Abstract
Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT), a potentially curative therapy for malignant and nonmalignant diseases, is being increasingly used in younger patients. Although allo-HSCT survivors have an established increased risk of cardiovascular disease, there is limited knowledge of the long-term effects on cardiac function in survivors.
Objectives
The purpose of this study was to describe left ventricular (LV) systolic function in long-term allo-HSCT survivors treated in childhood, adolescence, or early adulthood.
Methods
Our cross-sectional cohort study included 104 patients (56% women), age 18 ± 10 years at time allo-HSCT with 17 ± 6 years of follow-up. Echocardiography included 2-dimensional (2D) and 3-dimensional (3D) analyses and speckle tracking imaging. In total, 55 healthy control subjects with a similar age, sex, and body mass index were used for comparison. Left ventricular systolic dysfunction (LVSD) was defined as reduced 2D left ventricular ejection fraction (LVEF) of <52% in men and <54% in women, and/or a reduced global longitudinal strain (GLS) of ≥−17%. Multivariable linear regression was used to determine independent predictors of 2D-LVEF and GLS.
Results
Allo-HSCT survivors had significantly reduced LV systolic function compared with control subjects: 2D-LVEF (55.2 ± 5.8% vs. 59.0 ± 2.9%; p < 0.001), 3D LVEF (54.0 ± 5.1% vs. 57.6 ± 2.7%; p < 0.001), and GLS (−17.5 ± 2.2% vs. −19.8 ± 1.4%; p < 0.001). LVSD was found in 44.2%, of whom 28.3% were symptomatic. Clinical factors independently associated with 2D-LVEF and/or GLS included age, anthracyclines, graft versus host disease (GVHD), heart rate, and hypertension. In the 45% of survivors pre-treated with anthracyclines, the effect of anthracyclines on 2D-LVEF and GLS was dose-dependent.
Conclusions
LVSD is common in long-term survivors of allo-HSCT treated in their youth. Pre-HSCT therapies with anthracyclines, age, heart rate, hypertension, and graft versus host disease are associated with measures of LV function.
Key Words: anthracyclines, cardiovascular risk factors, echocardiography, graft versus host disease, heart failure, left ventricular systolic function
Abbreviations and Acronyms: allo-HSCT, allogeneic hematopoietic stem cell transplantation; CV, cardiovascular; GLS, global longitudinal strain; GVHD, graft versus host disease; LVEF, left ventricular ejection fraction; LVSD, left ventricular systolic dysfunction; NYHA, New York Heart Association
Central Illustration
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a complex and potentially curative therapy for malignant and nonmalignant diseases. It is increasingly being used, particularly in younger individuals (1). Improvements in protocols and supportive therapy have resulted in improved initial survival rates (2). However, long-term survivors of allo-HSCT face high rates of life-debilitating complications (3, 4, 5, 6). In patients that survive beyond 5 years, mortality rates are 4 to 9 times higher than the general population, corresponding with a 30% shorter life expectancy regardless of age at transplantation (7). The incidence of heart failure (HF) has been reported to be between 5.6% to 10.8% in those who have survived at least 10 years after HSCT (4,5,8,9), and the risk of cardiovascular (CV) related mortality is 2 to 4 times higher in HSCT survivors than in the general population (4). The relative role of different risk factors in the development of left ventricular systolic dysfunction (LVSD) in long-term allo-HSCT survivors is not well defined, especially in those treated during childhood, adolescence, and young adulthood. Furthermore, few studies have comprehensively evaluated heart function in long-term survivors of allo-HSCT. This observational study aimed to evaluate cardiac function in a population recruited nationwide who underwent allo-HSCT at a young age. Modern echocardiography techniques, including 3-dimensional (3D) imaging and speckle tracking echocardiography, were used to assess left ventricular (LV) systolic function.
Methods
Study design and inclusion criteria
This Norwegian cross-sectional study included all survivors of allo-HSCT performed at Oslo University Hospital who were <30 years of age at HSCT, were age >16 years (born prior to August 1998) at inclusion, and had a minimum of 5 years of follow-up after allo-HSCT. Our hospital has been the single national center for allo-HSCT, and a complete nationwide cohort was identified by browsing the quality registry. Indications for allo-HSCT included malignant and nonmalignant diseases. A total of 11 individuals with mucopolysaccharidosis type 1 (Hurler syndrome) were excluded, as these patients may have multiorgan pathology as part of their primary disease. Medical history was documented retrospectively, including: disease type, pre-/post-transplantation therapies, other medical illness, risk factors, symptoms, and current medication. Anthracycline cumulative dosage was converted to isotoxic doses of doxorubicin (10). Written informed consent was obtained from all participants, and the study was approved by the Regional Committee for Medical and Health Research Ethics.
Clinical assessment
Dyspnea was graded according to the New York Heart Association (NYHA) functional classification (11). Blood pressures (BPs) were acquired after echocardiography (>30 min), in the supine position as the average of 3 measurements. Blood samples were collected after overnight fasting and were analyzed at the hospital laboratory. N-terminal pro–B-type natriuretic peptide (NT-proBNP) concentrations were determined by an electrochemiluminescence immunoassay (Roche proBNP II, Roche Diagnostics, Basel Switzerland), and troponin was measured using a high-sensitive immunoassay (Roche hs-TnT). Manufacturer’s recommendations were used for classifying elevated NT-proBNP according to the age- and sex-specific cutoffs, and for classifying elevated troponin as >14 ng/l. Hypertension was defined as current use of antihypertensive drugs, systolic BP >140 mm Hg, or diastolic BP >90 mm Hg. Diabetes mellitus type II was identified by hemoglobin HbA1c >6.5% (48 mmol/mol), or use of glucose-lowering medication. Hypothyroidism was defined by the use of thyroid replacement medication or serum TSH >4 mg/l and fT4 <9 pmol/l. Hypercholesterolemia was defined as LDL >4.1 mmol/l (160 mg/dl) or use of lipid-lowering medication. Acute and chronic graft versus host disease (GVHD) were graded by the Glucksberg and Schulman scales, respectively (12,13).
Echocardiography
Transthoracic echocardiography studies were performed using Vivid E9 scanners and dedicated software (Echo-PAC version 113.1.3, GE-Vingmed Ultrasound, Horten, Norway). All echocardiograms were acquired and analyzed by the same experienced investigator (R.J.M) en-bloc, and in random order after the last patient inclusion. After acquisition and prior to analyses, all echocardiograms were deidentified, and the investigator was blinded to all of the patient’s medical records. Two-dimensional (2D) left ventricular ejection fraction (LVEF), 3D-LVEF, and global longitudinal strain (GLS) were measured on separate occasions to reduce bias. Studies followed current guidelines for evaluation of LV function and cardiotoxicity (14,15). Measurements were averaged from 3 consecutive heart cycles.
2D-LVEF was manually traced using the modified Simpson’s biplane method (14). 3D-LVEF was calculated with semiautomated software for endocardial detection, and was subsequently manually adjusted. A 3D pyramid volume acquisition was obtained from 4 to 6 cardiac cycles, adjusting for depth and sector width (60° to 70°), resulting in an average volume rate of 39 frames/s (range 29 to 51 frames/s). Sex-specific cutoffs for 2D-LVEF were used as recommended (14). GLS was performed on the 3 standard apical views of good acoustic quality, with similar frame rates (average 62 frames/s), sector depth, and heart rate. The region of interest was manually adjusted to correctly define the endocardial borders, apex, and mitral valve plane. The AFI software from GE calculated GLS as the average of peak systolic strain values in a 17-segment model. We considered GLS ≥−17% to be abnormal in young individuals, based on the lower limit of the 95% confidence interval (CI) found in our control group. LVSD was defined as reduced 2D-LVEF (men <52%, women <54%) and/or abnormal GLS (≥−17%).
Valve stenosis or regurgitation was categorized as mild, moderate, or severe according to guidelines (16). Pericardial thickness was assessed after careful adjustments of gain settings, and nonharmonic ultrasound set at 2.5 MHz. Pathology was defined as increased pericardial fluid >0.5 cm at end-diastole, and/or presence of abnormal thickening or fusion of the visceral and parietal membranes.
Measurement accuracy and intraobserver variability
In total, 25 recordings for 2D-LVEF and GLS from patients and control subjects were randomly selected for blinded intraobserver variability assessment. For intraobserver variability, the same images were analyzed >3 months apart by the same observer and software, blinded to the previous result. The average value of 3 repeated measurements was used to calculate intraclass correlation coefficient. The Cronbach's alpha and intraclass correlation coefficient from average measures, using 2-way mixed and absolute agreement for 2D-LVEF, was 0.95 (95% CI: 0.89 to 0.98) and GLS 0.96 (95% CI: 0.89 to 0.99).
Control group
A control group was recruited from healthy volunteers responding to advertisements. The sample size was estimated to detect differences between allo-HSCT patients and control subjects for the normally distributed variables 2D-LVEF and GLS. With 104 patients, an expected 10% SD in-group and α of 0.05, it was determined that 52 control subjects were required at a power of 0.83 to demonstrate a 5% difference in 2D-LVEF between groups. We included 55 control subjects to allow for a small buffer and still obtain a power >0.80. Control individuals were selected to obtain comparative group characteristics for race, ethnicity, sex, age, height, and body mass index (BMI). The only exclusion criterion was established CV disease.
Statistical analysis
Continuous data are presented as mean ± SD if normally distributed, or as median (25th, 75th percentile) if asymmetrically distributed. Categorical data are presented as numbers and percentages. Allo-HSCT survivors and control subjects were compared with the Student's t-test for continuous data, and chi-square or Fisher exact test for categorical data. To adjust for any differences between the allo-HSCT group and control subjects when analyzing cardiac function, inverse-probability weighting (propensity scoring) was performed. In general linear regression analyses, the observations were weighted in each group by the inverse of the probability of being in that group with a given set of covariates: age, BMI, heart rate, and diastolic blood pressure (DBP). Multivariable linear regression analyses were conducted to determine significant predictors for the primary outcome variables of 2D-LVEF and GLS in survivors. Covariates included: age, height, BMI, heart rate, cumulative anthracycline dosage, sex, mediastinal radiation, total body irradiation, malignancy, hypertension, diabetes mellitus, hypercholesterolemia, hypothyroidism, smoking, and GVHD (acute and/or chronic). All continuous variables were standardized, and p values, beta, and corresponding 95% confidence intervals were reported. The final regression models considered multicollinearity, and contained all variables with p < 0.20 in a univariable regression and/or variables considered as clinically important risk factors for LVSD. Likewise, deletion was used for the data omissions: 2 for smoking in the univariable test, and 2 for hypercholesterolemia (high triglycerides). To evaluate the effects of anthracycline dosage, patients were allocated into 3 groups according to cumulative anthracycline dosage: none, moderate (<300 mg/m2), and high (≥300 mg/m2). Chi-square, Kruskal-Wallis test, and 1-way analysis of covariance with Bonferroni post hoc analyses were used to identify group differences. Analysis of covariance was used to adjust for age, BMI, heart rate, and DBP. Statistical analyses used SPSS version 25 (SPSS Inc., Chicago, Illinois), and a p value <0.05 was considered significant.
Results
Patient demographics and allo-HSCT treatment characteristics
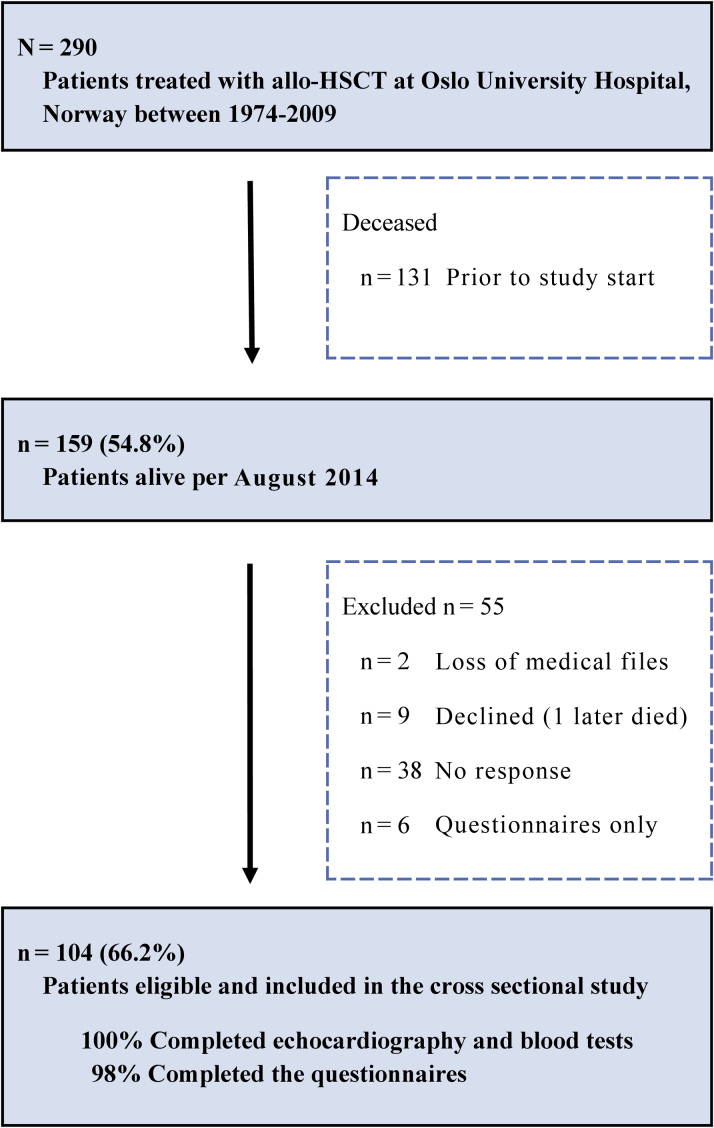
Patients characteristics are shown in Table 1. In total, 290 patients were treated during the time period specified for inclusion. Of these, 131 died prior to study start (total deaths 45.2%) and 2 were excluded due to incomplete patient files. Nonparticipants (n = 53) were younger (age 27.7 years vs. 34.3 years; p = 0.001), had shorter follow-up time (13.2 years vs. 16.5 years; p < 0.001), and were more likely men (37 men vs. 16 women; p = 0.005). A total of 104 participants (66.2% of eligible survivors) accepted the study invitation, provided informed consent, and completed clinical examinations (Figure 1). Malignant disease occurred in 74% (55.8% women), and was the indication for anthracycline chemotherapy in 45.2% (59.6% women) and mediastinal radiotherapy in 2 (1.9%). Median isotoxic cumulative anthracycline dosage was 270 mg/m2 (range 45 to 585 mg/m2). Stem cells were obtained from the bone marrow in 84.6%, and 29.8% had unrelated donors. In total, 95 (91.3%) received a myeloablative conditioning regimen consisting of busulfan in combination with cyclophosphamide. The total dosage of busulfan was 4 to 5 mg/kg/day administered orally over 4 days, combined with 50 mg/kg/day of cyclophosphamide administered intravenously over 4 days or 60 mg/kg/day over 2 days. In total, 23 (22.1%) received antilymphocyte globulin and 7 (6.7%) fractionated total body irradiation during conditioning. Cyclosporine was administrated in 99% as part of standard GVHD prophylaxis. Resolved GVHD was identified in 29 (43.3%) of those with prior acute and/or chronic GVHD.
Table 1.
Patient Characteristics
| Allo-HSCT (n = 104) | Control Subjects (n = 55) | p Value | |
|---|---|---|---|
| Female | 56 (53.8) | 29 (52.7) | 0.893 |
| Height, m | 1.72 ± 0.09 | 1.74 ± 0.09 | 0.053 |
| Weight, kg | 72.5 ± 17.5 | 73.3 ± 12.3 | 0.730 |
| Body surface area, m2 | 1.83 ± 0.23 | 1.88 ± 0.18 | 0.179 |
| Body mass index, kg/m2 | 24.5 ± 5.1 | 24.1 ± 3.4 | 0.530 |
| Age at allo-HSCT, yrs | 17.8 ± 9.6 | — | — |
| Age at examination, yrs | 35.0 ± 11.7 | 36.4 ± 10.6 | 0.460 |
| Years to follow-up | 17.2 ± 5.6 | — | — |
| Systolic blood pressure, mm Hg | 122 ± 19 | 117 ± 11 | 0.074 |
| Diastolic blood pressure, mm Hg | 72 ± 13 | 66 ± 8 | 0.002 |
| Heart rate, beats/min | 69 ± 11 | 68 ± 12 | 0.629 |
| Malignant disease | 77 (74.0) | ||
| Acute lymphoblastic leukemia | 12 (11.5) | ||
| Acute myeloid leukemia | 32 (30.8) | ||
| Chronic myeloid leukemia | 26 (25.0) | ||
| Other malignant | 7 (6.7) | ||
| Nonmalignant disease | 27 (26.0) | ||
| Severe aplastic anemia | 17 (16.3) | ||
| Metabolic/immunodeficiencies | 10 (9.6) | ||
| Pre-transplantation therapy | |||
| Mediastinal radiotherapy | 2 (1.9) | ||
| Anthracyclines | 47 (45.2) | ||
| Cumulative anthracycline dosage, mg/m2 | 270 (140, 435) | ||
| Myeloablative conditioning | |||
| Chemotherapy, busulfan/cyclophosphamide | 95 (91.3) | ||
| Chemotherapy + total body irradiation | 7 (6.7) | ||
| None | 2 (1.9) | ||
| GVHD | 67 (64.4) | ||
| Acute GVHD | 27 (26.2) | ||
| Chronic GVHD | 12 (11.5) | ||
| Both acute and chronic GVHD | 28 (26.9) |
Values are n (%), mean ± SD, or median (25th, 75th percentiles). Percentages may not equal 100% given rounding
allo-HSCT = allogeneic hematopoietic stem cell transplantation; GVHD = graft-versus-host-disease.
Figure 1.
CONSORT Diagram: Illustration of Patient Recruitment
In total, 104 (66.2%) of eligible survivors were evaluated with comprehensive echocardiography.
Clinical assessment and risk factors
Table 2 presents clinical assessment findings. Of 104 allo-HSCT survivors, LVSD was detected in 46 (44.2%) survivors, of whom 13 (12.7% of all survivors) had NYHA functional class II or III symptoms, and 33 (32.4% of all survivors) were asymptomatic (NYHA functional class I). NYHA functional class II or III symptoms were present in 28 (27.5%) of all survivors. Elevated NT-proBNP was more prevalent among survivors than control subjects (17 vs. 2 participants; p = 0.029), and was found in 10 (58.8%) with LVSD. Cardiovascular medications (predominantly antihypertensive therapies) were prescribed in 19 (41.3%) survivors with LVSD. Compared with survivors without hypertension, those with hypertension (n = 42) were older (age 42 years vs. 30 years; p < 0.001), had longer follow-up time (19.1 years vs. 16.0 years; p = 0.004), had higher BMI (26.2 kg/m2 vs. 23.4 kg/m2; p = 0.007), and were more likely to be on cardiac medications (76.2%). Compared with survivors without hypercholesterolemia, survivors with hypercholesterolemia had higher BMI (27.9 vs. 23.9 kg/m2; p = 0.004), were older (age 45 years vs. 33 years; p < 0.001), and had longer follow-up time (19.5 years vs. 16.6 years; p = 0.043). Among survivors, hypothyroidism was more frequent in women (9 women vs 1 man; p = 0.016). In comparing survivors with control subjects, no sex differences were observed for the risk factors: hypertension (p = 0.064), hypercholesterolemia (p = 0.374), GVHD (p = 0.206), or obesity (p = 0.375). At examination, allo-HSCT survivors had a slightly higher DBP (72 mm Hg vs. 66 mm Hg; p = 0.002) compared with the healthy control subjects.
Table 2.
Clinical Assessment
| Allo-HSCT (n = 104) | Control Subjects (n = 55) | p Value | |
|---|---|---|---|
| New York Heart Association functional class∗ | |||
| I | 74 (72.5) | 55 (100) | <0.001 |
| II | 16 (15.7) | 0 (0.0) | 0.002 |
| III | 12 (11.8) | 0 (0.0) | 0.009 |
| Comorbidities | |||
| Hypertension | 42 (40.4) | 1 (1.8) | <0.001 |
| Diabetes mellitus | 3 (2.9) | 0 (0.0) | 0.552 |
| Hypothyroidism | 10 (9.6) | 0 (0.0) | 0.016 |
| Hypercholesterolemia | 16 (15.4) | 0 (0.0) | 0.002 |
| Smoking (current/previous)∗ | 10 (9.8)/18 (17.6) | 2 (3.6)/11 (20.0) | 0.165/0.717 |
| Cardiovascular medications | 33 (31.7) | 0 (0.0) | <0.001 |
| Statins | 4 (3.8) | 0 (0.0) | — |
| Calcium-channel blockers | 13 (12.5) | 0 (0.0) | — |
| Beta-blockers | 13 (12.5) | 0 (0.0) | — |
| Angiotensin-converting enzyme inhibitors | 7 (6.7) | 0 (0.0) | — |
| Angiotensin receptor blockers | 13 (12.5) | 0 (0.0) | — |
| Laboratory parameters | |||
| Troponin T >14 ng/l | 3 (2.9) | 0 (0.0)† | 0.551 |
| Elevated NT-proBNP‡ | 17 (16.3) | 2 (4.0)† | 0.029 |
| HDL cholesterol, mmol/l§ | 1.5 ± 0.4 | 1.7 ± 0.5† | 0.051 |
| LDL cholesterol, mmol/l§ | 3.1 ± 0.8 | 3.0 ± 0.9† | 0.433 |
Values are n (%) or mean ± SD.
allo-HSCT = allogeneic hematopoietic stem cell transplantation; HDL = high-density lipids; LDL = low-density lipids; NT-proBNP = N-terminal pro–B-type natriuretic peptide.
2 absences in findings.
n = 50.
Elevated = age 18 to 44 years: men >86 ng/l, women >130 ng/l; age 45 to 54 years: men >121 ng/l, women >249 ng/l.
Conversion rate to mg/dl = mmol/l × 38.66 (Roche Diagnostics, Basel, Switzerland).
Echocardiography and LVSD
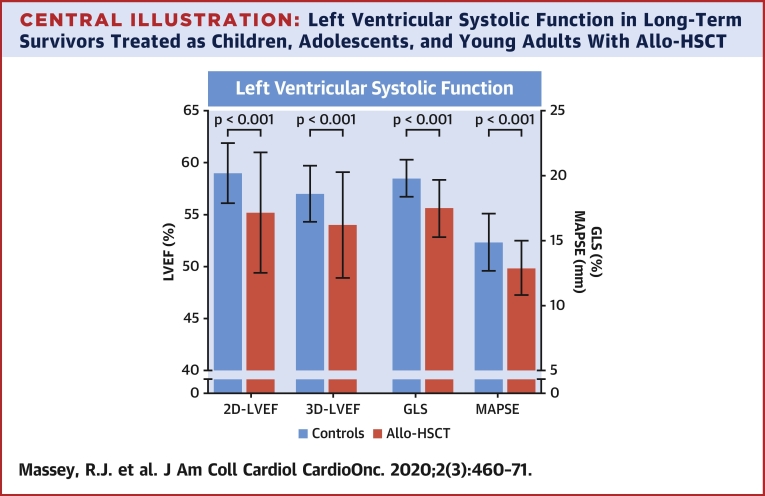
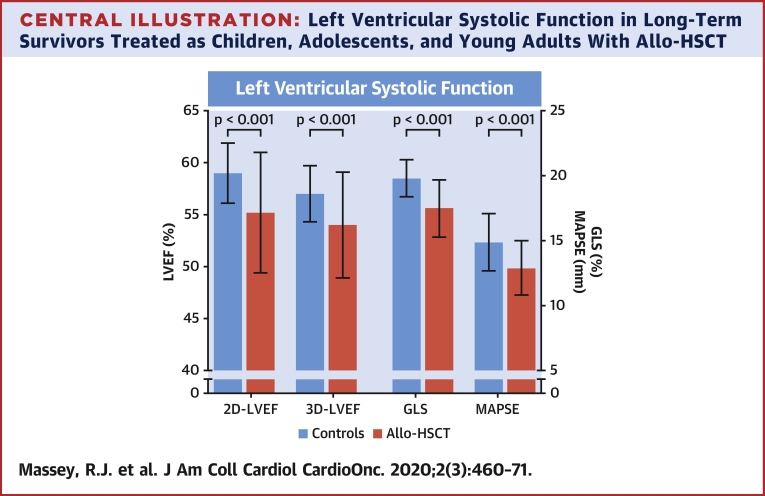
2D-LVEF was feasible in all patients, 3D-LVEF in 85%, and GLS in 96%. The majority of echocardiography parameters for systolic function were significantly reduced in allo-HSCT survivors compared with control subjects: 2D-LVEF (55.2 ± 5.8% vs. 59.0 ± 2.9%; p < 0.001), 3D-LVEF (54.0 ± 5.1% vs. 57.6 ± 2.7%; p < 0.001), GLS (−17.5 ± 2.2% vs. −19.8 ± 1.4%; p < 0.001), and MAPSE (12.9 ± 2.1 mm vs. 14.9 ± 2.2 mm; p < 0.001) was all reduced in survivors compared with control subjects (Table 3, Central Illustration). These group differences remained significant after controlling for baseline differences in age, BMI, heart rate, and DBP (Table 3). After exclusion of participants with established CV disease (45 survivors and 1 control), LVEF and GLS remained significantly impaired in survivors compared with control subjects, despite survivors being considerably younger (Supplemental Table 1). In total, 46 (44.2%) allo-HCST survivors had LVSD in contrast to none in the control group. No sex differences were found for the prevalence of reduced LVEF, GLS, or LVSD. Allo-HSCT survivors had significantly (p < 0.001) smaller LV end-diastolic volumes (indexed 2D LV end-diastolic volume) and smaller cardiac mass compared to controls. LVSD was described as mild to moderate global hypokinesis in most instances. Septal hypokinesis was described in three survivors (2.9%), including one symptomatic individual found to have significant coronary disease by angiography.
Table 3.
Echocardiography Assessment
| Allo-HSCT (n = 104) | Control Subjects (n = 55) | p Value | Inverse-Probability Weighting Beta (Standard Error), p Value∗ |
|
|---|---|---|---|---|
| IVSd, mm | 0.86 ± 0.17 | 0.89 ± 0.14 | 0.274 | |
| IVSd >12 mm | 4 (3.8) | 2 (3.6) | 0.957 | |
| Indexed LV mass index, g/m2† | 70.5 ± 18.0 | 73.5 ± 12.5 | 0.230 | |
| Relative wall thickness | 0.30 ± 0.06 | 0.29 ± 0.05 | 0.263 | |
| Indexed LVIDd, cm/m2 | 2.68 ± 0.32 | 2.69 ± 0.25 | 0.984 | |
| Indexed LVIDs, cm/m2 | 1.87 ± 0.30 | 1.84 ± 0.20 | 0.395 | |
| Indexed 2D-LVEDV, ml/m2 | 63.6 ± 13.3 | 71.9 ± 13.9 | 0.000 | |
| Indexed 2D-LVESV, ml/m2 | 28.8 ± 8.3 | 29.6 ± 6.5 | 0.562 | |
| Indexed 3D-LVEDV, ml/m2 | 71.5 ± 13.4 | 75.2 ± 12.8 | 0.083 | |
| Indexed 3D-LVESV, ml/m2 | 33.0 ± 8.6 | 31.7 ± 7.7 | 0.374 | |
| 3D sphericity index | 0.33 ± 0.7 | 0.33 ± 0.6 | 0.652 | |
| Cardiac index, l/min/m2 | 2.62 ± 0.44 | 2.76 ± 0.47 | 0.082 | — |
| Fractional shortening, % | 30.6 ± 5.7 | 31.6 ± 4.0 | 0.210 | 1.08 (0.78), 0.169 |
| 2D-LVEF, % | 55.2 ± 5.8 | 59.0 ± 2.9 | <0.001 | 3.80 (0.74), <0.001 |
| 2D-LVEF ♂<52%, ♀<54% | 33 (31.7) | 0 (0.0) | <0.001 | — |
| 2D-LVEF <50% | 17 (16.3) | 0 (0.0) | <0.001 | — |
| 3D-LVEF, % | 54.0 ± 5.1 | 57.6 ± 2.7 | <0.001 | 3.42 (0.68), <0.001 |
| 3D-LVEF ♂<52%, ♀<54% | 29 (27.9) | 0 (0.0) | <0.001 | — |
| MAPSE, mm | 12.9 ± 2.1 | 14.9 ± 2.2 | <0.001 | 2.02 (0.34), <0.001 |
| s′ velocity, cm/s | 8.0 ± 1.7 | 8.9 ± 1.7 | 0.002 | 7.72 (2.66), 0.004 |
| GLS, % | −17.5 ± 2.2 | −19.8 ± 1.4 | <0.001 | 2.13 (0.30), <0.001 |
| GLS ≥−17% | 34 (32.7) | 0 (0.0) | <0.001 | — |
| Pericardial pathology | 8 (7.7) | 0 (0.0) | 0.051 | — |
Values are mean ± SD or n (%), unless otherwise indicated. Indexed values to BSA.
allo-HSCT = allogeneic hematopoietic stem cell transplantation; GLS = global longitudinal strain; IVSd = interventricular septal end-diastolic dimension; LVEDV = left ventricular end-diastolic volume; LVEF = left ventricular ejection fraction; LVESV = left ventricular end-systolic volume; LVIDd = left ventricular internal end-diastolic dimension; LVIDs = left ventricular internal end-systolic dimension; MAPSE = mitral annular plane systolic excursion, average of septum and lateral; NT-proBNP = N-terminal pro–B-type natriuretic peptide; sʹ velocity = tissue Doppler peak systolic velocity, average of septum and lateral.
Inverse-probability weighting, covariates: age, heart rate, body mass index, and diastolic blood pressure.
ASE cube formula (14).
Central Illustration.
Left Ventricular Systolic Function in Long-Term Survivors Treated as Children, Adolescents, and Young Adults With Allo-HSCT
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) survivors were found to have significantly reduced left ventricular systolic function with echocardiography compared with healthy control subjects. Most cases were described as mild to moderate global hypokinesis with few cases of valve disease. Significant independent predictors of left ventricular systolic function were age, anthracyclines, graft versus host disease, heart rate, and hypertension. 2D = 2-dimensional; 3D = 3-dimensional; LVEF = left ventricular ejection fraction; GLS = global longitudinal strain; MAPSE = mitral annular plane systolic excursion.
Univariable and multivariable linear regression analyses are shown in Tables 4 and 5. Statistically significant independent predictors for 2D-LVEF were age, cumulative anthracycline dosage, and GVHD. Significant independent predictors for GLS were age, heart rate, cumulative anthracycline dosage and hypertension. Anthracycline therapy was found to be a strong predictor of impaired LVEF and GLS. Further analysis confirmed a dose dependent relationship between anthracycline dose and reduction in 2D-LVEF and GLS, after adjusting for age, BMI, heart rate, and DBP (Table 6). Those who received higher anthracycline dosages tended to have greater evidence of cardiac dysfunction and adverse remodeling.
Table 4.
Linear Regression Analysis for Predictors of 2D-LVEF in allo-HSCT Survivors (n = 104)
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Sex | 0.05 | −0.34 to 0.44 | 0.809 | −0.06 | −0.42 to 0.30 | 0.739 |
| Age, yrs∗ | 0.27 | 0.09 to 0.46 | 0.005 | 0.29 | 0.07 to 0.52 | 0.011 |
| Height, m | −0.08 | −0.28 to 0.17 | 0.423 | |||
| Body mass index, kg/m2 | 0.18 | −0.01 to 0.38 | 0.062 | 0.06 | −0.14 to 0.26 | 0.548 |
| Heart rate, beats/min | −0.01 | −0.20 to 0.19 | 0.952 | |||
| Mediastinal radiation | −0.64 | −2.07 to 0.77 | 0.370 | |||
| Cumulative anthracycline dosage, mg/m2 | −0.41 | −0.59 to −0.23 | <0.001 | −0.46 | −0.63 to −0.28 | <0.001 |
| Total body irradiation | 0.26 | −0.52 to 1.03 | 0.517 | −0.20 | −0.91 to 0.51 | 0.580 |
| Malignancy | −0.22 | −0.66 to 0.23 | 0.339 | |||
| Hypertension | 0.13 | −0.27 to 0.53 | 0.511 | −0.18 | −0.60 to −0.24 | 0.393 |
| Diabetes mellitus | −0.68 | −1.84 to 0.48 | 0.248 | |||
| Hypercholesterolemia | 0.06 | −0.48 to 0.61 | 0.822 | |||
| Hypothyroidism | 0.12 | −0.55 to 0.78 | 0.727 | |||
| Smoking, current/previous | 0.11 | −0.34 to 0.55 | 0.632 | |||
| Graft versus host disease, acute and/or chronic GVHD | 0.29 | −0.12 to 0.69 | 0.164 | 0.64 | 0.26 to 1.03 | 0.001 |
Linear regression was conducted with standardized continuous variables, including 2-dimensional left ventricular ejection fraction (2D-LVEF) and dichotomous variables.
allo-HSCT = allogeneic hematopoietic stem cell transplantation; GVHD = graft versus host disease.
Age at examination.
Table 5.
Linear Regression Analysis for Predictors of GLS in Allo-HSCT Survivors (n = 98)
| Univariable |
Multivariable |
|||||
|---|---|---|---|---|---|---|
| β | 95% CI | p Value | β | 95% CI | p Value | |
| Sex | 0.38 | −0.06 to 0.73 | 0.092 | 0.22 | −0.16 to 0.59 | 0.249 |
| Age, yrs∗ | −0.06 | −0.26 to 0.14 | 0.548 | −0.24 | −0.45 to −0.02 | 0.031 |
| Height, m | 0.07 | −0.14 to 0.27 | 0.523 | |||
| Body mass index, kg/m2 | 0.01 | −0.21 to 0.22 | 0.964 | |||
| Heart rate, beats/min | 0.33 | 0.13 to 0.52 | 0.001 | 0.34 | 0.15 to 0.53 | 0.001 |
| Mediastinal radiation | 0.68 | −0.74 to 2.10 | 0.343 | |||
| Cumulative anthracycline dosage, mg/m2 | 0.19 | −0.01 to 0.39 | 0.059 | 0.25 | 0.07 to 0.44 | 0.009 |
| Total body irradiation | 0.40 | −0.44 to 1.24 | 0.345 | 0.20 | −0.64 to 1.04 | 0.638 |
| Malignancy | 0.38 | −0.07 to 0.82 | 0.095 | |||
| Hypertension | 0.48 | 0.08 to 0.87 | 0.019 | 0.50 | 0.04 to 0.96 | 0.035 |
| Diabetes mellitus | 0.49 | 0.93 to 1.91 | 0.493 | |||
| Hypercholesterolemia | 0.66 | 0.11 to 1.22 | 0.020 | 0.44 | −0.14 to 1.02 | 0.134 |
| Hypothyroidism | −0.37 | −1.03 to 0.29 | 0.271 | |||
| Smoking, current/previous | 0.20 | −0.25 to 0.65 | 0.372 | |||
| GVHD, acute and/or chronic GVHD | −0.02 | −0.44 to 0.39 | 0.919 | −0.29 | −0.69 to 0.11 | 0.158 |
Linear regression was conducted with standardized continuous variables (including global longitudinal strain [GLS]) and dichotomous variables. GLS is a negative value. Increases in GLS (e.g., with hypertension) reflect worsening in longitudinal shortening (systolic function).
Abbreviations as in Table 4.
Age at examination.
Table 6.
Dose-Related Responses to Anthracycline Used in Pre-Treatment Therapies in allo-HSCT Survivors
| None (n = 57) | Low Dosage (<300 mg/m2) (n = 25) | High Dosage (≥ 300 mg/m2) (n = 22) | p Value | |
|---|---|---|---|---|
| Cumulative anthracycline dosage, mg/m2 | 0 (0, 0) | 170 (75, 200) | 435 (354, 464) | <0.001∗† |
| Total body irradiation | 4 (7.0) | 1 (4.0) | 2 (9.1) | 0.779‡ |
| Female | 28 (50.0) | 14 (56.0) | 14 (63.6) | 0.495‡ |
| Age, yrs | 35.1 ± 11.9 | 36.4 ± 11.7 | 32.8 ± 11.3 | 0.571‡ |
| Body mass index, kg/m2 | 25.0 ± 5.7 | 25.2 ± 4.6 | 22.7 ± 3.6 | 0.146‡ |
| Systolic blood pressure, mm Hg | 124 ± 19 | 127 ± 20 | 112 ± 15 | 0.016∗‖ |
| Diastolic blood pressure, mm Hg | 73 ± 12 | 77 ± 14 | 66 ± 12 | 0.015∗ |
| Heart rate, beats/min | 70 ± 11 | 70 ± 12 | 67 ± 11 | 0.511‡ |
| Hypertension | 23 (40.4) | 12 (48.0) | 7 (31.8) | 0.529‡ |
| Malignancy | 30 (52.6) | 25 (100.0) | 22 (100.0) | <0.001† |
| GVHD (acute and/or chronic GVHD) | 32 (56.1) | 15 (60.0) | 20 (90.9) | 0.013∗‖ |
| New York Heart Association functional class II or III | 10 (18.2) (n = 55) | 10 (40.0) | 8 (36.4) | 0.073‡ |
| NT-proBNP, ng/l | 33 (18, 58) | 52 (25, 148) | 110 (57, 182) | <0.001∗† |
| IVDd, cm | 0.90 ± 0.2 | 0.86 ± 0.1 | 0.79 ± 0.1 | 0.089‡§ |
| LVIDd, cm | 4.8 ± 0.5 | 4.9 ± 0.5 | 5.1 ± 0.4 | 0.002§‖ |
| LVIDs, cm | 3.2 ± 0.5 | 3.5 ± 0.6 | 3.7 ± 0.4 | <0.001†§ |
| LV mass, g¶ | 131.5 ± 49.9 | 133.1 ± 29.0 | 125.2 ± 36.2 | 0.264‡§ |
| Fractional shortening, % | 32.8 ± 4.7 | 29.2 ± 6.5 | 26.6 ± 4.4 | <0.001†§ |
| 2D-LVEF, % | 57.0 ± 4.7 | 54.6 ± 7.0 | 51.1 ± 5.0 | <0.001§‖ |
| 3D-LVEF, % | 55.9 ± 3.9 (n = 48) | 52.7 ± 6.2 (n = 21) | 50.8 ± 4.4 (n = 19) | 0.001†§ |
| s' velocity, cm/s | 8.2 ± 1.4 | 8.0 ± 1.9 | 7.4 ± 2.0 | 0.039§‖ |
| MAPSE, mm | 13.2 ± 2.1 | 12.6 ± 2.1 | 12.5 ± 2.1 | 0.242‡§ |
| GLS, % | −17.9 ± 2.1 (n = 55) | −17.1 ± 2.1 (n = 24) | −16.8 ± 2.2 (n = 21) | 0.023§‖ |
Values are median (25th, 75th percentiles), n (%), or mean ± SD.
Abbreviations as in Table 3.
Significant difference between anthracycline ≥300 mg/m2 and <300 mg/m2 in Bonferroni post hoc analysis (<0.05).
Significant difference with both treatment groups (anthracycline ≥300 mg/m2 and <300 mg/m2) with no anthracycline group in Bonferroni post hoc analysis (<0.05).
No significant difference between treatment groups in Bonferroni post hoc analysis.
Echocardiographical parameters are adjusted for covariates of age, heart rate, body mass index, and diastolic blood pressure.
Significant difference between anthracyclines ≥300 mg/m2 and no anthracycline in Bonferroni post hoc analysis (<0.05).
ASE cube formula (14).
Other pathology
Valvular heart disease was rare, and no lesions were severe. Pericardial pathology was found in 8 (7.7%) of the allo-HSCT survivors and none in the control group. In total, 7 cases of pericardial pathology occurred in those with acute and/or chronic GVHD (n = 67), although the difference was not statistically significant (p = 0.254).
Discussion
Our study is unique for several reasons. Allo-HSCT regimens in Norway have remained standardized without much radiation exposure. We have a complete hospital registry, long observation time (average 17 years), and applied contemporary echocardiographic techniques. To our knowledge, no prior studies have evaluated LV systolic function with comprehensive echocardiography in very long-term survivors of allo-HSCT in children, adolescents, and young adults. We found a high rate of LVSD, occurring in 44.2%, indicating that cardiac dysfunction in this patient group is more prevalent than previously documented. Symptomatic LVSD (NYHA functional class II or III) was found in 12.7% of the entire study population, which is higher than previously reported in other HSCT studies (4,5,8,9). An equally important finding was the high frequency of asymptomatic LVSD (NYHA functional class I), which was found in 32.3% of survivors that over time is likely to manifest as overt HF. In comparison, asymptomatic HF has previously been reported in 5.1% of long-term survivors of lymphoma when treated with autologous HSCT (9). Our study differed by defining LVSD with sex-specific 2D-LVEF cutoffs and GLS. Moreover, in our cohort, transplants were primarily of stem-cell origin, and although there were heterogeneous reasons for allo-HSCT, there were also few cases of radiotherapy. The lack of symptoms (NYHA functional class II or III) in a greater proportion of those with LVSD is unexpected. However, symptoms of dyspnea and fatigue are commonly accepted side effects of chemotherapy, possibly accounting for reduced recognition and lack of awareness. The absence of symptoms may also explain the infrequent prescription of cardioprotective medications in our cohort.
Plausible explanations for the high frequency of LVSD in this study population include exposure time, age at HSCT, pre-transplantation therapies, and post-transplant risk factors. Few previous studies have as long follow-up time as this present study; this is an important difference, because cardiac dysfunction after chemotherapy may worsen in parallel with observation time (4,8,17,18). However, this study cannot discern the precise timing of LVSD onset, and one could potentially speculate that cardiac injury occurred at time of therapy, with a further worsening in LVSD over time. Another possible explanation is that young patients are at higher risk of heart disease due to organ immaturity and growth disturbances caused by cardiotoxic therapies (19). This may explain the smaller LV size in survivors compared with control subjects, even after consideration for CV disease and confounders. Sex has previously been implicated as a risk factor for cardiotoxicity (8,19, 20, 21), but this association was not found in our study.
Cyclophosphamide has historically been noted to have cardiotoxic effects (22). However, published data linking alkylating agents to long-term heart disease is scarce. In contrast, anthracycline exposure is known to cause myocyte depletion, and is shown to increase the risk of HF by 5-fold in long-term survivors treated in their youth (23). The cardiotoxic effect of anthracycline was also confirmed in this study; anthracycline treatment was a significant independent predictor for the reduction of 2D-LVEF and GLS. Moreover, the reduction in systolic function and adverse LV remodeling were dose-dependent. These observations are in agreement with other studies that have used similar thresholds of cumulative anthracycline dosage (>250 to 300 mg/m2) (8,9,19,21,23).
In addition to cardiotoxic therapies, we found that traditional CV risk factors have a potential role in modifying the risk for LVSD in long-term survivors of allo-HSCT. CV risk factors are commonly reported in HSCT survivors, occurring more frequently in allo-HSCT compared with autologous-HSCT survivors, resulting in a higher prevalence of heart conditions in allo-HSCT survivors (6,18,24, 25, 26). Survivors in this study had a high prevalence of CV risk factors, but at a level comparable to other studies (6,18,20,21,24, 25, 26). Any potential differences in reported CV risk factor prevalence are likely associated with cardiotoxic exposures, recipient age, and observation time. In our study, hypertension and hypercholesterolemia were more frequently observed in survivors with older age and longer survival time, suggesting that risk profiles may alter with aging after HSCT. One concerning finding from our cross-sectional analyses was that hypertension was prevalent in 40.4%, but the use of antihypertensive therapy in only 30.8%. A similarly high prevalence of hypertension (24% to 45%) has been reported in long-term survivors (>10 years) treated with HSCT at various ages (5,9,20,21,24,25). The significance of hypertension in contributing to prevailing cardiac disease in HSCT survivors has previously been shown (5,21). However, in our study, given that the onset and duration of hypertension is unknown, it is unclear if the cumulative consequences of hypertension were fully manifested. Indeed, the echocardiograms showed little evidence of concentric remodeling beyond a small but significant difference in indexed LV end-diastolic volume. However, hypertension is known to cause myocardial fibrosis that leads to reduced longitudinal shortening of the heart (27). This may explain why hypertension was found to be an independent predictor of GLS.
Hypothyroidism has been reported in 30% of long-term survivors of HSCT with busulfan conditioning (28). Hypothyroidism was found in 9.6% in our cohort, although no association with LVSD was found in our data. Dyslipidemia has been reported in 13% to 52% of survivors of HSCT (5,6,18,20,24,25). Hypercholesterolemia occurred in 15% of our patients, and a trend between elevated cholesterol level and reduced GLS was observed. Overall, the contribution of CV risk factors to LVSD in allo-HSCT survivors stresses the importance of preventive strategies for reduction of risk factors and promotion of a healthy lifestyle. This is especially important in younger patients with potentially longer life expectancy.
In our study, GVHD was found to be a highly prevalent complication of allo-HSCT survivors. There is limited evidence that GVHD directly mediates myocardial damage. However, active chronic GVHD has been shown to be associated with a higher risk of CV death (5). It is thought that the chronic inflammation processes instigated by GVHD results in endothelial damage leading to accelerated atherosclerosis (29). We did not find GVHD (acute or chronic) to be consistently associated with LV systolic function, and found a very modest association with increased LVEF (β = 0.64; 95% CI: 0.26 to 1.03) and no association with GLS. GVHD may have an indirect role in altering cardiac function through the development of CV risk factors. Moreover, a higher proportion of pericardial pathology was observed in patients with GVHD. Although not statistically significant, it is numerically striking, may have clinical relevance possibly affecting systolic function, and supports the few previous reports on this matter (30). This is an area that deserves further study.
Echocardiography is a noninvasive, cost-effective, and readily available technique with a traditional reliance on 2D-LVEF to define systolic dysfunction. The limitations of traditional 2D-LVEF are well known, leading to 3D and speckle tracking echocardiography being recommended by expert consensus panels (15,31). The necessity for HSCT studies to use modern echocardiographic techniques to evaluate structural and functional changes in survivors has been promoted by oncology experts (32). Our study placed an emphasis on high-quality echocardiography with incorporation of modern techniques to ensure conclusive evidence of LVSD. We found a consistent difference between allo-HSCT survivors and control subjects in our measures of LV systolic function. These newer techniques can potentially lead to a higher reported incidence of cardiac dysfunction, but can also identify more patients at risk of developing HF. As is well-established, a relationship between LVEF and GLS exists, although these 2 parameters measure slightly different properties of systolic function. GLS reflects impaired longitudinal shortening and has been described as an earlier marker of myocardial damage, whereas reductions in LVEF (radial and circumferential contraction) are often first seen in more advanced remodeling (15,33).
A remaining challenge with GLS is the lack of consensus in defining absolute cutoffs for dysfunction, variability, and intervendor differences (27). The GLS cutoff used in our analysis was based on our normal data, using the same equipment and operator. This value (−17%) is conservative when compared with a meta-analysis performed by Yingchoncharoen et al. (27), which found the 95% CI for normal GLS to be −18.9% to −20.4% (27). The use of linear regression with a continuous outcome variable, however, allowed identification of predictors of LV function without dependency on cutoff values, and provided a specific analysis of incremental change.
Study strengths and limitations
The cross-sectional design of this study describes associations and not causality. This study cannot determine the timing of initial myocardial damage or conclude if deterioration of LV function is a part of a progressive continuum. The number of deaths attributed to solely CV reasons prior to study start is not known, as the CV status of nonstudy participants had not been recorded. Registry data showed nonparticipants to be younger with shorter follow-up, possibly resulting in overestimation of LVSD in our sample. Potential selection bias from recruitment of control subjects without CV disease was addressed by adjusting for baseline differences, and subgroup analyses focused on those without CV disease.
The strengths of this study are completeness of the cohort, nationwide patient inclusion, long follow-up, and standardized transplantation regimens with minimum confounding from radiation. We consider the data collection to be comprehensive, of high quality, and contemporary. All echocardiograms were performed and analyzed by the same investigator eliminating interobserver bias, were conducted blinded to medical status, and had excellent reproducibility.
Conclusions
In our study of 104 long-term allo-HSCT survivors treated in their youth, LV systolic function was found to be significantly reduced when compared with a healthy control group. We found 44% experienced LVSD, of whom 28.3% were symptomatic. Variables independently associated with LV systolic function were anthracyclines, age, heart rate, hypertension, and GVHD. Anthracyclines were found to have a significant dose-dependent effect on LV systolic function.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Long-term survivors treated in their youth with allo-HSCT are at increased risk of LVSD. Anthracyclines, age, heart rate, hypertension, and GVHD were found to be significant independent predictors of LV systolic function. A high prevalence of cardiac dysfunction and CV risk factors in young survivors after allo-HSCT suggests potential benefits from surveillance regimens that include echocardiography with strain imaging, and monitoring of modifiable risk factors.
TRANSLATIONAL OUTLOOK: Larger, prospective studies with contemporary imaging technologies are needed to understand the full impact of allo-HSCT on cardiac function.
Acknowledgment
We thank Maiju Pesonen, PhD, at Oslo Centre for Biostatistics and Epidemiology (OCBE), Oslo University Hospital Research Support Services.
Footnotes
This study is funded by the Norwegian Extra-foundation and the Norwegian Cancer foundation. The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
Appendix
For a supplemental table, please see the online version of this paper.
Appendix
References
- 1.Passweg J.R., Baldomero H., Bader P. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016;51:786–792. doi: 10.1038/bmt.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooley T.A., Chien J.W., Pergam S.A. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia S., Francisco L., Carter A. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow E.J., Mueller B.A., Baker K.S. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Chow E.J., Wong K., Lee S.J. Late cardiovascular complications after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:794–800. doi: 10.1016/j.bbmt.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eissa H.M., Lu L., Baassiri M. Chronic disease burden and frailty in survivors of childhood HSCT: a report from the St. Jude Lifetime Cohort Study. Blood Advances. 2017;1:2243–2246. doi: 10.1182/bloodadvances.2017010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin P.J., Counts G.W., Jr., Appelbaum F.R. Life expectancy in patients surviving more than 5 years after hematopoietic cell transplantation. J Clin Oncol. 2010;28:1011–1016. doi: 10.1200/JCO.2009.25.6693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armenian S.H., Sun C.L., Shannon T. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murbraech K., Smeland K.B., Holte H. Heart failure and asymptomatic left ventricular systolic dysfunction in lymphoma survivors treated with autologous stem-cell transplantation: a national cross-sectional study. J Clin Oncol. 2015;33:2683–2691. doi: 10.1200/JCO.2015.60.8125. [DOI] [PubMed] [Google Scholar]
- 10.Children's Oncology Group (COG) October 2018. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers, version 5.0, section 33: pages 40.www.survivorshipguidelines.org Available at: Accessed February 2020. [Google Scholar]
- 11.The Criteria Committee of the New York Heart Association . 9th edition. Little, Brown and Co.; Boston: 1994. Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. [Google Scholar]
- 12.Glucksberg H., Storb R., Fefer A. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Shulman H.M., Cardona D.M., Greenson J.K. NIH consensus development project on criteria for clinical trials in chronic graft-versus-host disease: II. The 2014 Pathology Working Group Report. Biol Blood Marrow Transplant. 2015;21:589–603. doi: 10.1016/j.bbmt.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2014;15:1063–1093. doi: 10.1093/ehjci/jeu192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgartner H., Falk V., Bax J. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. doi: 10.1093/eurheartj/ehx391. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong G.T., Kawashima T., Leisenring W. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–1227. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tichelli A., Bucher C., Rovo A. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 19.Lipshultz S.E., Lipsitz S.R., Mone S.M. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332:1738–1743. doi: 10.1056/NEJM199506293322602. [DOI] [PubMed] [Google Scholar]
- 20.Pophali P.A., Klotz J.K., Ito S. Male survivors of allogeneic hematopoietic stem cell transplantation have a long term persisting risk of cardiovascular events. Exp Hematol. 2014;42:83–89. doi: 10.1016/j.exphem.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Armenian S.H., Sun C.-L., Francisco L. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottdiener J.S., Appelbaum F.R., Ferrans V.J., Deisseroth A., Ziegler J. Cardiotoxicity associated with high-dose cyclophosphamide therapy. Arch Intern Med. 1981;141:758–763. [PubMed] [Google Scholar]
- 23.Mulrooney D.A., Yeazel M.W., Kawashima T. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chow E.J., Baker K.S., Lee S.J. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armenian S.H., Sun C.L., Vase T. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker K.S., Ness K.K., Steinberger J. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yingchoncharoen T., Agarwal S., Popovic Z.B., Marwick T.H. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–191. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Sanders J.E., Hoffmeister P.A., Woolfrey A.E. Thyroid function following hematopoietic cell transplantation in children: 30 years' experience. Blood. 2009;113:306–308. doi: 10.1182/blood-2008-08-173005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tichelli A., Gratwohl A. Vascular endothelium as 'novel' target of graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:139–148. doi: 10.1016/j.beha.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Karakulska-Prystupiuk E., Basak G., Dwilewicz-Trojaczek J., Paluszewska M., Boguradzki P., Jedrzejczak W. Pericarditis in patients with chronic graft-vs-host disease. Transplant Proc. 2018;50:2218–2222. doi: 10.1016/j.transproceed.2018.02.130. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong G.T., Joshi V.M., Ness K.K. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armenian S.H., Chemaitilly W., Chen M. National Institutes of Health hematopoietic cell transplantation late effects initiative: The Cardiovascular Disease and Associated Risk Factors Working Group Report. Biol Blood Marrow Transplant. 2017;23:201–210. doi: 10.1016/j.bbmt.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thavendiranathan P., Poulin F., Lim K.D., Plana J.C., Woo A., Marwick T.H. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.