Abstract
Background
In childhood cancer survivors (CCS) at risk for heart failure, echocardiographic surveillance recommendations are currently based on anthracyclines and chest-directed radiotherapy dose. Whether the ejection fraction (EF) measured at an initial surveillance echocardiogram can refine these recommendations is unknown.
Objectives
The purpose of this study was to assess the added predictive value of EF at >5 years after cancer diagnosis to anthracyclines and chest-directed radiotherapy dose in CCS, for the development of left ventricular dysfunction with an ejection fraction <40% (LVD40).
Methods
Echocardiographic surveillance was performed in 299 CCS from the Emma Children’s Hospital in the Netherlands. Cox regression models were built including cardiotoxic cancer treatment exposures with and without EF to estimate the probability of LVD40 at 10-year follow-up. Calibration, discrimination, and reclassification were assessed. Results were externally validated in 218 CCS.
Results
Cumulative incidences of LVD40 at 10-year follow-up were 3.7% and 3.6% in the derivation and validation cohort, respectively. The addition of EF resulted in an integrated area under the curve increase from 0.74 to 0.87 in the derivation cohort and from 0.72 to 0.86 in the validation cohort (likelihood ratio p < 0.001). Reclassification of CCS without LVD40 improved significantly (noncase continuous net reclassification improvement 0.50; 95% confidence interval [CI]: 0.40 to 0.60). A predicted LVD40 probability ≤3%, representing 75% of the CCS, had a negative predictive value of 99% (95% CI: 98% to 100%) for LVD40 within 10 years. However, patients with midrange EF (40% to 49%) at initial screening had an incidence of LVD40 of 11% and a 7.81-fold (95% CI: 2.07- to 29.50-fold) increased risk of LV40 at follow-up.
Conclusions
In CCS, an initial surveillance EF, in addition to anthracyclines and chest-directed radiotherapy dose, improves the 10-year prediction for LVD40. Through this strategy, both the identification of low-risk survivors in whom the surveillance frequency may be reduced and a group of survivors at increased risk of LVD40 could be identified.
Key Words: cardio-oncology, childhood cancer survivors, echocardiography, risk prediction model, surveillance
Abbreviations and Acronyms: CCS, childhood cancer survivors; CI, confidence interval; EF, ejection fraction; LVD40, left ventricular dysfunction with an ejection fraction <40%
Central Illustration
The survival of childhood cancer has increased considerably over the last decades, with 80% of children with cancer becoming long-term (≥5 year) survivors (1). However, the same treatment that successfully cured their childhood cancer places them at an increased risk of adverse events up to 40 years after childhood cancer diagnosis (2). Cardiotoxicity in childhood cancer survivors (CCS) is a well-known late effect after treatment with anthracyclines, mitoxantrone, or chest-directed radiotherapy (3, 4, 5). The cumulative incidence of symptomatic heart failure at 40 years past cancer diagnosis is 10.6% in CCS treated with cardiotoxic cancer therapies (5). In addition, asymptomatic left ventricular (LV) dysfunction is frequently present in CCS and is associated with an increased risk of developing symptomatic heart failure in the general population (6). When defined as an ejection fraction (EF) <50%, or as a fractional shortening <28%, asymptomatic LV dysfunction has been reported in 6% to 8% of CCS at a median of 9 to 23 years after cancer diagnosis (7, 8, 9, 10).
Currently, to detect and treat asymptomatic LV dysfunction early, the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG) recommends to perform an echocardiogram once every 5 years in all CCS treated with cardiotoxic cancer therapies (11,12). More frequent surveillance is thought reasonable in high-risk CCS treated with cumulative doses of anthracyclines ≥250 mg/m2, chest-directed radiotherapy ≥35 Gy, or a combination of the 2 (anthracycline ≥100 mg/m2 and chest-directed radiotherapy ≥15 Gy) (11). Referral to a cardiologist is recommended after asymptomatic LV dysfunction (EF <50%) is identified. However, the recommendation for pharmacological treatment is, due to a lack of evidence in CCS (13,14), based on guidelines for adults with asymptomatic LV dysfunction from other causes (11,15,16). These guidelines recommend treatment for symptomatic patients and for patients with asymptomatic LV systolic dysfunction, although their direct relevance to CCS is unknown (17,18).
The current IGHG surveillance guidelines do not include measurements of LV function in the risk stratification for cardiomyopathy (11). We hypothesized that EF measured at the first long-term follow-up echocardiogram may improve cardiomyopathy risk stratification and may further serve to personalize surveillance frequency recommendations. With the knowledge that an EF <40% is a strong and widely accepted indication to start heart failure medications (15), an optimal surveillance strategy should be directed to timely identify CCS with an EF <40%, regardless of the presence of heart failure symptoms.
In this study, we assessed and externally validated the added predictive value of EF at first long-term follow-up echocardiogram in asymptomatic CCS treated with cardiotoxic cancer therapies for the development of left ventricular dysfunction with ejection fraction <40% (LVD40).
Methods
Study population
The derivation cohort consisted of CCS from the Emma Children’s Hospital in Amsterdam, the Netherlands. This cohort included CCS with a primary childhood malignancy between 1966 and 1997, treated with anthracyclines, mitoxantrone, and/or chest-directed radiotherapy who were at least 5 years past cancer diagnosis (8).
The validation cohort consisted of CCS from the Radboud University Medical Center in Nijmegen, the Netherlands (19, 20, 21). CCS treated with anthracyclines, who were at least 5 years past cancer diagnosis and who visited the survivorship outpatient clinic between 2006 and 2012 were included in this cohort.
From both cohorts, we selected CCS >18 years of age at the first follow-up echocardiogram who were treated with anthracyclines, mitoxantrone and/or chest-directed radiotherapy. CCS with a history of heart failure before the first available follow-up echocardiogram ≥5 years after cancer diagnosis were excluded. Asymptomatic CCS with an EF <40% before or at the first echocardiogram were also excluded. For the longitudinal analysis, CCS with ≥2 follow-up echocardiograms were included when the time interval between each echocardiogram was ≤5 years and the total follow-up was ≥1 year. We chose the time interval of ≤5 years between each echocardiogram because this is the time interval recommended by the cardiomyopathy surveillance guidelines (11). Informed consent for participation in the late effects study cohort was previously obtained from all participants, and the study was approved by the medical ethics boards of the Emma Children’s Hospital/Academic Medical Center and the Radboud University Medical Center (8,19,20).
Data collection
Data were retrospectively collected from medical records, digitally archived echocardiograms, and the database of prior studies within these cohorts (8,19,20). Variables of interest included: sex, cancer diagnosis, age at cancer diagnosis, age at first echocardiogram (see “Echocardiograms” section), time since cancer diagnosis at first echocardiogram, cardiovascular risk factors (hypertension, dyslipidemia, and/or diabetes reported in questionnaires or diagnosed by a physician), heart failure medication prescriptions, cumulative doses of anthracycline (summed according to doxorubicin-equivalent ratios [22]), mitoxantrone, and chest-directed radiotherapy.
Chest-directed radiotherapy was defined as radiotherapy involving the heart region and included total body irradiation, left or whole abdominal irradiation, spinal irradiation, thoracic irradiation, and inverted Y-field irradiation. For the chest-directed radiotherapy dose, we used the maximum prescribed dose to the smallest field and added the total body irradiation dose (23).
Echocardiograms
The first available echocardiogram ≥5 years after cancer diagnosis was used to measure the initial EF. All subsequent echocardiograms after the first echocardiogram were systematically collected. All echocardiograms were performed by trained sonographers and supervised by an imaging cardiologist. Fractional shortening was measured in the parasternal long axis and calculated from the LV internal diameter at end-diastole and -systole at the base of the LV by M-mode echocardiography. Biplane EF was measured in the apical chamber views with the modified Simpson’s method (24). In cases where biplane EF could not be measured, EF was calculated using the Teichholz formula that has been shown to accurately estimate EF in the absence of dyssynchrony and wall motion abnormalities (25). We assessed the agreement between Teichholz and biplane-derived EF in 323 echocardiograms where both metrics were available (Supplemental Table 1 and Supplemental Figure 1). The overall agreement on the endpoint of EF <40% or EF ≥40% was 97% (26). In 30 randomly selected echocardiograms, the intraclass correlation coefficient for the intraobserver variability of biplane EF was 0.83 (95% confidence interval [CI]: 0.67 to 0.92) and the intraclass correlation coefficient for interobserver variability was 0.79 (95% CI: 0.61 to 0.90), which is comparable to values reported in the published data (27).
Statistical analyses
Continuous variables are presented as mean ± SD when normally distributed and as median (25th to 75th percentile) when asymmetrically distributed. Categorical variables are presented as number with percentages. Patient characteristics were compared between groups with the Student’s t-test for normally distributed continuous variables, the Kruskal-Wallis test for asymmetrically distributed continuous variables, and the chi-square or Fisher exact test for categorical variables.
The primary endpoint was the onset of LVD40 after the first follow-up echocardiogram. Time was considered from the point at which the initial EF was obtained. The cumulative incidence of LVD40 was estimated with death as a competing risk, and CCS with an EF 40% to 49% was compared with CCS with an EF ≥50% at first echocardiogram using the Fine and Gray’s test (28).
Hazard ratios (HRs) with 95% CIs were estimated with multivariable Cox regression models. Anthracycline and chest-directed radiotherapy dose that are currently used for risk stratification in the IGHG surveillance guideline (11) were entered in the model with and without the addition of initial EF. EF was categorized to estimate the risk associated with an EF 40% to 49% (midrange) compared with an EF ≥50% (preserved). Continuous EF was used in the prediction model development because continuous covariates have superior predictive power.
The proportionality assumption was tested with the Schoenfeld residual test and by inspecting the Schoenfeld residuals over time (29). Nonlinearity of the covariates was tested for with restricted cubic splines (see the Supplemental Appendix for results) (30).
Individual 10-year probabilities for LVD40 (LVD40prob) were estimated with the formula: LVD40prob(t = 10) = 1 − (H0[t = 10]exp(LP)) (31), with H0(t) representing baseline hazard with the Breslow estimator at 10-year follow-up in both cohorts, and LP the linear predictor with the coefficients derived from the model fitted in the derivation cohort.
Calibration was evaluated by plotting the observed versus the predicted 10-year probabilities for LVD40 in 5 groups. In the derivation cohort, improvement in model performance with the addition of initial EF was tested using the likelihood ratio test (32). Discrimination was quantified with the integrated area under the receiver-operating characteristic curve (iAUC), which represents a weighted average of time-dependent AUC measures (33,34). Bias and 95% CIs of the iAUCs were assessed using 2,000 bootstrap samples. The continuous net reclassification improvement (cNRI) was calculated of the model with addition of the initial EF value compared to the model without EF. The cNRI indicates the proportion of patients that accurately change in their predicted risk with the addition of EF to the model and can be calculated for cases and noncases (Supplemental Table 4) (35). Time-dependent accuracy measures (sensitivity, specificity, and negative and positive predictive values) of the model with EF were calculated with the “timeROC” package, which accounts for censoring (36).
To adjust for selection bias that might have resulted from the exclusion of CCS, we performed a sensitivity analysis in the derivation cohort where we weighted the HR estimates with the inverse of the sampling probability (37). To estimate the sampling probability, we used a logistic regression model with selection for this study (yes/no) as the outcome, and sex, age at cancer diagnosis (as a spline), cumulative anthracycline dose (as a spline), chest-directed radiotherapy dose, cumulative mitoxantrone dose, cancer diagnosis year, and LVD40 or heart failure (yes/no) as covariates. Additional sensitivity analyses were performed with heart failure medication use and cardiovascular risk factors (hypertension, dyslipidemia, and diabetes).
All analyses were performed in R version 3.5.1 (R Foundation, Vienna, Austria), and a 2-sided p value <0.05 was considered statistically significant. No missing data was present in the predictor variables.
Results
Characteristics of CCS in the derivation and validation cohort
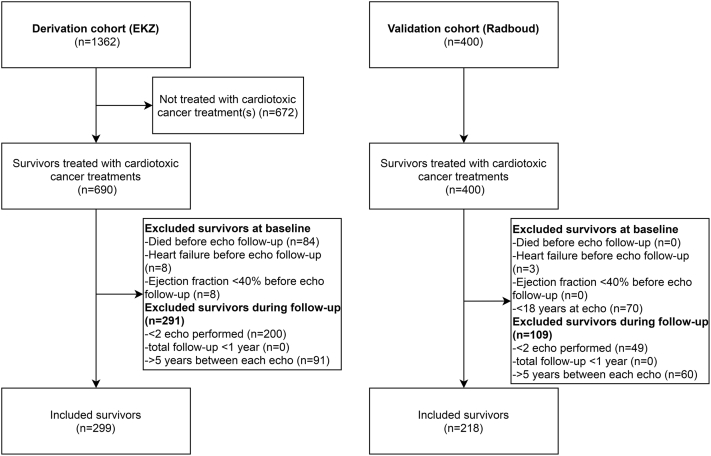
In the derivation cohort, 690 CCS received cardiotoxic cancer treatment and survived ≥5 years after diagnosis (Figure 1). A total of 84 CCS died before available echocardiographic follow-up (4 of heart failure). Other reasons for exclusion included: heart failure or an EF <40% before the first follow-up echocardiogram (n = 16), <2 follow-up echocardiograms performed (n = 200), or ≥5 years between the follow-up echocardiograms (n = 91). In total, 299 CCS were eligible for this study. Compared with the CCS that were excluded, those included were more often women (56.2% vs. 33.7%; p < 0.001) and were treated with higher anthracycline doses (median 280 mg/m2 [25th to 75th percentile: 180 to 400 mg/m2] vs. 200 mg/m2 [25th to 75th percentile: 150 to 360 mg/m2]; p = 0.013) (Supplemental Table 2).
Figure 1.
Flowchart of Patient Inclusion
Flowchart describing the inclusion of childhood cancer survivors (CCS) in the derivation and validation cohort. Adult survivors who were previously treated with cardiotoxic cancer treatments with at least 2 surveillance echocardiograms performed at more than 5 years from cancer diagnosis and with <5 years between each echocardiogram were selected. Survivors with heart failure or an ejection fraction <40% before or at the first surveillance echocardiogram were excluded. echo = echocardiogram; EKZ = Emma Children’s Hospital; Radboud = Radboud University Medical Center.
In the validation cohort, 400 CCS were treated with cardiotoxic cancer treatments and survived ≥5 years after diagnosis, and 218 of them were eligible for inclusion (Figure 1). Reasons for exclusion were age <18 years during echocardiographic follow-up (n = 70), >5 years between the follow-up echocardiograms (n = 60), <2 follow-up echocardiograms performed (n = 49), and heart failure before echocardiographic follow-up (n = 3).
Patient characteristics of both cohorts are presented in Table 1. Compared with the derivation cohort, CCS in the validation cohort were more often treated with anthracyclines at lower doses (derivation cohort 280 mg/m2 [180 to 400 mg/m2]; validation cohort 180 mg/m2 [150 to 301 mg/m2]) and had a higher initial EF (derivation cohort mean 61.6 ± 7.1% vs. validation cohort mean 57.1 ± 6.9%). A midrange initial EF (EF 40% to 49%) was present at baseline in 13.7% CCS in the derivation cohort and in 5.5% of the patients in the validation cohort. CCS with a midrange EF were exposed to higher anthracycline doses compared with CCS with a preserved EF (EF ≥50%) (Supplemental Table 3). Follow-up after the first echocardiogram was longer in the derivation cohort (median 10.9 years [25th to 75th percentile: 8.2 to 13.1 years]) compared with the validation cohort (median 8.9 years [25th to 75th percentile: 5.2 to 10.9 years]) (Table 1).
Table 1.
Characteristics of the CCS in the Derivation and Validation Cohort
| Derivation Cohort: Amsterdam (n = 299) | Validation Cohort: Nijmegen (n = 218) | p Value | |
|---|---|---|---|
| Female | 168 (56.2) | 109 (50.0) | 0.192 |
| Age at cancer diagnosis, yrs | 7.22 (4.01–11.71) | 7.02 (4.00–12.46) | 0.625 |
| Time since cancer diagnosis at first follow-up echo, yrs | 16.74 (11.83–23.15) | 16.95 (12.99–21.70) | 0.512 |
| Age at first follow-up echo, yrs | 24.06 (19.60–30.71) | 22.63 (20.05–28.06) | 0.399 |
| Tumor | <0.001 | ||
| ALL | 55 (18.4) | 71 (32.6) | |
| AML | 14 (4.7) | 15 (6.9) | |
| Hodgkin lymphoma | 23 (7.7) | 30 (13.8) | |
| Non-Hodgkin lymphoma | 61 (20.4) | 37 (17.0) | |
| Nephroblastoma | 46 (15.4) | 14 (6.4) | |
| Soft-tissue sarcoma | 28 (9.4) | 7 (3.2) | |
| Ewing sarcoma | 18 (6.0) | 14 (6.4) | |
| Osteosarcoma | 24 (8.0) | 13 (6.0) | |
| CNS tumor | 17 (5.7) | 4 (1.8) | |
| Germ cell tumor | 4 (1.3) | 1 (0.5) | |
| Neuroblastoma | 2 (0.7) | 9 (4.1) | |
| Other | 7 (2.3) | 2 (0.9) | |
| Anthracyclines | 239 (79.9) | 214 (98.2) | <0.001 |
| Cumulative anthracycline dose, mg/m2 | 280.0 (180.0–400.0) | 180.0 (150.0–301.4) | <0.001 |
| Chest RT | 105 (35.1) | 59 (27.1) | 0.065 |
| Chest RT dose, Gy | 25.0 (18.0–33.3) | 20.0 (18.0–30.0) | 0.406 |
| Anthracyclines and chest RT | 45 (15.1) | 56 (25.7) | 0.004 |
| Mitoxantrone | 12 (4.0) | 7 (4.2) | 1.000 |
| Cumulative mitoxantrone dose, mg/m2 | 12.0 (12.0–16.0) | 40.0 (20.0–40.0) | 0.003 |
| EF at first follow-up echo | 57.1 ± 6.9 | 61.6 ± 7.1 | <0.001 |
| EF 40%–49% at first follow-up echo | 41 (13.7) | 12 (5.5) | 0.004 |
| Hypertension | 15 (5.0) | — | |
| Dyslipidemia | 4 (1.34) | — | |
| Diabetes mellitus | 2 (0.7) | — | |
| Heart failure medication(s) use at first echo | 4 (1.3) | 3 (1.4) | 1.000 |
| Follow-up after the first follow-up echo, yrs | 10.90 (8.19–13.05) | 8.86 (5.22–10.86) | <0.001 |
| Number of follow-up echoes per patient | 5 (3–6) | 3 (2–4) | <0.001 |
| Echocardiographic surveillance rate, per 5 yrs | 2.26 (1.96–2.67) | 1.93 (1.57–2.52) | <0.001 |
| Left ventricular dysfunction with EF <40% during follow-up | 11 (3.7) | 7 (3.2) | 0.965 |
Values are n (%), median (25th to 75th percentile), or mean ± SD.
ALL = acute lymphocytic leukemia; AML = acute myeloid leukemia; chest RT = chest-directed radiotherapy; CNS = central nervous system; Gy = gray.
Incidence of LVD40 and characteristics of CCS with LVD40 in the derivation cohort
In the derivation cohort, the cumulative incidence of LVD40 at 10-year follow-up after the first echocardiogram was 3.7% (11 events; 95% CI: 1.4% to 5.9%). In 6 patients, LVD40 was accompanied by symptomatic heart failure, and 10 patients were treated with heart failure medications. At a median follow-up of 7.2 years (25th to 75th percentile: 6.2 to 9.7 years) after the initial EF, 12 CCS died: 10 deaths were due to cancer, 1 death was due to nervous system disease in a patient with a cerebral drain, and 1 had unexplained sudden death without a known cardiomyopathy diagnosis.
The cumulative LVD40 incidence 10 years after the initial EF was significantly higher in CCS with an initial midrange EF (11.0%) compared with CCS with an initial preserved EF (2.6%; Gray’s test p = 0.012). In CCS with LVD40, the median time from first echocardiogram to LVD40 onset was 7.2 years (25th to 75th percentile 3.8 to 8.4 years; range 1.2 to 12.2 years) and was not significantly different between CCS with a midrange EF and those with a preserved EF (median 7.2 years [25th to 75th percentile: 3.3 to 8.9 years] vs. 6.6 years [25th to 75th percentile: 4.7 to 7.7 years]; p = 0.085). In multivariable analysis adjusted for anthracycline and chest-directed radiotherapy, CCS with an initial midrange EF had a higher risk of LVD40 compared with CCS with a preserved EF (HR: 7.8; 95% CI: 2.1 to 29.5) (Table 2).
Table 2.
Multivariable Cox Regression of Potential Risk Factors for LVD40 During Follow-Up
| HR (95% CI) | p Value | |
|---|---|---|
| Model without first EF | ||
| Anthracycline dose (per 100-mg/m2 increment) | 1.71 (1.21–2.40) | 0.002 |
| Chest-directed radiotherapy dose (per 10-Gy increment) | 1.65 (1.20–2.26) | 0.002 |
| Model with continuous first EF | ||
| EF at first echocardiogram (per 10-point decrease) | 9.62 (2.84–32.57) | <0.001 |
| Anthracycline dose (per 100-mg/m2 increment) | 1.43 (1.04–1.98) | 0.026 |
| Chest-directed radiotherapy dose (per 10-Gy increment) | 1.67 (1.21–2.30) | 0.002 |
| Model with categorized first EF | ||
| Midrange versus preserved EF at first echocardiogram | 7.81 (2.07–29.50) | 0.002 |
| Anthracycline dose (per 100-mg/m2 increment) | 1.70 (1.22–2.36) | 0.002 |
| Chest-directed radiotherapy dose (per 10-Gy increment) | 1.91 (1.34–2.72) | <0.001 |
CI = confidence interval; EF = ejection fraction; HR = hazard ratio; LVD40 = left ventricular dysfunction with ejection fraction <40%.
All CCS who developed LVD40 were treated with cumulative anthracycline doses ≥100 mg/m2 or chest-directed radiotherapy doses ≥15 Gy, corresponding to a moderate or high risk according to the cardiomyopathy surveillance guideline (11).
Prediction model development
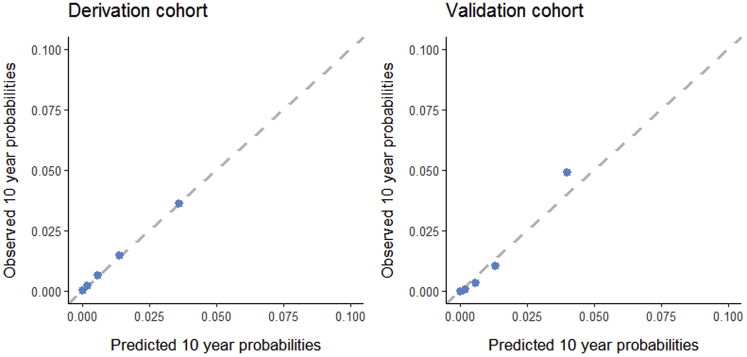
Lower initial EF increased the risk of LVD40 during follow-up (HR: 9.6 per 10-point EF decrease; 95% CI: 2.8 to 32.6) (Table 2). Addition of initial EF to the prediction model with anthracycline and chest-directed radiotherapy dose increased the iAUC from 0.74 (bias 0.018; 95% CI: 0.55 to 0.84) to 0.87 (bias 0.009; 95% CI: 0.71 to 0.98). The likelihood ratio test comparing the predictive performance of the model with EF with the model without EF was highly significant (p < 0.001). The model with EF showed good calibration at 10-year follow-up (Figure 2). Net reclassification of cases who developed LVD40 did not improve significantly with the addition of initial EF (case cNRI 0.15; 95% CI: −0.55 to 0.84) (Supplemental Table 4). However, for noncases (who did not develop LVD40), reclassification improved significantly (non-case cNRI 0.50; 95% CI: 0.40 to 0.60).
Figure 2.
Calibration Plots
Agreement between the predicted 10-year probabilities of a left ventricular ejection fraction <40% (LVD40) obtained from the Cox regression model compared with the observed 10-year LVD40 probabilities in the derivation and the validation cohorts. Predictions from the final multivariable Cox regression model including ejection fraction are shown.
A 10-year predicted risk ≤3% was present in 76.3% of CCS and achieved a high sensitivity (89.8%; 95% CI: 70.6% to 100%) and negative predictive value (99.5%; 95% CI: 98.6% to 100%) with a specificity of 76.2% (95% CI: 70.0% to 82.5%) and a positive predictive value of 12.0% (95% CI: 4.0% to 20.0%) (Table 3).
Table 3.
Time-Dependent Accuracy Measures of the Multivariable Model Including Continuous EF at Different Predicted Risks Cut-Offs for LVD40 at 10-Year Follow-Up After the First Echocardiogram
| Predicted Risk Cut-Off∗ (%) | Actual Risk∗ (%) | % of Cohort With Risk Above Cut-Off | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| Derivation cohort: Amsterdam | ||||||
| 1.0 | 1.1 | 47.8 | 89.8 (70.6–100.0) | 47.5 (40.2–54.8) | 5.8 (1.9–9.8) | 99.2 (97.7–100.0) |
| 2.0 | 2.1 | 34.1 | 89.8 (70.6–100.0) | 63.0 (55.9–70.0) | 8.0 (2.6–13.5) | 99.4 (98.3–100.0) |
| 3.0 | 3.1 | 23.7 | 89.8 (70.6–100.0) | 76.2 (70.0–82.5) | 12.0 (4.0–20.0) | 99.5 (98.6–100.0) |
| 4.0 | 4.0 | 18.7 | 89.8 (70.6–100.0) | 81.8 (76.1–87.4) | 15.1 (5.2–25.0) | 99.6 (98.7–100.0) |
| 5.0 | 4.9 | 15.4 | 56.0 (23.4–88.6) | 85.1 (79.9–90.3) | 11.9 (1.9–22.0) | 98.2 (96.4–100.0) |
| Validation cohort: Nijmegen | ||||||
| 1.0 | 0.7 | 47.2 | 100.0 (100.0–100.0) | 59.0 (48.4–69.6) | 8.3 (1.7–15.0) | 100.0 (100.0–100.0) |
| 2.0 | 1.9 | 31.7 | 100.0 (100.0–100.0) | 71.1 (61.3–80.9) | 11.4 (2.3–20.5) | 100.0 (100.0–100.0) |
| 3.0 | 3.3 | 25.2 | 85.1 (57.8–100.0) | 77.1 (68.0–86.2) | 12.2 (1.6–22.8) | 99.3 (97.9–100.0) |
| 4.0 | 4.9 | 20.2 | 85.1 (57.8–100.0) | 81.9 (73.6–90.2) | 14.9 (2.0–27.9) | 99.3 (98.0–100.0) |
| 5.0 | 6.6 | 17.0 | 85.1 (57.8–100.0) | 88.0 (80.9–95.0) | 20.8 (3.0–38.7) | 99.4 (98.1–100.0) |
NPV = negative predictive value; PPV = positive predictive value; other abbreviations as in Table 2.
Predicted and actual cumulative incidences of LVD40 at 10 years from the first echocardiogram.
Results of the inverse probability-weighted sensitivity analysis, performed to adjust for selection bias that might have resulted from the exclusion of CCS, were comparable to the main results and are shown in Supplemental Table 5. In another sensitivity analysis, heart failure medication use and presence of cardiovascular risk factors (hypertension, dyslipidemia, and diabetes) at time of the initial EF were not associated with LVD40 and did not attenuate the association of initial EF with LVD40 (Supplemental Table 6).
External validation
In the validation cohort, the cumulative incidence of LVD40 at 10-year follow-up after the first echocardiogram was 3.6% (7 events; 95% CI: 0.7% to 6.4%). With the model developed in the derivation cohort, individual 10-year LVD40 probabilities were calculated. The model showed good calibration up to a LVD40 probability of 5%, which represented 83.0% of the CCS (Figure 2). The iAUC increased from 0.72 (bias −0.003; 95% CI: 0.70 to 0.77) to 0.86 (bias −0.003; 95% CI: 0.83 to 0.89) in the model containing initial EF versus the model containing only anthracycline and chest-directed radiotherapy dose. A predicted 10-year probability ≤3% was present in 74.8% of the CCS and resulted in a sensitivity of 85.1% (95% CI: 57.8% to 100%), specificity of 77.1% (95% CI: 68.0% to 86.2%), positive predictive value of 12.2% (95% CI: 1.6% to 22.8%), and negative predictive value of 99.3% (95% CI: 97.9% to 100%) (Table 3).
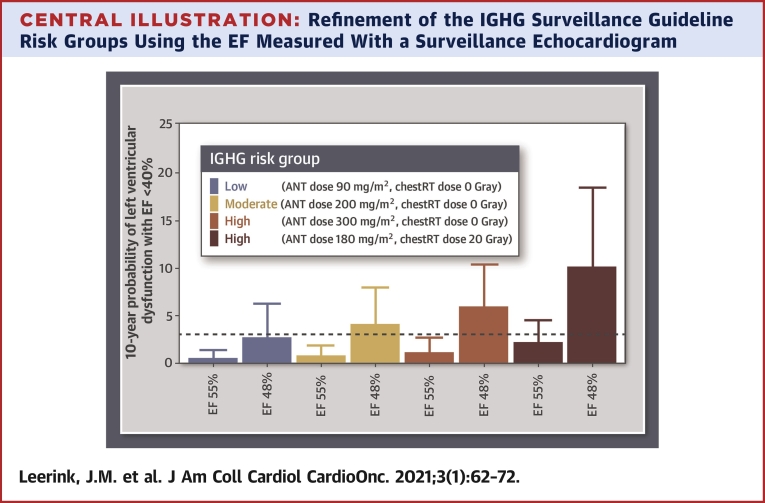
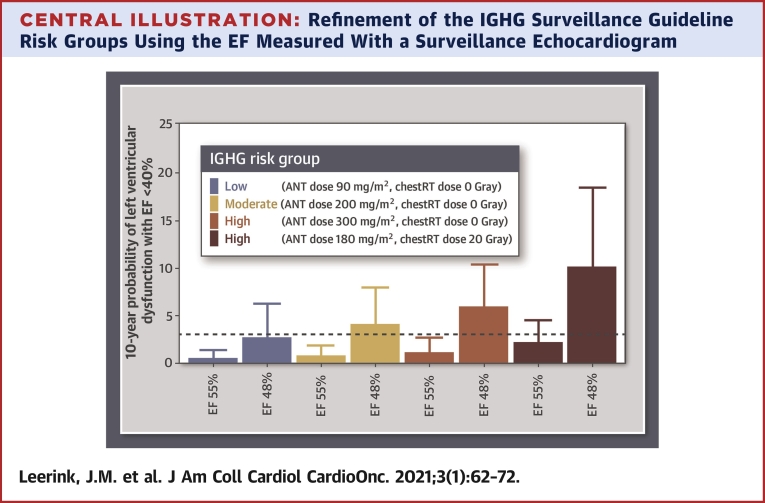
Predicted probabilities of LVD40 within 10 years in individual survivors with different predictor value combinations are shown in the Central Illustration. Survivors in the low- and moderate-risk group according to the IGHG surveillance guidelines with a preserved initial EF (EF 55%) had a predicted LVD40 probability ≤3.0%. In contrast, survivors in the low- and moderate-IGHG risk group with a midrange EF (EF 48%) had a predicted LVD40 probability, where the upper 95% CI was >3.0%. Our validated prediction model including a surveillance EF, cumulative anthracycline, and chest-directed radiotherapy dose is accessible online (38).
Central Illustration.
Refinement of the IGHG Surveillance Guideline Risk Groups Using the EF Measured With a Surveillance Echocardiogram
Predicted probabilities for developing left ventricular dysfunction with ejection fraction (EF) <40% within 10 years in individual fictional survivors. In colors the risk categories (low, moderate, or high) are presented according to the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG). In each IGHG risk category, the 10-year probability of left ventricular dysfunction with EF <40% is compared between a survivor with an initial surveillance EF of 48% and a survivor with an initial EF of 55%. Bars represent the risk estimate; error bars represent the 95% confidence interval.
Discussion
In this echocardiographic follow-up study of long-term CCS, we show in 2 independent cohorts that addition of an initial surveillance EF improves the 10-year prediction of LVD40 in CCS and accurately identifies low-risk survivors who are unlikely to develop LVD40 within 10 years. This may improve the current IGHG recommended risk-stratification for cardiomyopathy, which is based solely on anthracycline and chest-directed radiotherapy dose to estimate risk (11).
Previous echocardiographic follow-up studies in long-term CCS were generally limited in sample size (range n = 28 to 115) and did not assess the additive predictive value of echocardiography together with cancer treatment exposures (39, 40, 41, 42, 43, 44, 45). We demonstrate for the first time in a relatively large cohort of CCS with long-term follow-up, that a midrange EF (EF 40% to 49%) is associated with an almost 8-fold increased risk for LVD40 compared with those with a preserved first EF (EF ≥50%), a finding that is in line with the risk of asymptomatic LV dysfunction for development of symptomatic heart failure from other causes in the general population (relative risk: 4.6; 95% CI: 2.2 to 9.8) (6). The fact that 13.7% of the CCS in the derivation cohort and 5.5% of the CCS in the validation cohort had a midrange EF, considerably higher than the LV dysfunction (EF <50%) prevalence of 1.7% to 3.6% in the general population at age 50 to 62 years (6), suggests that a large group of relatively young CCS are already at increased risk.
Implications for surveillance
The IGHG cardiomyopathy surveillance guidelines recommend echocardiographic surveillance once every 5 years in CCS treated with anthracyclines and/or chest-directed radiotherapy (11). In the absence of long-term longitudinal echocardiographic follow-up data, these recommendations were based on simulation studies with relative risks of asymptomatic LV dysfunction for heart failure and treatment effects obtained from the general population (46,47).
Recently, it has been suggested in a simulation model that cardiomyopathy surveillance is cost-ineffective in the IGHG low-risk group, representing ∼40% of the survivors (48). Our results in 2 independent CCS cohorts also suggest that LVD40 is very unlikely in low-risk survivors according to the IGHG surveillance guideline, as no LVD40 events occurred in this risk group during a median follow-up of 10.9 years in the derivation cohort and 8.9 years in the validation cohort.
In addition, we demonstrate that a surveillance EF obtained at a median of 17 years (25th to 75th percentile 13 to 22 years) after cancer diagnosis and a median age of 23 years (25th to 75th percentile 20 to 28 years), in addition to anthracycline and chest-directed radiotherapy dose, accurately reclassifies 50% (95% CI: 40% to 60%) of the CCS who will not develop LVD40 to a lower-risk category. This means that an initial surveillance EF can refine the risk stratification as recommended by the IGHG surveillance guideline, resulting in reclassification of survivors in the IGHG moderate-risk group to a group at low risk of LVD40 within 10 years (Central Illustration).
We were able to identify a large subgroup representing at least 75% of CCS in the derivation and validation cohort with a predicted risk ≤3% who were unlikely to develop LVD40 within 10 years (NPV 99%; 95% CI: 98% to 100%). This finding implicates that for low-risk CCS with a predicted risk ≤3%, obtaining the next surveillance echocardiogram within 10 years may be sufficient. It also means that only ∼25% of the CCS population determined to be at higher risk needs to be screened according to the current surveillance protocol, and that the yield of patients with LVD40 within the 10-year follow-up period will be higher from 1 of 30 patients to 1 of 8 patients screened.
Study limitations
First, echocardiograms obtained before 1999 were unavailable for analysis, and therefore, the initial echocardiogram was obtained at varying time points after cancer diagnosis (25th to 75th percentile: 12 to 23 years) and age of the CCS (25th to 75th percentile: 20 to 30 years). This makes our results applicable to survivors with echocardiograms performed at these ages and years after diagnosis. Of note, age at baseline was not associated with LVD40 onset (HR: 0.99; p = 0.859). Second, the Teichholz EF is currently not preferred for calculating EF, and limits of agreement with biplane EF were relatively large. However, there was 97% agreement between Teichholz EF and biplane EF on the outcome (LVD40) in our study. Third, the number of CCS who developed LVD40 was low, which resulted in broad confidence intervals of our HR estimates. Fourth, selection bias may have been present in our study, as the CCS included in the derivation cohort were treated with higher anthracycline doses compared with the CCS not included in the study. However, we confirmed our findings in a validation cohort of 218 CCS who received lower anthracycline doses (median 180 mg/m2) and in a sensitivity analysis that adjusted for the possible influence of selection bias. This underlines the generalizability of our findings to lower-risk survivors. Last, other echocardiographic measurements, such as diastolic dysfunction, valvular abnormalities, and myocardial strain parameters, were not evaluated in this study, although they may be useful (49,50). This is currently being assessed in the Dutch LATER (Late Effects After Childhood Cancer) cohort study (51).
Conclusions
Our results demonstrate that EF measured with a surveillance echocardiogram at a median of 17 years (25th to 75th percentile 13 to 22 years) from cancer diagnosis and a median age of 23 years (25th to 75th percentile 20 to 28 years) has additional predictive value in the risk stratification for a therapeutically relevant decreased EF <40%. Our validated model and 10-year risk calculator can be used to classify 75% of CCS as low-risk for LVD40 within 10 years; less frequent surveillance may be appropriate in these survivors.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: In CCS at risk for heart failure, a prediction model that includes EF at approximately 13 to 22 years from cancer diagnosis, in addition to anthracycline and chest-directed radiotherapy dose, improves the 10-year prediction of LVD40. In addition to the use of this model to identify a large subgroup of CCS with a predicted risk ≤3% for LVD40 within 10 years, we determined that a midrange EF (EF 40% to 49%) is associated with an almost 8-fold increased risk for LVD40 compared with those with a preserved first EF (EF ≥50%).
TRANSLATIONAL OUTLOOK: Larger studies with longer follow-up are needed to assess whether follow-up surveillance echocardiograms can be performed at 10-year intervals or even longer periods of time. Moreover, other echocardiographic parameters, such as myocardial strain, should be studied to understand their predictive value in this population.
Funding Support and Author Disclosures
This study was funded by a Dutch Heart Foundation Grant (CVON2015-21). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For an expanded Methods section as well as a supplemental figure and tables, please see the online version of this paper.
Appendix
References
- 1.Gatta G., Botta L., Rossi S. Childhood cancer survival in Europe 1999-2007: results of EUROCARE-5—a population-based study. Lancet Oncol. 2014;15:35–47. doi: 10.1016/S1470-2045(13)70548-5. [DOI] [PubMed] [Google Scholar]
- 2.Oeffinger K.C., Mertens A.C., Sklar C.A. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Pal H.J., van Dalen E.C., van Delden E. High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol. 2012;30:1429–1437. doi: 10.1200/JCO.2010.33.4730. [DOI] [PubMed] [Google Scholar]
- 5.Feijen E.A.M., Font-Gonzalez A., van der Pal H.J.H. Risk and temporal changes of heart failure among 5-year childhood cancer survivors: a DCOG-LATER Study. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.118.009122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echouffo-Tcheugui J.B., Erqou S., Butler J., Yancy C.W., Fonarow G.C. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure: a systematic overview and meta-analysis. J Am Coll Cardiol HF. 2016;4:237–248. doi: 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Mulrooney D.A., Armstrong G.T., Huang S. Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: a cross-sectional study. Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Pal H.J., van Dalen E.C., Hauptmann M. Cardiac function in 5-year survivors of childhood cancer: a long-term follow-up study. Arch Intern Med. 2010;170:1247–1255. doi: 10.1001/archinternmed.2010.233. [DOI] [PubMed] [Google Scholar]
- 9.Armenian S.H., Mertens L., Slorach C. Prevalence of anthracycline-related cardiac dysfunction in long-term survivors of adult-onset lymphoma. Cancer. 2018;124:850–857. doi: 10.1002/cncr.31110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong G.T., Joshi V.M., Ness K.K. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armenian S.H., Hudson M.M., Mulder R.L. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/S1470-2045(14)70409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sieswerda E., Postma A., van Dalen E.C. The Dutch Childhood Oncology Group guideline for follow-up of asymptomatic cardiac dysfunction in childhood cancer survivors. Ann Oncol. 2012;23:2191–2198. doi: 10.1093/annonc/mdr595. [DOI] [PubMed] [Google Scholar]
- 13.Silber J.H., Cnaan A., Clark B.J. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22:820–828. doi: 10.1200/JCO.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Cheuk D.K., Sieswerda E., van Dalen E.C., Postma A., Kremer L.C. Medical interventions for treating anthracycline-induced symptomatic and asymptomatic cardiotoxicity during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD008011.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ponikowski P., Voors A.A., Anker S.D. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 16.Yancy C.W., Jessup M., Bozkurt B. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Yusuf S., Pitt B., Davis C.E., Hood W.B., Jr., Cohn J.N. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 18.Jong P., Yusuf S., Rousseau M.F., Ahn S.A., Bangdiwala S.I. Effect of enalapril on 12-year survival and life expectancy in patients with left ventricular systolic dysfunction: a follow-up study. Lancet. 2003;361:1843–1848. doi: 10.1016/S0140-6736(03)13501-5. [DOI] [PubMed] [Google Scholar]
- 19.Pourier M.S., Mavinkurve-Groothuis A.M.C., Loonen J. Is screening for abnormal ECG patterns justified in long-term follow-up of childhood cancer survivors treated with anthracyclines? Pediatr Blood Cancer. 2017;64 doi: 10.1002/pbc.26243. [DOI] [PubMed] [Google Scholar]
- 20.Mavinkurve-Groothuis A.M., Groot-Loonen J., Bellersen L. Abnormal NT-pro-BNP levels in asymptomatic long-term survivors of childhood cancer treated with anthracyclines. Pediatr Blood Cancer. 2009;52:631–636. doi: 10.1002/pbc.21913. [DOI] [PubMed] [Google Scholar]
- 21.Mavinkurve-Groothuis A.M., Groot-Loonen J., Marcus K.A. Myocardial strain and strain rate in monitoring subclinical heart failure in asymptomatic long-term survivors of childhood cancer. Ultrasound Med Biol. 2010;36:1783–1791. doi: 10.1016/j.ultrasmedbio.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Feijen E.A.M., Leisenring W.M., Stratton K.L. Derivation of anthracycline and anthraquinone equivalence ratios to doxorubicin for late-onset cardiotoxicity. JAMA Oncol. 2019;5:864–871. doi: 10.1001/jamaoncol.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kok J.L., Teepen J.C., van der Pal H.J. Incidence of and risk factors for histologically confirmed solid benign tumors among long-term survivors of childhood cancer. JAMA Oncol. 2019;5:671–680. doi: 10.1001/jamaoncol.2018.6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folland E.D., Parisi A.F., Moynihan P.F., Jones D.R., Feldman C.L., Tow D.E. Assessment of left ventricular ejection fraction and volumes by real-time, two-dimensional echocardiography. a comparison of cineangiographic and radionuclide techniques. Circulation. 1979;60:760–766. doi: 10.1161/01.cir.60.4.760. [DOI] [PubMed] [Google Scholar]
- 25.Teichholz L.E., Kreulen T., Herman M.V., Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7–11. doi: 10.1016/0002-9149(76)90491-4. [DOI] [PubMed] [Google Scholar]
- 26.de Vet H.C.W., Mokkink L.B., Terwee C.B., Hoekstra O.S., Knol D.L. Clinicians are right not to like Cohen’s κ. BMJ. 2013;346:f2125. doi: 10.1136/bmj.f2125. [DOI] [PubMed] [Google Scholar]
- 27.Stanton T., Leano R., Marwick T.H. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 28.Gray R.J. A Class of K-Sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 29.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. [Google Scholar]
- 30.Marrie R.A., Dawson N.V., Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62:511–517.e1. doi: 10.1016/j.jclinepi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Royston P., Altman D.G. External validation of a Cox prognostic model: principles and methods. BMC Med Res Methodol. 2013;13:33. doi: 10.1186/1471-2288-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vickers A.J., Cronin A.M., Begg C.B. One statistical test is sufficient for assessing new predictive markers. BMC Med Res Methodol. 2011;11:13. doi: 10.1186/1471-2288-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heagerty P.J., Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 34.Harrell F.E., Jr., Califf R.M., Pryor D.B., Lee K.L., Rosati R.A. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 35.Pencina M.J., D'Agostino R.B., Sr., Steyerberg E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanche P., Dartigues J.-F., Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32:5381–5397. doi: 10.1002/sim.5958. [DOI] [PubMed] [Google Scholar]
- 37.Hernan M.A., Hernandez-Diaz S., Robins J.M. A structural approach to selection bias. Epidemiology. 2004;15:615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 38.Leerink J. Risk calculator for left ventricular dysfunction with ejection fraction <40% in childhood cancer survivors. https://risk-of-cardiomyopathy.netlify.com/ Available at:
- 39.Elbl L., Hrstkova H., Tomaskova I., Blazek B., Michalek J. Long-term serial echocardiographic examination of late anthracycline cardiotoxicity and its prevention by dexrazoxane in paediatric patients. Eur J Pediatr. 2005;164:678–684. doi: 10.1007/s00431-005-1732-x. [DOI] [PubMed] [Google Scholar]
- 40.Elbl L., Hrstkova H., Tomaskova I., Michalek J. Late anthracycline cardiotoxicity protection by dexrazoxane (ICRF-187) in pediatric patients: echocardiographic follow-up. Support Care Cancer. 2006;14:128–136. doi: 10.1007/s00520-005-0858-8. [DOI] [PubMed] [Google Scholar]
- 41.Sorensen K., Levitt G.A., Bull C., Dorup I., Sullivan I.D. Late anthracycline cardiotoxicity after childhood cancer: a prospective longitudinal study. Cancer. 2003;97:1991–1998. doi: 10.1002/cncr.11274. [DOI] [PubMed] [Google Scholar]
- 42.Rathe M., Carlsen N.L., Oxhoj H., Nielsen G. Long-term cardiac follow-up of children treated with anthracycline doses of 300 mg/m2 or less for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2010;54:444–448. doi: 10.1002/pbc.22302. [DOI] [PubMed] [Google Scholar]
- 43.Johnson G.L., Moffett C.B., Geil J.D., Greenwood M.F., Noonan J.A. Late echocardiographic findings following childhood chemotherapy with normal serial cardiac monitoring. J Pediatr Hematol Oncol. 1996;18:72–75. doi: 10.1097/00043426-199602000-00014. [DOI] [PubMed] [Google Scholar]
- 44.Border W.L., Sachdeva R., Stratton K.L. Longitudinal changes in echocardiographic parameters of cardiac function in pediatric cancer survivors. J Am Coll Cardiol CardioOnc. 2020;2:26–37. doi: 10.1016/j.jaccao.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lipshultz S.E., Lipsitz S.R., Sallan S.E. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–2636. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 46.Yeh J.M., Nohria A., Diller L. Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: a model-based estimation of the clinical and economic effects. Ann Intern Med. 2014;160:661–671. doi: 10.7326/M13-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wong F.L., Bhatia S., Landier W. Cost-effectiveness of the children's oncology group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med. 2014;160:672–683. doi: 10.7326/M13-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrhardt M.J., Ward Z.J., Liu Q. Cost-Effectiveness of the International Late Effects of Childhood Cancer Guideline Harmonization Group screening guidelines to prevent heart failure in survivors of childhood cancer. J Clin Oncol. 2020 doi: 10.1200/JCO.20.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gori M., Redfield M.M., Calabrese A. Is mild asymptomatic left ventricular systolic dysfunction always predictive of adverse events in high-risk populations? Insights from the DAVID-Berg study. Eur J Heart Fail. 2018;20:1540–1548. doi: 10.1002/ejhf.1298. [DOI] [PubMed] [Google Scholar]
- 50.Kalam K., Otahal P., Marwick T.H. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 51.Leerink J.M., Feijen E.L.A.M., van der Pal H.J.H. Diagnostic tools for early detection of cardiac dysfunction in childhood cancer survivors: methodological aspects of the Dutch Late Effects After Childhood Cancer (LATER) cardiology study. Am Heart J. 2020;219:89–98. doi: 10.1016/j.ahj.2019.10.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.