Abstract
Cardiac masses are rare, but remain an important component of cardio-oncology practice. These include benign tumors, malignant tumors (primary and secondary) and tumor-like conditions (e.g., thrombus, Lambl’s excrescences, and pericardial cyst). The advent of multimodality imaging has enabled identification of the etiology of cardiac masses in many cases, especially in conjunction with information from clinical settings. This paper provides a comprehensive review of the epidemiology, clinical presentation, imaging, diagnosis, management, and outcomes of cardiac masses.
Key Words: cardiac masses, cardiac metastases, cardiac myxoma, cardiac sarcoma, cardiac tumors, papillary fibroelastoma, pericardial cyst, pericardial mesothelioma, pericardial tumors
Abbreviations and Acronyms: CMR, cardiovascular magnetic resonance imaging; CT, computed tomography; FDG, fluorodeoxyglucose; PCT, primary cardiac tumor; PET, positron emission tomography; RT3DE, real-time 3-dimensional echocardiography; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; UEA, ultrasound-enhancing agent
Central Illustration
Highlights
-
•
Cardiac tumors are rare and should be considered as part of the differential diagnosis of any space-occupying mass noted on cardiovascular and/or thoracic imaging modalities.
-
•
It may be possible to get close to a diagnosis without biopsy using a structured imaging approach.
-
•
The prognosis and treatment of each tumor is different, although early diagnosis is usually associated with a better outcome.
Despite being exceedingly rare, cardiac tumors form an important component of cardio-oncology practice in which diagnosis and management are vital (1). Tumors encompass a broad set of lesions and/or masses that can be categorized as neoplastic or non-neoplastic. Neoplastic lesions can be further classified into primary and secondary tumors (i.e., metastasis to the heart). Up to 90% of primary neoplastic tumors [referred to here as primary cardiac tumors (PCTs)] are benign (2) and may originate from the pericardium or myocardium. The incidence of clinically diagnosed PCTs is approximately 1,380/100 million individuals (3). Compared with PCTs, secondary cardiac tumors are 22 to 132 times more common and are by definition malignant (4,5). The classification of these lesions as benign or malignant is an important predictor of prognosis; however, any cardiac tumor, even if histologically benign, can have substantial hemodynamic or arrhythmic consequences depending on its size and location within the heart.
Over the past decade, there has been a notable increase in the incidence of PCTs (6). Part of this increase may be attributed to the advances in imaging techniques, with multimodality imaging being more widespread and accessible (7). Multimodality imaging is often necessary to help determine the etiology of PCTs. Several factors that are helpful in establishing a diagnosis before open biopsy include location of the mass, imaging characteristics, and age at presentation.
Clinical Manifestations
Cardiac tumors may be symptomatic or found incidentally during evaluation for a seemingly unrelated problem or physical finding. Symptoms are usually related to its cardiac location, although some may produce systemic symptoms. In general, tumors may present in 1 of 3 ways:
-
1.
Systemic: constitutional (fever, arthralgias, weight loss, fatigue) and paraneoplastic syndromes (PCTs).
-
2.
Cardiac: mass effect interfering with myocardial function or blood flow, resultant arrhythmias, interference with heart valves causing regurgitation, or pericardial effusion with or without tamponade. Typical symptoms include dyspnea, chest discomfort, pre-syncope, or syncope.
-
3.
Embolic: pulmonary and/or systemic thromboembolic phenomenon from the tumor.
Diagnostic Approach
The clinical setting provides critical diagnostic clues in helping establish the etiology of a cardiac mass or lesion. For example, cardiac thrombi are most likely associated with arrhythmias such as atrial fibrillation or left ventricular apical infarct with apical hypokinesis or akinesis, whereas vegetations may be seen in patients with native valve disease or prosthetic valves who present with fevers, bacteremia, and abnormal inflammatory markers. However, if a cardiac mass represents a tumor, its etiology can often be determined by considering 4 factors:
-
1.
Age of the patient at time of presentation; for example, rhabdomyomas and fibromas are the most common benign cardiac tumors in children.
-
2.
Epidemiologic likelihood and clinical probability; for example, a 70-year-old man who recently experienced an anterior myocardial infarction with an akinetic left ventricular apex and associated cardiac mass on echocardiogram most likely has an intracardiac thrombus.
-
3.
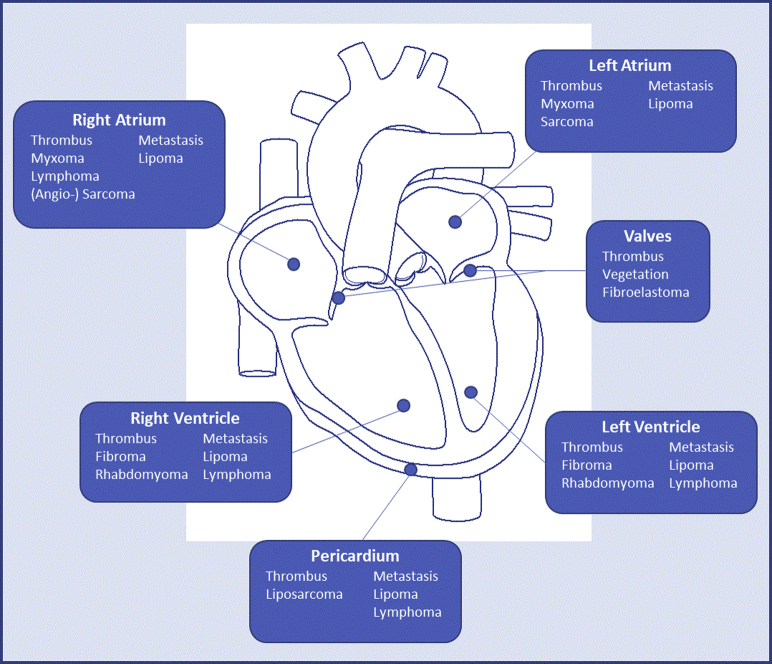
Location of tumor (Figure 1).
-
4.
Noninvasive tissue characterization with cardiovascular magnetic resonance (CMR) imaging (Table 1).
Figure 1.
Outline of the Distribution of Subtypes of Cardiac Masses by Anatomic Location
The most typical locations of where different subtypes of cardiac masses are found. However, many cardiac tumours can occur in any chamber.
Adapted with permission from Dujardin KS et al. (8).
Table 1.
Typical Noninvasive Tissue Characterization of Common Cardiac Masses With Cardiovascular Magnetic Resonance Imaging
| LGE Enhancement | T1-Weighted Imaging | T2-Weighted Imaging | |
|---|---|---|---|
| Malignant tumors | |||
| Angiosarcoma | ++ | ↑ / ←→ / ↓ | ↑ / ←→ / ↓ |
| Rhabdomyosarcoma | +++ | ←→ | ↑↑↑ |
| Undifferentiated Sarcoma | ++ | ←→ | ↑↑↑ |
| Lymphoma | −/+ | ←→ | ←→ |
| Metastasis∗ | ++ | ↓↓ | ↑↑ |
| Benign tumors | |||
| Myxoma | ++ | ←→ | ↑↑ |
| Lipoma | − | ↑↑ | ↑↑ |
| Fibroma | ++++ | ←→ | ↓ |
| Rhabdomyoma | −/+ | ←→ | ←→/↑ |
| Non-tumor masses | |||
| Thrombus | − | ↓ / ↑ | ↓ / ↑ |
| Cysts | − | ↓↓ | ↑↑ |
All tumors can have atypical appearances because of altered tissue composition. T1- and T2-weighted imaging signal intensity is relative to myocardium.
↓ = low; ←→ = isointense; ↑ = high; − = no uptake; + = minimal late gadolinium enhancement (LGE) uptake; ++ = heterogenous LGE uptake; +++ = homogenous LGE uptake; ++++ = hyperenhancement; CMR = cardiovascular magnetic resonance.
Metastatic melanoma has a high T1-weighted and a low T2-weighted signal intensity. Adapted with permission from Motwani et al. (9).
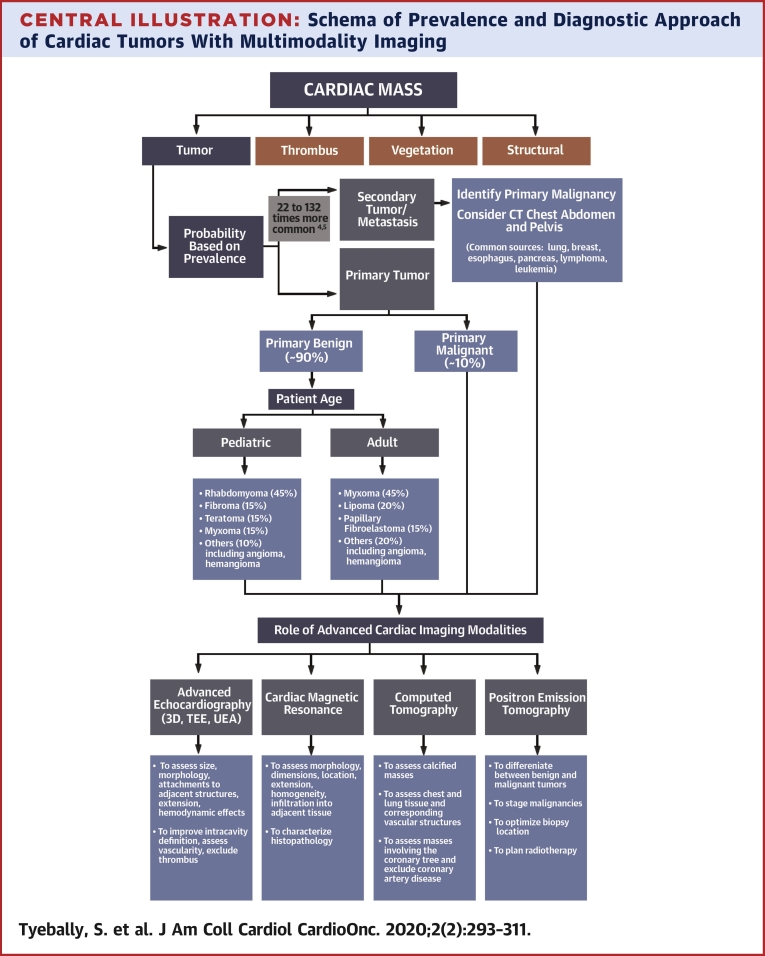
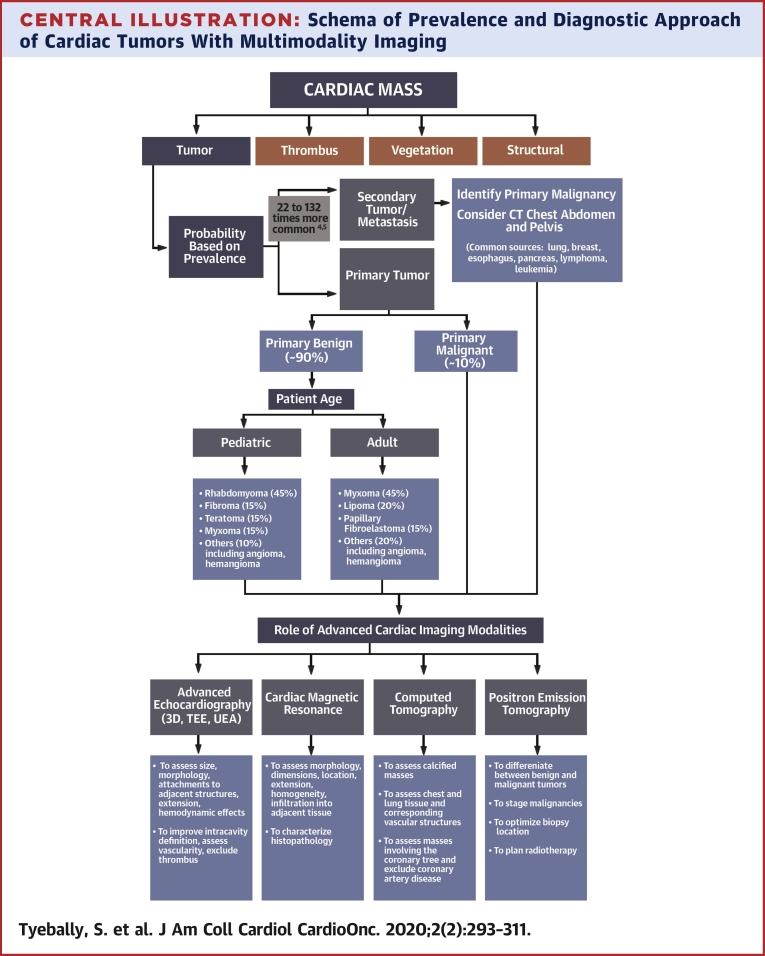
Using this approach, and integrating the clinical data, an accurate diagnosis and treatment strategy is usually possible without the need for percutaneous or open surgical biopsy (10) (Central Illustration).
Central Illustration.
Schema of Prevalence and Diagnostic Approach of Cardiac Tumors With Multimodality Imaging
Diagnostic approach when a cardiac mass is first identified. 3D = 3-dimensional; CT = computed tomography; TEE = transesophageal echocardiography; UEA = ultrasound-enhancing agent.
Role of Multimodality Imaging in Establishing the Diagnosis
The goals of the initial evaluation are to ascertain the presence of a cardiac tumor, the location of the lesion within cardiac structures, and when possible, whether a tumor is benign or malignant. This information is crucial in planning further evaluation and management. Although obtaining a histopathological specimen is the gold standard for diagnosis, multimodality imaging may be able to identify the etiology of a cardiac mass in many cases.
2-dimensional transthoracic echocardiography
Two-dimensional transthoracic echocardiography (TTE) is often the first diagnostic approach in terms of imaging modality because of its wide availability. Additional benefits include lack of radiation, low cost, and portability. TTE allows for the assessment of size, location, mobility, and the pericardial involvement of a tumor. Furthermore, it has good spatial resolution and is useful for understanding the hemodynamic significance of the mass (e.g., vascular or valvular obstruction secondary to the mass). Echocardiography is also the optimal imaging modality for imaging small highly mobile masses (<1 cm) or masses arising from valves.
The limitations of TTE include poor acoustic windows, particularly in obese patients and those with chronic lung disease (11), and lack of potential for tissue characterization. In addition, the extent and origin of the mass may not be distinguishable by echocardiography if it arises from outside of standard imaging views (e.g., in the superior vena cava or inferior vena cava or branch pulmonary vessels).
Advanced echocardiographic techniques
Transesophageal echocardiography (TEE) is commonly used when a valvular lesion is suspected, particularly in patients with atrial masses or with mobile valvular lesions. It may also become necessary to better characterize a cardiac tumor in terms of size, morphology, attachment site, extension, and hemodynamic affects. When used in combination with real-time 3-dimensional echocardiography (RT3DE), TEE offers incremental value for the evaluation of intracardiac masses by providing a more accurate assessment of the anatomical relationships, size, and shape of the mass (12).
The use of ultrasound-enhancing agents (UEAs) in echocardiography is an important tool for the assessment of myocardial perfusion and can also be used for the evaluation of the relative perfusion of a cardiac mass. It allows for improved definition of intracavity structures and assessment of vascularity (13). The difference in perfusion of cardiac masses may help distinguish between vascular and nonvascular tumors or thrombus because there are both qualitative and quantitative differences in perfusion among the various pathologies (14). For example, malignant tumors, which are highly vascular due to abnormal neovascularization, demonstrate greater enhancement than the adjacent myocardium. In contrast, benign tumors (myxomas) often have poor blood supply and therefore demonstrate lower perfusion on visual inspection and have quantitatively less perfusion than the surrounding myocardium. Thrombi are avascular and therefore do not perfuse with UEA echocardiography; therefore, UEA echocardiography perfusion imaging may aid in the early identification of thrombi and guide subsequent diagnostic and/or treatment strategies.
Cardiovascular magnetic resonance imaging
Once a cardiac mass is suspected (generally following TTE), patients may be referred for CMR for further evaluation of the mass. This technique provides a complete multiplanar and noninvasive evaluation of the mass and its potential involvement with the cardiac chambers and pericardium. Because it also provides information about the extracardiac structures and its surrounding anatomy, this often proves useful in surgical planning.
Characteristics that can be assessed by CMR include morphology, dimensions, location, extension, homogeneity, presence of infiltration in the surrounding tissues, and signal characteristics that can help with histopathological characterization (fatty infiltration, necrosis, hemorrhage, calcification, vascularity, and so forth) (15). T1-weighted black blood imaging with and without fat saturation, pre-contrast T2-weighted imaging, resting first-pass perfusion (to assess for vascularity), early gadolinium imaging (particularly useful for diagnosis of thrombi), and late gadolinium enhancement imaging should be performed (16). T1 and T2 mapping are recent developments in CMR and allow instant quantitative detection of myocardial abnormalities beyond conventional qualitative assessment.
The main limitation of CMR compared with echocardiography is its lower temporal resolution; for this reason, CMR is generally not indicated for evaluation of valvular vegetations (17). Other disadvantages include long acquisition times (30 min to 1 h), limited availability compared with echocardiography, inability to be performed in hemodynamically unstable patients, and contraindications that include claustrophobia and older generation cardiac devices (18).
Cardiac computed tomography
Cardiac computed tomography (CT) has become an increasingly used modality for the assessment of cardiac masses, especially when other imaging modalities are nondiagnostic or contraindicated. With high isotropic spatial and temporal resolution, multiplanar image reconstruction capabilities, and fast acquisition times, cardiac CT offers an alternative to CMR in many patients (19). Electrocardiographic gating minimizes motion-related artifacts and allows a more precise delineation of the lesion margins and relationship to tissue planes, which is particularly valuable for surgical planning. Compared with other cardiac imaging modalities, CT is optimal for the evaluation of calcified masses, global assessment of chest and lung tissue and corresponding vascular structures, and exclusion of obstructive coronary artery disease or masses in proximity or that cause obstruction to the coronary arteries, which may be helpful in surgical planning (19). Cardiac CT is also useful in tumor staging because of its ability to detect metastases in cases of suspected malignancies.
Disadvantages of CT include radiation exposure, a small risk of contrast-induced nephropathy, and limited soft tissue and temporal resolutions compared with magnetic resonance imaging.
Positron emissions tomography
Positron emissions tomography (PET) offers an accurate evaluation of the metabolic activity of tumors using fluorodeoxyglucose (18F-FDG). FDG-PET is helpful for staging malignancies while also revealing potential myocardial and pericardial involvement. It is also useful in the evaluation of early responses to cancer therapy planning of radiation therapy, and optimization of biopsy location.
The extent of FDG uptake by tumors is useful for differentiation between benign and malignant tumors; this is usually based on maximum standardized uptake value evaluation (20). In a study of 20 patients who underwent hybrid magnetic resonance/PET scanning, 18F-FDG-PET scanning had a sensitivity of 100% and specificity of 92% for the differentiation of malignant cardiac masses versus benign cardiac masses (21). Limitations included the need for dietary preparation, especially in patients with intramyocardial tumors and who had radiation exposure (22).
PCTs
Introduction to PCTs
PCTs are classified into 3 main classes: benign, malignant, and intermediate with uncertain biological behavior. The latter class was newly featured in the 2015 World Health Organization classification (23). Benign etiologies include most of all primary tumors (>90%), whereas <10% are malignant and only 1% are intermediate (24,25).
Benign PCTs
Most PCTs are benign and include myxomas, rhabdomyomas, papillary fibroelastoma, fibromas, hemangiomas, lipoma, and leiomyomas (Table 2). Myxoma is by far the predominant pathological subtype (24). Non-myxoma subtypes, which mostly occur in children and adolescents, are less reported (24,25). Benign tumors exhibit favorable prognosis with a 30-day mortality of only 1% (24). They are generally more common in older women (24,25), and according to their size and location, benign PCTs manifest with a wide array of symptoms. However, 13.3% to 27.7% of cases occur in asymptomatic individuals and are detected incidentally (24).
Table 2.
Histopathology, Role of Biopsy, and Role of Surgery in the Management of Benign Cardiac Tumors
| Tumor | Histopathology | Role of Biopsy | Role of Surgery | Ref. # |
|---|---|---|---|---|
| Myxoma | Spindle or stellate cells, pseudo-vascular structure, myxoid matrix, hemorrhage Dystrophic calcification can be present. |
Not usually needed | Complete resection recommended to prevent embolic complications | (26,27) |
| Lipoma | Mature adipocytes, occasionally with entrapped myocytes at the periphery | Not usually needed | Not required in most cases; can be considered for severely symptomatic cases | (28,29) |
| Fibroma | Fibroblasts and collagen bundles, some elastic fibers, calcification is a common finding | Not usually needed | Can be considered for severely symptomatic cases | (26,30) |
| Rhabdomyoma | Spider cell (vacuolated enlarged cardiac myocyte with clear cytoplasm due to abundant glycogen). | Not usually needed | Usually not needed due to spontaneous regression; can be considered for severely symptomatic cases | (26,31) |
| Papillary fibroelastoma | Endocardium-coated fronds with an avascular collagenous core containing mucopolysaccharide and elastin | Not usually needed | Recommended for large left-sided lesions to reduce the risk of embolic complications | (8,28,32) |
Classes of benign PCTs
Cardiac myxoma
The most common benign PCT, often found in the left atrium, is best visualized by echocardiography. Myxomas can present with embolic symptoms. Complete surgical resection confers the best clinical outcome.
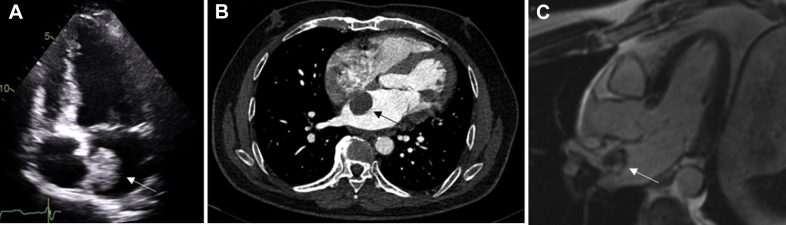
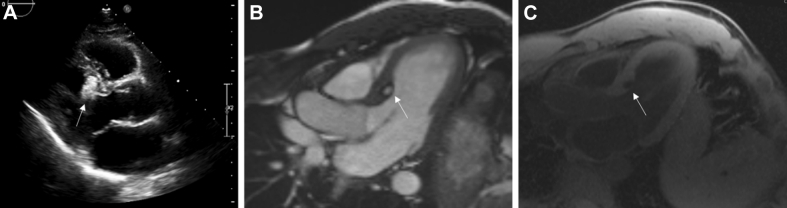
Cardiac myxomas (Figure 2) are the most common PCT (25) and are believed to be derived from mesenchymal cell precursors (33). They form intracavitary masses, which are most commonly found in the left atrium attached by a stalk to the fossa ovalis, but may be seen in the right atrium in children (33,34). Other anatomical origins include the atrial free wall and mitral valve leaflets; however, these are less likely (35). Association with Carney complex (a multiple neoplasia and lentiginosis syndrome) is well established in 7% of patients found to have a cardiac myxoma on presentation (36). Mean age at diagnosis is 50 years, and approximately 70% occur in women (37).
Figure 2.
Myxoma
Left atrial myxoma in a 53-year-old man who presented with embolic events. (A) Large mobile mass (white arrow) seen on transthoracic echocardiography attached to the interatrial septum. (B) Low-attenuated, well-circumscribed mass with a smooth surface (black arrow) seen on cardiac computed tomography (CT). (C) Heterogeneous uptake in left atrial mass (white arrow) on late gadolinium imaging on cardiac magnetic resonance (CMR).
Myxomas are morphologically classified into polypoid and papillary. The former, when large, may present with obstructive symptoms with a “tumor plop” being occasionally heard on auscultation. In contrast, papillary myxoma tends to cause embolic events. In both variants, constitutional symptoms like fatigue, fever, and weight loss have also been reported.
On echocardiography, cardiac myxomas typically appear as a mobile mass attached to the endocardial surface by a stalk, usually arising from the fossa ovalis (8). RT3DE can aid with both stalk analysis and mass heterogeneity using the cropping function and carefully using digital analysis to dissect the lesion (12). TEE may be needed to better visualize the implantation site and identify any potential extension into the pulmonary or caval vein. On cardiac CT, myxomas often manifest as a low-attenuation intracavitary mass with a smooth or slightly villous surface (38). Calcification is seen in approximately 14% of patients and is more commonly associated in right-sided lesions (38). Arterial-phase contrast enhancement is usually not present (39), but heterogeneous enhancement is recognized on studies performed with a longer time delay (38). Reconstruction of cine images can assist in assessment of lesion mobility and attachment; however, this is often unreliable, especially for tumors with a short stalk (39). On CMR, a heterogeneous appearance on T1- and T2-weighted images is often found due to the composition of myxomas, which tend to have varying amounts of myxoid, hemorrhagic, ossific, and necrotic tissue (40). Delayed enhancement is normally patchy in nature. Prolapse through the mitral or tricuspid valve in diastole on steady-state free precession sequences may suggest the attachment point of a pedunculated lesion.
Resection is required for a histological diagnosis but mainly to prevent major complications. Surgical resection is associated with a low operative mortality and good long-term outcome (27). Annual follow-up with TTE is recommended for a minimum of 4 years because approximately 10% to 15% of these tumors recur, most often at the site of the original tumor (41).
Lipoma
Lipomas are slightly less common and have been mostly discovered incidentally because they are often asymptomatic. They are best visualized on CMR with application of additional fat-saturation pre-pulses. Surgical removal is recommended only in severely symptomatic cases.
Lipoma (Figure 3) is the second most common primary benign cardiac neoplasm (8% to 12%) and most commonly occurs in middle-aged and older adults (30). Approximately 50% of lipomas originate from the subendocardial layer, and the other half arise from the subepicardial or myocardial layers and grow into the pericardial sac (30). They are typically asymptomatic but may cause arrhythmias or valvular dysfunction (29). Subepicardial lipomas can compress the coronary arteries, which leads to ischemic chest pain.
Figure 3.
Lipoma
Incidental finding of a cardiac mass in a 65-year-old man. (A) Ovoid mass (white arrow) on long-axis view on transthoracic echocardiography. (B) Well-circumscribed mass (white arrow) in the basal anteroseptum of the left ventricle not causing any left ventricular outflow tract obstruction on steady-state free precession (SSFP) sequences on CMR. (C) Complete signal suppression of mass (white arrow) on fat-water suppression imaging on CMR. Other abbreviation as in Figure 2.
On TTE, lipomas tend to be broad-based, immobile, without a pedicle, and well circumscribed. They are homogeneous without evidence of calcification and hyperechoic in the cavity but hypoechoic in the pericardium (29). Cardiac CT may reveal homogeneous, nonenhancing masses with fat attenuation located within the cardiac chamber, the myocardium, or in the pericardium (30).On T1-weighted imaging on CMR, lipomas have a characteristic homogeneous high-signal intensity relative to the myocardium that is markedly suppressed with the application of additional fat-saturation pre-pulses (10). On T2 imaging, lipomas appear hyperintense; on fat-saturated imaging, lipomas exhibit a markedly low signal. With contrast administration, lipomas do not demonstrate perfusion (40,42). Surgical removal is generally not indicated, except in severely symptomatic cases (29).
Papillary fibroelastoma
Papillary fibroelastomas are rare and often found on the downstream side of valves. They are best visualized on echocardiography and can present with embolic complications. Surgical excision is reserved for large left-sided tumors.
Papillary fibroelastoma (Figure 4) is more rare and constitutes 11.5% of all PCTs (25). It is composed of collagen and elastic fibers with an endothelial covering and a short connective tissue pedicle (43). They are found on the valvular endocardium of the aortic and mitral valves, followed by the tricuspid valve and the endocardium, which consists of 75% of all valvular tumors (38). Unlike vegetations, fibroelastomas are often found downstream of the valve (left ventricular side of the mitral valve, aortic side of the aortic valve) and usually do not commonly cause valvular dysfunction (44). Patients are typically middle-aged and present with embolic complications, particularly cerebral; this is related to the tumor’s predilection for left-sided valves (45).
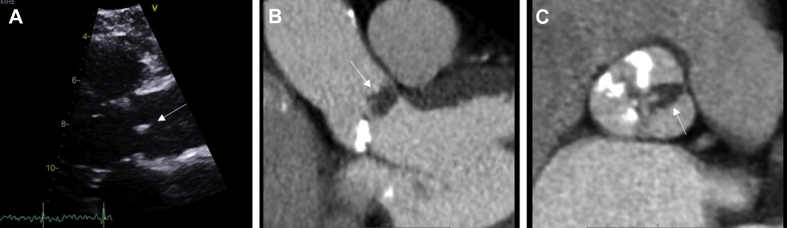
Figure 4.
Papillary Fibroelastoma
Papillary fibroelastoma in a 78-year-old woman presenting with embolic events. (A) Long-axis, zoomed-in view of a small independent mobile structure (white arrow) attached to the aortic valve. (B and C) Hypodense mass (white arrows) between the right and left leaflets with frondlike papillary appearance on cardiac CT. Abbreviation as in Figure 2.
The echocardiographic features of a papillary fibroelastoma are its small size, with independent motion and attachment to an endocardial surface. On TEE, the borders may appear slightly stippled or shimmering, which is due to vibration at the tumor–blood interface because of its finger-like projection (46). TEE is more sensitive in identifying papillary fibroelastomas compared with TTE because of the typical small size of these tumors, which reinforces the role of TEE in the evaluation of an embolic event (32). Occasionally, on electrocardiographically gated cardiac CT, they appear as a focal low attenuation mass arising from a valve surface. On T1-weighted imaging on CMR, fibroelastomas demonstrate an isointense pattern (10). On T2-weighted imaging, fibroelastomas appear hypointense due to their high fibrous content, and in other cases, they may also demonstrate a hyperintense signal (10).
Surgical excision is recommended for larger (≥1 cm) left-sided papillary fibroelastomas in patients who are deemed appropriate surgical candidates with low risk, or at the time of cardiac surgery for another cardiovascular condition (8). This dramatically reduces the risk of stroke (32). Right-sided papillary fibroelastomas are usually managed conservatively unless they are associated with hemodynamically significant obstruction or risk of paradoxical embolism (e.g., in cases of intracardiac shunts) (32). If surgery is not undertaken due to high surgical risk or patient preference, treatment with antiplatelet agents may be considered, despite limited supporting data (32).
Rhabdomyoma
Rhabdomyomas are mostly found in the pediatric population, with no predilection for any specific cardiac chamber. They can present with arrhythmias and heart failure symptoms. Rhabdomyomas usually spontaneously regress; therefore, serial echocardiography is recommended as a follow-up.
Rhabdomyoma (Figure 5) is the most prevalent pediatric PCT and typically presents during the first year of life (47). These tumors are multiple in 90% of patients and often involve the atria and ventricles with equal distribution on the right and left side of the heart (31). Association with tuberous sclerosis is well established (48). Rhabdomyomas are arrhythmogenic and can present with palpitations and syncopal symptoms. Cavity protrusion that results in flow obstruction may result in heart failure symptoms (47).
Figure 5.
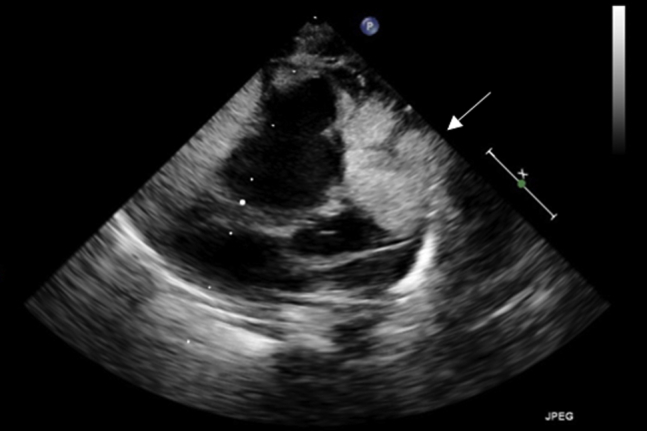
Rhabdomyoma
An echocardiogram in a newborn who had an antenatal diagnosis of multiple cardiac rhabdomyomas and who was subsequently diagnosed as having tuberous sclerosis. Large cardiac masses extending to the right ventricular wall laterally, posteriorly, and anteriorly without evidence of right ventricular outflow tract obstruction.
On TTE, they may appear as small, well-circumscribed (multiple) nodules or a pedunculated mass in a cardiac cavity. Occasionally, myocardial embedding is seen and will appear as a lobulated homogeneous and hyperechogenic mass, often brighter than the surrounding myocardium (29). Cardiac CT will usually reveal an intramural lesion with homogeneous low attenuation and intracavitary extension. The presence of multiple masses and other features of tuberous sclerosis distinguish rhabdomyomas from fibromas. On T1-weighted imaging on CMR, they can appear isointense, and on T2-weighted imaging, they tend to show hyperintensity compared with that of the myocardium (42). There is no delayed gadolinium enhancement exhibited by rhabdomyomas (42).
Rhabdomyomas typically undergo spontaneous regression; therefore, serial echocardiographic follow-up is key and often sufficient (49). Surgery is usually reserved for patients with intractable arrhythmias or heart failure (31).
Fibroma
Fibromas are more common in infants, are often located in the ventricle, and can be associated with a wider syndrome. They are best seen on CT as a homogeneous mass with central calcification. Surgical resection is recommended due to risk of sudden cardiac death.
Cardiac fibroma is the second most common PCT in infants. They are composed of fibroblasts and collagen fibers of variable sizes with central calcifications (50). They are commonly located in the ventricular myocardium, usually in the left ventricular free wall or interventricular septum, and can often mimic hypertrophic cardiomyopathy (51). They may also be associated with familial adenomatous polyposis and Gorlin syndrome, in which case an atrial origin is more common. Although histologically benign, patients may present with ventricular arrhythmias and sudden death secondary to interference with conduction pathways (52). Large tumors can also cause dyspnea (53).
On TTE, they usually appear as a distinct well-demarcated, noncontractile, and solid highly echogenic mass within the myocardium with central calcifications. Typically, their size ranges from 1 to 10 cm. Cardiac CT typically shows an intramyocardial, homogeneous low-attenuation mass with occasional central calcification with variable margins and little to no contrast enhancement (54). On T1-weighted imaging on CMR, fibromas appear isointense compared with that of muscle. On T2-weighted images, they are known to be homogeneous and hypointense, unlike other tumors, and typically show minimal or no enhancement with gadolinium (55).
Because of the increased risk for fatal arrhythmias and sudden death, coupled with the fact that these tumors do not spontaneously regress (unlike rhabdomyomas), surgical resection is recommended, regardless of symptoms (30).
Cardiac paraganglioma
Cardiac paraganglioma is a rare neuroendocrine tumor usually located in the left atrium. It is positive on PET imaging. Surgical resection is recommended, with pre-operative medical optimization to prevent catecholamine surges.
Cardiac paraganglioma (Figure 6) is an exceptionally rare tumor that originates from the paraganglionic cells located in relation to the great arteries, the coronary arteries, or the atria. It is commonly located in the left atrium (55%), followed by the interatrial septum (16%) and the anterior surface of the heart (10%) (56). They can either be secretory or nonsecretory tumors. The former produces endogenous catecholamines that lead to an excessive sympathetic discharge, which results in a plethora of symptoms (e.g., palpitations, flushing, and tremors). Like any cardiac tumor, patients may also present with symptoms of dyspnea and angina related to compression of the cardiac chambers or coronary arteries.
Figure 6.
Cardiac Paraganglioma
Cardiac paraganglioma in a 72-year-old man presenting with flushing and palpitations. (A) A well-circumscribed heterogeneous mass with contrast uptake (white arrow) on cardiac CT at the superior-septal aspect of the right atrium, with significant compression of the right upper pulmonary vein. The mass is well vascularized and supplied by a branch of the right coronary artery. (B) Heterogeneous appearance on SSFP imaging on CMR (white arrow) on. (C) Avid right atrial mass (white arrow) on fluorodeoxyglucose−positron emission tomography (FDG-PET) CT. Abbreviations as in Figures 2 and 3.
On echocardiography, paragangliomas usually appear as echogenic masses that have a broad base (50). On cardiac CT, they appear as heterogeneous masses with low attenuation on unenhanced imaging (50), with marked enhancement on contrast-enhanced imaging (57). On CMR, they usually show high-signal intensity on T2-weighted images (50). They are expected to be positive on PET imaging (58). Definitive diagnosis remains histopathological examination.
Pre-operatively, in a secreting type of cardiac paraganglioma, medical optimization with alpha-blockade must be carried out, followed by beta-blockade to avoid hemodynamic changes during surgery. The definitive treatment of a symptomatic cardiac paraganglioma is surgical. However, because these tumors are highly vascular, they tend to involve the coronary arteries; therefore, surgical resection is often difficult (59).
Rare benign PCTs and masses mimicking benign PCTs
Rare benign PCTs and masses mimicking benign PCTs are further discussed in the Supplemental Appendix.
Malignant PCTs
Introduction to malignant PCTs
Malignant tumors (Table 3) are extremely rare and represent only 5% to 6% of PCTs (3,6). Sarcomas are most common (64.8%), followed by lymphomas (27%) and mesotheliomas (8%) (3). Evidence of rapid growth, local invasion, presence of feeding vessels, hemorrhage, or necrosis within the mass, involvement of >1 cardiac chamber, and pericardial effusion are all features suggestive of malignancy. However, no single feature is both sensitive and specific (40,52). As with benign cardiac tumors, the clinical presentation can vary but typically is related to the location of the tumor.
Table 3.
Histopathology, Role of Biopsy and Role of Surgery in the Management of Malignant Cardiac Tumors
| Tumor | Histopathology | Role of Biopsy | Role of Surgery | Ref. # |
|---|---|---|---|---|
| Angiosarcoma | Highly vascularized, myocardial infiltration, pleomorphism, necrosis, and mitosis | Usually required, if possible | Complete surgical resection, if possible Surgical resection/debulking to improve symptoms |
(26,60) |
| Leiomyosarcoma | Compact bundles of spindled cells with blunt nuclei, regions of necrosis, and mitotic figures with epithelioid regions are often present | Usually required, if possible | Complete surgical resection, if possible Surgical resection/debulking to improve symptoms |
(28) |
| Rhabdomyosarcoma | Embryonal type with rhabdomyoblasts containing abundant glycogen and expressing desmin, myoglobin, and myogenin | Usually required, if possible | Complete surgical resection, if possible Surgical resection/debulking to improve symptoms |
(28) |
| Osteosarcoma | Histologically heterogeneous, most composed of spindle cell lesions or malignant fibrous histiocytoma, with microscopic foci of osteosarcoma and chondrosarcoma in the spindle regions | Usually required, if possible | Complete surgical resection if possible Surgical resection/debulking to improve symptoms |
(61,62) |
| Undifferentiated sarcoma | Consist of undifferentiated plump spindle cells with frequent mitotic activity and (often) nuclear pleomorphism | Usually required, if possible | Complete surgical resection, if possible Surgical resection/debulking to improve symptoms |
(60,63) |
| Primary cardiac lymphoma | Diffuse large B-cell lymphoma is the most common subtype, although Burkitt lymphoma, low-grade B-cell lymphoma, and T-cell lymphoma have also been described | Required | No role for surgery | (61,64) |
| Mesothelioma | Epithelioid (plump, rounded), sarcomatoid (spindled), or a combination of either | Pericardial biopsy or pericardial fluid analysis | Complete surgical resection, if possible Pericardiectomy to relieve constriction |
(65,66) |
| Metastasis | Largely dependent on primary tumor | Typically required at site of primary tumor | Surgical debulking only in symptomatic cases | (61,67) |
Generally, the survival of patients diagnosed with malignant primary tumors are poor. In a retrospective study conducted by Sultan et al. (68), in which 747 patients with malignant primary tumors were analyzed, the survival was 81.2% at 1 month, 45.3% at 12 months, and 11.5% at 60 months from the time of diagnosis. Patients treated with surgery or surgery with chemotherapy and/or radiotherapy had better outcomes compared with those who had no surgery, recognizing a risk of selection bias (68). The greatest risk factors for mortality were advanced age, higher comorbidity index, and the presence of metastases (68).
Classes of malignant primary tumors
Primary cardiac sarcomas
Cardiac sarcomas represent more than two-thirds of all malignant primary tumors (3). Compared with extracardiac soft tissue sarcomas, cardiac sarcomas are seen in younger patients in their 40s and who have worse prognosis with a 5-year survival rate of 14% (3).
Histopathological subtypes of primary cardiac sarcomas include angiosarcomas, leiomyosarcomas, liposarcomas, rhabdomyosarcomas, synovial sarcomas, myxofibrosarcomas, and undifferentiated pleomorphic sarcomas. Angiosarcomas, followed by leiomyosarcomas, are the most common differentiated sarcomas (69).
Angiosarcoma
Angiosarcoma is an aggressive sarcoma that often originates in the right atrium and has a high likelihood of metastases at the time of presentation. Complete surgical resection confers the best long-term outcome; however, this is rarely possible due to anatomical considerations and metastatic spread.
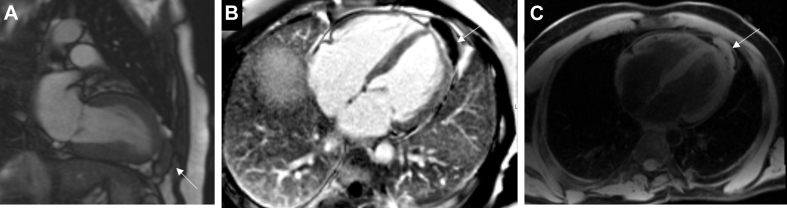
Angiosarcomas (Figure 7) are highly aggressive tumors consisting of irregularly shaped vascular channels lined by anaplastic epithelial cells with sizeable areas of necrosis and hemorrhage. They preferentially affect men and have a peak incidence in the fourth decade of life. They are of right atrial origin in approximately 75% of cases and typically fill this chamber and then infiltrate into the pericardium, tricuspid valve, right ventricle, and right coronary artery (70). Metastases develop in 47% to 89% of patients, most commonly to the lungs but also to the bone, colon, and brain (71). Patients typically present with symptoms of right heart failure, shortness of breath due to hemopericardium, and palpitations secondary to supraventricular arrhythmias.
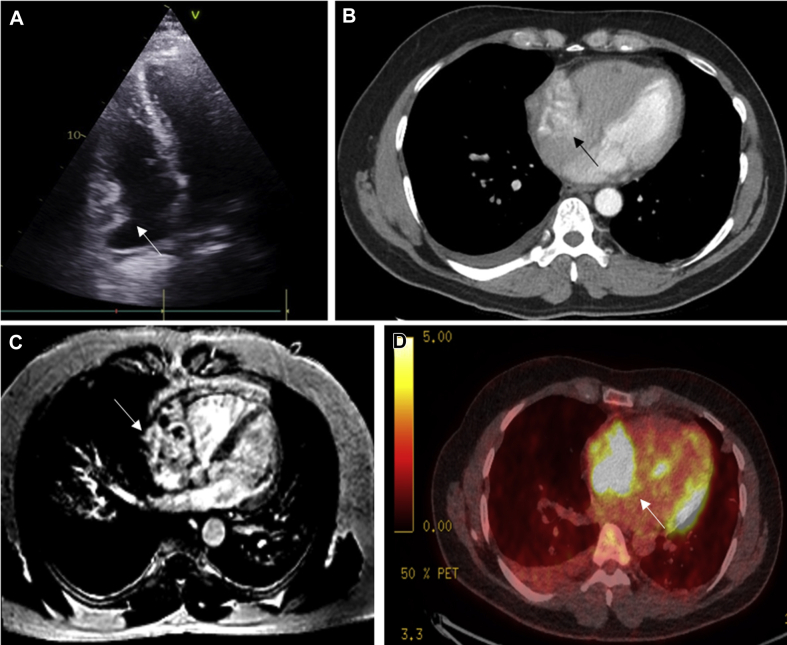
Figure 7.
Angiosarcoma
Cardiac angiosarcoma with lung metastases in a 58-year-old man presenting with hemoptysis. (A) Heterogeneous irregular mass (white arrow) in the right atrium on echocardiography. (B) Mass involving the right atrium (black arrow), which is heterogeneous in appearance on enhanced cardiac CT with scattered areas of nonenhancing necrosis. Right-sided pleural effusion is present. (C) Large mass involving the right atrium (white arrow) that crosses tissue boundaries (infiltrating the right atrium and across into the pericardial space and into the extracardiac fat). The mass is highly vascular with additional hemorrhagic and necrotic components seen on late enhancement imaging on CMR. (D) Irregular area of intense metabolic activity (white arrow) in the right atrium on PET CT. Abbreviations as in Figures 2 and 6.
On TTE, angiosarcomas typically appear as an echogenic nodular or lobulated mass in the right atrium, with pericardial effusion or direct pericardial extension (72). Cardiac CT allows for evaluation of the tumor burden and vascularity. The tumor is grossly hemorrhagic and often heterogeneous in appearance because of the scattered areas of nonenhancing necrosis (19). Invasion into adjacent structures at the time of diagnosis is common, with pericardial and pleural effusions readily observed (19). When invasion into the pericardium is present, there is usually a “sheet-like” thickening due to the distribution and arrangement of the tumor cells, which is in contrast to the nodular appearance seen in rhabdomyosarcomas (19). On T1-weighted imaging on CMR, they appear as isointense lesions with multiple nodular areas of high intensity, often portrayed as having a “cauliflower” appearance (72,73). Late gadolinium enhancement characteristics of the tumor show heterogeneous enhancement and may show a large necrotic core without enhancement (74). When invasion into the pericardium is present, it is often described as having a “sunray” appearance due to avid late gadolinium enhancement along the prominent vasculature of the tumor (72,75).
Cardiac angiosarcomas have an especially poor prognosis, with a mean survival of 3.8 ± 2.5 months without surgical resection (71). There are no clear prognostic factors. Negative margins resection confers the best long-term outcome but is often precluded due to anatomical considerations. Neoadjuvant chemotherapy in combination with surgical debulking may confer some survival advantage. However, because most of the literature on cardiac angiosarcomas is based on clinical cases or single institution studies, no standardized treatment algorithms exist (76).
Leiomyosarcoma
Leiomyosarcomas (Figure 8) are rare but highly aggressive. They are usually only symptomatic once the tumor has reached an advanced stage. Complete resection and adjunctive chemotherapy or radiotherapy may help to marginally improve prognosis.
Figure 8.
Leiomyosarcoma
Paracardiac leiomyosarcoma in a 42-year-old man presenting with chest pain. (A) Paracardiac mass (white arrow) adjacent to the left ventricular apex that appears to be external to but closely adherent to the pericardium on SSFP imaging on CMR. (B) Paracardiac mass (white arrow) showing no late gadolinium enhancement on CMR. (C) Increased signal intensity on fat-water suppression imaging on CMR. Abbreviations as in Figures 2 and 3.
Leiomyosarcomas include 8% to 9% of all cardiac sarcomas (77) and are a rare and highly aggressive cardiac sarcoma with no more than 200 cases reported in the literature (78). These tumors are usually seen in the posterior left atrium and present as sessile masses that can have a mucoid appearance (79). This tumor has also been described in all other cardiac chambers. Histologically, it is composed of compact bundles of spindled cells with blunt nuclei and regions of necrosis, and mitotic figures with epithelioid regions are often present (80).
The clinical presentation depends on the location and size of the tumor (81); however, it is usually asymptomatic until it reaches an advanced stage. The tumors that infiltrate the myocardium are frequently associated with arrhythmias (81). Other less common presentations include features of venous and intra-aortic thrombosis (82). On cardiac CT, leiomyosarcomas appear as irregular lobulated low-attenuated masses with pericardial effusion (83). They usually invade mitral valve and pulmonary veins and appear as a filling defect (83). Dystrophic calcification may also be seen (72).
Primary cardiac leiomyosarcomas have a poor prognosis, and the mean survival time without treatment is reported to be 6 months from the time of diagnosis. Despite the high incidence of distant metastases, surgical resection relieves symptoms adequately and offers palliation. A few cases of palliative chemotherapy alone or surgery followed by palliative chemotherapy have been reported, with survival of 10 to 18 months (77).
Rhabdomyosarcoma
Rhabdomyosarcomas are the most common pediatric malignancy and often affect the valves. These tumors present with symptoms related to cardiac invasion or obstruction and are managed with a combination of surgery, chemotherapy, and radiotherapy. Prognosis is dismal.
Rhabdomyosarcomas are malignant tumors of striated muscle that account for 4% to 7% of cardiac sarcomas and remain the most common pediatric cardiac malignancy (57). These tumors arise from the myocardium, have a tendency toward valvular involvement, and show no chamber predilection (84).They are often bulky and invasive, >10 cm in diameter, and are usually of the embryonal type, with rhabdomyoblasts containing abundant glycogen and expressing desmin, myoglobin, and myogenin (85). They typically present with symptoms related to cardiac invasion or obstruction. Because the growth rate is high before clinical presentation, rhabdomyosarcomas are difficult to treat and are often rapidly fatal (86).
Cardiac CT may show a smooth (87) or irregular (88) low-attenuation mass in a cardiac chamber. On CMR, they appear isointense on T1-weighted images, hyperintense on T2-weighted images, and generally demonstrate homogeneous contrast enhancement, and in some instances, with regions of hypointensity due to central necrosis (55,57). Extracardiac extension is clearly depicted at CT and CMR imaging. Obstructive pulmonary artery metastasis of rhabdomyosarcoma has been described as markedly hyperintense (89).
The standard therapy for rhabdomyosarcoma is a combination of surgery, chemotherapy, and radiation therapy, an approach that has been developed over the past several decades through a series of large-scale trials (90). Complete surgical resection is still an important prognostic factor; however, due to the aggressive nature of the tumor, 46% of patients present with metastatic disease (91). Therefore, the most important component of the combined modality approach is systemic chemotherapy. Vincristine, dactinomycin, and cyclophosphamide are the standard regimen for rhabdomyosarcoma (92). Radiation therapy is administered to achieve local control in patients with residual microscopic or gross disease following surgery and chemotherapy (93). Unfortunately, despite treatment, patients with primary cardiac rhabdomyosarcoma are expected to survive <1 year in most cases (94).
Osteosarcoma
Osteosarcoma is extremely rare, predominantly found in the left atrium, and presents with arrhythmias and heart failure symptoms. This tumor is best seen on CT as low attenuated masses with dense mineralization. It should be treated with surgical resection, if possible.
Primary cardiac osteosarcomas are extremely rare, consisting of 3% to 9% of all cardiac sarcomas (80). They consist of sheets of atypical spindle or ovoid cells, mingled with osteoid, bone, or chondroid substances. They may be predominantly osteoblastic but still possess the potentiality of chondroblastic or fibroblastic differentiation (95).
Unlike metastatic osteosarcoma, which most often occurs in the right atrium, primary cardiac osteosarcomas arise in the left atrium in most cases. They are highly invasive and frequently infiltrate into the adjacent myocardium, which results in arrhythmias and impaired ventricular function. They can often infiltrate into the pericardium, causing pericardial effusion.
On echocardiography, cardiac osteosarcomas often appear as asymmetrical internal echoes. The characteristic CT findings of cardiac osteosarcomas have been reported as a low attenuation mass with dense mineralization (72). Osteosarcomas often have dense calcification and form stone masses, or may also have minimal calcification in the early stages. Technetium-99 MDP (methylene diphosphonate) single-photon emission CT is also useful in detecting bone-origin osteosarcoma and bone metastasis because of its exclusive superiority in imaging the skeletal system.
Because of its invasion, size, site, and attachment, complete resection is impossible for most cases (62), which results in an ominous prognosis. Location in the left atrium, absence of necrosis, and complete surgical excision are associated with better survival rates.
Undifferentiated sarcoma
Undifferentiated sarcomas have no specific histological pattern, are invasive, and are often seen in the left atrium. Most patients present with pulmonary congestion. The prognosis is poor, despite surgery and palliative chemotherapy.
Sarcomas without specific histological patterns, ultrastructural features, or specific immunohistochemical findings are regarded as undifferentiated and constitute <24% of sarcomas (80). Undifferentiated high-grade pleiomorphic sarcoma (previously called pleiomorphic malignant fibrous histiocytoma) has a mean age of presentation of 44 years and affects either sex equally (80). They predominate in the left atrium and are typically invasive with a characteristic diffuse wall involvement (96). Undifferentiated pleiomorphic sarcoma is a diagnosis of exclusion, after thorough sampling and judicious use of ancillary diagnostic techniques. The tumors have diverse clinical manifestations, although the most common is pulmonary congestion (80). Similar to angiosarcomas, they may appear as focal or infiltrative masses with necrosis and hemorrhage on CMR. However, unlike angiosarcomas, which are usually found in the right atrium, undifferentiated sarcomas have a predilection for the left atrium (81%) (97). This is an extremely aggressive form of cancer, and the prognosis is very poor. Surgery and palliative chemotherapy are the mainstay of treatment (63,98).
Primary cardiac lymphomas
Primary cardiac lymphomas are mainly aggressive B-cell lymphomas seen more commonly in immunocompromised individuals. These tumors have a predilection for the right side of the heart and can present with nonspecific symptoms, including constitutional symptoms. Treatment is with chemotherapy and immunotherapy.
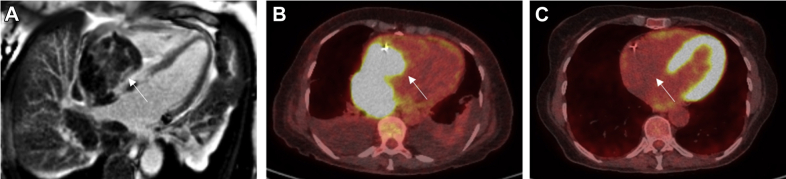
Primary cardiac lymphomas (Figure 9) in immunocompetent patients are an uncommon malignancy, accounting for 1.3% of PCTs (99), whereas cardiac metastases from extracardiac forms of lymphoma are far more common (approximately 25% of patients with lymphoma have cardiac involvement). Nearly all primary cardiac lymphomas are aggressive B-cell lymphomas, with an increasing incidence secondary to lymphoproliferative disorders related to Epstein-Barr virus in patients with AIDS and in patients who have received transplants. The mean age at diagnosis is 63 years of age (99). They most commonly involve the right side of the heart, particularly the right atrium, but any chamber can be involved. There are frequently multiple lesions.
Figure 9.
Primary Cardiac Lymphoma
Primary cardiac lymphoma in a 72-year-old woman presenting with shortness of breath and worsening peripheral edema. (A) Cardiac mass (white arrow) shows patchy heterogeneous late gadolinium enhancement on CMR. (B) FDG PET CT pre-treatment. Increased FDG uptake within the mass (white arrow), which is replacing the right atrium and extending into the right ventricle. (C) FDG PET CT post-treatment. Complete metabolic resolution in the right atrial cardiac mass (white arrow). Abbreviations as in Figures 2 and 6.
The symptoms of primary cardiac lymphoma are nonspecific. It can manifest as a heart rhythm disturbances, including heart block, syncopal episodes, or even as a restrictive cardiomyopathy (100). Constitutional complaints (fever, chills, sweats, and weight loss), chest pain, and dyspnea are also common. Approximately 20% of patients may develop acute heart failure before the presentation of other symptoms (101).
On echocardiography, these tumors can appear as homogeneous, infiltrating masses that lead to wall thickening and restrictive physiology, or as nodular masses intruding into the heart chambers, with a predilection for the right heart chambers (especially, the right atrium). The atrioventricular groove can be affected as well, encasing the right coronary artery, as well as the pericardium with effusion or enfacement. Cardiac CT may show a large focal mass, diffuse soft tissue infiltration, or multiple nodules with heterogeneous enhancement. Mediastinal lymphadenopathy may also be present. On CMR, tissue appears isointense on T1-weighted imaging, although some cases report hypointense lesions. On T2-weighted imaging, lesions are mildly hyperintense due to diffuse edema. Scattered regions of hypointensity suggestive of central necrosis may also be appreciated (102). 18F-FDG PET provides information on the metabolism and degree of cell proliferation in the lesions through the intensity of uptake of 18F-FDG, which is directly proportional to the degree of malignancy of the lesion (103). 18F-FDG PET is also performed after completion of chemotherapy to evaluate the final treatment response.
The diagnosis can be confirmed via analysis of pericardial effusion, endomyocardial biopsy tissue, or surgical specimens. Treatment is primarily with anthracycline-based chemotherapy and the monoclonal anti-CD20 antibody rituximab (23,64). The overall response rates to chemotherapy are 79% to 87%; however, the recurrence rate is as high as 55%, and recurrence usually develops at extracardiac extranodal sites (101). Multimodality treatment may also include autologous stem cell transplantation. Cardiac lymphoma typically follows an aggressive clinical course. The prognoses of patients with primary cardiac lymphomas are poor. More than 60% of patients die within 2 months of diagnosis (104). Predictors for a worse outcome are immune status, left ventricular involvement, presence of extracardiac disease, and arrhythmia (105).
Mesothelioma
Mesothelioma is rare and arises from the pericardium in most cases. It often presents with a pericardial effusion with or without tamponade. It is a highly aggressive disease with an extremely poor prognosis, often treated palliatively only.
Mesotheliomas consist of most of the cases of primary malignant pericardial tumors, and they arise from the pericardial mesothelial cell layer. Association with asbestos exposure is assumed, but a definitive causal relationship has not yet been established (60). They are rare, accounting for <2% of all mesotheliomas. By definition, there must be a lack of pleural involvement or most of the tumor must be within the pericardial space (23).
Male patients dominate the population of patients with primary pericardial mesothelioma, with a median age of 46 years, and a preponderance of patients in their fifth to seventh decades (106). Clinical symptoms and signs are closely correlated with the accumulation of tumor, and therefore, can include chest pain, dyspnea, systemic symptoms (e.g., weight loss, fever, and nights sweats), and arrhythmias, as well as facial, pedal, or low-extremity edema (57,107,108).
Echocardiography typically reveals a pericardial effusion and a tumor encasing the heart; a discrete mass may not be seen. CMR and CT show multiple enhancing and coalescing pericardial masses that envelop the pericardial space (109). Integrated PET/CT imaging with co-registration of functional and anatomic imaging data improves the localization of regions of increased FDG uptake and the accuracy of staging (110,111). This is valuable for monitoring the efficiency of disease management and the direction of subsequent therapy (110,111).
Microscopic evaluation is needed to establish the diagnosis. A cytological sample may be obtained by pericardiocentesis but is commonly unrevealing, and only clues of hemorrhage and/or inflammation are found (112,113). Histopathological analysis is therefore required (114). A tissue sample can be obtained through open biopsy, pericardiectomy, or mass resection (114). Histologically, mesotheliomas can be epithelial, sarcomatous, or biphasic.
Primary pericardial mesothelioma is a highly aggressive disease with a uniformly negative prognosis due to its late presentation, diagnostic uncertainties, difficulty in complete resection, and poor response to therapies (66). The median post-diagnosis survival is 6 months in patients with pericardial mesothelioma (107). No standard treatment strategy has been established; however, surgery with or without chemotherapy are often used. With regard to surgery, complete resection is hardly achieved because of the wide myocardial infiltration and invasion of adjacent structures; however, long-term survival is expected in cases of localized mass and complete resection (66). Palliative surgery and pericardectomy are most commonly used as methods to relieve constriction, whereas the creation of a pericardial window can be helpful for the treatment of instilling chemotherapy (66).
Secondary Cardiac Tumors
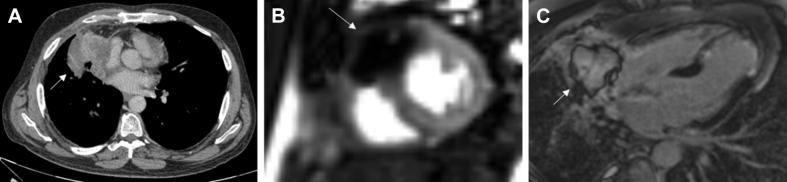
Cardiac metastases (Figure 10) are 20 to 40 times more common than PCTs (60). Up to 12% of oncology patients have metastases to the heart or pericardium at autopsy, although most remain clinically silent (115). Melanomas have the greatest propensity for cardiac involvement (116), whereas carcinomas of the thorax, including breast, lung, and esophageal, are the most common carcinomas that metastasize to the heart. Malignant tumors use 1 of 4 routes to reach the heart (117), including hematogeneous, lymphatic, transvenous spread, or direct invasion. In addition, the metastatic route significantly determines the target tissue (117). For instance, masses that spread through the lymphatics often seed the pericardium or epicardium (117).
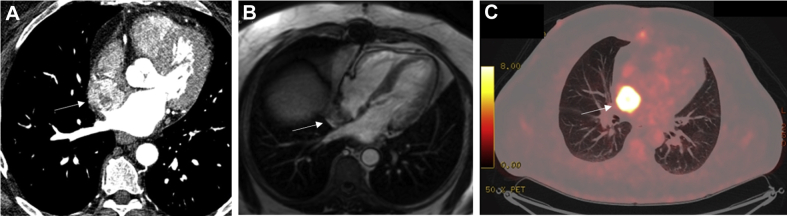
Figure 10.
Secondary Cardiac Tumor
Non-small cell lung cancer with cardiac metastasis in a 48-year-old man presenting with acute anterior myocardial infarction. (A) Necrotic mass in the right middle lobe (white arrow) on cardiac CT, invading into the pericardium. (B) On resting perfusion on CMR, there is a large perfusion defect within the soft tissue mass (white arrow). The perfusion defect extends down the interventricular septum, where it likely represents the recent left anterior descending artery infarct. The mass did not enhance on first pass perfusion (not shown). (C) On late gadolinium enhancement imaging on CMR, there is an avascular core within the mass (white arrow), surrounded by high signal enhancement. Abbreviations as in Figure 2.
Cardiac involvement should be suspected or sought in any patient with a known malignancy who develops new cardiovascular signs or symptoms. Pericardial involvement may give rise to pericardial effusion and tamponade (72,73). Myocardial metastasis can result in conduction disturbances that trigger arrhythmias that may be resistant to standard antiarrhythmic medications (72,77). Myocardial replacement with neoplastic cells may eventually lead to heart failure (73). Intracavitary masses impede blood flow and cause valvular dysfunction (72). Multiple masses or nodules are typical of metastases, although diffuse infiltration has also been recognized.
Echocardiography should be undertaken as the initial diagnostic test to evaluate for the presence of cardiac metastatic disease. On CMR, most secondary cardiac malignancies exhibit a low-signal intensity on T1-weighted imaging and high-signal intensity on T2-weighted imaging (118,119). A notable exception to this is metastatic melanoma, which appears hyperintense on T1-weighted imaging due to the paramagnetic T1-shortening effects of melanin. Importantly, the presence of cardiac metastases by CMR, even if isolated, confers a similar prognosis to stage IV cancers (120). FDG-PET/CT imaging is commonly used to evaluate for metastatic lesions. In most extracardiac tissues, delineation of an FDG-avid focus is relatively simple. In cardiac metastases, this task is more challenging. The myocardium, because of its high metabolic activity, exhibits FDG uptake similar to a tumor (121,122). Patients should undergo adequate dietary preparation before FDG PET imaging (22).
Management is individualized and directed toward the primary tumor. Resection is usually not indicated, except in select conditions (74): 1) in patients with intracavitary metastases that result in significant hemodynamic complications progressing to cardiac decompensation (67,122); or 2) patients with solitary cardiac disease when the primary tumor is controlled and good prognosis is expected (121).
Conclusions
Although cardiac tumors are rare, they can lead to serious complications, such as intracardiac obstruction and fatal arrhythmias, even when benign. Imaging plays a central role in the evaluation of a suspected tumor, and with multimodality imaging being more readily available, we are better able to accurately distinguish cardiac masses to provide optimal medical management. Imaging not only helps to identify a likely etiology but also to plan approaches for further diagnostic investigations and guide management, be it medical, surgical, or both.
Acknowledgments
The authors would like to thank Dr. Leon Menezes and Dr. Reena Tanna for their assistance in image acquisition for this review.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For a discussion of rare benign primary cardiac tumors, please see the online version of this paper.
Appendix
References
- 1.Ghosh A.K., Malcolm Walker J. Cardio-oncology. Br J Hosp Med. 2017;78:C11–C13. doi: 10.12968/hmed.2017.78.1.C11. [DOI] [PubMed] [Google Scholar]
- 2.McAllister H.A. Primary tumors and cysts of the heart and pericardium. Curr Probl Cardiol. 1979;4:1–51. doi: 10.1016/0146-2806(79)90008-2. [DOI] [PubMed] [Google Scholar]
- 3.Cresti A., Chiavarelli M., Glauber M. Incidence rate of primary cardiac tumors: a 14-year population study. J Cardiovasc Med. 2016;17:37–43. doi: 10.2459/JCM.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 4.Lam King Yin, Dickens P., Chan A.C.L. Tumors of the heart: a 20-year experience with a review of 12 485 consecutive autopsies. Arch Pathol Lab Med. 1993;117:1027–1031. [PubMed] [Google Scholar]
- 5.Butany J., Leong S.W., Carmichael K., Komeda M. A 30-year analysis of cardiac neoplasms at autopsy. Can J Cardiol. 2005;21:675–680. [PubMed] [Google Scholar]
- 6.Oliveira G.H., Al-Kindi S.G., Hoimes C., Park S.J. Characteristics and survival of malignant cardiac tumors a 40-year analysis of >500 patients. Circulation. 2015;132:2395–2402. doi: 10.1161/CIRCULATIONAHA.115.016418. [DOI] [PubMed] [Google Scholar]
- 7.Biersmith M.A., Tong M.S., Guha A., Simonetti O.P., Addison D. Multimodality cardiac imaging in the era of emerging cancer therapies. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dujardin K.S., Click R.L., Oh J.K. The role of intraoperative transesophageal echocardiography in patients undergoing cardiac mass removal. J Am Soc Echocardiogr. 2000;13:1080–1083. doi: 10.1067/mje.2000.107157. [DOI] [PubMed] [Google Scholar]
- 9.Motwani M., Kidambi A., Herzog B.A., Uddin A., Greenwood J.P., Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. 2013;268:26–43. doi: 10.1148/radiol.13121239. [DOI] [PubMed] [Google Scholar]
- 10.Bruce C.J. Cardiac tumours: diagnosis and management. Heart. 2011;97:151–160. doi: 10.1136/hrt.2009.186320. [DOI] [PubMed] [Google Scholar]
- 11.Buckley O., Madan R., Kwong R., Rybicki F.J., Hunsaker A. Cardiac masses, part 1: imaging strategies and technical considerations. AJR Am J Roentgenol. 2011;197:W837−41. doi: 10.2214/AJR.10.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaragosa-Macias E., Chen M.A., Gill E.A. Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography. 2012;29:207–219. doi: 10.1111/j.1540-8175.2011.01627.x. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya S., Khattar R., Senior R. Characterisation of intra-cardiac masses by myocardial contrast echocardiography. Int J Cardiol. 2013;163 doi: 10.1016/j.ijcard.2012.06.098. e11−3. [DOI] [PubMed] [Google Scholar]
- 14.Kirkpatrick J.N., Wong T., Bednarz J.E. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412–1419. doi: 10.1016/j.jacc.2003.09.065. [DOI] [PubMed] [Google Scholar]
- 15.Beroukhim R.S., Prakash A., Valsangiacomo Buechel E.R. Characterization of cardiac tumors in children by cardiovascular magnetic resonance imaging: a multicenter experience. J Am Coll Cardiol. 2011;58:1044–1054. doi: 10.1016/j.jacc.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Parwani P., Co M., Ramesh T. Differentiation of cardiac masses by cardiac magnetic resonance imaging. Curr Cardiovasc Imaging Rep. 2020;13 [Google Scholar]
- 17.Zatorska K., Michalowska I., Duchnowski P., Szymanski P., Kusmierczyk M., Hryniewiecki T. The usefulness of magnetic resonance imaging in the diagnosis of infectious endocarditis. J Heart Valve Dis. 2015;24:767–775. [PubMed] [Google Scholar]
- 18.Hoey E., Ganeshan A., Nader K., Randhawa K., Watkin R. Cardiac neoplasms and pseudotumors: imaging findings on multidetector CT angiography. Diagnostic Interv Radiol. 2012;18:67–77. doi: 10.4261/1305-3825.DIR.4215-11.2. [DOI] [PubMed] [Google Scholar]
- 19.Kassop D., Donovan M.S., Cheezum M.K. Cardiac masses on cardiac CT: a review. Curr Cardiovasc Imaging Rep. 2014;7:1–13. doi: 10.1007/s12410-014-9281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahbar K., Seifarth H., Schäfers M. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53:856–863. doi: 10.2967/jnumed.111.095364. [DOI] [PubMed] [Google Scholar]
- 21.Nensa F., Tezgah E., Poeppel T.D. Integrated 18F-FDG PET/MR imaging in the assessment of cardiac masses: a pilot study. J Nucl Med. 2015;56:255–260. doi: 10.2967/jnumed.114.147744. [DOI] [PubMed] [Google Scholar]
- 22.Osborne M.T., Hulten E.A., Murthy V.L. Patient preparation for cardiac fluorine-18 fluorodeoxyglucose positron emission tomography imaging of inflammation. J Nucl Cardiol. 2017;24:86–99. doi: 10.1007/s12350-016-0502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke A., Tavora F. The 2015 WHO classification of tumors of the heart and pericardium. J Thorac Oncol. 2016;11:441–452. doi: 10.1016/j.jtho.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 24.Wu H.M., Chen Y., Xiao Z.B. Clinical and pathological characteristics of cardiac tumors: analyses of 689 cases at a single medical center. Chinese J Pathol. 2019;48:293–297. doi: 10.3760/cma.j.issn.0529-5807.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Habertheuer A., Laufer G., Wiedemann D. Primary cardiac tumors on the verge of oblivion: a European experience over 15 years. J Cardiothorac Surg. 2015;10:56. doi: 10.1186/s13019-015-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basso C., Rizzo S., Valente M., Thiene G. Cardiac masses and tumours. Heart. 2016;102:1230–1245. doi: 10.1136/heartjnl-2014-306364. [DOI] [PubMed] [Google Scholar]
- 27.Keeling I.M., Oberwalder P., Anelli-Monti M. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardio-Thoracic Surg. 2002;22:971–977. doi: 10.1016/s1010-7940(02)00592-4. [DOI] [PubMed] [Google Scholar]
- 28.Maleszewski J.J., Anavekar N.S., Moynihan T.J., Klarich K.W. Pathology, imaging, and treatment of cardiac tumours. Nat Rev Cardiol. 2017;14:536–549. doi: 10.1038/nrcardio.2017.47. [DOI] [PubMed] [Google Scholar]
- 29.Mankad R., Herrmann J. Cardiac tumors: Echo assessment. Echo Res Pract. 2016;3:R65–R77. doi: 10.1530/ERP-16-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajiah P., Kanne J.P., Kalahasti V., Schoenhagen P. Computed tomography of cardiac and pericardiac masses. J Cardiovasc Comput Tomogr. 2011;5:16–29. doi: 10.1016/j.jcct.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 31.O’Donnell D.H., Abbara S., Chaithiraphan V. Cardiac tumors: optimal cardiac MR sequences and spectrum of imaging appearances. Am J Roentgenol. 2009;193:377–387. doi: 10.2214/AJR.08.1895. [DOI] [PubMed] [Google Scholar]
- 32.Tamin S.S., Maleszewski J.J., Scott C.G. Prognostic and bioepidemiologic implications of papillary fibroelastomas. J Am Coll Cardiol. 2015;65:2420–2429. doi: 10.1016/j.jacc.2015.03.569. [DOI] [PubMed] [Google Scholar]
- 33.Türkmen N., Eren B., Fedakar R., Çomunoglu N. An unusual cause of sudden death: Cardiac myxoma. Adv Ther. 2007;24:529–532. doi: 10.1007/BF02848775. [DOI] [PubMed] [Google Scholar]
- 34.Freedom R.M., Lee K.J., MacDonald C., Taylor G. Selected aspects of cardiac tumors in infancy and childhood. Pediatr Cardiol. 2000;21:299–316. doi: 10.1007/s002460010070. [DOI] [PubMed] [Google Scholar]
- 35.Wang J.G., Wang B., Hu Y. Clinicopathologic features and outcomes of primary cardiac tumors: a 16-year-experience with 212 patients at a Chinese medical center. Cardiovasc Pathol. 2018;33:45–54. doi: 10.1016/j.carpath.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Carney J.A., Hruska L.S., Beauchamp G.D., Gordon H. Dominant inheritance of the complex of myxomas, spotty pigmentation, and endocrine overactivity. Mayo Clin Proc. 1986;61:165–172. doi: 10.1016/s0025-6196(12)61843-6. [DOI] [PubMed] [Google Scholar]
- 37.Pinede L., Duhaut P., Loire R. Clinical presentation of left atrial cardiac myxoma: a series of 112 consecutive cases. Medicine (Baltimore) 2001;80:159–172. doi: 10.1097/00005792-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 38.Grebenc M.L., Rosado-De-Christenson M.L., Green C.E., Burke A.P., Galvin J.R. From the archives of the AFIP: cardiac myxoma: imaging features in 83 patients. Radiographics. 2002;22:673–689. doi: 10.1148/radiographics.22.3.g02ma02673. [DOI] [PubMed] [Google Scholar]
- 39.Scheffel H., Baumueller S., Stolzmann P. Atrial myxomas and thrombi: comparison of imaging features on CT. Am J Roentgenol. 2009;192:639–645. doi: 10.2214/AJR.08.1694. [DOI] [PubMed] [Google Scholar]
- 40.Sparrow P.J., Kurian J.B., Jones T.R., Sivananthan M.U. MR imaging of cardiac tumors. Radiographics. 2005;25:1255–1276. doi: 10.1148/rg.255045721. [DOI] [PubMed] [Google Scholar]
- 41.Elbardissi A.W., Dearani J.A., Daly R.C. Survival after resection of primary cardiac tumors: a 48-year experience. Circulation. 2008;118(14 Suppl):S7–S15. doi: 10.1161/CIRCULATIONAHA.107.783126. [DOI] [PubMed] [Google Scholar]
- 42.Jeong D., Patel A., François C.J., Gage K.L., Fradley M.G. Cardiac magnetic resonance imaging in oncology. Cancer Control. 2017;24:147–160. doi: 10.1177/107327481702400207. [DOI] [PubMed] [Google Scholar]
- 43.Ngaage D.L., Mullany C.J., Daly R.C. Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg. 2005;80:1712–1718. doi: 10.1016/j.athoracsur.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 44.Capotosto L., Elena G., Massoni F. Cardiac tumors: echocardiographic diagnosis and forensic correlations. Am J Forensic Med Pathol. 2016;37:306–316. doi: 10.1097/PAF.0000000000000271. [DOI] [PubMed] [Google Scholar]
- 45.Cianciulli T.F., Soumoulou J.B., Lax J.A. Papillary fibroelastoma: clinical and echocardiographic features and initial approach in 54 cases. Echocardiography. 2016;33:1811–1817. doi: 10.1111/echo.13351. [DOI] [PubMed] [Google Scholar]
- 46.Klarich K.W., Enriquez-Sarano M., Gura G.M., Edwards W.D., Tajik A.J., Seward J.B. Papillary fibroelastoma: echocardiographic characteristics for diagnosis and pathologic correlation. J Am Coll Cardiol. 1997;30:784–790. doi: 10.1016/s0735-1097(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 47.Tzani A., Doulamis I.P., Mylonas K.S., Avgerinos D.V., Nasioudis D. Cardiac tumors in pediatric patients: a systematic review. World J Pediatr Congenit Heart Surg. 2017;8:624–632. doi: 10.1177/2150135117723904. [DOI] [PubMed] [Google Scholar]
- 48.Kocabaş A., Ekici F., Çetin Cardiac rhabdomyomas associated with tuberous sclerosis complex in 11 children: presentation to outcome. Pediatr Hematol Oncol. 2013;30:71–79. doi: 10.3109/08880018.2012.734896. [DOI] [PubMed] [Google Scholar]
- 49.Smythe J.F., Dyck J.D., Smallhorn J.F., Freedom R.M. Natural history of cardiac rhabdomyoma in infancy and childhood. Am J Cardiol. 1990;66:1247–1249. doi: 10.1016/0002-9149(90)91109-j. [DOI] [PubMed] [Google Scholar]
- 50.Araoz P.A., Mulvagh S.L., Tazelaar H.D., Julsrud P.R., Breen J.F. CT and MR imaging of benign primary cardiac neoplasms with echocardiographic correlation. Radiographics. 2000;20:1303–1309. doi: 10.1148/radiographics.20.5.g00se121303. [DOI] [PubMed] [Google Scholar]
- 51.Basso C., Buser P.T., Rizzo S., Lombardi M., Thiene G. Benign cardiac tumours. In: Lombardi M., Plein S., Petersen S., editors. The EACVI Textbook of Cardiovascular Magnetic Resonance. Oxford University Press; Oxford, UK: 2018. pp. 469–473. [Google Scholar]
- 52.Burke A.P., Rosado-de-Christenson M., Templeton P.A., Virmani R. Cardiac fibroma: clinicopathologic correlates and surgical treatment. J Thorac Cardiovasc Surg. 1994;108:862–870. [PubMed] [Google Scholar]
- 53.Agrawal S.K.B., Rakhit D.J., Livesey S., Pontefract D., Harden S.P. Large intra-cardiac benign fibrous tumour presenting in an adult patient identified using MRI. Clin Radiol. 2009;64:637–640. doi: 10.1016/j.crad.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 54.Stéphant E., Ana S., Philippe D. Inter-ventricular septal cardiac fibroma in an adult: MR and MDCT features with pathologic correlation. Eur J Radiol Extra. 2008;67:e13–e106. [Google Scholar]
- 55.Kiaffas M.G., Powell A.J., Geva T. Magnetic resonance imaging evaluation of cardiac tumor characteristics in infants and children. Am J Cardiol. 2002;89:1229–1233. doi: 10.1016/s0002-9149(02)02314-7. [DOI] [PubMed] [Google Scholar]
- 56.Mandak J.S., Benoit C.H., Starkey R.H., Nassef L.A. Echocardiography in the evaluation of cardiac pheochromocytoma. Am Heart J. 1996;132:1063–1066. doi: 10.1016/s0002-8703(96)90027-7. [DOI] [PubMed] [Google Scholar]
- 57.Grebenc M.L., Rosado De Christenson M.L., Burke A.P., Green C.E., Galvin J.R. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073–1103. doi: 10.1148/radiographics.20.4.g00jl081073. [DOI] [PubMed] [Google Scholar]
- 58.Wang J.G., Han J., Jiang T., Li Y.J. Cardiac paragangliomas. J Card Surg. 2015;30:55–60. doi: 10.1111/jocs.12455. [DOI] [PubMed] [Google Scholar]
- 59.Brown M.L., Zayas G.E., Abel M.D., Young W.F., Schaff H.V. Mediastinal paragangliomas: the Mayo clinic experience. Ann Thorac Surg. 2008;86:946–951. doi: 10.1016/j.athoracsur.2008.04.105. [DOI] [PubMed] [Google Scholar]
- 60.Butany J., Nair V., Naseemuddin A., Nair G.M., Catton C., Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 61.Hoey E.T.D., Mankad K., Puppala S., Gopalan D., Sivananthan M.U. MRI and CT appearances of cardiac tumours in adults. Clin Radiol. 2009;64:1214–1230. doi: 10.1016/j.crad.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 62.Wang J.G., Liu B., Gao H., Li Y.J., Zhao P., Liu X.P. Primary cardiac osteosarcoma. Heart Lung Circ. 2016;25:698–704. doi: 10.1016/j.hlc.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 63.Watson R., Frye J., Trieu M., Yang M.X. Primary undifferentiated pleomorphic cardiac sarcoma with MDM2 amplification presenting as acute left-sided heart failure. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2018-226073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neragi-Miandoab S., Kim J., Vlahakes G.J. Malignant tumours of the heart: a review of tumour type, diagnosis and therapy. Clin Oncol. 2007;19:748–756. doi: 10.1016/j.clon.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 65.Maleszewski J.J., Bois M.C., Bois J.P., Young P.M., Stulak J.M., Klarich K.W. Neoplasia and the heart: pathological review of effects with clinical and radiological correlation. J Am Coll Cardiol. 2018;72:202–227. doi: 10.1016/j.jacc.2018.05.026. [DOI] [PubMed] [Google Scholar]
- 66.Cao S., Jin S., Cao J. Malignant pericardial mesothelioma: a systematic review of current practice. Herz. 2018;43:61–68. doi: 10.1007/s00059-016-4522-5. [DOI] [PubMed] [Google Scholar]
- 67.Bussani R., De-Giorgio F., Abbate A., Silvestri F. Cardiac metastases. J Clin Pathol. 2007;60:27–34. doi: 10.1136/jcp.2005.035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sultan I., Bianco V., Habertheuer A. Long-term outcomes of primary cardiac malignancies: multi-institutional results from the National Cancer Database. J Am Coll Cardiol. 2020;75:2338–2347. doi: 10.1016/j.jacc.2020.03.041. [DOI] [PubMed] [Google Scholar]
- 69.Ramlawi B., Leja M.J., Abu Saleh W.K. Surgical treatment of primary cardiac sarcomas: review of a single-institution experience. Ann Thorac Surg. 2016;101:698–702. doi: 10.1016/j.athoracsur.2015.07.087. [DOI] [PubMed] [Google Scholar]
- 70.Best A.K., Dobson R.L., Ahmad A.R. Best cases from the AFIP: cardiac angiosarcoma. Radiographics. 2003;23:S141−5. doi: 10.1148/rg.23si035140. [DOI] [PubMed] [Google Scholar]
- 71.Butany J., Yu W. Cardiac angiosarcoma: two cases and a review of the literature. Can J Cardiol. 2000;16:197–205. [PubMed] [Google Scholar]
- 72.Araoz P.A., Eklund H.E., Welch T.J., Breen J.F. CT and MR imaging of primary cardiac malignancies. Radiographics. 1999;19:1421–1434. doi: 10.1148/radiographics.19.6.g99no031421. [DOI] [PubMed] [Google Scholar]
- 73.Vander Salm T.J. Unusual primary tumors of the heart. Semin Thorac Cardiovasc Surg. 2000;12:89–100. doi: 10.1053/ct.2000.5080. [DOI] [PubMed] [Google Scholar]
- 74.Palaskas N., Thompson K., Gladish G. Evaluation and management of cardiac tumors. Curr Treat Options Cardiovasc Med. 2018;20:29. doi: 10.1007/s11936-018-0625-z. [DOI] [PubMed] [Google Scholar]
- 75.Tüdös Z., Köcher M., Černá M. Sun ray appearance in a case of cardiac angiosarcoma: a comparison of MRI and PET/CT. Magn Reson Med Sci. 2017;16:176–180. doi: 10.2463/mrms.cr.2015-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Look Hong N.J., Pandalai P.K., Hornick J.L. Cardiac angiosarcoma management and outcomes: 20-year single-institution experience. Ann Surg Oncol. 2012;19:2707–2715. doi: 10.1245/s10434-012-2334-2. [DOI] [PubMed] [Google Scholar]
- 77.Jellis C., Doyle J., Sutherland T., Gutman J., MacIsaac A. Cardiac epithelioid leiomyosarcoma and the role of cardiac imaging in the differentiation of intracardiac masses. Clin Cardiol. 2010;33:E6−9. doi: 10.1002/clc.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang J.G., Cui L., Jiang T., Li Y.J., Wei Z.M. Primary cardiac leiomyosarcoma: an analysis of clinical characteristics and outcome patterns. Asian Cardiovasc Thorac Ann. 2015;23:623–630. doi: 10.1177/0218492315574197. [DOI] [PubMed] [Google Scholar]
- 79.Sarjeant J.M., Butany J., Cusimano R.J. Cancer of the heart: epidemiology and management of primary tumors and metastases. Am J Cardiovasc Drugs. 2003;3:407–421. doi: 10.2165/00129784-200303060-00004. [DOI] [PubMed] [Google Scholar]
- 80.Malaurie E., Petitjean C., Traversat J., Chapelon-Abric C., Vacheron A., Arrivé L. Tumors of the heart and great vessels. In: Buthiau D., Khayat D., editors. CT and MRI in Oncology. Springer; New York: 1998. pp. 179–184. [Google Scholar]
- 81.Gierlak W., Syska-Sumińska J., Zieliński P., Dłuzniewski M., Sadowski J. Cardiac tumors: leiomyosarcoma - a case report. Kardiochir Torakochirurgia Pol. 2015;12:251–254. doi: 10.5114/kitp.2015.54464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida M., Ando S.I., Naito Y., Yano H. Mediastinal leiomyosarcoma concurrent with intra-aortic thrombosis. BMJ Case Rep. 2013 doi: 10.1136/bcr-2012-007527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hassan M., Khattak M., Abdullah H.M.A., Nasib B. Primary cardiac leiomyosarcoma presenting as haemoptysis in a 22-year-old patient: an unusual presentation of a rare condition. BMJ Case Rep. 2017;2017 doi: 10.1136/bcr-2017-219416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gilkeson R.C., Chiles C. MR evaluation of cardiac and pericardial malignancy. Magn Reson Imaging Clin N Am. 2003;11:173–186. doi: 10.1016/s1064-9689(02)00047-8. [DOI] [PubMed] [Google Scholar]
- 85.Tavora F., Burke A. Pathology of cardiac tumours and tumour-like conditions. Diagnostic Histopathol. 2010;16:1–9. [Google Scholar]
- 86.Tavil Y., Turkoglu S., Tacoy G., Cemri M. Huge biatrial cardiac rhabdomyosarcoma resulting in bilateral atrioventricular valve obstruction. Cardiovasc Pathol. 2006;15:354–355. doi: 10.1016/j.carpath.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 87.Rheeder P., Stmson I.W., Mentis H., Karusseit V.O.L. Cardiac rhabdomyosarcoma in a renal transplant patient. Transplantation. 1995;60:204–205. [PubMed] [Google Scholar]
- 88.Jack C.M., Cleland J., Geddes J.S. Left atrial rhabdomyosarcoma and the use of digital gated computed tomography in its diagnosis. Br Heart J. 1986;55:305–307. doi: 10.1136/hrt.55.3.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Suehiro S., Matsuda M., Hirata T. Primary cardiac rhabdomyosarcoma developed after receiving radiotherapy for left breast cancer 18 years prior. J Cardiol Cases. 2017;15:181–183. doi: 10.1016/j.jccase.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Panda S.P., Chinnaswamy G., Vora T. Diagnosis and management of rhabdomyosarcoma in children and adolescents: ICMR consensus document. Indian J Pediatr. 2017;84:393–402. doi: 10.1007/s12098-017-2315-3. [DOI] [PubMed] [Google Scholar]
- 91.Siontis B.L., Zhao L., Leja M. Primary cardiac sarcoma: a rare, aggressive malignancy with a high propensity for brain metastases. Sarcoma. 2019;2019:1960593. doi: 10.1155/2019/1960593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orlandi A., Ferlosio A., Roselli M., Chiariello L., Spagnoli L.G. Cardiac sarcomas: an update. J Thorac Oncol. 2010;5:1483–1489. doi: 10.1097/JTO.0b013e3181e59a91. [DOI] [PubMed] [Google Scholar]
- 93.Beverly Raney R., Maurer H.M., Anderson J.R. The Intergroup Rhabdomyosarcoma Study Group (IRSG): major lessons from the IRS-I through IRS-IV studies as background for the current IRS-V treatment protocols. Sarcoma. 2001;5:9–15. doi: 10.1080/13577140120048890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simsek H., Sahin M., Gumrukcuoglu H.A., Tuncer M., Gunes Y. Recurrence of primary cardiac rhabdomyosarcoma without methastasis two years after surgery. Eur J Gen Med. 2012;9:146–148. [Google Scholar]
- 95.Dell’Amore A., Asadi N., Caroli G., Dolci G., Bini A., Stella F. Recurrent primary cardiac osteosarcoma: a case report and literature review. Gen Thorac Cardiovasc Surg. 2014;62:175–180. doi: 10.1007/s11748-013-0236-2. [DOI] [PubMed] [Google Scholar]
- 96.Lestuzzi C., De Paoli A., Baresic T., Miolo G., Buonadonna A. Malignant cardiac tumors: diagnosis and treatment. Future Cardiol. 2015;11:485–500. doi: 10.2217/fca.15.10. [DOI] [PubMed] [Google Scholar]
- 97.Perchinsky M.J., Lichtenstein S.V., Tyers G.F.O. Primary cardiac tumors: forty years’ experience with 71 patients. Cancer. 1997;79:1809–1815. doi: 10.1002/(sici)1097-0142(19970501)79:9<1809::aid-cncr25>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 98.Xu G., Shi X., Shao G. An unusual case of metastasis of a pulmonary undifferentiated pleomorphic sarcoma to the right ventricle: a case report. J Med Case Rep. 2013;7:165. doi: 10.1186/1752-1947-7-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gowda R.M., Khan I.A. Clinical perspectives of primary cardiac lymphoma. Angiology. 2003;54:599–604. doi: 10.1177/000331970305400510. [DOI] [PubMed] [Google Scholar]
- 100.Zhong L., Yang S., Lei K., Jia Y. Primary cardiac lymphoma: a case report and review of the literature. Chinese-German J Clin Oncol. 2013;12:43–45. [Google Scholar]
- 101.Carras S., Berger F., Chalabreysse L. Primary cardiac lymphoma: diagnosis, treatment and outcome in a modern series. Hematol Oncol. 2017;35:510–519. doi: 10.1002/hon.2301. [DOI] [PubMed] [Google Scholar]
- 102.Wang S., Li M., Zhang L., Xie M. Multimodal imaging evaluation of a primary cardiac lymphoma in an immunocompetent patient. Echocardiography. 2018;35:2121–2123. doi: 10.1111/echo.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castelli J.B., Alexandre L., Futuro G., Scanavacca M., Júnior J.S. Primary cardiac lymphoma detected by 18F-FDG PET scan: a case report. J Nucl Cardiol. 2011;18:974–977. doi: 10.1007/s12350-011-9418-4. [DOI] [PubMed] [Google Scholar]
- 104.Lamba G., Frishman W.H. Cardiac and pericardial tumors. Cardiol Rev. 2012;20:237–252. doi: 10.1097/CRD.0b013e31825603e7. [DOI] [PubMed] [Google Scholar]
- 105.Petrich A., Cho S.I., Billett H. Primary cardiac lymphoma. Cancer. 2011;117:581–589. doi: 10.1002/cncr.25444. [DOI] [PubMed] [Google Scholar]
- 106.Eren N.T., Akar A.R. Primary pericardial mesothelioma. Curr Treat Options Oncol. 2002;3:369–373. doi: 10.1007/s11864-002-0002-7. [DOI] [PubMed] [Google Scholar]
- 107.Restrepo C.S., Vargas D., Ocazionez D., Martínez-Jiménez S., Betancourt Cuellar S.L., Gutierrez F.R. Primary pericardial tumors. Radiographics. 2013;33:1613–1630. doi: 10.1148/rg.336135512. [DOI] [PubMed] [Google Scholar]
- 108.Patel J., Sheppard M.N. Primary malignant mesothelioma of the pericardium. Cardiovasc Pathol. 2011;20:107–109. doi: 10.1016/j.carpath.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Vogel H.J.P., Wondergem J.H.M., Falke T.H.M. Mesothelioma of the pericardium: CT and MR findings. J Comput Assist Tomogr. 1989;13:543–544. doi: 10.1097/00004728-198905000-00041. [DOI] [PubMed] [Google Scholar]
- 110.Aga F., Yamamoto Y., Norikane T., Nishiyama Y. A case of primary pericardial mesothelioma detected by 18F-FDG PET/CT. Clin Nucl Med. 2012;37:522–523. doi: 10.1097/RLU.0b013e3182478c13. [DOI] [PubMed] [Google Scholar]
- 111.Ost P., Rottey S., Smeets P., Boterberg T., Stragier B., Goethals I. F-18 fluorodeoxyglucose PET/CT scanning in the diagnostic work-up of a primary pericardial mesothelioma a case report. J Thorac Imaging. 2008;23:35–38. doi: 10.1097/RTI.0b013e318156eb39. [DOI] [PubMed] [Google Scholar]
- 112.Mezei G., Chang E.T., Mowat F.S., Moolgavkar S.H. Epidemiology of mesothelioma of the pericardium and tunica vaginalis testis. Ann Epidemiol. 2017;27:348–359.e11. doi: 10.1016/j.annepidem.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 113.McGehee E., Gerber D.E., Reisch J., Dowell J.E. Treatment and outcomes of primary pericardial mesothelioma: a contemporary review of 103 published cases. Clin Lung Cancer. 2019;20:e152–e157. doi: 10.1016/j.cllc.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 114.Klein A.L., Abbara S., Agler D.A. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26:965–1012.e15. doi: 10.1016/j.echo.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 115.Klatt E.C., Heitz D.R. Cardiac metastases. Cancer. 1990;65:1456–1459. doi: 10.1002/1097-0142(19900315)65:6<1456::aid-cncr2820650634>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 116.Patel J.K., Didolkar M.S., Pickren J.W., Moore R.H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am J Surg. 1978;135:807–810. doi: 10.1016/0002-9610(78)90171-x. [DOI] [PubMed] [Google Scholar]
- 117.Goldberg A.D., Blankstein R., Padera R.F. Tumors metastatic to the heart. Circulation. 2013;128:1790–1794. doi: 10.1161/CIRCULATIONAHA.112.000790. [DOI] [PubMed] [Google Scholar]
- 118.Esposito A., De Cobelli F., Ironi G. CMR in the assessment of cardiac masses: primary malignant tumors. J Am Coll Cardiol Img. 2014;7:1057–1061. doi: 10.1016/j.jcmg.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 119.Hoey E.T.D., Shahid M., Ganeshan A., Baijal S., Simpson H., Watkin R.W. MRI assessment of cardiac tumours: part 2, spectrum of appearances of histologically malignant lesions and tumour mimics. Quant Imaging Med Surg. 2014;4:489–497. doi: 10.3978/j.issn.2223-4292.2014.11.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pun S.C., Plodkowski A., Matasar M.J. Pattern and prognostic implications of cardiac metastases among patients with advanced systemic cancer assessed with cardiac magnetic resonance imaging. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katalinic D., Stern-Padovan R., Ivanac I. Symptomatic cardiac metastases of breast cancer 27 years after mastectomy: a case report with literature review - pathophysiology of molecular mechanisms and metastatic pathways, clinical aspects, diagnostic procedures and treatment modalities. World J Surg Oncol. 2013;11:14. doi: 10.1186/1477-7819-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Allen B.C., Mohammed T.L., Tan C.D., Miller D.V., Williamson E.E., Kirsch J.S. Metastatic melanoma to the heart. Curr Probl Diagn Radiol. 2012;41:159–164. doi: 10.1067/j.cpradiol.2011.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.