Abstract
Objectives
The purpose of this study was to evaluate whether immune checkpoint inhibitors (ICIs) are associated with an increased risk of major adverse cardiovascular events (MACE) compared with non-ICI therapies in patients with lung cancer.
Background
ICIs activate the host immune system to target cancer cells. Though uncommon, cardiovascular immune-related adverse events can be life-threatening.
Methods
A retrospective single-institution cohort study of 252 patients with pathologically confirmed lung cancer who received ICI or non-ICI therapy was analyzed. The primary endpoint was MACE, defined as a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure.
Results
During a median follow-up of 6 months, MACE occurred in 13.3% of ICI-treated patients, with a median time to event of 51 days, compared with 10.3% and 64 days in non-ICI patients. ICIs were not associated with MACE (hazard ratio [HR]: 1.18; 95% confidence interval [CI]: 0.57 to 2.43; p = 0.66) in a univariable Fine-Gray regression analysis incorporating noncardiovascular death as a competing risk. Multivariable regression analyses determined that patients treated with ICIs with elevated serum troponin I >0.01 ng/ml (HR: 7.27; 95% CI: 2.72 to 19.43; p < 0.001) and B-type natriuretic peptide (BNP) >100 pg/ml (HR: 2.65; 95% CI: 1.01 to 6.92; p = 0.047) had an increased risk of MACE. Patients pre-treated or receiving combined immunotherapy with ICIs and vascular endothelial growth factor inhibitors (VEGFIs) or tyrosine kinase inhibitors (TKIs) had an increased risk of MACE (HR: 2.15; 95% CI: 1.05 to 4.37; p = 0.04).
Conclusions
ICIs were not independently associated with an increased risk of MACE in patients with lung cancer, although power is an important limitation in these analyses. ICI-associated cardiotoxicity was associated with elevations in serum troponin and BNP, and combined immunotherapy with VEGFIs or TKIs. Future studies are needed to further define the role of cardiac biomarkers as a monitoring strategy with ICI therapy.
Key Words: BNP, cardiotoxicity, immune checkpoint inhibitors, lung cancer, MACE, troponin
Abbreviations and Acronyms: BNP, B-type natriuretic peptide; CI, confidence interval; HR, hazard ratio; ICI, immune checkpoint inhibitor; IQR, interquartile range; LVEF, left ventricular ejection fraction; MACE, major adverse cardiovascular events; PD, programmed cell death protein; PD-L1, programmed cell death-ligand 1; TKI, tyrosine kinase inhibitor; TnI, troponin I; VEGFI, vascular endothelial growth factor inhibitor
Central Illustration
Immune checkpoint inhibitors (ICIs) are a newer class of monoclonal antibody agents that activate the host immune system for the targeted killing of cancer cells. Increasingly used for the treatment of lung cancer, immunotherapy agents directed at programmed cell death protein (PD)-1 and its associated ligand have demonstrated an overall survival benefit as front-line therapy in non–small-cell lung cancer and in combination with cytotoxic chemotherapy for small-cell lung cancer (1, 2, 3, 4). However, the unchecked systemic inflammatory response leads to immune-related adverse events affecting various organ systems, including the cardiovascular system (5, 6, 7).
Although randomized clinical trials of ICIs for lung cancer rarely reported cardiovascular immune-related adverse events before their approval by the U.S. Federal Drug Administration, these events have been more frequently observed in the community since their widespread use (8, 9, 10, 11). A French case series detected 30 cases of ICI-related cardiotoxicity over 2 years, predominantly in the form of cardiomyopathy and conduction abnormalities with a median time to event of 65 days after starting ICIs (12). A multicenter case-control study of patients treated with ICIs detected a 1.14% prevalence of myocarditis with a median time of onset of 34 days and a 46% incidence of major adverse cardiovascular events (MACE) among cases of ICI-associated myocarditis (13). Similarly, the patient-reported World Health Organization VigiBase global database of drug-related adverse events found that 75% of the total number of drug-related cardiotoxicity cases reported in 2017 involved ICIs, with myocarditis, pericardial disease, supraventricular arrhythmias, and vasculitis more commonly attributed to ICIs (14). This discrepancy between the incidence of ICI-associated cardiotoxicity reported in clinical trials and the community may be due to a lack of standardization in methods and quality assurance in evaluating cardiovascular immune-related adverse events, resulting in an underestimation of their actual incidence (15).
Our knowledge of ICI-associated cardiotoxicity has been significantly enhanced by case series and pharmacovigilance databases, though limitations in study design, potentially biased with cases and controls derived from different source populations, impair the assessment of ICI-related cardiovascular toxicity. For example, the 2 most extensive retrospective studies differed in their conclusions on the associations of left ventricular systolic dysfunction, elevated serum B-type natriuretic peptide (BNP), and elevated serum troponin levels with ICI (16). Furthermore, it is unknown whether specific ICI agents have a higher risk of cardiotoxicity and whether this risk is dose-dependent.
To further evaluate ICI-related cardiotoxicity, we performed a retrospective cohort study of patients with lung cancer who received either ICI- or non-ICI–based therapy to determine the relative risk of major and nonmajor cardiovascular events. The objectives of this study were to evaluate: 1) the relationship between ICIs and MACE; 2) the risk of MACE according to baseline clinical or treatment factors, including prior or concurrent treatment with vascular endothelial growth factor inhibitors (VEGFIs) or tyrosine kinase inhibitors (TKIs); and 3) the risk of MACE in patients receiving ICIs with an abnormal cardiac biomarker during ICI therapy. We hypothesized that there would be a higher incidence of MACE in patients treated with ICIs compared with non-ICI therapies, and cardiotoxicity would be associated with abnormal levels of cardiac biomarkers in patients receiving ICI therapy.
Methods
Patients
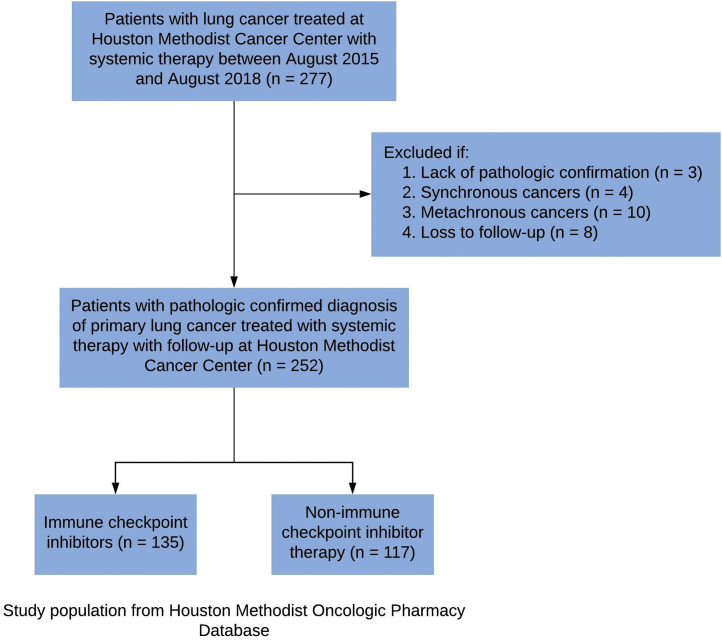
The study population was selected from the Houston Methodist Oncologic Pharmacy Registry, which includes information regarding treatments provided to patients at 7 hospitals and affiliated cancer centers located within the Houston, Texas metropolitan area (Supplemental Figure 1). Patients with a clinical diagnosis of primary lung cancer by an oncologist who received systemic therapy (chemotherapy or immunotherapy) between August 1, 2015, and August 1, 2018, were included in the study (Figure 1).
Figure 1.
Patient Cohort Selection
Cases lacking pathological confirmation and lost to follow-up were excluded from the analysis. To reduce confounding related to treatment selection and preserve the independence of observations, the study excluded patients with synchronous (2 or more primary malignancies diagnosed simultaneously) and metachronous (second malignancy diagnosed within 2 years of the primary malignancy) cancers (Figure 1). The study was approved by the institutional review board at all participating sites, per federal and local standards.
Covariates
Data collected retrospectively from electronic health records included cardiovascular risk factors, medical comorbidities, medications, cancer diagnosis and stage, past or concurrent chemotherapy, immunotherapy, radiotherapy, pre- and post-treatment left ventricular ejection fraction (LVEF), post-treatment cardiac biomarkers (serum BNP and troponin I levels), ICI (including specific agent and dose), and cardiovascular outcomes. Elevated troponin was defined as a serum troponin I (TnI) concentration higher than 0.01 ng/ml. Elevated BNP was defined as a serum level higher than 100 pg/ml.
ICIs comprised 3 different classes of agents in the study: PD-1 inhibitors, programmed cell death-ligand 1 (PD-L1) inhibitors, and cytotoxic T-lymphocyte-associated protein 4 (CTLA4) inhibitors. Specific agents included in the study were nivolumab and pembrolizumab (PD-1 inhibitors), atezolizumab and durvalumab (PD-L1 inhibitors), and ipilimumab (CTLA4 inhibitor).
Definitions and outcomes of interest
The primary endpoint was MACE, defined as a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, and hospitalization for heart failure. Secondary outcomes were cardiovascular adverse events as defined by the Common Terminology Criteria for Adverse Events (CTCAE), version 5.0, which included all new cardiac and vascular disorders occurring during treatment, graded from 1 to 5 based on pre-specified clinical severity (17). All events were adjudicated independently by an events committee consisting of cardiologists not directly involved in the study. Reviewers were not blinded to the specific treatment received by each patient. Cardiologists also reviewed all electrocardiograms, echocardiograms, stress tests, and angiography when available, because these were studies obtained at clinician discretion.
In terms of biomarker assessment, serum TnI and BNP levels were measured at clinician discretion either at baseline or after initial ICI or non-ICI therapy for lung cancer and not at regular pre-specified time intervals. All biomarker data included for analysis was obtained from the predefined study period (August 1, 2015, to August 1, 2018). Continuous variables included the timing and number of laboratory measurements and the maximum level of each biomarker per patient. Categorical variables were created for elevations above specific thresholds in the serum concentration of each biomarker after initiation of antineoplastic therapy. The Siemens Centaur XP1 immunoassay platform (Siemens Healthineers, Erlangen, Germany) was used to quantify biomarker levels within each hospital laboratory.
Statistical analysis
Continuous variables are presented as mean ± SD or median (interquartile range [IQR]), and categorical variables are presented as number (percentage). Statistical comparisons between groups used the independent Student’s t-test or Mann-Whitney test for continuous data, and chi-square or Fisher exact test for categorical data, whenever appropriate. Time to MACE was defined as the elapsed duration in days from the initial infusion of ICI or non-ICI therapy to the first event within the composite outcome (if any). Cumulative incidence analysis for MACE was adjusted for the competing risk of death from other causes due to the high incidence of cancer-related mortality. Univariable and multivariable Fine and Gray regression models (18) with consideration for noncardiovascular death as a competing risk were performed to evaluate MACE associations between ICI and non-ICI therapies and other clinical risk factors.
We first determined the association of ICI therapy alone or ICI with other clinical risk factors (e.g., ICI + prior ischemic cardiomyopathy) and MACE in unadjusted Fine and Gray regression analysis. We then examined the risk of MACE in univariable and multivariable Fine and Gray regressions (with noncardiovascular death as a competing risk), including treatment exposure (ICI or non-ICI therapy), age, sex, and cardiac biomarkers as categorical variables in each model. Elevated serum TnI was defined as elevations >0.01 ng/ml, and elevated serum BNP was defined as >100 pg/ml in the categorical variables analyses. Lastly, we assessed ICI therapy and time to elevated serum TnI or time to elevated serum BNP levels (both continuous variables) with the risk of MACE in the multivariable model. Time to elevated serum TnI and time to elevated serum BNP levels were defined as the time-to-first elevation (measured in months) after initial treatment. We explored potential interactions between ICI therapy and cardiac biomarkers (elevated TnI or elevated BNP and time to elevated TnI or time to elevated BNP) in separate multivariable Fine and Gray regression models incorporating noncardiovascular death as a competing risk event. The proportional subdistribution hazards assumption was assessed (19), and there was no evidence for the violation of any covariates. All statistical tests were 2-sided, and 0.05 was set as the level of significance. Statistical analyses were performed using Stata version 16 (StataCorp LLC, College Station, Texas) and IBM SPSS Statistics version 25 (IBM, Armonk, New York).
Results
Two hundred fifty-two patients with lung cancer were included in the study with baseline clinical characteristics by treatment group shown in Table 1. Patients treated with ICIs were more likely to have had a prior myocardial infarction or exposed to prior or concurrent VEGFI or TKI therapy. Patients who received non-ICI therapies had a higher prevalence of diabetes and chronic kidney disease Stage 3 or higher and were more likely to have small-cell lung cancer.
Table 1.
Baseline Characteristics by Treatment (N = 252)
| Non-ICI (n = 117) | ICI (n = 135) | p Value | |
|---|---|---|---|
| Age at start of treatment, yrs | 69.3 ± 8.9 | 68.5 ±11.6 | 0.57 |
| Female | 56 (47.9) | 56 (41.5) | 0.31 |
| Body mass index, kg/m2 | 25.7 ± 5.8 | 24.6 ± 5.1 | 0.12 |
| Cardiovascular risk factors | |||
| Current or prior smoking | 99 (84.6) | 114 (84.4) | 1.00 |
| Hypertension | 81 (69.2) | 91 (67.4) | 0.79 |
| Diabetes mellitus | 44 (37.6) | 29 (21.5) | 0.005 |
| Pre-treatment comorbidities | |||
| Coronary artery disease | 35 (29.9) | 32 (23.7) | 0.32 |
| Prior myocardial infarction | 3 (2.6) | 15 (11.1) | 0.01 |
| Prior coronary stenting | 12 (10.3) | 10 (7.4) | 0.50 |
| Coronary artery bypass graft | 13 (11.1) | 9 (6.7) | 0.27 |
| Stroke | 6 (5.1) | 10 (7.4) | 0.61 |
| Heart failure | 10 (8.5) | 10 (7.4) | 0.82 |
| Chronic obstructive pulmonary disease | 55 (47.0) | 47 (34.8) | 0.05 |
| Chronic kidney disease∗ | 19 (16.2) | 11 (8.1) | 0.05 |
| Baseline left ventricular ejection fraction† | 60.3 ± 7.5 | 58.0 ± 9.3 | 0.32 |
| Pre-treatment cardiovascular medications | |||
| Aspirin | 43 (36.8) | 44 (32.6) | 0.51 |
| Statin | 50 (42.7) | 60 (44.4) | 0.80 |
| Beta-blockers | 47 (40.2) | 50 (37.0) | 0.70 |
| ACE inhibitor or ARB | 37 (31.6) | 35 (25.9) | 0.33 |
| Aldosterone receptor antagonist | 2 (1.7) | 4 (3.0) | 0.69 |
| Primary lung cancer type | |||
| Non–small-cell lung cancer | 78 (66.7) | 121 (89.6) | <0.001 |
| Small-cell lung cancer | 39 (33.3) | 14 (10.4) | |
| Prior or concurrent chemotherapy, immunotherapy, and radiotherapy | |||
| Chemotherapy‡ | 117 (100) | 110 (81.5) | <0.001 |
| Vascular endothelial growth factor or tyrosine kinase inhibitors§ | 15 (12.8) | 37 (27.4) | 0.005 |
| Radiation | 70 (59.8) | 88 (65.2) | 0.43 |
| Thoracic radiation | 54 (46.2) | 58 (43.0) | 0.61 |
| Thoracic radiation dose, Gy | 23.6 ± 27.6 | 22.5 ± 30.3 | 0.30 |
Values are mean ± SD or n (%), unless otherwise indicated.
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blockers; ICI = immune checkpoint inhibitor.
Chronic kidney disease = glomerular filtration rate <60 ml/min/1.73 m2.
Baseline echocardiography available in 47 of 117 non-ICI– and 46 of 135 ICI-treated patients.
Chemotherapy agents consisted of, in decreasing frequency: carboplatin, pemetrexed, paclitaxel, etoposide, cisplatin, gemcitabine, docetaxel, and vinorelbine.
VEGFI and TKI agents consisted of, in decreasing frequency: bevacizumab, erlotinib, and afatinib.
Major adverse cardiovascular events
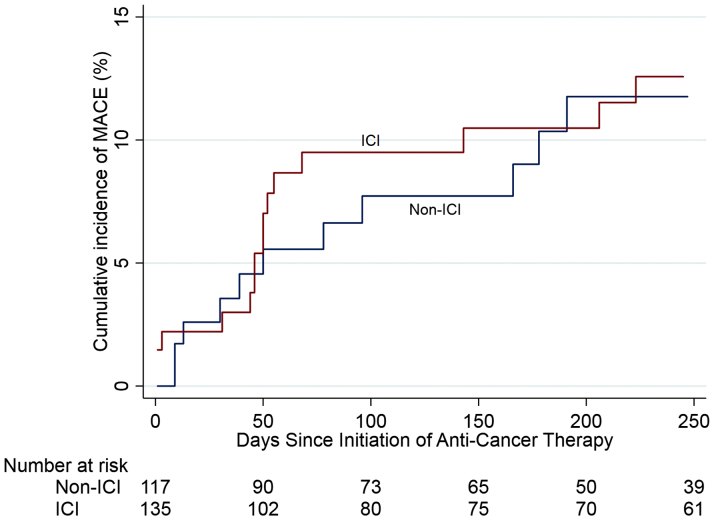
At a median follow-up of 6 months (IQR: 1.7 to 13.7 months), the incidence of MACE was 13.3% in the ICI group with a median time to event of 51 days compared with 10.3% in the non-ICI group with a median time to event of 64 days (p = 0.56 for incidence) (Table 2). This difference between the 2 groups was not statistically significant (hazard ratio [HR]: 1.18, 95% confidence interval [CI]: 0.57 to 2.43; p = 0.66) for the incidence of MACE (Figures 2 and 3).
Table 2.
Cardiovascular Biomarkers and Outcomes According to Treatment (N = 252)
| Non-ICI (n = 117) | ICI (n = 135) | p Value | |
|---|---|---|---|
| Post-treatment LVEF∗ | 55.6 ± 11.6 | 53.8 ± 14.1 | 0.46 |
| Elevated serum troponin I† | 7 (6.0) | 22 (16.3) | 0.01 |
| Initial, ng/ml | 2.6 ± 4.9 | 3.3 ± 10.8 | 0.87 |
| Peak, ng/ml | 2.8 ± 4.9 | 9.2 ± 18.1 | 0.37 |
| Median time to elevation, days | 38 (12–157) | 36 (6–152) | 0.88 |
| Number of measurements | 3 (0–7) | 4 (1–8) | 0.01 |
| Elevated serum BNP‡ | 34 (28.2) | 47 (34.8) | 0.261 |
| Level, pg/ml | 515 ± 908 | 453 ± 456 | 0.687 |
| Median time to elevation, days | 54 (9–161) | 57 (22–245) | 0.42 |
| Number of measurements | 2 (0–3) | 2 (1–4) | 0.02 |
| All-cause death | 33 (28.2) | 51 (37.8) | 0.14 |
| Major adverse cardiovascular events§ | 12 (10.3) | 18 (13.3) | 0.45 |
| Cardiovascular death | 7 (6.0) | 13 (9.6) | 0.95 |
| Nonfatal myocardial infarction | 1 (0.9) | 1 (0.7) | 0.92 |
| Nonfatal stroke | 1 (0.9) | 0 (0.0) | 0.28 |
| Hospitalization for heart failure | 3 (2.6) | 4 (3.0) | 0.85 |
| Cardiovascular adverse events per CTCAE (version 5.0) | 42 (35.9) | 51 (37.8) | 0.76 |
| Arrhythmia | 31 (26.5) | 25 (18.5) | 0.13 |
| Cardiac-related chest pain | 12 (10.3) | 25 (18.5) | 0.07 |
| Valvular heart disease | 4 (3.4) | 2 (1.5) | 0.31 |
| Cardiomyopathy | 13 (11.1) | 20 (14.8) | 0.39 |
| Myopericardial disease | 11 (9.4) | 9 (6.7) | 0.42 |
Values are mean ± SD, n (%), or median (interquartile range).
BNP = B-type natriuretic peptide; CTCAE = Common Terminology Criteria of Adverse Events, version 5.0; ICI = immune checkpoint inhibitor; LVEF = left ventricular ejection fraction; MACE = major adverse cardiac events.
Post-treatment LVEF was determined by the most recent transthoracic echocardiogram in 47 of 117 of non-ICI– and 47 of 135 of ICI-treated patients.
Serum TnI was measured in 203 of 252 patients.
Serum BNP was measured in 193 of 252 patients.
MACE is defined as the first event within the composite endpoint to occur. Some patients developed multiple events during their treatment course though only the first event occurring during therapy is listed in this table.
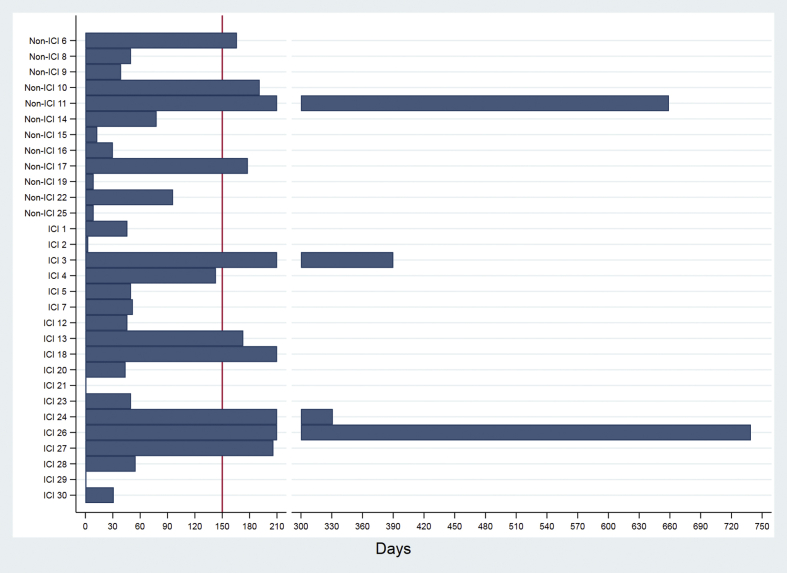
Figure 2.
Time From Initiation of Anti-Cancer Treatment for Lung Cancer to MACE
Horizontal histogram depicting total elapsed days from initial dose of ICI or non-ICI therapy for treatment of lung cancer and MACE: X-axis: Time elapsed (in days) from initial dose of ICI or non-ICI therapy for lung cancer and MACE. Y-axis: Treatment type (ICI or non-ICI) and individual subject number for 30 of 252 total cohort patients who sustained MACE in the study. ICI = immune checkpoint inhibitor; MACE = major adverse cardiac events.
Figure 3.
Cumulative Incidence of MACE After Initiation of Anti-Cancer Treatment
Analyses adjusted for the competing risk of death. The hazard ratio for the association between ICI and MACE is 1.18, 95% confidence interval: 0.57 to 2.43; p = 0.66. Abbreviations as in Figure 2.
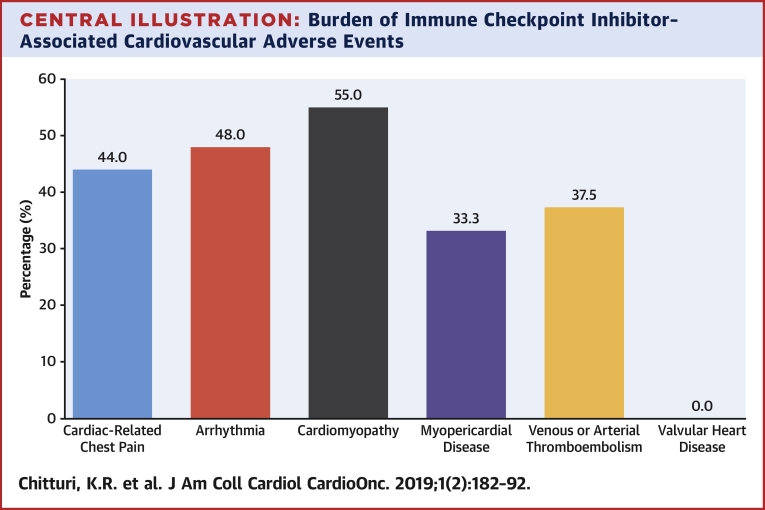
Multiple events within the composite MACE occurred in some patients as denoted by the event breakdown in Table 2, although only the first event was included in the primary endpoint for time-to-event analysis. MACE among patients treated with ICIs (n = 18) predominantly involved cardiovascular death (n = 13) related to fatal myocardial infarction (n = 7) and cardiac arrest (n = 6), and hospitalizations for heart failure (n = 4) with 1 patient having a nonfatal myocardial infarction. Cardiovascular adverse events (graded from 1 to 5 in severity), as defined by the Common Terminology Criteria for Adverse Events, was clinically diverse among patients receiving ICIs, including arrhythmias and conduction disturbances (n = 25), cardiac-related chest pain (n = 25), cardiomyopathy and heart failure (n = 20), pericardial disease (n = 8), myocarditis (n = 1), valvular disease (n = 2), and venous or arterial thromboembolic events (n = 8) with a higher proportion of cardiomyopathy, arrhythmia, and cardiac-related chest pain being life-threatening (Central Illustration).
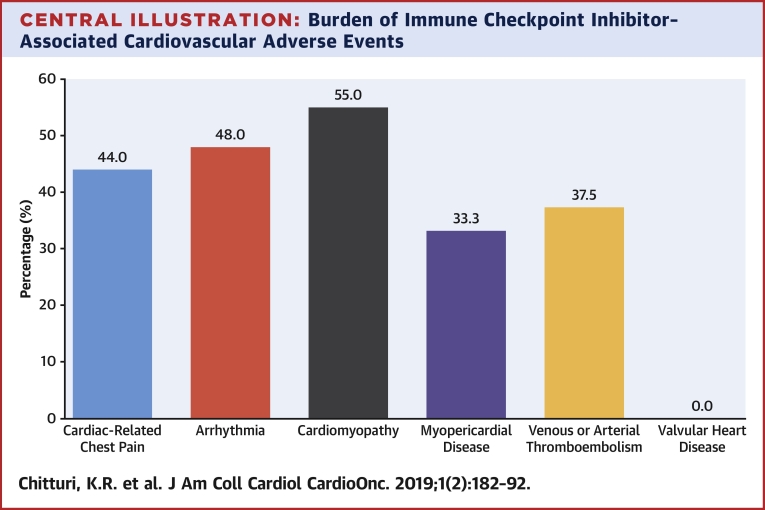
Central Illustration.
Burden of Immune Checkpoint Inhibitor–Associated Cardiovascular Adverse Events
According to the Common Terminology Criteria of Adverse Events (CTCAE) version 5.0, 37.8% of patients receiving immune checkpoint inhibitors (ICIs) developed cardiovascular adverse events. This bar graph depicts the distribution of cardiovascular adverse events, identified by CTCAE, that were adjudicated as major adverse cardiac events. For example, 25 patients on ICI had chest pain by CTCAE, and in 11 patients (44%), these were adjudicated as a major adverse cardiac event. Some patients developed multiple major adverse cardiac events, and each event is counted in this figure. Of note, myopericardial disease includes both pericardial disease and myocarditis.
Baseline characteristics, including sex, cardiovascular risk factors, comorbidities, LVEF, prior or concurrent systemic therapy, and radiotherapy, were not associated with MACE (Table 3). No pre-treatment cardiovascular medications were associated with a decreased risk of MACE. No specific ICI agent was associated with MACE (Table 4). Cardiotoxicity was not dose-dependent for nivolumab (Supplemental Figure 2) or pembrolizumab (Supplemental Figure 3), the most frequently used agents in the study. Few patients received dual ICI therapy (n = 8), an independent risk factor for fulminant myocarditis associated with MACE (11). Patients receiving ICI with concurrent or previous VEGFI or TKI immunotherapy had an increased risk of MACE compared with their counterparts (HR: 2.15; 95% CI: 1.05 to 4.37; p = 0.04) (Table 5).
Table 3.
Baseline Characteristics by MACE (N = 252)
| No MACE (n = 222) | MACE (n = 30) | p Value | |
|---|---|---|---|
| Age at start of treatment, yrs | 69.1 ± 10.1 | 67.2 ± 12.5 | 0.34 |
| Female | 99 (44.6) | 13 (43.3) | 1.00 |
| Body mass index, kg/m2 | 25.2 ± 5.4 | 24.8 ± 5.5 | 0.74 |
| Cardiovascular risk factors | |||
| Current or prior smoking | 187 (84.2) | 26 (86.7) | 1.00 |
| Hypertension | 151 (68.0) | 21 (70.0) | 1.00 |
| Diabetes mellitus | 63 (28.4) | 10 (33.3) | 0.67 |
| Pre-treatment comorbidities | |||
| Coronary artery disease | 55 (24.8) | 12 (40.0) | 0.08 |
| Prior myocardial infarction | 15 (6.8) | 3 (10.0) | 0.46 |
| Prior coronary stenting | 20 (9.0) | 2 (6.7) | 1.00 |
| Coronary artery bypass graft | 20 (9.0) | 2 (6.7) | 1.00 |
| Stroke | 14 (6.3) | 2 (6.7) | 1.00 |
| Heart failure | 17 (7.7) | 3 (10.0) | 0.72 |
| Chronic obstructive pulmonary disease | 90 (40.5) | 12 (40.0) | 1.00 |
| Chronic kidney disease∗ | 24 (10.8) | 6 (20.0) | 0.14 |
| Baseline left ventricular ejection fraction† | 58.9 ± 8.4 | 60.3 ± 9.1 | 0.86 |
| Pre-treatment cardiovascular medications | |||
| Aspirin | 72 (32.4) | 15 (50.0) | 0.07 |
| Statin | 95 (42.8) | 15 (50.0) | 0.56 |
| Beta-blockers | 83 (37.4) | 14 (46.7) | 0.33 |
| ACE inhibitor or ARB | 66 (29.7) | 6 (20.0) | 0.39 |
| Aldosterone receptor antagonist | 4 (1.8) | 2 (6.7) | 0.15 |
| Primary lung cancer type | |||
| Non–small-cell lung cancer | 172 (77.5) | 27 (90.0) | 0.15 |
| Small-cell lung cancer | 50 (22.5) | 3 (10.0) | |
| Prior or concurrent chemotherapy or radiation | |||
| Chemotherapy‡ | 200 (90.1) | 27 (90.0) | 1.00 |
| Radiation | 137 (61.7) | 21 (70.0) | 0.43 |
| Thoracic radiation | 103 (46.4) | 9 (30.0) | 0.12 |
| Thoracic radiation dose, Gy | 23.7 ± 28.8 | 17 ± 30.6 | 0.63 |
| Vascular endothelial growth factor or tyrosine kinase inhibitors§ | 45 (20.3) | 7 (23.3) | 0.64 |
Values are mean ± SD or n (%).
Abbreviations as in Table 1.
Chronic kidney disease = glomerular filtration rate <60 ml/min/1.73 m2.
Baseline echocardiography available in 47 of 117 non-ICI– and 46 of 135 ICI-treated patients.
Chemotherapy agents consisted of, decreasing frequency: carboplatin, pemetrexed, paclitaxel, etoposide, cisplatin, gemcitabine, docetaxel, and vinorelbine.
VEGFI and TKI agents consisted of, in decreasing frequency: bevacizumab, erlotinib, and afatinib.
Table 4.
Comparison of MACE by Treatment (N = 252)
| No MACE (n = 222) | MACE (n = 30) | p Value | |
|---|---|---|---|
| Type of treatment | |||
| ICI | 117 (52.7) | 18 (60.0) | 0.56 |
| Non-ICI | 105 (47.3) | 12 (40.0) | |
| Number of ICI doses | 4.4 ± 8.2 | 2.4 ± 3.8 | 0.03 |
| Specific ICI | |||
| Nivolumab | 61 (27.5) | 10 (33.3) | 0.52 |
| Pembrolizumab | 39 (17.6) | 6 (20.0) | 0.80 |
| Ipilimumab | 7 (3.2) | 0 (0.0) | 1.00 |
| Atezolizumab | 19 (8.6) | 2 (6.7) | 1.00 |
| Durvalumab | 3 (1.4) | 0 (0.0) | 1.00 |
| Combination ICI (anti-PD-1 + anti-CTLA4) | 8 (3.2) | 0 (0.0) | 1.00 |
| Total dose of ICI, mg | |||
| Nivolumab | 548 ± 1665 | 255 ± 615 | 0.35 |
| Pembrolizumab | 249 ± 721 | 213 ± 624 | 0.80 |
| Atezolizumab | 630 ± 3,983 | 240 ± 1,110 | 0.60 |
| Elevated serum troponin I | 13 (5.9) | 17 (56.7) | <0.001 |
| Troponin I, ng/ml | |||
| Initial troponin I | 0.51 ± 0.21 | 5.28 ± 12.8 | 0.16 |
| Peak serum troponin I | 1.21 ± 1.28 | 12.9 ± 20.4 | 0.04 |
| Elevated serum BNP | 59 (26.6) | 20 (66.7) | <0.001 |
| Peak serum BNP, pg/ml | 369.1 ± 433.3 | 803.9 ± 1,094.9 | 0.10 |
Values are n (%) or mean ± SD.
CTLA4 = cytotoxic T-lymphocyte-associated protein 4; PD1 = programmed cell death protein 1; other abbreviations as in Table 2.
Table 5.
ICI-Associated MACE Univariable Analysis of Clinical Risk Factors with Noncardiovascular Death as a Competing Event
| Major Adverse Cardiovascular Events | |||
|---|---|---|---|
| Immune Checkpoint Inhibitors | Hazard Ratio∗ | 95% Confidence Interval | p Value |
| ICI | 1.18 | 0.57–2.43 | 0.66 |
| ICI + prior ischemic heart disease | 2.08 | 0.91–4.73 | 0.08 |
| ICI + prior heart failure | 0.73 | 0.10–5.39 | 0.76 |
| ICI + SCLC diagnosis | 0.58 | 0.08–4.36 | 0.60 |
| ICI + thoracic radiotherapy | 0.32 | 0.10–1.06 | 0.06 |
| ICI + VEGFI or TKI | 2.15 | 1.05–4.37 | 0.04 |
| ICI + BNP elevation | 2.73 | 1.33–5.60 | 0.01 |
| ICI + troponin elevation | 5.20 | 2.59–10.47 | <0.001 |
SCLC = small-cell lung cancer; TKI = tyrosine kinase inhibitor; VEGFI = vascular endothelial growth factor inhibitor; other abbreviations as in Table 2.
Hazard ratios presented in comparison with patients on non-ICI therapies and without concurrent risk factors.
Biomarkers and MACE
Biomarkers were evaluated in most patients, as 80.6% (n = 203 of 252) had TnI levels measured, and 76.6% (n = 193 of 252) had BNP levels measured during the study period (Table 2). There was a statistically significant median difference in the number of TnI (p = 0.01) or BNP (p = 0.02) measurements between patients receiving ICI versus non-ICI therapy, though minimal absolute difference (Supplemental Appendix). A multivariable Fine and Gray competing risk regression model found no significant interactions between ICI therapy and elevated TnI (p = 0.34) or elevated BNP (p = 0.61). In the ICI group, the median time to biomarker elevation for TnI was 36 (IQR: 6 to 152) days, and for BNP was 57 (IQR: 22 to 245) days. Patients who suffered from MACE were more likely to have an elevated TnI or BNP (Table 4). Among the 203 patients with biomarkers measured at baseline or any time during lung cancer therapy, an elevated TnI >0.01 ng/ml (HR: 7.27; 95% CI: 2.72 to 19.43; p < 0.001) or BNP >100 pg/ml (HR: 2.65; 95% CI: 1.01 to 6.92; p = 0.047) was associated with an increased risk of MACE in multivariable analyses (Table 6).
Table 6.
Risk of MACE in Univariable and Multivariable Fine-Gray Hazard Model With Noncardiovascular Death as a Competing Event
| Univariable |
Multivariable∗ |
|||
|---|---|---|---|---|
| Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age at start | 0.98 (0.95–1.02) | 0.44 | ||
| Female | 0.92 (0.45–1.89) | 0.82 | ||
| ICI | 1.18 (0.57–2.43) | 0.66 | 0.63 (0.29–1.35) | 0.23 |
| Nivolumab | 1.16 (0.54–2.51) | 0.70 | ||
| Pembrolizumab | 1.14 (0.47–2.75) | 0.77 | ||
| Atezolizumab | 0.71 (0.17–3.03) | 0.64 | ||
| Elevated troponin I (>0.01 ng/ml) | 9.82 (4.91–19.66) | <0.001 | 7.27 (2.72–19.43) | <0.001 |
| Initial troponin (in ng/ml) | 1.01 (0.99–1.02) | 0.54 | ||
| Peak troponin (in ng/ml) | 1.01 (0.99–1.02) | 0.20 | ||
| Elevated BNP (>100 pg/ml) | 5.14 (2.36–11.20) | <0.001 | 2.65 (1.01–6.92) | 0.047 |
CI = confidence interval; other abbreviations as in Table 2.
Adjusted for ICI, elevated serum troponin I, and elevated serum BNP categorical variables.
The multivariable Fine and Gray regression including noncardiovascular death as a competing risk and quantity of ICI doses and time to elevated TnI and BNP as continuous variables found that each month increase in time to an elevated TnI significantly decreased the risk of MACE (HR: 0.83, 95% CI: 0.73 to 0.94; p = 0.004). There was no association between MACE and time to an elevated BNP (HR: 0.98, 95% CI: 0.84 to 1.13; p = 0.75) (Supplemental Table 1).
Discussion
As guidelines for the management of ICI-related cardiotoxicity are beginning to emerge, screening protocols for prevention and early recognition remain in progress (20,21). Early recognition is vital, because the majority of MACE in the ICI-treated group occurred early during therapy, with a median time to event of 51 days, consistent with previous case-control and cross-sectional studies, which reported a median onset ranging from 30 to 65 days (12, 13, 14, 15, 16). Increasing knowledge regarding risk factors and clinical manifestations of ICI-related cardiovascular toxicity is critical for identifying patients at highest risk who may benefit from closer monitoring during treatment, initial cardiac testing, or possibly consideration of alternative therapies depending upon the risk-benefit ratio.
In this retrospective cohort study, we observed that the incidence of MACE was 13.3% in the ICI group compared with the 10.3% in the non-ICI group. Regardless of treatment status, patients with lung cancer have an elevated risk of cardiovascular disease, especially in association with comorbid heart failure, myocardial infarction, and arrhythmias (22). We did not observe an association between ICIs and MACE. However, the lack of statistical significance for MACE with ICIs may be due to limited power from a small sample size, because a 2-sided log-rank test demonstrated only 12% power at α = 0.05 significance level to detect a difference in the incidence of MACE between the 2 groups. Indeed, a U.S. Food and Drug Administration pooled analysis of 21,664 patients within 59 trials found ICI therapy was associated with higher rates of myocarditis, vasculitis, ischemia, arrhythmia, and pericardial disease compared with non-ICI therapies (23). Although ICI-treated patients did not have a statistically significant increase in the risk of MACE compared with non-ICI–treated patients, patients on ICI did have a high burden of cardiovascular events. As noted in Table 2 and the Central Illustration, a high percentage of patients who met CTCAE version 5 criteria for cardiac events such as cardiomyopathy, chest pain, and arrhythmia subsequently developed high-grade, severe events and MACE.
Dual ICI blockade with nivolumab and ipilimumab is a known risk factor for MACE secondary to immune-related myocarditis, though our study was not adequately powered for this assessment because only 8 patients received concurrent nivolumab and ipilimumab (10,11). Hemodynamically significant pericardial effusions requiring urgent pericardial window occurred in 2 patients on ICIs, consistent with findings from a cohort study that found a higher relative risk for pericardial effusions necessitating urgent intervention in patients treated with ICIs for lung cancer compared with systemic therapies (24). Previous or concurrent thoracic radiotherapy with ICIs was not associated with MACE, contrary to a recent retrospective analysis that found increased risk of MACE with cardiac radiation dose exposure, and a murine model of concurrent thoracic irradiation and PD-1 blockade that demonstrated increased radiation-induced cardiotoxicity and reduced LVEF, though there was no significant difference between cumulative thoracic radiation dose between the ICI and non-ICI groups (25,26).
However, we did determine that therapy with an ICI combined with prior or concurrent treatment with VEGFI or TKI was associated with an increased risk of MACE. These findings warrant further investigation because angiogenesis inhibitors are known to increase the risk of hypertension, cardiomyopathy, and pulmonary hypertension (27, 28, 29, 30, 31). A large meta-analysis comprising 77 studies of patients treated with VEGFIs and TKIs found severe hypertension in 7.4% of patients, arterial thromboembolism in 1.8%, cardiac ischemia in 1.7%, and myocardial dysfunction in 2.3%, and the overall risk of MACE was only 0.25% (29). Individual studies found baseline coronary artery disease as a risk factor for heart failure in patients receiving VEGFIs or TKIs, indicating that these agents may worsen underlying ischemic cardiomyopathy (27,28). A recent observational study found that patients receiving ICIs were at increased risk of acute vascular events within 6 months of initiation, with the risk doubled in patients with lung cancer (32). In our study population, prior ischemic heart disease approached statistical significance for a MACE association, although pre-existing cardiomyopathy was not associated with MACE in patients receiving ICIs.
Rather than assume that ICI-associated cardiotoxicity is dose-independent, our study directly demonstrated that escalating doses of nivolumab and pembrolizumab were not associated with MACE in the dose-response analyses for each ICI agent (Supplemental Figures 2 and 3). Patients who tolerated ICIs in the first 2 months of treatment were less likely to develop MACE, suggesting ICI-associated cardiotoxicity may be related to host immunobiology rather than the medication itself. Traditional pharmacokinetics and safety parameters such as maximum tolerated dose and dose-limiting toxicity do not apply to ICIs because these agents are minimally diffused out of the vascular space and rely on receptor-mediated clearance. Furthermore, interpatient variability in tumor burden and synthesis of PD-L1, alterations in proteolytic function, and genetic polymorphisms in the neonatal Fc receptor may predispose to ICI cardiotoxicity in individual patients (33,34). Further mechanistic studies in this area are needed.
In this study, we determined that elevated serum TnI and BNP levels were associated with an increased risk of MACE in ICI-treated patients with lung cancer compared with non-ICI therapies. Previous studies varied in their conclusions regarding cardiac enzymes and their predictive value of cardiovascular immune-related adverse events during ICI therapy. One multicenter case-control study found elevated BNP associated with ICI-related cardiomyopathy and arrhythmias (12). Elevated serum troponin level had previously been found to be a predictor of ICI-mediated myocarditis and associated MACE, though the association of elevated troponin with MACE comprising atherosclerotic vasculo-occlusive events such as myocardial infarction was not directly evaluated in this study (13). Bias and heterogeneity of these studies may account for their varying conclusions because cases and controls were derived from different source populations in these studies. A unique feature of our study compared with these pharmacovigilance studies is our study comprised patients of similar comorbidities (albeit with some differences as noted in Table 1) treated at the same institution, minimizing potential confounding factors (16).
Study limitations
Due to a retrospective design, selection bias remains a concern as there was no prospective cardiovascular screening protocol across all sites, and screening for cardiac biomarkers and other tests were left at the discretion of the individual providers. However, a uniform platform was used in measuring cardiac biomarkers at all sites, and an independent events committee adjudicated all events. Moreover, as noted in the preceding text, we were limited by power and sample size to detect statistically significant differences in MACE. Furthermore, the median follow-up time of 6 months (IQR: 1.7 to 13.7 months) limits the conjecture of cardiotoxicity beyond this time frame; further studies are required to assess for ICI-associated cardiovascular late effects.
Another limitation is the impact of unmeasured confounding of our associations, because the ICI group comprised more patients with prior MI, and the non-ICI group had a higher rate of baseline diabetes and chronic kidney disease. However, patients were otherwise well matched demographically regarding age and sex as well as smoking history and hypertension. Additionally, incorporating hospitalization for heart failure as a surrogate for immune-related myocarditis within MACE rather than a standard definition due to a lack of biopsy-proven cases is another limitation.
Conclusions
ICIs were not independently associated with an increased risk of MACE, although power is an important limitation in these analyses. ICI-associated cardiotoxicity was dose-independent, occurred early during treatment, and was associated with elevated serum troponin and BNP. Future studies could consider cardiac biomarker assessment as a monitoring strategy with ICI therapy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: The incidence of major adverse cardiovascular events was 13.3% in lung cancer patients treated with ICIs. The risk of MACE was increased in patients who had received therapy with vascular endothelial growth factor inhibitors or tyrosine kinase inhibitors. Elevated serum troponin and BNP levels before or during therapy were associated with an increased risk of MACE in patients receiving ICIs.
TRANSLATIONAL OUTLOOK: Additional research is needed to determine the clinical utility of cardiovascular biomarker evaluation in identifying lung cancer patients at increased risk of adverse cardiovascular outcomes. Moreover, understanding the mechanisms of ICI-associated cardiotoxicity, including host immunobiological factors and interactions with other agents such as tyrosine kinase inhibitors, are important priorities.
Footnotes
Dr. Bhimaraj has been a consultant for Abbott and Abiomed. Dr. Bernicker has served on an advisory board for Guardant Health. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
For an expanded Methods section as well as supplemental figures and a table, please see the online version of this paper.
Appendix
References
- 1.Borghaei H., Paz-Ares L., Horn L. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herbst R.S., Baas P., Kim D.W. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 3.Horn L., Mansfield A.S., Szczesna A. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 4.Rittmeyer A., Barlesi F., Waterkamp D. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson D.B., Chandra S., Sosman J.A. Immune checkpoint inhibitor toxicity in 2018. JAMA. 2018;320:1702–1703. doi: 10.1001/jama.2018.13995. [DOI] [PubMed] [Google Scholar]
- 6.Postow M.A., Sidlow R., Hellmann M.D. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018;378:158–168. doi: 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
- 7.Zimmer L., Goldinger S.M., Hofmann L. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210–225. doi: 10.1016/j.ejca.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan A., Burkeen G., Houranieh J., Arnold S., Anthony L. Immune checkpoint-associated cardiotoxicity: case report with systematic review of literature. Ann Oncol. 2017;28:2034–2038. doi: 10.1093/annonc/mdx213. [DOI] [PubMed] [Google Scholar]
- 9.Ederhy S., Cautela J., Ancedy Y., Escudier M., Thuny F., Cohen A. Takotsubo-like syndrome in cancer patients treated with immune checkpoint inhibitors. J Am Coll Cardiol Img. 2018;11:1187–1190. doi: 10.1016/j.jcmg.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 10.Heinzerling L., Ott P.A., Hodi F.S. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J Immunother Cancer. 2016;4:50. doi: 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson D.B., Balko J.M., Compton M.L. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escudier M., Cautela J., Malissen N. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 13.Mahmood S.S., Fradley M.G., Cohen J.V. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salem J.E., Manouchehri A., Moey M. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol. 2018;19:1579–1589. doi: 10.1016/S1470-2045(18)30608-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gujral D.M., Cleator S.J., Bhattacharyya S. Cardiac safety evaluation in cancer clinical trials. Eur J Cancer. 2018;103:143–146. doi: 10.1016/j.ejca.2018.07.141. [DOI] [PubMed] [Google Scholar]
- 16.Lyon A.R., Yousaf N., Battisti N.M.L., Moslehi J., Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. 2018;19:e447–e458. doi: 10.1016/S1470-2045(18)30457-1. [DOI] [PubMed] [Google Scholar]
- 17.Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. U.S. Department of Health and Human Services; Bethesda, MD: 2017. [Google Scholar]
- 18.Fine J.P., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Zhou B., Fine J., Laird G. Goodness-of-fit test for proportional subdistribution hazards model. Statist Med. 2013;32:3804–3811. doi: 10.1002/sim.5815. [DOI] [PubMed] [Google Scholar]
- 20.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Totzeck M., Schuler M., Stuschke M., Heusch G., Rassaf T. Cardio-oncology - strategies for management of cancer-therapy related cardiovascular disease. Int J Cardiol. 2019;280:163–175. doi: 10.1016/j.ijcard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Kravchenko J., Berry M., Arbreev K., Lyerly H.K., Yashin A., Akushevich I. Cardiovascular comorbidities and survival of lung cancer patients: Medicare data based analysis. Lung Cancer. 2015;88:85–93. doi: 10.1016/j.lungcan.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amiri-Kordestani L., Moslehi J., Cheng J. Cardiovascular adverse events in immune checkpoint inhibitor clinical trials: a U.S. Food and Drug Administration pooled analysis. J Clin Oncol. 2018;36:3009. [Google Scholar]
- 24.Palaskas N., Morgan J., Daigle T. Targeted cancer therapies with pericardial effusions requiring pericardiocentesis focusing on immune checkpoint inhibitors. Am J Cardiol. 2019;123:1351–1357. doi: 10.1016/j.amjcard.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Atkins K.M., Rawal B., Chaunzwa T.L. Cardiac radiation dose, cardiac disease, and mortality in patients with lung cancer. J Am Coll Cardiol. 2019;73:2976–2987. doi: 10.1016/j.jacc.2019.03.500. [DOI] [PubMed] [Google Scholar]
- 26.Du S., Zhou L., Alexander G.S. PD-1 modulates radiation-induced cardiac toxicity through cytotoxic t lymphocytes. J Thorac Oncol. 2018;13:510–520. doi: 10.1016/j.jtho.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu T.F., Rupnick M.A., Kerkela R. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370:2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Di Lorenzo G., Autorino R., Bruni G. Cardiovascular toxicity following sunitinib therapy in metastatic renal cell carcinoma: a multicenter analysis. Ann Oncol. 2009;20:1535–1542. doi: 10.1093/annonc/mdp025. [DOI] [PubMed] [Google Scholar]
- 29.Nazer B., Humphreys B.D., Moslehi J. Effects of novel angiogenesis inhibitors for the treatment of cancer on the cardiovascular system: focus on hypertension. Circulation. 2011;124:1687–1691. doi: 10.1161/CIRCULATIONAHA.110.992230. [DOI] [PubMed] [Google Scholar]
- 30.Touyz R.M., Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Onc. 2018;2:13. doi: 10.1038/s41698-018-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waliany S., Sainani K.L., Park L.S., Zhang C.A., Srinivas S., Witteles R.M. Increase in blood pressure associated with tyrosine kinase inhibitors targeting vascular endothelial growth factor. J Am Coll Cardiol CardioOnc. 2019;1:24–36. doi: 10.1016/j.jaccao.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bar J., Markel G., Gottfried T. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122–131. doi: 10.1016/j.ejca.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 33.Centanni M., Moes D., Troconiz I.F., Ciccolini J., van Hasselt J.G. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet. 2019;58:835–857. doi: 10.1007/s40262-019-00748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheng J., Srivastava S., Sanghavi K. Clinical pharmacology considerations for the development of immune checkpoint inhibitors. J Clin Pharmacol. 2017;57:S26–S42. doi: 10.1002/jcph.990. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.