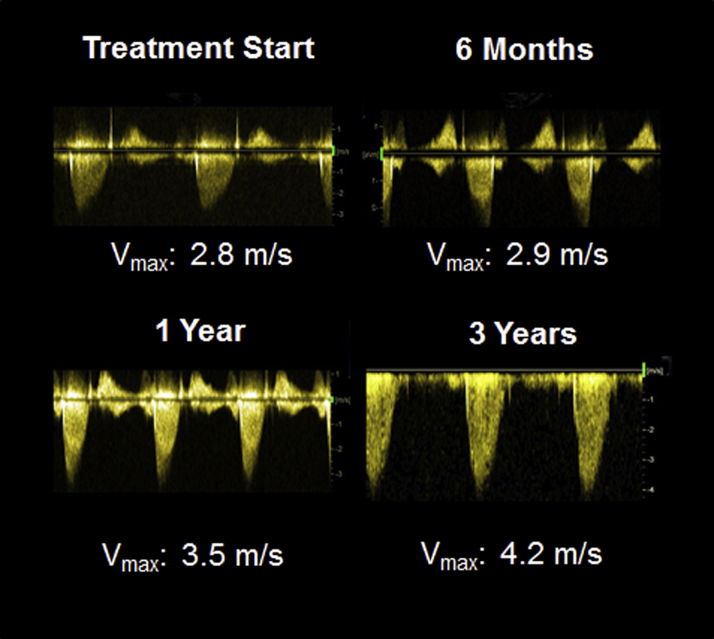
A 61-year-old woman diagnosed with chronic myeloid leukemia (CML) (Sokal intermediate risk) was treated with the tyrosine kinase inhibitor (TKI) nilotinib 600 mg/day. The patient was a nonsmoker and had no cardiovascular history, diabetes, or other relevant diseases. Blood tests revealed hypercholesterolemia with a total cholesterol of 263 mg/dl and a low-density lipoprotein of 186 mg/dl. Renal function was normal. Echocardiography performed at diagnosis showed moderate septal hypertrophy (18 mm) with mild outflow obstruction. The aortic valve was tricuspid with some sclerosis but had a normal opening. The maximum velocity (Vmax) across the aortic valve was 2.8 m/s (Figure 1), and the Vmax in the left ventricular outflow tract was 2.1 m/s, which did not indicate any significant aortic stenosis, with a calculated aortic valve area of 2.0 cm2 by the continuity equation (indexed aortic valve area of 1.2 cm2/cm2).
Figure 1.
Echocardiography Demonstrating Rapid Progression of Aortic Valve Stenosis in a Patient With CML Treated With Nilotinib
Aortic valve maximum velocity (Vmax) increased from 2.8 m/s at treatment initiation to 4.2 m/s after 3 years of nilotinib treatment. CML = chronic myeloid leukemia.
The patient responded favorably and rapidly to nilotinib, achieving a deep/complete molecular response within 6 months of TKI treatment. Follow-up echocardiography demonstrated stable aortic valve sclerosis at 6 months (Figure 1). However, 12 months after nilotinib therapy initiation, there was a marked increase in aortic valve velocity, with a Vmax of 3.5 m/s (Figure 1), which corresponded to an increase in Vmax of 0.7 m/s/year. During the third treatment year, the patient developed dyspnea and chest pain on exertion. Echocardiography showed progression of aortic valve disease, fulfilling the criteria of severe aortic stenosis, with a Vmax of 4.2 m/s (Figure 1) without any increases in the left ventricular outflow tract parameters. Because of the uncertainty of the continuity equation in the setting of left ventricular outflow tract obstruction (1), severe aortic stenosis was confirmed by transesophageal echocardiography, with an aortic valve area by planimetry of 0.8 cm2 (0.5 cm2/m2). The patient underwent surgical aortic valve replacement with a bioprothesis and concomitant subvalvular myectomy, after which symptom relief was achieved.
The unexpectedly rapid progression into symptomatic severe valvular aortic stenosis that required valve replacement surgery within 3 years after initiation of nilotinib in a relatively young patient with CML with a tricuspid aortic valve prompted us to investigate mechanisms of possible TKI-associated valvular disease.
Clinical Perspective
In addition to myocardial damage and heart failure, direct vascular effects of both chemotherapy and radiotherapy are also important considerations in cardio-oncology (2). Specifically in patients with CML, recent evidence has raised concerns of cardiovascular adverse events associated with the use of second-generation TKIs (3). Imatinib is a first-generation TKI used in CML that resulted in dramatic increases in patient survival. However, to overcome imatinib resistance or intolerance, more potent second-generation inhibitors, such as nilotinib, were developed. Cardiovascular concerns for nilotinib were initially raised based on observations of peripheral arterial disease (4). However, recent observational data also pointed to an increased incidence of myocardial infarction and other vascular events in patients treated with TKIs (5). Importantly, this might not be a general drug class effect but might specifically apply to the second-generation TKIs (3,6). Differential cardiovascular effects between first- and second-generation TKIs were supported by a previous study in patients with CML in which the incidence of myocardial infarction was approximately 3.6-fold higher among patients treated with nilotinib compared with those treated with imatinib (5). This led to the introduction of cardiovascular risk monitoring and prevention in hematological treatment recommendations for TKI (6).
In our case, the annual progression of the aortic valve Vmax was 0.7 m/s. Rapid aortic stenosis progression is defined by an increase in Vmax of ≥0.3 m/s/year (1), which suggested accelerated progression of aortic stenosis after initiation of nilotinib in this patient. Close monitoring of the patient allowed the appropriate diagnosis of valvular aortic stenosis. Aortic valve replacement surgery was performed in accordance with guideline recommendations for severe, symptomatic aortic stenosis. Because of concern that the accelerated valvular disease was a result of nilotinib treatment, the patient was switched to imatinib treatment before cardiac surgery.
Translational Perspective
Calcification and thickening of the aortic valve, accompanied by inflammation, extracellular matrix remodeling, and intra-leaflet hemorrhages, are part of the underlying pathological processes of aortic valve stenosis. Several contributors to aortic stenosis incidence and progression have been identified, including age, traditional cardiovascular risk factors, genetic susceptibility, and certain comorbidities. In addition, direct exposure to ionizing radiation during radiotherapy increases aortic valve calcification and stenosis (7). However, the potential effects of targeted therapies (e.g., TKI) on valvular heart disease have received less attention in cardio-oncology. Understanding the direct valvular effects of cancer therapies and determining their mechanistic implications in valve calcification may be fundamental for understanding valvular heart disease in various cancer populations.
With the aim of understanding if there is a potential role of first- and second-generation TKIs in aortic valve stenosis, we applied an experimental approach. Our protocol was approved by the local ethical committee, and informed consent was obtained from all subjects. Human aortic valves were obtained from 7 patients who underwent aortic valve replacement surgery. Valvular interstitial cells (VICs) were isolated as previously described (8). Each aortic valve was used to generate an individual cell culture, which was exposed to either nilotinib (10 μM) or the first-generation TKI imatinib (10 μM). A corresponding volume of dimethyl sulfoxide vehicle was administered to control cultures. In vitro calcification was assessed after 9 days of culture in the presence of 2.6-μM inorganic phosphate according to previously described protocols (9). After 24 h of culture, mRNA was isolated for the determination of bone morphogenic protein(BMP)-2 expression levels with ThermoFisher 7900HT fast real-time polymerase chain reaction using primers and probes from Thermo Fischer Scientific (Waltham, Massachusetts (Hs00154192_m1) and normalized to glyceraldehyde 3-phosphate dehydrogenase (Hs99999905_m1) as a housekeeping gene. Statistically significant differences were determined using a 1-way analysis of variance followed by a recommended post hoc test. Statistical significance was assigned at p < 0.05. Statistical analysis was performed using GraphPad Prism 8 (GraphPad Software, San Diego, California).
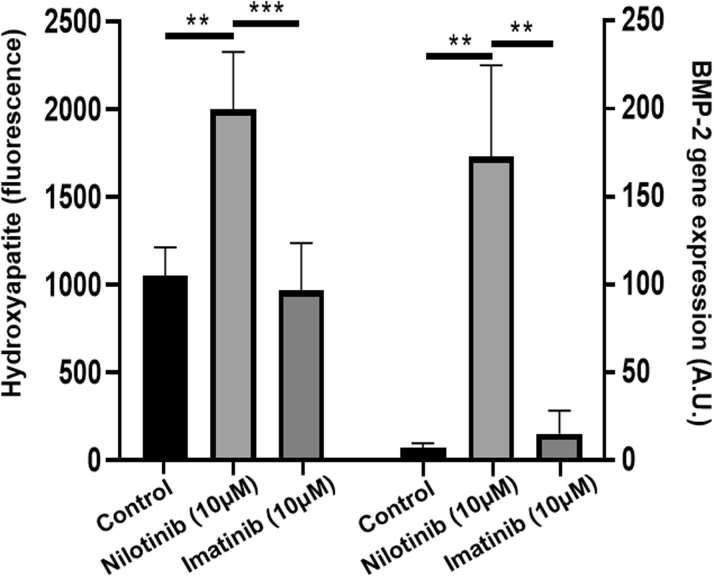
These experiments revealed that nilotinib (10 μM) significantly increased phosphate-induced VIC calcification compared with that in control cultures (Figure 2). Likewise, nilotinib induced a 24.0 ± 5.1-fold increase in BMP-2 expression (Figure 2). Because BMP-2, through downstream pSMAD 1/5/8 signaling, was shown to drive valvular calcification (10), these findings supported a direct pro-calcifying effect of nilotinib in the aortic valve. In contrast, the first-generation TKI imatinib did not significantly alter either calcification or BMP-2 expression in human VICs (Figure 2).
Figure 2.
Nilotinib Enhanced Valvular Interstitial Cell Calcification and Osteogenic Signaling
Treatment of human valvular interstitial cells with nilotinib increased phosphate-induced calcification after 9 days and mRNA expression of the osteogenic activator bone morphogenic protein (BMP)-2 after 24 h. In contrast, treatment with imatinib was not significantly different from vehicle control (dimethyl sulfoxide). Data represented as mean ± SD. **p < 0.01; ***p < 0.001.
Discussion
In summary, a possible causal relation behind the reported case of rapid aortic valve stenosis development during nilotinib treatment was supported by the capacity of nilotinib to increase calcification and induce osteogenic activation in human VICs. The present case report and the associated experimental data therefore suggest the direct valvular toxic effects of nilotinib. Similar effects were not observed for imatinib. The effects of other TKIs remain to be established.
However, it might be premature to make recommendations for routine echocardiographic screening in TKI-treated patients with CML. It should also be emphasized that the main purpose of TKI treatment in CML is the antileukemic effect, which should not be compromised by concerns for adverse effects without cause, as indicated in hematological treatment recommendations (6). However, an awareness of a possible link between TKI and aortic stenosis could potentially lead to earlier diagnosis and treatment of valve disease in patients with CML.
Studies are ongoing to decipher the mechanisms involved in nilotinib-induced valvular effects. Furthermore, we are currently exploring the incidence of aortic valve stenosis in larger populations with CML. Patient registries, such as that previously used to monitor myocardial infarction in CML and nilotinib treatment (5), might provide further insights into a possible increased risk of aortic stenosis associated with nilotinib treatment.
Conclusions
These observations provide the first indication of a possible link between TKI and valvular heart disease in CML.
Footnotes
Dr. Bäck was supported by grants from the Swedish Heart and Lung Foundation (grant 20180571), the King Gustaf V and Queen Victoria Freemason Foundation, the Stockholm County Council (grant 20170365), and the Marianne and Marcus Wallenberg Foundation (grant 2015.0104). Dr. Franco-Cereceda was supported by a donation from Fredrik Lundberg. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Baumgartner H., Hung J., Bermejo J. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Herrmann J., Yang E.H., Iliescu C.A. Vascular toxicities of cancer therapies: the old and the new--an evolving avenue. Circulation. 2016;133:1272–1289. doi: 10.1161/CIRCULATIONAHA.115.018347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herrmann J. Tyrosine kinase inhibitors and vascular toxicity: impetus for a classification system? Current Oncol Rep. 2016;18:33. doi: 10.1007/s11912-016-0514-0. [DOI] [PubMed] [Google Scholar]
- 4.Kim T.D., Rea D., Schwarz M. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27:1316–1321. doi: 10.1038/leu.2013.70. [DOI] [PubMed] [Google Scholar]
- 5.Dahlen T., Edgren G., Lambe M. Cardiovascular events associated with use of tyrosine kinase inhibitors in chronic myeloid leukemia: a population-based cohort study. Ann Intern Med. 2016;165:161–166. doi: 10.7326/M15-2306. [DOI] [PubMed] [Google Scholar]
- 6.Steegmann J.L., Baccarani M., Breccia M. European LeukemiaNet recommendations for the management and avoidance of adverse events of treatment in chronic myeloid leukaemia. Leukemia. 2016;30:1648–1671. doi: 10.1038/leu.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnellan E., Masri A., Johnston D.R. Long-term outcomes of patients with mediastinal radiation-associated severe aortic stenosis and subsequent surgical aortic valve replacement: a matched cohort study. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laguna-Fernandez A., Carracedo M., Jeanson G. Iron alters valvular interstitial cell function and is associated with calcification in aortic stenosis. Eur Heart J. 2016;37:3532–3535. doi: 10.1093/eurheartj/ehw122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carracedo M., Artiach G., Witasp A. The G-protein coupled receptor ChemR23 determines smooth muscle cell phenotypic switching to enhance high phosphate-induced vascular calcification. Cardiovasc Res. 2019;115:1557–1566. doi: 10.1093/cvr/cvy316. [DOI] [PubMed] [Google Scholar]
- 10.Gomez-Stallons M.V., Wirrig-Schwendeman E.E., Hassel K.R. Bone morphogenetic protein signaling is required for aortic valve calcification. Arterioscler Thromb Vascul Biol. 2016;36:1398–1405. doi: 10.1161/ATVBAHA.116.307526. [DOI] [PMC free article] [PubMed] [Google Scholar]