding Risk
Thrombocytopenia
Thrombocytopenia, defined as a platelet count of <100 × 109/l, is a common complication in patients with cancer, affecting a large majority of patients receiving certain chemotherapy regimens, especially those with hematologic malignancies undergoing hematopoietic stem cell transplantation. Despite the higher bleeding risk, thrombocytopenia is not associated with a reduction of thromboembolic risk. In addition, prolonged thrombocytopenia (over 30 days) is associated with a 4-fold increased risk of recurrent VTE, as showed in a retrospective study (100).
The main challenge for CAT risk management in the setting of recurrent VTE is balancing the opposing risks of bleeding and VTE recurrence. Several aspects should be considered for assessing individual risk of recurrence, including thrombosis burden (size, location), time from event, history of VTE, and etiology. For instance, catheter-related thrombosis is associated with lower rates of recurrence or PE than other thromboembolic events. Similarly, distal DVT and incidental SSPE appear to be lower-risk events (101). On the other hand, bleeding is more frequent in the case of allogeneic hematopoietic stem cell transplantation, history of concurrent coagulopathy, and liver or renal impairment. However, the risk of bleeding is poorly and inconclusively defined for this population, especially for platelet counts between 10 × 109/l and 50 × 109 /l.
According to the recent recommendations from the Scientific and Standardization Committee (SSC) of the ISTH (102), because of the higher risk of VTE recurrence during the acute phase (<30 days from the event), full-dose anticoagulation is recommended for patients with a platelet count of ≥50 × 109/l. However, once platelet counts decline below this level, alternative strategies should be considered.
For patients with symptomatic SPE or more proximal PE, proximal DVT, or history of recurrence, full anticoagulation associated with platelet transfusion (threshold ≥40 × 109/l) may be indicated. Conversely, for distal DVT, incidental SSPE, and catheter-related thrombosis, a dose-modification strategy using 50% or prophylactic-dose LMWH is a feasible option for a platelet count between 25 × 109/l and 50 × 109/l. Generally, with this strategy, anticoagulation is discontinued under 25 × 109/l, although in some special situations, prophylactic doses could be used at ≥10 × 109/l.
The dose modification strategy is derived from expert consensus and uses different approaches, including empirical reductions with relatively weaker data. A recent systematic review by Samuelson et al. (103) also addressed this issue and did not identify any evidence for the superiority of one approach over another (103).
The risk of recurrence decreases after the initial 30-day period. Therefore, in this subacute or chronic period, anticoagulation could be reduced to lower the risk of bleeding and avoid unnecessary transfusions. In particular, decreased dosing (50% or prophylactic doses of LMWH) is suggested for platelet counts between 25 and 50 × 109/l, whereas temporary discontinuation should be considered for platelet counts of <25 × 109/l. In certain patients at low risk of recurrent thrombosis, withholding anticoagulation for the entire period of thrombocytopenia (platelet count of <50 × 109/l) may be effective and safe.
LMWH is currently the preferred anticoagulant among patients with thrombocytopenia. Data on the use of DOACs in CAT patients with severe thrombocytopenia (<50 × 109/l) are lacking, even though some evidence is emerging (104). So far, based on current data, inferior vena cava filter insertion should be considered only in patients with absolute contraindications to anticoagulation (105). According to guidelines, patients who experienced VTE and low platelet count (<50 × 109/l) should be treated with full anticoagulation and possibly with platelet transfusion during the first 30 days after diagnosis of VTE. Prophylactic anticoagulant dose may be effective and safe during the chronic phase of VTE for platelet counts between 25 × 109/l and 50 × 109/l.
Intracranial lesions
Patients with brain tumors have among the highest VTE rates of all patients with cancer, with a similar incidence as those with pancreatic and gynecologic malignancies. Symptomatic VTE develops in 19% to 29% of patients with gliomas, the most common type of primary brain tumor. Systematic reviews on the association between intracranial lesions and incidence of VTE have not been performed. Confidence in the actual estimate is low, but a small, retrospective study with major methodological weaknesses that evaluated the VTE rate in 42 patients treated at a single center from 1992 to 2001 indicated that the VTE rate could be as high as 60% (25 of 42) in primary central nervous system (CNS) lymphoma (106), significantly higher compared to the 6% to 7% rate observed in other types of lymphoma (107). Moreover, it is much more common is cerebral metastases, in which case approximately 20% develop VTE. Although the majority of thrombotic events occur post-operatively, VTE risk persists throughout the clinical course. In a prospective study by Brandes et al. (108) of 77 patients with CNS tumors that had been followed up for more than 2 years after surgery, the risk of DVT was up to 32% at 24 months. Primary prophylaxis is not currently recommended in this population.
The management of VTE in these patients is complicated by multiple factors, including comorbidities and poor performance status, drug interactions, and, primarily, the possibility of intracranial hemorrhage (ICH), which can be life threatening. Little data exist to help decision making because large prospective trials about anticoagulation generally have excluded patients with intracranial tumors. In the CLOT trial, only 27 patients had brain tumors, and 2 of them developed intracranial bleeding complications. Caution in prescribing anticoagulation in the presence of brain metastases is warranted based on the high rate of spontaneous ICH, especially in certain tumor types such as non–small-cell lung cancer or renal cell carcinoma (109).
A retrospective case-control study by Donato et al. (110) tried to specifically determine whether a therapeutic dose of anticoagulation increased the risk of ICH. They analyzed 104 patients with parenchymal CNS metastasis from solid tumors and VTE receiving therapeutic enoxaparin, matched with 189 control individuals without any anticoagulant therapy. Primary brain tumor and hematologic malignancies were excluded. ICH was defined as measurable when the event was >1 ml in volume and as trace for <1 ml. Furthermore, each bleeding was classified as significant if >10 ml in volume, as symptomatic (the presence of neurologic deficit, headache, or nausea or change in cognitive function), or as requiring surgical intervention, according to current definitions (111). Results from this study described a cumulative incidence of measurable ICHs at 1 year of 19% in the enoxaparin cohort and 21% in the control cohort, with no statistical difference (HR: 1.02; 90% CI: 0.66 to 1.59; p = 0.97). No statistical differences were observed when considering individual malignancies, with a similar rate of events in the enoxaparin and control groups (42% vs. 33% for total bleeds, respectively; p = 0.23) for non–small-cell lung cancer. Similarly, overall survival was similar in the enoxaparin and control groups (8.4 vs. 9.7 months; p = 0.65). Data derived from this study provide reassurance that LMWH may be safely administered to patients with metastatic brain tumors, without increasing the likelihood of ICH.
Current ASCO guidelines do not consider intracranial lesions as an absolute contraindication for anticoagulation but recommend a case-by-case choice for the best therapeutic strategy.
Arterial Thrombosis Treatment
There are limited data that sufficiently address the management of cardiac ischemic disease in patients with cancer. Medical therapy (including antiplatelet agents) and catheter-based revascularization (i.e., percutaneous coronary intervention) are the cornerstones for ATE treatment in patients both with and without cancer. Attention should be paid to the bleeding risk, because thrombocytopenia is more common in patients with cancer because of chemotherapy or bone marrow failure. In a study of patients without cancer presenting with an acute coronary syndrome, baseline thrombocytopenia was associated with a higher rate of complications compared to patients without thrombocytopenia (30-day death rate: 6.2% vs. 2.1%; major bleeding: 11.9% vs. 7%; major cardiac events 9.6% vs. 5.2%; major cardiac events plus major bleeding: 18.5% vs. 10.8%) (112). For these reasons, the standard approaches to treating a MI, such as antiplatelet, anticoagulant, and thrombolytic therapies exacerbate bleeding risk and, consequently, are typically withheld from patients with thrombocytopenia. However, considering the high mortality rate of ATE, the Society for Cardiovascular Angiography and Interventions Expert Consensus Statement (113), encourages a reduced platelet count threshold for cardiovascular therapies, recommending aspirin initiation in patients if platelet counts are >10,000/ml and dual antiplatelet therapy initiation (with aspirin and clopidogrel) if platelet counts are >30,000/ml. Because of a lack of evidence, prasugrel, ticagrelor, and glycoprotein IIb/IIIa inhibitors should not be used in patients with platelet counts of <50,000/ml.
Revascularization is imperative in the setting of critical ischemia or infarction. Based on the Society for Cardiovascular Angiography and Interventions expert consensus, there is no platelet count limit for diagnostic left heart catheterization (66). Moreover, platelet transfusion is not recommended prophylactically in patients with cancer undergoing cardiac catheterization with thrombocytopenia, unless platelet counts are <20,000/ml and the multidisciplinary discussion, including the oncology/hematology team, recommends transfusion.
There are numerous opportunities for further investigations into ATE in patients with cancer. One important question that should be addressed is whether antiplatelet therapy or anticoagulation can be effective in the prevention of ATE. Aspirin, for example, has been shown to decrease the rates of arterial thrombosis in polycythemia vera and MM (114,115). However, whether we can prevent arterial thrombi in other cancers or prevent treatment-related ATE is unknown. Recent subgroup data from the CASSINI trial show that rivaroxaban is also effective in reducing ATE (0.5% in rivaroxaban group vs. 1.2% in the placebo group; HR: 0.39; 95% CI: 0.08 to 2.03). This finding potentially strengthens the case for primary prophylaxis in high-risk patients with cancer. Optimal surveillance strategies for arterial thromboembolic disease remain unclear. There are many imaging modalities for identifying arterial disease; the role of positron emission tomography–computed tomography scanning, for instance, has been assessed to try to identify patients who should be started on a statin before chemotherapy based on the presence of coronary calcium, which may potentially be predictive of cardiac events (116). However, which patients should be screened and at what time interval is unknown and warrants further investigation. At present, a multidisciplinary approach with the oncologist and cardiologist, together with a precise identification and evaluation of traditional cardiovascular risk factors, is the current recommendation until more studies and guidelines are performed.
Regarding ATE management in patients with cancer, no specific guidelines are available because of a lack of cancer-specific data, and usual care is advised.
Summary and Future Directions
Clinical practice is rapidly changing in the prevention and management of CAT, and many therapeutic options are becoming available both for prevention and treatment in patients with cancer. The one-size-fits-all approach based on LMWH is being replaced by individualized decision making because of emerging evidence on the efficacy and safety of DOACs. Primary prevention with DOACs is a new recommendation by most major guidelines and represents a paradigm shift in this setting. However, this also means greater complexity and new challenges. Physicians, indeed, will be called to carefully evaluate the best antithrombotic drug, bleeding and recurrence risk, potential drug interactions, and patient preferences for determining the best strategy for each individual. Moreover, improvements in risk stratification are also needed; active investigations into biomarkers, including genetic and microRNA profiles, are ongoing. Implementation science focused on translating clinical trial and translational research into health system–based practice approaches are urgently needed so that the benefit of this large body of investigative work is optimized to reduce the public health burden of cancer-associated VTE.
Funding Support and Author Disclosures
Dr. Khorana has received research support from the Sondra and Stephen Hardis Chair in Oncology Research and the Consortium Linking Oncology with Thrombosis (CLOT) grant from the National Heart, Lung and Blood Institute (U01 HL143402); and has a consulting relationship with and receives honoraria from Janssen, Bayer, Sanofi, and Bristol Myers Squibb, not directly related to this manuscript. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Khorana A.A. Malignancy, thrombosis and Trousseau: the case for an eponym. J Thromb Haemost. 2003;1:2463–2465. doi: 10.1111/j.1538-7836.2003.00501.x. [DOI] [PubMed] [Google Scholar]
- 2.Donnellan E., Khorana A.A. Cancer and venous thromboembolic disease: a review. Oncologist. 2017;22:199–207. doi: 10.1634/theoncologist.2016-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohoon K.P., Ransom J.E., Leibson C.L. Direct medical costs attributable to cancer-associated venous thromboembolism: a population-based longitudinal study. Am J Med. 2016;129:1000.e15–1000.e25. doi: 10.1016/j.amjmed.2016.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim A.S., Johnston S.C. Global variation in the relative burden of stroke and ischemic heart disease. Circulation. 2011;124:314–323. doi: 10.1161/CIRCULATIONAHA.111.018820. [DOI] [PubMed] [Google Scholar]
- 5.McSweeney J.C., Rosenfeld A.G., Abel W.M. Preventing and experiencing ischemic heart disease as a woman: state of the science: a scientific statement from the American Heart Association. Circulation. 2016;133:1302–1331. doi: 10.1161/CIR.0000000000000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khorana A.A., Francis C.W., Culakova E., Kuderer N.M., Lyman G.H. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5:632–634. doi: 10.1111/j.1538-7836.2007.02374.x. [DOI] [PubMed] [Google Scholar]
- 7.Prandoni P., Lensing A.W., Piccioli A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–3488. doi: 10.1182/blood-2002-01-0108. [DOI] [PubMed] [Google Scholar]
- 8.Mukai M., Oka T. Mechanism and management of cancer-associated thrombosis. J Cardiol. 2018;72:89–93. doi: 10.1016/j.jjcc.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Patel H.K., Khorana A.A. Anticoagulation in cancer patients: a summary of pitfalls to avoid. Curr Oncol Rep. 2019;21:18. doi: 10.1007/s11912-019-0767-5. [DOI] [PubMed] [Google Scholar]
- 10.Navi B.B., Singer S., Merkler A.E. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33. doi: 10.1212/WNL.0000000000000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore R.A., Adel N., Riedel E. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29:3466–3473. doi: 10.1200/JCO.2011.35.5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker A.J., Card T.R., West J., Crooks C., Grainge M.J. Incidence of venous thromboembolism in patients with cancer—a cohort study using linked United Kingdom databases. Eur J Cancer. 2013;49:1404–1413. doi: 10.1016/j.ejca.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 13.Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res. 2016;140(Suppl 1):S12–S17. doi: 10.1016/S0049-3848(16)30092-5. [DOI] [PubMed] [Google Scholar]
- 14.Navi B.B., Reiner A.S., Kamel H. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938. doi: 10.1016/j.jacc.2017.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donadini M.P., Ageno W. Unusual site thrombosis. Semin Hematol. 2011;48:264–270. doi: 10.1053/j.seminhematol.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Hicks A.M., DeRosa A., Raj M. Visceral thromboses in pancreas adenocarcinoma: systematic review. Clin Colorectal Cancer. 2018;17:e207–e216. doi: 10.1016/j.clcc.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menapace L.A., Peterson D.R., Berry A., Sousou T., Khorana A.A. Symptomatic and incidental thromboembolism are both associated with mortality in pancreatic cancer. Thromb Haemost. 2011;106:371–378. doi: 10.1160/TH10-12-0789. [DOI] [PubMed] [Google Scholar]
- 18.Key N.S., Khorana A.A., Kuderer N.M. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2020;38:496–520. doi: 10.1200/JCO.19.01461. [DOI] [PubMed] [Google Scholar]
- 19.Farge D., Frere C., Connors J.M. 2019 international clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. Lancet Oncol. 2019;20:e566–e581. doi: 10.1016/S1470-2045(19)30336-5. [DOI] [PubMed] [Google Scholar]
- 20.Mandalà M., Falanga A., Roila F., ESMO Guidelines Working Group Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(Suppl 6):vi85–vi92. doi: 10.1093/annonc/mdr392. [DOI] [PubMed] [Google Scholar]
- 21.NCCN Guidelines . 2020. Cancer-associated venous thromboembolic disease. Version 1.2021. Available at: https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Accessed March 17, 2021. [Google Scholar]
- 22.Lyman G.H., Carrier M., Ay C. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowe G.D. Common risk factors for both arterial and venous thrombosis. Br J Haematol. 2008;140:488–495. doi: 10.1111/j.1365-2141.2007.06973.x. [DOI] [PubMed] [Google Scholar]
- 24.Khorana A.A., Connolly G.C. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27:4839–4847. doi: 10.1200/JCO.2009.22.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khorana A.A., Francis C.W., Culakova E., Kuderer N.M., Lyman G.H. Frequency, risk factors, and trends for venous thromboembolism among hospitalized cancer patients. Cancer. 2007;110:2339–2346. doi: 10.1002/cncr.23062. [DOI] [PubMed] [Google Scholar]
- 26.Vergati M., Della-Morte D., Ferroni P. Increased risk of chemotherapy-associated venous thromboembolism in elderly patients with cancer. Rejuvenation Res. 2013;16:224–231. doi: 10.1089/rej.2013.1409. [DOI] [PubMed] [Google Scholar]
- 27.White R.H., Dager W.E., Zhou H., Murin S. Racial and gender differences in the incidence of recurrent venous thromboembolism. Thromb Haemost. 2006;96:267–273. doi: 10.1160/TH06-07-0365. [DOI] [PubMed] [Google Scholar]
- 28.Connolly G.C., Khorana A.A. Emerging risk stratification approaches to cancer-associated thrombosis: risk factors, biomarkers and a risk score. Thromb Res. 2010;125(Suppl 2):S1–S7. doi: 10.1016/S0049-3848(10)00227-6. [DOI] [PubMed] [Google Scholar]
- 29.Franco R.F., Reitsma P.H. Genetic risk factors of venous thrombosis. Hum Genet. 2001;109:369–384. doi: 10.1007/s004390100593. [DOI] [PubMed] [Google Scholar]
- 30.Blom J.W., Doggen C.J., Osanto S., Rosendaal F.R. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722. doi: 10.1001/jama.293.6.715. [DOI] [PubMed] [Google Scholar]
- 31.Dickmann B., Ahlbrecht J., Ay C. Regional lymph node metastases are a strong risk factor for venous thromboembolism: results from the Vienna Cancer and Thrombosis Study. Haematologica. 2013;98:1309–1314. doi: 10.3324/haematol.2012.073338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connolly G.C., Francis C.W. Cancer-associated thrombosis. Hematology Am Soc Hematol Educ Program. 2013;2013:684–691. doi: 10.1182/asheducation-2013.1.684. [DOI] [PubMed] [Google Scholar]
- 33.Li W., Garcia D., Cornell R.F. Cardiovascular and thrombotic complications of novel multiple myeloma therapies: a review. JAMA Oncol. 2017;3:980–988. doi: 10.1001/jamaoncol.2016.3350. [DOI] [PubMed] [Google Scholar]
- 34.Kristinsson S.Y., Pfeiffer R.M., Björkholm M. Arterial and venous thrombosis in monoclonal gammopathy of undetermined significance and multiple myeloma: a population-based study. Blood. 2010;115:4991–4998. doi: 10.1182/blood-2009-11-252072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roopkumar J., Poudel S.K., Gervaso L. Risk of thromboembolism in patients with ALK and EGFR-mutant lung cancer: a cohort study. J Thromb Haemost. 2021;19:822–829. doi: 10.1111/jth.15215. [DOI] [PubMed] [Google Scholar]
- 36.Agnelli G., Bolis G., Capussotti L. A clinical outcome-based prospective study on venous thromboembolism after cancer surgery: the @RISTOS project. Ann Surg. 2006;243:89–95. doi: 10.1097/01.sla.0000193959.44677.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khorana A.A., Dalal M., Lin J., Connolly G.C. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–655. doi: 10.1002/cncr.27772. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham D., Starling N., Rao S. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 39.Cavo M., Zamagni E., Cellini C. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood. 2002;100:2272–2273. doi: 10.1182/blood-2002-06-1674. [DOI] [PubMed] [Google Scholar]
- 40.Tamborini Permunian E., Gervaso L., Gerdes V., Moja L., Guasti L., Squizzato A. Direct-acting antiviral drugs for chronic hepatitis C and risk of major vascular events: a systematic review. Intern Emerg Med. 2018;13:775–790. doi: 10.1007/s11739-018-1828-8. [DOI] [PubMed] [Google Scholar]
- 41.Schutz F.A., Je Y., Azzi G.R., Nguyen P.L., Choueiri T.K. Bevacizumab increases the risk of arterial ischemia: a large study in cancer patients with a focus on different subgroup outcomes. Ann Oncol. 2011;22:1404–1412. doi: 10.1093/annonc/mdq587. [DOI] [PubMed] [Google Scholar]
- 42.Choueiri T.K., Schutz F.A., Je Y., Rosenberg J.E., Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–2285. doi: 10.1200/JCO.2009.27.2757. [DOI] [PubMed] [Google Scholar]
- 43.Roopkumar J., Swaidani S., Kim A.S. Increased incidence of venous thromboembolism with cancer immunotherapy. Med. 2012;2:423–434. doi: 10.1016/j.medj.2021.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khorana A.A., Francis C.W., Blumberg N., Culakova E., Refaai M.A., Lyman G.H. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khorana A.A., Francis C.W., Culakova E., Lyman G.H. Risk factors for chemotherapy-associated venous thromboembolism in a prospective observational study. Cancer. 2005;104:2822–2829. doi: 10.1002/cncr.21496. [DOI] [PubMed] [Google Scholar]
- 46.Khorana A.A. Cancer-associated thrombosis: updates and controversies. Hematology Am Soc Hematol Educ Program. 2012;2012:626–630. doi: 10.1182/asheducation-2012.1.626. [DOI] [PubMed] [Google Scholar]
- 47.Ay C., Vormittag R., Dunkler D. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the Vienna Cancer and Thrombosis Study. J Clin Oncol. 2009;27:4124–4129. doi: 10.1200/JCO.2008.21.7752. [DOI] [PubMed] [Google Scholar]
- 48.Ay C., Dunkler D., Marosi C. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377–5382. doi: 10.1182/blood-2010-02-270116. [DOI] [PubMed] [Google Scholar]
- 49.Thaler J., Ay C., Mackman N. Microparticle-associated tissue factor activity, venous thromboembolism and mortality in pancreatic, gastric, colorectal and brain cancer patients. J Thromb Haemost. 2012;10:1363–1370. doi: 10.1111/j.1538-7836.2012.04754.x. [DOI] [PubMed] [Google Scholar]
- 50.van Es N., Di Nisio M., Cesarman G. Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica. 2017;102:1494–1501. doi: 10.3324/haematol.2017.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khorana A.A., Kuderer N.M., Culakova E., Lyman G.H., Francis C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–4907. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mulder F.I., Candeloro M., Kamphuisen P.W. The Khorana score for prediction of venous thromboembolism in cancer patients: a systematic review and meta-analysis. Haematologica. 2019;104:1277–1287. doi: 10.3324/haematol.2018.209114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verso M., Agnelli G., Barni S., Gasparini G., LaBianca R. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the PROTECHT score. Intern Emerg Med. 2012;7:291–292. doi: 10.1007/s11739-012-0784-y. [DOI] [PubMed] [Google Scholar]
- 54.Cella C.A., Di Minno G., Carlomagno C. Preventing venous thromboembolism in ambulatory cancer patients: the ONKOTEV study. Oncologist. 2017;22:601–608. doi: 10.1634/theoncologist.2016-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gerotziafas G.T., Taher A., Abdel-Razeq H. A predictive score for thrombosis associated with breast, colorectal, lung, or ovarian cancer: the prospective COMPASS-cancer-associated thrombosis study. Oncologist. 2017;22:1222–1231. doi: 10.1634/theoncologist.2016-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pabinger I., van Es N., Heinze G. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5:e289–e298. doi: 10.1016/S2352-3026(18)30063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanfilippo K.M., Luo S., Wang T.F. Predicting venous thromboembolism in multiple myeloma: development and validation of the IMPEDE VTE score. Am J Hematol. 2019;94:1176–1184. doi: 10.1002/ajh.25603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li A., Wu Q., Luo S. Derivation and validation of a risk assessment model for immunomodulatory drug-associated thrombosis among patients with multiple myeloma. J Natl Compr Canc Netw. 2019;17:840–847. doi: 10.6004/jnccn.2018.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lustig D.B., Rodriguez R., Wells P.S. Implementation and validation of a risk stratification method at The Ottawa Hospital to guide thromboprophylaxis in ambulatory cancer patients at intermediate-high risk for venous thrombosis. Thromb Res. 2015;136:1099–1102. doi: 10.1016/j.thromres.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 60.Khorana A.A., Francis C.W., Kuderer N.M. Dalteparin thromboprophylaxis in cancer patients at high risk for venous thromboembolism: a randomized trial. Thromb Res. 2017;151:89–95. doi: 10.1016/j.thromres.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Kunapareddy G., Switzer B., Jain P. Implementation of an electronic medical record tool for early detection of deep vein thrombosis in the ambulatory oncology setting. Res Pract Thromb Haemost. 2019;3:226–233. doi: 10.1002/rth2.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.White R.H., Zhou H., Romano P.S. Incidence of symptomatic venous thromboembolism after different elective or urgent surgical procedures. Thromb Haemost. 2003;90:446–455. doi: 10.1160/TH03-03-0152. [DOI] [PubMed] [Google Scholar]
- 63.Fagarasanu A., Alotaibi G.S., Hrimiuc R., Lee A.Y., Wu C. Role of extended thromboprophylaxis after abdominal and pelvic surgery in cancer patients: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23:1422–1430. doi: 10.1245/s10434-016-5127-1. [DOI] [PubMed] [Google Scholar]
- 64.Burleigh E., Wang C., Foster D. Thromboprophylaxis in medically ill patients at risk for venous thromboembolism. Am J Health Syst Pharm. 2006;63:S23–S29. doi: 10.2146/ajhp060390. [DOI] [PubMed] [Google Scholar]
- 65.Zwicker J.I., Roopkumar J., Puligandla M. Dose-adjusted enoxaparin thromboprophylaxis in hospitalized cancer patients: a randomized, double-blinded multicenter phase 2 trial. Blood Adv. 2020;4:2254–2260. doi: 10.1182/bloodadvances.2020001804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barbar S., Noventa F., Rossetto V. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost. 2010;8:2450–2457. doi: 10.1111/j.1538-7836.2010.04044.x. [DOI] [PubMed] [Google Scholar]
- 67.Di Nisio M., Carrier M., Lyman G.H., Khorana A.A. Subcommittee on Haemostasis and Malignancy. Prevention of venous thromboembolism in hospitalized medical cancer patients: guidance from the SSC of the ISTH. J Thromb Haemost. 2014;12:1746–1749. doi: 10.1111/jth.12683. [DOI] [PubMed] [Google Scholar]
- 68.Patell R., Rybicki L., McCrae K.R., Khorana A.A. Predicting risk of venous thromboembolism in hospitalized cancer patients: utility of a risk assessment tool. Am J Hematol. 2017;92:501–507. doi: 10.1002/ajh.24700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldhaber S.Z., Leizorovicz A., Kakkar A.K. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365:2167–2177. doi: 10.1056/NEJMoa1110899. [DOI] [PubMed] [Google Scholar]
- 70.Cohen A.T., Spiro T.E., Buller H.R. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368:513–523. doi: 10.1056/NEJMoa1111096. [DOI] [PubMed] [Google Scholar]
- 71.Ageno W., Lopes R.D., Yee M.K. Extended prophylaxis of venous thromboembolism with betrixaban in acutely ill medical patients with and without cancer: insights from the APEX trial. J Thromb Thrombolysis. 2020;49:214–219. doi: 10.1007/s11239-019-01943-5. [DOI] [PubMed] [Google Scholar]
- 72.Hull R.D., Schellong S.M., Tapson V.F. Extended-duration venous thromboembolism prophylaxis in acutely ill medical patients with recently reduced mobility: a randomized trial. Ann Intern Med. 2010;153:8–18. doi: 10.7326/0003-4819-153-1-201007060-00004. [DOI] [PubMed] [Google Scholar]
- 73.Spencer F.A., Lessard D., Emery C., Reed G., Goldberg R.J. Venous thromboembolism in the outpatient setting. Arch Intern Med. 2007;167:1471–1475. doi: 10.1001/archinte.167.14.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyman G.H., Eckert L., Wang Y., Wang H., Cohen A. Venous thromboembolism risk in patients with cancer receiving chemotherapy: a real-world analysis. Oncologist. 2013;18:1321–1329. doi: 10.1634/theoncologist.2013-0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Levine M., Hirsh J., Gent M. Double-blind randomised trial of a very-low-dose warfarin for prevention of thromboembolism in stage IV breast cancer. Lancet. 1994;343:886–889. doi: 10.1016/s0140-6736(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 76.Agnelli G., George D.J., Kakkar A.K. Semuloparin for thromboprophylaxis in patients receiving chemotherapy for cancer. N Engl J Med. 2012;366:601–609. doi: 10.1056/NEJMoa1108898. [DOI] [PubMed] [Google Scholar]
- 77.Di Nisio M., Porreca E., Candeloro M., De Tursi M., Russi I., Rutjes A.W. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2016;12:CD008500. doi: 10.1002/14651858.CD008500.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Riess H., Pelzer U., Hilbig A. Rationale and design of PROSPECT-CONKO 004: a prospective, randomized trial of simultaneous pancreatic cancer treatment with enoxaparin and chemotherapy) BMC Cancer. 2008;8:361. doi: 10.1186/1471-2407-8-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maraveyas A., Waters J., Roy R. Gemcitabine versus gemcitabine plus dalteparin thromboprophylaxis in pancreatic cancer. Eur J Cancer. 2012;48:1283–1292. doi: 10.1016/j.ejca.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 80.Larocca A., Cavallo F., Bringhen S. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–939. doi: 10.1182/blood-2011-03-344333. [DOI] [PubMed] [Google Scholar]
- 81.Khorana A.A., Soff G.A., Kakkar A.K. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. 2019;380:720–728. doi: 10.1056/NEJMoa1814630. [DOI] [PubMed] [Google Scholar]
- 82.Carrier M., Abou-Nassar K., Mallick R. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. 2019;380:711–719. doi: 10.1056/NEJMoa1814468. [DOI] [PubMed] [Google Scholar]
- 83.Lee A.Y., Levine M.N., Baker R.I. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–153. doi: 10.1056/NEJMoa025313. [DOI] [PubMed] [Google Scholar]
- 84.Lee A.Y.Y., Kamphuisen P.W., Meyer G. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314:677–686. doi: 10.1001/jama.2015.9243. [DOI] [PubMed] [Google Scholar]
- 85.Kahale L.A., Hakoum M.B., Tsolakian I.G. Anticoagulation for the long-term treatment of venous thromboembolism in people with cancer. Cochrane Database Syst Rev. 2018;6:CD006650. doi: 10.1002/14651858.CD006650.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khorana A.A., Yannicelli D., McCrae K.R. Evaluation of US prescription patterns: are treatment guidelines for cancer-associated venous thromboembolism being followed? Thromb Res. 2016;145:51–53. doi: 10.1016/j.thromres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 87.Sabatino J., De Rosa S., Polimeni Direct oral anticoagulants in patients with active cancer: a systematic review and meta-analysis. J Am Coll Cardiol CardioOnc. 2020;2:428–440. doi: 10.1016/j.jaccao.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raskob G.E., van Es N., Verhamme P. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 89.Young A.M., Marshall A., Thirlwall J. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 90.van der Hulle T., den Exter P.L., van den Hoven P. Cohort study on the management of cancer-associated venous thromboembolism aimed at the safety of stopping anticoagulant therapy in patients cured of cancer. Chest. 2016;149:1245–1251. doi: 10.1016/j.chest.2015.10.069. [DOI] [PubMed] [Google Scholar]
- 91.Francis C.W., Kessler C.M., Goldhaber S.Z. Treatment of venous thromboembolism in cancer patients with dalteparin for up to 12 months: the DALTECAN Study. J Thromb Haemost. 2015;13:1028–1035. doi: 10.1111/jth.12923. [DOI] [PubMed] [Google Scholar]
- 92.Jara-Palomares L., Solier-Lopez A., Elias-Hernandez T. Tinzaparin in cancer associated thrombosis beyond 6months: TiCAT study. Thromb Res. 2017;157:90–96. doi: 10.1016/j.thromres.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 93.Bach A.G., Schmoll H.J., Beckel C. Pulmonary embolism in oncologic patients: frequency and embolus burden of symptomatic and unsuspected events. Acta Radiol. 2014;55:45–53. doi: 10.1177/0284185113491569. [DOI] [PubMed] [Google Scholar]
- 94.Singh R., Sousou T., Mohile S., Khorana A.A. High rates of symptomatic and incidental thromboembolic events in gastrointestinal cancer patients. J Thromb Haemost. 2010;8:1879–1881. doi: 10.1111/j.1538-7836.2010.03929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaturvedi S., Sidana S., Elson P., Khorana A.A., McCrae K.R. Symptomatic and incidental venous thromboembolic disease are both associated with mortality in patients with prostate cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Di Nisio M., Carrier M. Incidental venous thromboembolism: is anticoagulation indicated? Hematology Am Soc Hematol Educ Program. 2017;2017:121–127. doi: 10.1182/asheducation-2017.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Galanaud J.P., Sevestre M.A., Pernod G. Long-term outcomes of cancer-related isolated distal deep vein thrombosis: the OPTIMEV study. J Thromb Haemost. 2017;15:907–916. doi: 10.1111/jth.13664. [DOI] [PubMed] [Google Scholar]
- 98.Dentali F., Pegoraro S., Barco S. Clinical course of isolated distal deep vein thrombosis in patients with active cancer: a multicenter cohort study. J Thromb Haemost. 2017;15:1757–1763. doi: 10.1111/jth.13761. [DOI] [PubMed] [Google Scholar]
- 99.Carrier M., Khorana A.A., Zwicker J., Noble S., Lee A.Y. Subcommittee on Haemostasis and malignancy for the SSC of the ISTH. Management of challenging cases of patients with cancer-associated thrombosis including recurrent thrombosis and bleeding: guidance from the SSC of the ISTH. J Thromb Haemost. 2013;11:1760–1765. doi: 10.1111/jth.12338. [DOI] [PubMed] [Google Scholar]
- 100.Kopolovic I., Lee A.Y., Wu C. Management and outcomes of cancer-associated venous thromboembolism in patients with concomitant thrombocytopenia: a retrospective cohort study. Ann Hematol. 2015;94:329–336. doi: 10.1007/s00277-014-2198-6. [DOI] [PubMed] [Google Scholar]
- 101.Barco S., Corti M., Trinchero A. Survival and recurrent venous thromboembolism in patients with first proximal or isolated distal deep vein thrombosis and no pulmonary embolism. J Thromb Haemost. 2017;15:1436–1442. doi: 10.1111/jth.13713. [DOI] [PubMed] [Google Scholar]
- 102.Samuelson Bannow B.T., Lee A., Khorana A.A. Management of cancer-associated thrombosis in patients with thrombocytopenia: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1246–1249. doi: 10.1111/jth.14015. [DOI] [PubMed] [Google Scholar]
- 103.Samuelson Bannow B.R., Lee A.Y.Y., Khorana A.A. Management of anticoagulation for cancer-associated thrombosis in patients with thrombocytopenia: a systematic review. Res Pract Thromb Haemost. 2018;2:664–669. doi: 10.1002/rth2.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kearon C., Akl E.A., Ornelas J. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–352. doi: 10.1016/j.chest.2015.11.026. [DOI] [PubMed] [Google Scholar]
- 105.Mismetti P., Laporte S., Pellerin O. Effect of a retrievable inferior vena cava filter plus anticoagulation vs anticoagulation alone on risk of recurrent pulmonary embolism: a randomized clinical trial. JAMA. 2015;313:1627–1635. doi: 10.1001/jama.2015.3780. [DOI] [PubMed] [Google Scholar]
- 106.Sawaya R., Zuccarello M., Elkalliny M., Nishiyama H. Postoperative venous thromboembolism and brain tumors: part I. Clinical profile. J Neurooncol. 1992;14:119–125. doi: 10.1007/BF00177615. [DOI] [PubMed] [Google Scholar]
- 107.Goldschmidt N., Linetsky E., Shalom E., Varon D., Siegal T. High incidence of thromboembolism in patients with central nervous system lymphoma. Cancer. 2003;98:1239–1242. doi: 10.1002/cncr.11623. [DOI] [PubMed] [Google Scholar]
- 108.Brandes A.A., Scelzi E., Salmistraro G. Incidence of risk of thromboembolism during treatment high-grade gliomas: a prospective study. Eur J Cancer. 1997;33:1592–1596. doi: 10.1016/s0959-8049(97)00167-6. [DOI] [PubMed] [Google Scholar]
- 109.Srivastava G., Rana V., Wallace S. Risk of intracranial hemorrhage and cerebrovascular accidents in non-small cell lung cancer brain metastasis patients. J Thorac Oncol. 2009;4:333–337. doi: 10.1097/JTO.0b013e318194fad4. [DOI] [PubMed] [Google Scholar]
- 110.Donato J., Campigotto F., Uhlmann E.J. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. 2015;126:494–499. doi: 10.1182/blood-2015-02-626788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schulman S., Kearon C. Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. 2005;3:692–694. doi: 10.1111/j.1538-7836.2005.01204.x. [DOI] [PubMed] [Google Scholar]
- 112.Hakim D.A., Dangas G.D., Caixeta A. Impact of baseline thrombocytopenia on the early and late outcomes after ST-elevation myocardial infarction treated with primary angioplasty: analysis from the Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction (HORIZONS-AMI) trial. Am Heart J. 2011;161:391–396. doi: 10.1016/j.ahj.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 113.Iliescu C.A., Grines C.L., Herrmann J. SCAI Expert consensus statement: evaluation, management, and special considerations of cardio-oncology patients in the cardiac catheterization laboratory (endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologıa Intervencionista) Catheter Cardiovasc Interv. 2016;87:E202–E223. doi: 10.1002/ccd.26379. [DOI] [PubMed] [Google Scholar]
- 114.Landolfi R., Marchioli R., Kutti J. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–124. doi: 10.1056/NEJMoa035572. [DOI] [PubMed] [Google Scholar]
- 115.Palumbo A., Cavo M., Bringhen S. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized trial. J Clin Oncol. 2011;29:986–993. doi: 10.1200/JCO.2010.31.6844. [DOI] [PubMed] [Google Scholar]
- 116.Detrano R., Guerci A.D., Carr J.J. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]