Abstract
Background
Heart failure (HF) and breast cancer are 2 of the leading causes of death in postmenopausal women. The temporal association between HF and breast cancer in postmenopausal women has not been described.
Objectives
This study sought to examine the temporal association between HF and breast cancer.
Methods
Postmenopausal women within the WHI (Women’s Health Initiative) cohort were studied. All prevalent HF and prevalent breast cancer at enrollment were self-reported. Incident hospitalized HF and breast cancer diagnoses were adjudicated through 2017.
Results
Among a cohort of 44,174 women (mean age 63 ± 7 years), 2,188 developed incident invasive breast cancer and 2,416 developed incident hospitalized HF over a median follow-up of 14 and 15 years, respectively. When compared with a breast cancer- and HF-free cohort, there was no association between prevalent HF and incident invasive breast cancer and similarly, there was no association between prevalent breast cancer and incident hospitalized HF. Across the entire cohort, the median survival after incident hospitalized HF was worse compared with an incident invasive breast cancer diagnosis (5 and 19 years, respectively). In women with incident invasive breast cancer, prevalent HF was associated with an increased risk of mortality (hazard ratio: 2.28; 95% confidence interval: 1.31 to 3.95). In women with incident hospitalized HF, prevalent breast cancer was associated with an increased risk of mortality (hazard ratio: 1.66; 95% confidence interval: 1.03 to 2.68). Cause of death after incident HF was different only in women with prevalent and interim breast cancer compared with those without prevalent and interim breast cancer.
Conclusions
In postmenopausal women, prevalent HF was not associated with a higher incidence of breast cancer and vice versa. However, the presence of incident invasive breast cancer or incident HF in those with prevalent HF or prevalent breast cancer, respectively, was associated with increased mortality.
Key Words: breast cancer, heart failure, incidence, mortality
Abbreviations and Acronyms: BMI, body mass index; CI, confidence interval; HF, heart failure; HR, hazard ratio; WHR, waist-hip ratio; WHI, Women’s Health Initiative
Central Illustration
Heart failure (HF) and cancer are 2 of the leading causes of death in the United States (1, 2, 3). HF and cancer share risk factors such as obesity, hypertension, diabetes mellitus, and the use of tobacco and alcohol, as well as pathophysiologic alterations such as heightened inflammation, oxidative stress, and immune dysregulation (4, 5, 6, 7, 8). Although cancer therapies, including certain chemotherapies and radiation, can cause myocardial dysfunction and may predispose patients to the development of incident HF (4, 5, 6,9), there have been conflicting results as to whether HF is associated with a higher risk of incident cancer (10, 11, 12, 13).
Prior studies have evaluated the association of breast cancer with the development of cardiovascular disease in postmenopausal women (14, 15, 16). To our knowledge, no study to date has examined the association between HF and the subsequent development of invasive and noninvasive breast cancer in postmenopausal women. Aside from the above-mentioned risk factors, unique to women, hormonal factors such as early menarche, late menopause, and nulliparity, are also shared risk factors for both breast cancer and HF (17,18). In this study, we sought to further expand our understanding of the association between HF and breast cancer and subsequent outcomes in a cohort of postmenopausal women using the WHI (Women’s Health Initiative) cohort. Specifically, we aimed to determine first, whether prevalent HF is a risk factor for incident breast cancer and whether prevalent breast cancer is a risk factor for incident hospitalized HF. Second, we aimed to determine whether prevalent HF increases the risk of mortality after incident breast cancer and whether prevalent breast cancer increases the risk of mortality after incident HF.
Methods
Data source and study population
The design of the WHI National Health Study has been previously described (19). Briefly, it is a large U.S.-based preventative study that enrolled 161,808 postmenopausal women (ages 50 to 79 years) between 1993 and 1998 at 40 U.S. clinical centers (19). Women were enrolled into either the clinical trial arm (2 hormone therapy trials: a trial of dietary modification and a trial of calcium and vitamin D supplementation; n = 68,132) or the observational study arm (n = 93,676). In 2010, a subcohort of 44,174 participants, including all women who had participated in the WHI Hormone Trials and additionally oversampled African Americans and Hispanic/Latina women, were evaluated both retrospectively and prospectively until February 28, 2017, for incident hospitalized HF events by trained physician adjudicators (20,21). Institutional review boards at all WHI clinical centers approved the WHI study, and all participants provided written informed consent.
Baseline demographic and clinical information
Each participant completed self-administered questionnaires, an interview, and a physical examination at the time of enrollment in WHI.
Definition and adjudication of breast cancer and HF
Prevalent breast cancer and prevalent HF were defined as participants self-reporting the presence of these diseases at the time of enrollment. Incident breast cancer diagnosis and incident hospitalized HF were centrally adjudicated. For all cases of breast cancer, documentation was sent to the clinical coordinating center for centralized review and coding by trained cancer coders under the supervision of a cancer epidemiologist and physician. These include hospital discharge summaries, operative reports, history and physical examination, radiology reports, oncology consultation reports, and estrogen and progesterone hormone receptor results for breast cancers (22). The WHI adjudication criteria for incident HF have been previously described in detail elsewhere (20). Briefly, hospital records of suspected HF were abstracted to include evidence of new onset of symptoms, history of HF, general medical history, physical examination, diagnostic tests, biomarkers, and medications (21). Physician adjudicators reviewed this information for evidence of acute decompensated HF.
HF was further classified as definite acute decompensated HF, possible acute decompensated HF, chronic stable HF, unclassifiable, or HF unlikely (23). Only definite and possible decompensated HF cases were classified as incident hospitalized HF. Only the first acute decompensated HF event was considered incident. When computing incident HF, we excluded women who had chronic HF on their first adjudication. All follow-up forms and outcomes were updated through February 28, 2017 (18).
Cause of death
Cause of death in the study was centrally adjudicated and included death from cardiovascular disease, death from cancer, death from other causes, and death from unknown causes. Mortality information and cause of death was enhanced by serial National Death Index queries.
Statistical analyses
Baseline characteristics at enrollment are presented as mean ± SD or count with percentage. Differences in baseline characteristics across the 4 groups: 1) breast cancer- and HF-free cohort; 2) prevalent breast cancer; 3) prevalent HF; and 4) prevalent breast cancer and HF—were compared using analysis of variance for continuous variables and chi-square test for categorical variables. We performed 3 pairwise comparisons (with Bonferroni adjustment): 1) prevalent HF versus breast cancer– and HF-free cohort; 2) prevalent breast cancer versus breast cancer– and HF-free cohort; and 3) prevalent HF versus prevalent breast cancer. The cumulative incidence of HF, invasive breast cancer, and all incident breast cancer (invasive and noninvasive) was presented as events per 1,000 person-years with 95% confidence interval (CI). Multivariable Cox proportional hazards regression was used to examine the risk factors associated with incident HF, incident invasive breast cancer, and incident breast cancer. For Cox proportional hazards models of incident HF, incident invasive breast cancer, and all incident breast cancer, we adjusted for the following baseline covariates: age; race; body mass index (BMI); waist-hip ratio (WHR); diabetes mellitus; hypertension; myocardial infarction; coronary artery disease; atrial fibrillation; pulse; systolic blood pressure; smoking; alcohol use; total physical activity (metabolic equivalent–hours per week); hemoglobin; menopausal hormone therapy trial participation and menopausal hormone therapy trial arm; age at menarche; parity; oophorectomy; and hysterectomy. Only variables that were statistically significant (p < 0.05) in univariable analysis were subsequently included in the multivariable Cox proportional hazards regression.
The associations between prevalent HF with incident breast cancer and prevalent breast cancer with incident HF were determined using the Kaplan-Meier estimate with log-rank statistics and Cox proportional hazards model. To evaluate the association between incident breast cancer (invasive and all breast cancer) and all-cause mortality in participants with prevalent HF or prevalent and interim HF, we defined interim HF as a HF diagnosis at any time prior to the development of incident breast cancer in the HF-free population. Similarly, to evaluate the association between incident HF and all-cause mortality in participants with prevalent breast cancer or prevalent and interim breast cancer, we defined interim breast cancer as a diagnosis of breast cancer at any time prior to the development of incident hospitalized HF in the breast cancer–free population. Cox proportional hazards model adjusting for age was performed. However, additional covariates were not included in a multivariable model given the lack of updated comorbidities data at the time of development of incident breast cancer or incident heart failure. Formal hypothesis testing comparing survival after incident breast cancer to survival after incident HF was not performed. Numerical median and overall survival of the 2 conditions were reported. In a sensitivity analysis, we used the Fine and Gray Cox proportional subdistribution models to examine the association of incident breast cancer or HF by prevalent disease with all-cause mortality accounting for the competing risk of death. We also performed a sensitivity analysis modeling HF and breast cancer as time-varying covariates to determine its effect on incident events and mortality. All analyses were 2-sided and a p value of 0.05 was considered statistically significant. Analyses were conducted with STATA (version 12, StataCorp. LP, College Station, Texas).
Results
Overall participant characteristics at enrollment
The mean age at enrollment of the study cohort of 44,174 women was 63 ± 7 years with 50% White and 33% Black participants. Participants were overweight and had abdominal obesity (average BMI was 29.6 ± 6.3 kg/m2 and average WHR was 0.82 ± 0.08). Nearly one-half smoked tobacco and 39% had hypertension (23% had a systolic blood pressure >140 mm Hg at enrollment); other cardiovascular comorbidities were less common (Table 1). Prevalence of breast cancer at enrollment was 1.6% (n = 692 of 44,174) and prevalence of HF was 1.5% (n = 684 of 44,174). Nineteen women had both breast cancer and HF at enrollment. A total of 42,817 women constituted the breast cancer– and HF-free population.
Table 1.
Baseline Characteristics of Participants by Prevalent Breast Cancer and Heart Failure
| Breast Cancer- and Heart Failure-Free Population (n = 42,817) | Prevalent Breast Cancer (n = 673) | Prevalent Heart Failure (n = 665) | Prevalent Breast Cancer and Heart Failure (n = 19) | p Value | |
|---|---|---|---|---|---|
| Age, yrs | 63 ± 7 | 63 ± 7 | 65 ± 7∗† | 64 ± 9 | <0.001 |
| Race | <0.001 | ||||
| White | 21,810 (51) | 28 (4)‡ | 191 (29)∗† | 1 (5) | |
| Black | 13,736 (32) | 491 (73)‡ | 375 (56)∗† | 16 (84) | |
| Other | 7,271 (17) | 154 (23)‡ | 99 (15)∗† | 2 (11) | |
| Coronary artery disease | 3,846 (9) | 74 (11) | 413 (62)∗† | 12 (63) | <0.001 |
| Myocardial infarction | 900 (2) | 15 (2) | 232 (35)∗† | 8 (44) | <0.001 |
| Angina | 2,400 (6) | 46 (7) | 316 (49)∗† | 6 (33) | <0.001 |
| CABG | 324 (1) | 4 (1) | 71 (11)∗† | 1 (6) | <0.001 |
| Atrial fibrillation | 1,575 (4) | 27 (4) | 118 (19)∗† | 3 (17) | <0.001 |
| Hyperlipidemia | 5,561 (14) | 122 (19)‡ | 192 (31)∗† | 3 (16) | <0.001 |
| Hypertension | 16,259 (38) | 330 (50)‡ | 492 (76)∗† | 17 (90) | <0.001 |
| Peripheral artery disease | 927 (2) | 28 (4)‡ | 87 (13)∗† | 2 (12) | <0.001 |
| Diabetes | 3,774 (9) | 95 (14)‡ | 210 (32)∗† | 4 (21) | <0.001 |
| Stroke | 634 (2) | 22 (3)‡ | 58 (9)∗† | 5 (26) | <0.001 |
| Transient ischemic attack | 885 (2) | 20 (3) | 64 (10)∗† | 3 (16) | <0.001 |
| Hormone therapy | 21,610 (52) | 279 (42)‡ | 323 (49)† | 6 (32) | <0.001 |
| Pregnancy | 16,049 (93) | 228 (91) | 268 (94) | 6 (100) | 0.483 |
| Hysterectomy | 18,721 (44) | 368 (55)‡ | 381 (57)∗ | 14 (74) | <0.001 |
| Oophorectomy | 11,536 (28) | 240 (37)‡ | 238 (38)∗ | 8 (50) | 0.001 |
| Nulliparity | 4,640 (11) | 114 (17)‡ | 59 (9)† | 2 (11) | 0.001 |
| Age at menarche, yrs | 13 | 13 | 13 | 12 | 0.467 |
| Age at menopause, yrs | 47 ± 7 | 46 ± 7‡ | 46 ± 8∗ | 46 ± 9 | 0.001 |
| Smoking status | 0.064 | ||||
| Never | 21,776 (52) | 295 (46) | 354 (53) | 8 (50) | |
| Past | 16,128 (38) | 281 (43) | 237 (36) | 7 (44) | |
| Current | 4,264 (10) | 237 (36) | 72 (11) | 1 (6) | |
| Pack-year smoking | 9 ± 18 | 7 ± 15‡ | 13 ± 23∗† | 9 ± 15 | <0.001 |
| Alcohol status | <0.001 | ||||
| Never | 6,034 (14) | 130 (19) | 133 (20) | 3 (16) | |
| Past | 9,932 (23) | 222 (33) | 291 (44) | 8 (42) | |
| Current | 26,851 (63) | 321 (48) | 241 (36) | 8 (42) | |
| Pulse, beats/min | 70 ± 12 | 72 ± 13‡ | 71 ± 11 | 74 ± 16 | 0.005 |
| Systolic blood pressure, mm Hg | 129 ± 18 | 130 ± 17 | 135 ± 21∗† | 133 ± 20 | <0.001 |
| Diastolic blood pressure, mm Hg | 76 ± 9 | 77 ± 9 | 76 ± 11 | 75 ± 10 | 0.475 |
| Body mass index, kg/m2 | 29.6 ± 6.2 | 30.2 ± 6.2‡ | 32.6 ± 7.7∗† | 34.3 ± 7.1 | <0.001 |
| Height, cm | 161 ± 7 | 161 ± 7 | 161 ± 7 | 159 ± 8 | 0.359 |
| Weight, kg | 77 ± 17 | 79 ± 18 | 85 ± 21∗† | 87 ± 19 | <0.001 |
| Waist-hip ratio | 0.82 ± 0.08 | 0.83 ± 0.07 | 0.85 ± 0.08∗† | 0.86 ± 0.07 | <0.001 |
| Total physical activity, MET-h/week | 6.4 ± 10.5 | 6.5 ± 10.4 | 4.8±9.4∗† | 4.7 ± 7.2 | <0.001 |
| Hemoglobin, g/dl | 13.4 ± 1.17 | 13.0 ± 1.11‡ | 13.1 ± 1.36∗ | 12.5 ± 1.28 | <0.001 |
Values are mean ± SD or n (%), unless otherwise indicated.
CABG = coronary artery bypass graft; MET = metabolic equivalent.
Significance (p < 0.05) when comparing prevalent heart failure to the breast cancer– and heart failure–free population.
Significance (p < 0.05) when comparing prevalent heart failure to prevalent breast cancer.
Significance (p < 0.05) when comparing prevalent breast cancer to the breast cancer– and heart failure–free population.
Baseline clinical characteristics of participants by prevalent breast cancer and HF
Baseline characteristics of the 4 groups are shown in Table 1. Women with prevalent breast cancer or prevalent HF were more likely to be Black and had a greater prevalence of cardiovascular risk factors compared with the breast cancer– and HF-free cohort. Compared with patients with prevalent breast cancer, patients with prevalent HF were older, heavier, had a higher burden of cardiovascular comorbidities, and were less active.
Breast cancer incidence
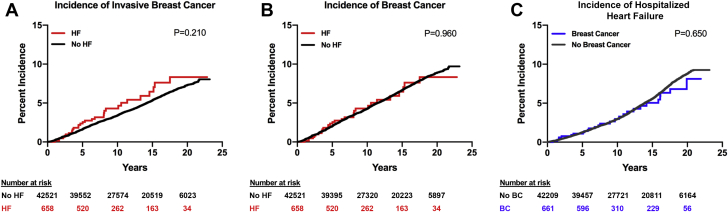
Over a median follow-up of 14 years (maximum of 23 years), 43,482 women were at risk for breast cancer. There was no difference in the incidence of invasive or all breast cancer between women with or without prevalent HF. Cumulative incidence and incidence rates by group are shown in Figures 1A and 1B and Table 2. The number of incident breast cancer events by prevalent HF are shown in Supplemental Table 1.
Figure 1.
Cumulative Incidence of Invasive Breast Cancer, All Breast Cancer, and HF
Cumulative incidence of invasive breast cancer, all breast cancer (invasive and noninvasive), and heart failure (HF) among women with and without prevalent HF or prevalent breast cancer. (A) Cumulative incidence of invasive breast cancer among women with and without prevalent HF. (B) Cumulative incidence of all breast cancer among women with and without prevalent HF. (C) Cumulative incidence of HF among women with and without prevalent breast cancer.
Table 2.
Unadjusted Incidence Rates of Incident Disease in Women With and Without Breast Cancer and Heart Failure
| Outcome | Group | Unadjusted Incidence Rate (per 1,000 Person-Years) | 95% CI | p Value |
|---|---|---|---|---|
| Incident invasive breast cancer | Prevalent heart failure | 4.5 | 3.2–6.5 | 0.210 |
| No heart failure | 3.7 | 3.5–3.8 | ||
| Incident breast cancer | Prevalent heart failure | 4.5 | 3.2–6.5 | 0.960 |
| No heart failure | 4.6 | 4.4–4.7 | ||
| Incident heart failure | Prevalent breast cancer | 3.5 | 2.4–5.1 | 0.650 |
| No breast cancer | 4.1 | 3.9–4.2 |
CI = confidence interval.
HF incidence
Over a median follow-up of 15 years (maximum of 23 years), 43,173 women were at risk for HF. There was no difference in the incidence of hospitalized HF between women with or without prevalent breast cancer. Cumulative incidence and incidence rates by group are shown in Figure 1C and Table 2. The number of incident HF events by prevalent breast cancer are shown in Supplemental Table 1.
Associations with incident breast cancer and HF
Independent predictors of incident invasive breast cancer were age, BMI, WHR, alcohol use, age at menarche, parity, history of bilateral oophorectomy, physical inactivity, and low hemoglobin (Table 3). The results were similar when data included all incident breast cancer (Table 4). Independent predictors of incident HF were age, white race, BMI, WHR, smoking, alcohol use, diabetes, hypertension, coronary artery disease, atrial fibrillation, physical inactivity, heart rate, systolic blood pressure, low hemoglobin, and trial participation (but not randomization to menopausal hormone therapy trial) (Table 5). Prevalent breast cancer was not a predictor of incident HF and similarly, prevalent HF was not a predictor of incident breast cancer in univariable- and multivariable-adjusted analyses. Shared predictors of both incident breast cancer and incident HF were age, BMI, WHR, alcohol use, physical inactivity, and heart rate (Tables 3, 4, and 5).
Table 3.
Multivariable Associations With Incident Invasive Breast Cancer
| Covariate | HR∗ | Lower 95% CI | Upper 95% CI | p Value |
|---|---|---|---|---|
| Age, yrs | 1.01 | 1.01 | 1.02 | <0.001 |
| Body mass index, kg/m2 | 1.02 | 1.02 | 1.03 | <0.001 |
| Waist-hip ratio | 1.81 | 1.06 | 3.09 | 0.029 |
| Alcohol use | 1.04 | 1.01 | 1.08 | 0.004 |
| Menarche | 0.96 | 0.93 | 0.99 | 0.011 |
| Parity | 0.97 | 0.94 | 0.99 | 0.008 |
| Bilateral oophorectomy | 0.80 | 0.71 | 0.91 | 0.001 |
| Total energy expenditure, MET-h/week | 1.00 | 0.99 | 1.00 | 0.031 |
| Hemoglobin, g/dl | 1.04 | 1.01 | 1.08 | 0.025 |
Table 4.
Multivariable Associations With Incident Breast Cancer
| Covariate | HR∗ | Lower 95% CI | Upper 95% CI | p Value |
|---|---|---|---|---|
| Age, yrs | 1.01 | 1.00 | 1.01 | 0.008 |
| Body mass index, kg/m2 | 1.02 | 1.02 | 1.03 | <0.001 |
| Waist-hip ratio | 2.09 | 1.31 | 3.34 | 0.002 |
| Alcohol use | 1.03 | 1.01 | 1.06 | 0.013 |
| Menarche | 0.97 | 0.95 | 1.00 | 0.03 |
| Parity | 0.97 | 0.95 | 0.99 | 0.011 |
| Bilateral oophorectomy | 0.84 | 0.75 | 0.94 | 0.003 |
| Total energy expenditure, MET-h/week | 1.00 | 0.99 | 1.00 | 0.018 |
| Pulse, beats/min | 1.00 | 1.00 | 1.01 | 0.022 |
| Hemoglobin, g/dl | 1.04 | 1.00 | 1.07 | 0.044 |
Table 5.
Multivariable Associations With Incident Hospitalized Heart Failure
| Covariate | HR∗ | Lower 95% CI | Upper 95% CI | p Value |
|---|---|---|---|---|
| Age, yrs | 1.10 | 1.09 | 1.10 | <0.001 |
| White race | 1.35 | 1.17 | 1.56 | <0.001 |
| Body mass index, kg/m2 | 1.04 | 1.03 | 1.05 | <0.001 |
| Waist-hip ratio | 3.03 | 1.88 | 4.88 | <0.001 |
| Smoking history | 1.54 | 1.44 | 1.65 | <0.001 |
| Alcohol use | 0.95 | 0.93 | 0.98 | 0.002 |
| Diabetes mellitus | 2.32 | 2.07 | 2.61 | <0.001 |
| Hypertension | 1.41 | 1.28 | 1.56 | <0.001 |
| Myocardial infarction | 1.92 | 1.58 | 2.33 | <0.001 |
| Coronary artery disease (excluding myocardial infarction) | 1.47 | 1.27 | 1.69 | <0.001 |
| Atrial fibrillation | 1.54 | 1.30 | 1.84 | <0.001 |
| Randomized to the hormone replacement therapy trial | 1.40 | 1.20 | 1.64 | <0.001 |
| Total energy expenditure, MET-h/week | 0.99 | 0.99 | 1.00 | 0.008 |
| Systolic blood pressure, mm Hg | 1.01 | 1.01 | 1.02 | <0.001 |
| Pulse, beats/min | 1.00 | 1.00 | 1.01 | 0.003 |
| Hemoglobin, g/dl | 0.93 | 0.89 | 0.97 | <0.001 |
Mortality in the breast cancer– and HF-free population
A total of 37,654 participants were free of prevalent or incident breast cancer and HF, of whom 5,996 died over a median follow-up of 15 years after enrollment (incidence rate of 11 deaths per 1,000 person-years).
Mortality after incident breast cancer
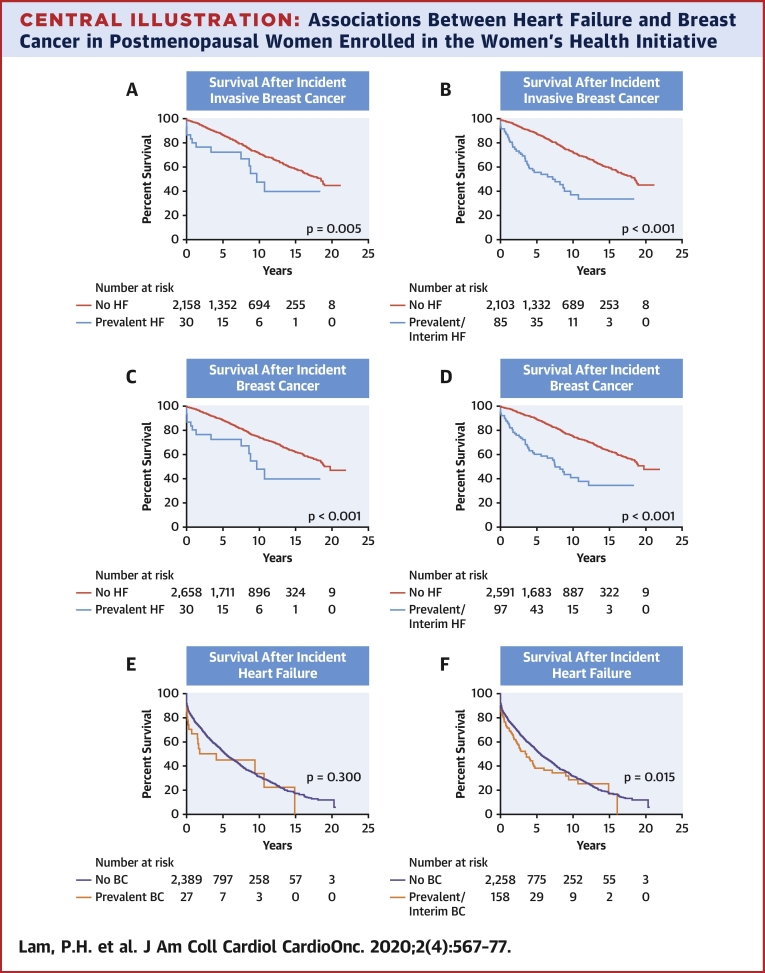
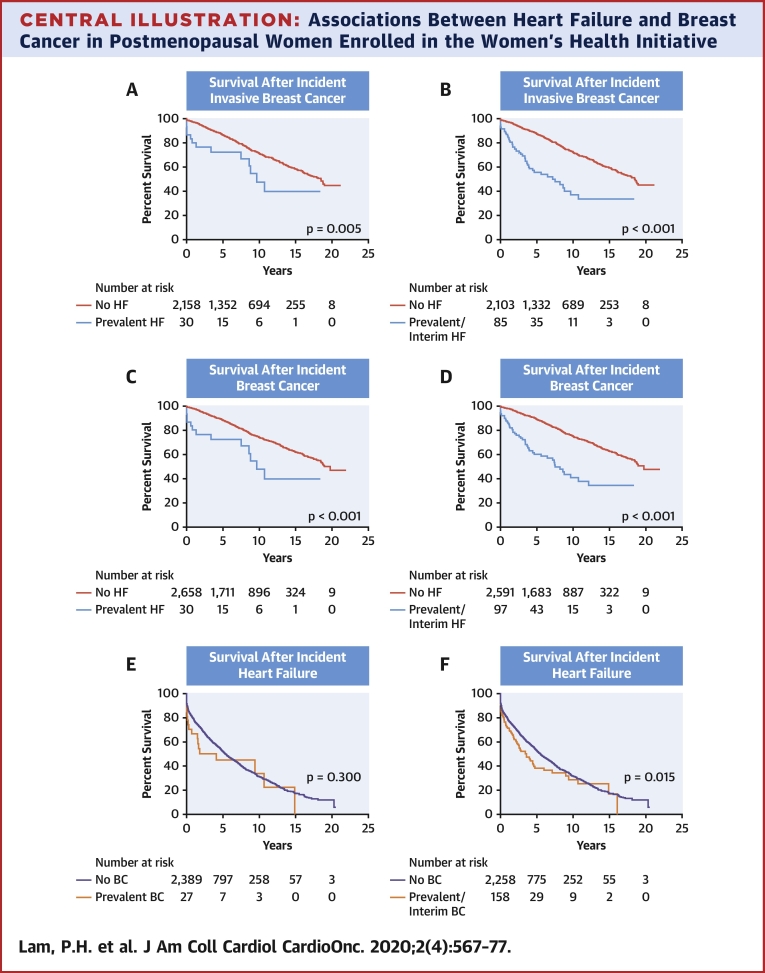
A total of 2,188 participants developed incident invasive breast cancer, of whom 573 died over a median follow-up of 7 years. A total of 2,688 participants developed all incident breast cancer, of whom 639 died over a median follow-up of 7 years. Median survival after incident invasive breast cancer was 19 years (25th percentile was 9 years) and after all incident breast cancer was 19 years (25th percentile was 10 years) (Central Illustration). Women with prevalent HF or prevalent and interim HF had a higher risk of mortality after incident invasive breast cancer compared with women without HF (age-adjusted hazard ratio [HR]: 2.28; 95% CI: 1.31 to 3.95; and age-adjusted HR: 2.58; 95% CI: 1.88 to 3.55, respectively) (Table 6). The results were similar when the data included both invasive and noninvasive breast cancer (Table 6). The association of incident breast cancer by prevalent HF with all-cause mortality was unaffected by the competing risk of death (p = 0.212).
Central Illustration.
Associations Between Heart Failure and Breast Cancer in Postmenopausal Women Enrolled in the Women’s Health Initiative
Although prevalent heart failure (HF) was not associated a higher incidence of breast cancer (BC) and vice versa, pre-existing BC or HF was associated with an increased mortality in those who develop an incident of the other. Kaplan-Meier survival curves with log-rank statistics in women with incident BC and HF grouped by prevalent or prevalent and interim HF and BC, respectively, are displayed. (A) Survival after an incident invasive BC diagnosis in women with and without prevalent HF. (B) Survival after an incident invasive BC diagnosis in women with and without prevalent and interim HF. (C) Survival after an incident invasive or noninvasive BC diagnosis in women with and without prevalent HF. (D) Survival after an incident invasive or noninvasive BC diagnosis in women with and without prevalent and interim HF. (E) Survival after incident hospitalized HF in women with and without prevalent BC. (F) Survival after incident hospitalized HF in women with and without prevalent and interim BC.
Table 6.
Associations With Prevalent and Interim Breast Cancer and Heart Failure With All-Cause Mortality After Development of Incident Disease
| Outcome | Subgroup | HR (95% CI)∗ | p Value |
|---|---|---|---|
| Mortality after incident invasive breast cancer | Prevalent heart failure | ||
| Univariable | 2.16 (1.25–3.75) | 0.005 | |
| Age-adjusted | 2.28 (1.31–3.95) | <0.001 | |
| Prevalent and interim heart failure | |||
| Univariable | 3.20 (2.33–4.38) | <0.001 | |
| Age-adjusted | 2.58 (1.88–3.55) | <0.001 | |
| Mortality after incident breast cancer | Prevalent heart failure | ||
| Univariable | 2.46 (1.42–4.26) | 0.001 | |
| Age-adjusted | 2.57 (1.48–4.45) | 0.001 | |
| Prevalent and interim heart failure | |||
| Univariable | 3.30 (2.44–4.46) | <0.001 | |
| Age-adjusted | 2.67 (1.20–3.60) | <0.001 | |
| Mortality after incident heart failure | Prevalent breast cancer | ||
| Univariable | 1.29 (0.80–2.08) | 0.300 | |
| Age-adjusted | 1.66 (1.03–2.68) | 0.038 | |
| Prevalent and interim breast cancer | |||
| Univariable | 1.30 (1.05–1.61) | 0.015 | |
| Age-adjusted | 1.27 (1.03–1.58) | <0.001 |
Mortality after incident HF
A total of 2,416 participants developed incident hospitalized HF, of whom 1,362 died over a median follow-up of 3 years. Median survival after incident HF was 5 years (25th percentile was 1 year). Survival curves by log-rank statistics are shown in the Central Illustration. Women with prevalent breast cancer or prevalent and interim breast cancer had a higher risk of mortality after incident HF compared with women without breast cancer (age-adjusted HR: 1.66; 95% CI: 1.03 to 2.68; and age-adjusted HR: 1.27; 95% CI: 1.03 to 1.58, respectively) (Table 6). The association of incident HF by prevalent breast cancer with all-cause mortality was unaffected by the competing risk of death (p = 0.335).
Sensitivity analysis using time-varying covariates
When analyzing incident HF with breast cancer events modeled as a time-varying covariate, prevalent breast cancer was a significant predictor of incident HF only in univariable analysis. Breast cancer was no longer a significant predictor of HF after adjusting for age or the other significant predictors of incident HF included in Tables 3, 4, and 5. Similarly, when analyzing incident breast cancer with HF events modeled as a time-varying covariate, prevalent HF was not a significant predictor of incident breast cancer in univariate analysis or when adjusting for other covariates. When HF and breast cancer events were modeled as time-varying covariates, there was no difference in its effect on mortality. This was consistent with the analysis using prevalent and prevalent and interim events.
Cause of death
Cause of death after incident HF was different in women with prevalent and interim breast cancer compared with in those without prevalent breast cancer (Table 7). Otherwise, there was no significant difference in the cause of death between the different groups.
Table 7.
Cause of Death by Incident Event
| Subgroup | Cancer Death | Cardiovascular Death | Other Cause of Death | Unknown Cause of Death | p Value | |
|---|---|---|---|---|---|---|
| Incident heart failure | ||||||
| No prevalent breast cancer | 166 (12) | 703 (52) | 332 (25) | 144 (11) | 0.496 | |
| Prevalent breast cancer | 4 (24) | 9 (53) | 3 (18) | 1 (6) | ||
| Incident heart failure | ||||||
| No prevalent and interim breast cancer | 147 (12) | 670 (53) | 317 (25) | 138 (11) | 0.002 | |
| Prevalent and interim breast cancer | 23 (26) | 42 (47) | 18 (20) | 7 (8) | ||
| Incident breast cancer | ||||||
| No prevalent heart failure | 312 (50) | 143 (23) | 94 (15) | 77 (12) | 0.967 | |
| Prevalent heart failure | 7 (54) | 3 (23) | 2 (15) | 1 (8) | ||
| Incident breast cancer | ||||||
| No prevalent and interim heart failure | 301 (51) | 131 (22) | 91 (15) | 70 (12) | 0.18 | |
| Prevalent and interim heart failure | 18 (39) | 15 (33) | 5 (11) | 8 (17) |
Values are n (%), representing the absolute number of cause-specific deaths and the percentage of all deaths, unless otherwise noted.
Discussion
In this cohort of postmenopausal women enrolled in the WHI—despite shared risk predictors such as age, obesity, alcohol use, physical inactivity, and elevated resting heart rates—we found no significant association between the development of incident hospitalized HF and incident breast cancer diagnosis in participants with prevalent breast cancer and prevalent HF, respectively. Across the entire cohort, the median survival after incident hospitalized HF was worse compared with that after incident invasive breast cancer (5 and 19 years, respectively). Furthermore, having prevalent breast cancer or prevalent HF was associated with increased mortality in participants who subsequently developed incident breast cancer or incident hospitalized HF, respectively. We found no significant difference in the cause of death between the different groups, except among patients with incident HF in those with prevalent and interim breast cancer compared with in those without prevalent and interim breast cancer. To the best of our knowledge, this is the first study to examine the temporal association between breast cancer and HF in a large prospective cohort of postmenopausal women.
The relationship between risk factors for HF and cancer is complex and intersects at many levels. Prior studies have suggested systemic pathological processes such as inflammation, oxidative stress, and immune dysregulation that may be involved in the pathogenesis of both HF and malignancy (7,8,24). Age is an established risk factor for both HF and cancer, whereas lifestyle, obesity, and comorbidities (including diabetes mellitus and hypertension) are consistently linked to HF, but their relationship to cancer is site-specific (7,8,24). Furthermore, cancer therapies (radiation and chemotherapy) may constitute a risk for development of HF (7,8,24,25). In our analysis, age, obesity, alcohol use, physical inactivity, and heart rate were shared risk factors for both incident HF and incident breast cancer. Unique risk factors for breast cancer were reproductive attributes and for HF were cardiovascular comorbidities (including diabetes mellitus, hypertension, atrial fibrillation, and coronary artery disease). The lack of association between HF and breast cancer suggests that whereas there may be shared risk factors, neither disease increases the likelihood of developing the other and thus each may have its own distinct pathophysiology. Recent translational studies have shown that certain cytokines, such as serpinA3, that are up-regulated in patients with HF can increase the proliferation of intestinal cancer cells in vitro (26). Whether these cytokines promote the development and/or proliferation of all cancers, including breast cancer, needs to be further examined. Because we did not have data for breast cancer therapies, the lack of association between prevalent breast cancer and the development of incident hospitalized HF in our study needs to be interpreted with caution. Moreover, only HF hospitalizations were considered, and it is possible that there were less severe cases of incident disease.
Our findings are in line with a recent analysis that demonstrated no increase in cancer risk among male physicians with HF (13). However, our findings are inconsistent with prior studies by Hasin et al. (10,11) and Banke et al. (12) that demonstrated a higher incidence of cancer in HF patients. The latter study provided a subgroup analysis for patients with breast cancer and showed an HR of 1.36 for incident breast cancer in HF patients (12). Although the results of our study are unlikely to be due to lack of power given the superimposed incidence curves through 20 years of follow-up, this is still a possibility due to the small number of prevalent HF and breast cancer cases at baseline and the small number of incident cases. When the analysis was restricted to invasive breast cancer, there was some separation of the breast cancer incidence curves in patients with HF versus no HF, but the difference did not reach statistical significance.
The incidence of breast cancer in our population was higher than the national average, likely due to enrichment of our population with older, postmenopausal women (27). However, the incidence of HF in our population was comparable to community studies (28,29), as well as studies that have described incident hospitalized HF (30,31). Several studies have evaluated the association between prevalent HF and cancer (10, 11, 12, 13). Most of these studies were limited by their small sample size, single-center registry, and inclusion of all types of cancer, which limits pathophysiologic understanding and generalizability. Our study is distinguished by its focus on the association between breast cancer and HF and outcomes in a large national cohort of postmenopausal women.
Despite the lack of association between HF and breast cancer incidence, incident invasive or all breast cancer in women with prevalent HF and similarly, incident hospitalized HF in women with prevalent breast cancer, were associated with an increased risk of all-cause mortality. Not surprisingly, women with incident HF had a poor prognosis with a median survival of 5 years and in comparison, women with incident invasive breast cancer fared better with a median survival of 19 years (32, 33, 34). These findings underscore the burden of HF in postmenopausal women and also highlight the significant impact of both disease processes on prognosis, which are consistent with prior published reports (10). Importantly, our results demonstrate that the additive prognostic implication of a prevalent comorbidity is affected by the mortality rate of the incident disease. For instance, prevalent and incident HF had a greater impact on mortality than prevalent and incident breast cancer, potentially secondary to a higher mortality from HF in and of itself irrespective of breast cancer. This knowledge may inform diagnostic and therapeutic decisions for women with HF and breast cancer and guide preventative and early detection strategies for women at risk. For example, postmenopausal women with either breast cancer or HF should undergo early counseling and aggressive screening and preventive measures for HF or breast cancer, respectively.
Study limitations
Incident HF in this cohort of the WHI was defined as a HF hospitalization, which may underestimate the incidence of HF as a result of exclusion of outpatient HF (13). In our study, prevalent HF and breast cancer were self-reported, which may result in misclassification and over- or under-reporting (35). As mentioned earlier, we did not have data on cancer therapies, and the lack of association does not rule out interactions between therapies and risk of HF. Prevalent breast cancer cases in the WHI were diagnosed prior to the advent of trastuzumab, which is the current mainstay for HER2-positive disease and an important risk factor for cardiotoxicity (7,8,24,25). Similarly, it is plausible that the risk of cardiotoxicity was mitigated by use of neurohormonal antagonists. However, we are unable to ascertain this as information on HF therapies was not available. We did not have information regarding receptor status and staging of the prevalent breast cancer cases within the WHI, which may affect generalizability and interpretation. Similarly, data on comorbidities at the time of incident disease were not available, which may have had an impact on overall survival. It is well established that age is the strongest predictor of mortality in the general population. The HRs for mortality after incident breast cancer or HF were similar in unadjusted and age-adjusted analyses. This lends support to the strength of the associations observed. Lastly, this study was restricted to postmenopausal women, and hormonal factors in premenopausal women may play a role in the relationship between HF and breast cancer. Nonetheless, there was no association between the 2 conditions despite adjustment for hormone therapy.
Conclusions
Among a large cohort of postmenopausal women, prevalent HF was not associated with incident breast cancer and similarly, prevalent breast cancer was not associated with incident hospitalized HF. Importantly, the median survival time after incident hospitalized HF was worse than that of incident breast cancer. Despite the lack of association, the presence of prevalent HF or prevalent breast cancer in participants with incident breast cancer or incident HF, respectively, was associated with an increased risk of all-cause mortality.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Prevalent HF was not associated with a higher incidence of breast cancer and vice versa. However, the presence of incident breast cancer or an incident hospitalized HF in those with prevalent HF or prevalent breast cancer, respectively, was associated with an increased risk of all-cause mortality. Importantly, the median survival time after an incident hospitalized HF was substantially worse than after an incident invasive breast cancer diagnosis.
TRANSLATIONAL OUTLOOK: These findings may inform diagnostic and therapeutic decisions for women with HF and breast cancer and guide preventative and early detection strategies for women at risk.
Author Disclosures
Dr. Nohria has received research support from Amgen, Inc.; and has consulted for Takeda Oncology. Dr. Fonarow has consulted for Abbott, Amgen, Bayer, Janssen, Novartis, and Medtronic. Dr. Chlebowski has consulted for Novartis, AstraZeneca, Genentech, Merck, and Immunomedics; and has received honorarium from Novartis and AstraZeneca. Dr. Mohammed serves on the Advisory Board for Pfizer; and has received research support from CardioCell, Abbott, Actelion, Corvia, and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
W. Gregory Hundley, MD, served as Guest Editor for this paper.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Weir H.K., Anderson R.N., Coleman King S.M. Heart disease and cancer deaths—trends and projections in the United States, 1969–2020. Prev Chronic Dis. 2016;13:E157. doi: 10.5888/pcd13.160211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloom M.W., Hamo C.E., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamo C.E., Bloom M.W., Cardinale D. Cancer therapy-related cardiac dysfunction and heart failure: part 2: prevention, treatment, guidelines, and future directions. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saiki H., Petersen I.A., Scott C.G. Risk of heart failure with preserved ejection fraction in older women after contemporary radiotherapy for breast cancer. Circulation. 2017;135:1388–1396. doi: 10.1161/CIRCULATIONAHA.116.025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaes A., Prizment A., Koene R.J., Konety S. Cardio-oncology related to heart failure: common risk factors between cancer and cardiovascular disease. Heart Fail Clin. 2017;13:367–380. doi: 10.1016/j.hfc.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koene R.J., Prizment A.E., Blaes A., Konety S.H. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114. doi: 10.1161/CIRCULATIONAHA.115.020406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Versace V.L., Berry N.M., Chowdhury M.H. Characteristics of patients with haematological and breast cancer (1996-2009) who died of heart failure-related causes after cancer therapy. ESC Heart Fail. 2016;3:253–260. doi: 10.1002/ehf2.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasin T., Gerber Y., McNallan S.M. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881–886. doi: 10.1016/j.jacc.2013.04.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasin T., Gerber Y., Weston S.A. Heart failure after myocardial infarction is associated with increased risk of cancer. J Am Coll Cardiol. 2016;68:265–271. doi: 10.1016/j.jacc.2016.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banke A., Schou M., Videbaek L. Incidence of cancer in patients with chronic heart failure: a long-term follow-up study. Eur J Heart Fail. 2016;18:260–266. doi: 10.1002/ejhf.472. [DOI] [PubMed] [Google Scholar]
- 13.Selvaraj S., Bhatt D.L., Claggett B. Lack of association between heart failure and incident cancer. J Am Coll Cardiol. 2018;71:1501–1510. doi: 10.1016/j.jacc.2018.01.069. [DOI] [PubMed] [Google Scholar]
- 14.Bradshaw P.T., Stevens J., Khankari N., Teitelbaum S.L., Neugut A.I., Gammon M.D. Cardiovascular disease mortality among breast cancer survivors. Epidemiology. 2016;27:6–13. doi: 10.1097/EDE.0000000000000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buttros D.A.B., Branco M.T., Orsatti C.L., Almeida-Filho B.S., Nahas-Neto J., Nahas E.A.P. High risk for cardiovascular disease in postmenopausal breast cancer survivors. Menopause. 2019;26:1024–1030. doi: 10.1097/GME.0000000000001348. [DOI] [PubMed] [Google Scholar]
- 16.Abdel-Qadir H., Thavendiranathan P., Austin P.C. The risk of heart failure and other cardiovascular hospitalizations after early stage breast cancer: a matched cohort study. J Natl Cancer Inst. 2019;111:854–862. doi: 10.1093/jnci/djy218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13:1141–1151. doi: 10.1016/S1470-2045(12)70425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall P.S., Nah G., Howard B.V. Reproductive factors and incidence of heart failure hospitalization in the Women's Health Initiative. J Am Coll Cardiol. 2017;69:2517–2526. doi: 10.1016/j.jacc.2017.03.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The Women's Health Initiative Study Group Design of the Women's Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 20.Eaton C.B., Pettinger M., Rossouw J. Risk factors for incident hospitalized heart failure with preserved versus reduced ejection fraction in a multiracial cohort of postmenopausal women. Circ Heart Fail. 2016;9 doi: 10.1161/CIRCHEARTFAILURE.115.002883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eaton C.B., Abdulbaki A.M., Margolis K.L. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women's Health Initiative. Circulation. 2012;126:688–696. doi: 10.1161/CIRCULATIONAHA.111.066688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curb J.D., McTiernan A., Heckbert S.R., for the WHI Morbidity and Mortality Committee Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(Suppl 9):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 23.Rosamond W.D., Chang P.P., Baggett C. Classification of heart failure in the Atherosclerosis Risk in Communities (ARIC) study: a comparison of diagnostic criteria. Circ Heart Fail. 2012;5:152–159. doi: 10.1161/CIRCHEARTFAILURE.111.963199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertero E., Canepa M., Maack C., Ameri P. Linking heart failure to cancer. Circulation. 2018;138:735–742. doi: 10.1161/CIRCULATIONAHA.118.033603. [DOI] [PubMed] [Google Scholar]
- 25.Mehta L.S., Watson K.E., Barac A. Cardiovascular disease and breast cancer: where these entities intersect: a scientific statement from the American Heart Association. Circulation. 2018;137:e30–e66. doi: 10.1161/CIR.0000000000000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meijers W.C., Maglione M., Bakker S.J.L. Heart failure stimulates tumor growth by circulating factors. Circulation. 2018;138:678–691. doi: 10.1161/CIRCULATIONAHA.117.030816. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention United States Cancer Statistics. 2015. https://gis.cdc.gov/Cancer/USCS/DataViz.html Available at: Accessed May 13, 2019.
- 28.Levy D., Kenchaiah S., Larson M.G. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 29.Gerber Y., Weston S.A., Redfield M.M. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med. 2015;175:996–1004. doi: 10.1001/jamainternmed.2015.0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loehr L.R., Rosamond W.D., Chang P.P., Folsom A.R., Chambless L.E. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 31.Jackson S.L., Tong X., King R.J., Loustalot F., Hong Y., Ritchey M.D. National burden of heart failure events in the United States, 2006 to 2014. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chlebowski R.T., Aragaki A.K., Anderson G.L. Association of low-fat dietary pattern with breast cancer overall survival: a secondary analysis of the Women's Health Initiative randomized clinical trial. JAMA Oncol. 2018;4 doi: 10.1001/jamaoncol.2018.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg R.J., Ciampa J., Lessard D., Meyer T.E., Spencer F.A. Long-term survival after heart failure: a contemporary population-based perspective. Arch Intern Med. 2007;167:490–496. doi: 10.1001/archinte.167.5.490. [DOI] [PubMed] [Google Scholar]
- 34.Taylor C.J., Ordonez-Mena J.M., Roalfe A.K. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000–2017: population based cohort study. BMJ. 2019;364:l223. doi: 10.1136/bmj.l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Writing Group Members. Mozaffarian D., Benjamin E.J. Heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–e360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.