There are reports of some patients with myeloproliferative disease (MPD) developing high-output heart failure (HOHF) (1). Another hematological disorder, multiple myeloma, is also known to be related to HOHF (2). According to the common pathological conditions in these 2 hematologic diseases, possible mechanisms for high-output states are increased oxygen demand secondary to splenomegaly and hepatomegaly caused by extramedullary hematopoiesis, and a reduction of systemic vascular resistance caused by elevated circulatory proinflammatory and angiogenic cytokines, such as interleukin (IL)-6, IL-8, tissue necrosis factor-alpha, and vascular endothelial growth factor (1,3,4).
Ruxolitinib, a JAK1/2 inhibitor, has recently emerged as a new therapeutic agent for MPD and is reported to improve splenomegaly and its associated symptoms by reducing extramedullary hematopoiesis (5). This has raised the possibility of JAK1/2 inhibition for the amelioration of HOHF symptoms associated with MPD by reducing extramedullary hematopoiesis and circulatory cytokines. Herein, we present a case that supports this possibility.
Case
A 75-year-old male patient with an 11-year history of MPD, which was initially diagnosed as polycythemia vera and later developed into myelofibrosis, was referred to our cardiovascular clinic for suspected heart failure. Except for hypertension, for which he was prescribed calcium-channel blockers, he had no prior history of cardiovascular disease. He was treated with hydroxyurea 6 years after the MPD diagnosis and developed shortness of breath during walking and peripheral edema 1 year later. Plasma brain natriuretic peptide (BNP) levels were 19.4 pg/ml at MPD diagnosis and increased to 143.9 pg/ml at the time of referral. Echocardiography identified dilatation of all 4 cardiac chambers (left ventricular end-diastolic diameter 61 mm; right ventricular end-diastolic diameter 48 mm; left atrial volume 74 ml; and right atrial volume 76 ml) along with hyperdynamic left ventricular function with mild mitral regurgitation and tricuspid regurgitation. The left ventricular end-diastolic diameter had increased from 52 mm at diagnosis to 61 mm at referral. HOHF associated with MPD was suspected, and right heart catheterization (RHC) was performed. The cardiac output was estimated using the Fick’s method, and the LaFarge equation was used for estimating oxygen consumption (6). Systemic vascular resistance was calculated by dividing the difference between mean arterial pressure and mean right atrial pressure by cardiac output. The findings were consistent with a high-output state: an elevated cardiac output (9.03 l/min), slightly elevated pulmonary capillary wedge pressure (11 mm Hg) and right atrial pressure (10 mm Hg), elevated mixed venous oxygen saturation (80%), and a decreased systemic vascular resistance (753.0 dynes•s•cm−5). A diagnosis of HOHF associated with MPD was made based on RHC results and marked splenomegaly, which provided evidence of extramedullary hematopoiesis. Other causes of high cardiac output state, namely, anemia, hyperthyroidism, vitamin insufficiency, systemic arteriovenous shunts, and cirrhotic liver disease, were excluded by appropriate blood analyses and a whole-body computed tomography scan. Oximetry analysis conducted during RHC was used to exclude intracardiac shunts. Existence of borderline pulmonary hypertension (PH), with a mean pulmonary artery pressure of 23 mm Hg, low diastolic pressure gradient (2 mm Hg), and low pulmonary vascular resistance (106.3 dynes•s•cm−5) were also detected.
The patient’s treatment was modified by replacing hydroxyurea with ruxolitinib after consultation with hematologists. Diuretic agents were not added as the cardiac filling pressure was only slightly elevated at rest.
The patient’s symptoms gradually improved, and plasma BNP levels were reduced to 51.2 pg/ml after 6 months, then normalized to 14.4 pg/ml after 1 year. Echocardiography 1 year after presentation to our clinic demonstrated a slight reduction in chamber sizes (left ventricular end-diastolic diameter 60 mm; right ventricular end-diastolic diameter 46 mm; left atrial volume 66 ml; and right atrial volume 64 ml). On RHC, there was a reduction in cardiac output (8.04 l/min), mean pulmonary capillary wedge pressure (9 mm Hg), and mean right atrial pressure (3 mm Hg). The mixed venous oxygen saturation was 71.1%, and systemic vascular resistance was 955.2 dynes•s•cm−5. Abdominal computed tomography demonstrated a reduction in spleen size (anterior to posterior diameter, from 195 to 185 mm; left to right diameter, from 105 to 99 mm; and longitudinal diameter, from 214 to 207 mm). PH also improved from a mean pulmonary artery pressure of 23 to 18 mm Hg, with a slight reduction in pulmonary vascular resistance (89.6 dynes•s•cm−5).
Discussion
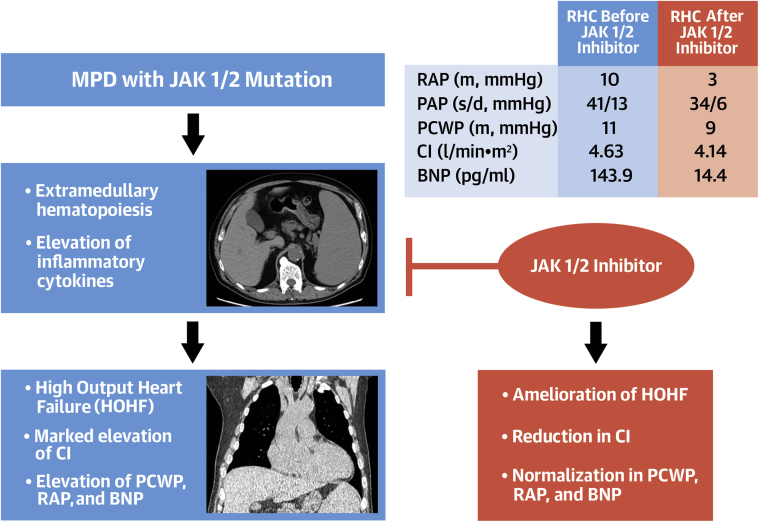
This case highlights the potential disease-modifying effect of JAK1/2 inhibition on HOHF associated with MPD (Figure 1). Reportedly, MPD accounts for 8% of HOHF, with poor survival in affected patients (1). Treatment of HOHF consists of supportive oxygen supplementation, diuretic agents, and control of its underlying cause. There is currently no established treatment, except supportive therapy for HOHF associated with MPD.
Figure 1.
JAK1/2 Inhibitor May Ameliorate High-Output Heart Failure Associated With Myeloproliferative Disease
MPD with JAK1/2 mutation often causes elevation of inflammatory cytokines and extramedullary hematopoiesis reflected in marked splenomegaly in the middle left panel. These 2 factors sometimes result in HOHF (typically presented with cardiomegaly shown in the lower left panel). Theoretically, JAK1/2 inhibition prohibits this pathway, and may ameliorate HOHF associated with MPD. Improved RHC data after the initiation of JAK1/2 inhibitor in our case (upper right table) support this hypothesis. BNP = brain natriuretic peptide; CI = cardiac index; HOHF = high-output heart failure; MPD = myeloproliferative disease; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; RAP = right atrial pressure; RHC = right heart catheterization.
Our observation regarding an improvement in HOHF with JAK1/2 inhibition may not be conclusive as it originates from a single case report. However, clinical improvement along with the concomitant reduction in plasma BNP level in the absence of any other intervention suggests that JAK1/2 inhibition may have contributed to the amelioration of HOHF. We used RHC to document an 11% reduction in cardiac output at rest following JAK1/2 inhibition treatment.
Our case report also suggests a hypothesis regarding the potential multifactorial pathophysiology of hemodynamic abnormalities in patients with MPD. In HOHF, a concomitant reduction in splenic size may be evidence of splenomegaly as a causal factor. This hypothesis is in line with a previous report that suggested the cause of HOHF related to other hematological disorders, including multiple myeloma, was increased blood circulation due to splenomegaly (2). However, in our case, the observed change in the spleen size was relatively small compared with that of a previous report (5). Although complete normalization of HOHF was not achieved, the normalization of plasma BNP level indicates that the partial reduction in cardiac output was clinically beneficial.
Interestingly, we also observed a reduction in mixed venous oxygen saturation and an increase in systemic vascular resistance after JAK1/2 inhibition. This improvement in vasodilatation disproportionate to the oxygen demand could be secondary to the effect of JAK1/2 inhibition on circulating inflammatory cytokines. In patients with MPD, plasma levels of pro-inflammatory and angiogenic cytokines, such as IL-6, IL-8, tissue necrosis factor-alpha, and vascular endothelial growth factor are reported to be elevated (4). In addition, the reduction of these cytokines after the initiation of ruxolitinib has been reported (7). Overall, these observations suggest the stabilization of inflammatory and angiogenic states with ruxolitinib may have a beneficial effect on the high-output state. Treatment with ruxolitinib also seemed to ameliorate PH. According to the low diastolic pressure gradient and pulmonary vascular resistance, the cause of PH in this patient was believed to be mainly due to a high cardiac output state. We observed a decrease in pulmonary vascular resistance, while systemic vascular resistance increased. Patients with MPD often develop pre-capillary PH, secondary to chronic thromboembolism in the pulmonary vasculature, portal hypertension, toxic effects of chemotherapeutic agents, and extramedullary hematopoiesis in the lungs. Although this needs further confirmation, our hypothesis is that a reduction in extramedullary hematopoiesis and cytokines by ruxolitinib may be beneficial for improvement of pre-capillary PH and the high-output state, and may have contributed to the reduction in BNP level. Last, we also observed the progression of anemia in this patient. The reduction of serum hemoglobin level after the introduction of ruxolitinib is known (5). Worsening of anemia is generally recognized as a contributor to the deterioration of the high-output state; however, here, the high-output state was improved even with the concurrent worsening of anemia. This observation suggests the risk of worsening anemia by ruxolitinib administration may be outweighed by the hemodynamic benefit.
Study limitations
The first limitation of our observation is that it is inferred from a single case report. Second, we did not formally assess exercise tolerance and its correlation with reported symptoms in this patient. A previous report analyzing 10 patients with HOHF associated with MPD reported a mean pulmonary wedge pressure of 16 mm Hg, cardiac output of 8.8 l/min, and a median systemic vascular resistance of 660 dynes•s•cm−5 (1). In light of these values, it appears that our patient had a milder hemodynamic disturbance. However, an apparent elevation in cardiac output and the patient’s self-reported shortness of breath during mild physical activity may have suggested an elevation of filling pressure during physical activity. Third, we were unable to analyze the long-term effect of JAK1/2 inhibition on cardiac output, pulmonary hypertension, exercise tolerance, and prognosis. Thus, to confirm our hypothesis, an adequately powered, double-blind, randomized controlled trial with endpoints including both short- and long-term clinical outcomes pertaining to heart failure, mortality, and exercise tolerance is required. However, given the relatively small number of patients with MPD-related HOHF or PH, a clinical trial may not be feasible, and retrospective and prospective cohort studies are needed. Fourth, we were unable to analyze circulating cytokines in this patient. Because one of the proposed mechanisms of HOHF is disproportionally reduced systemic vascular resistance secondary to elevated circulatory cytokines, these data may provide important translational information in elucidating the role of JAK1/2 inhibition in ameliorating the high-output state in MPD. In summary, JAK1/2 inhibitor may improve hemodynamic parameters and symptoms in patients with HOHF associated with MPD.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Reddy Y.N.V., Melenovsky V., Redfield M.M., Nishimura R.A., Borlaug B.A. High-output heart failure: a 15-year experience. J Am Coll Cardiol. 2016;68 doi: 10.1016/j.jacc.2016.05.043. 473–2. [DOI] [PubMed] [Google Scholar]
- 2.Robin J., Fintel B., Pikovskaya O., Davidson C., Cilley J., Flaherty J. Multiple myeloma presenting with high-output heart failure and improving with anti-angiogenesis therapy: two case reports and a review of the literature. J Med Case Rep. 2008;2:229. doi: 10.1186/1752-1947-2-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tefferi A., Vaidya R., Caramazza D., Finke C., Lasho T., Pardanani A. Circulating interleukin (IL)-8, IL-2R, IL-12, and IL-15 levels are independently prognostic in primary myelofibrosis: a comprehensive cytokine profiling study. J Clin Oncol. 2011;29:1356–1363. doi: 10.1200/JCO.2010.32.9490. [DOI] [PubMed] [Google Scholar]
- 4.Hoermann G., Greiner G., Valent P. Cytokine regulation of microenvironmental cells in myeloproliferative neoplasms. Mediators Inflamm. 2015;2015:869242. doi: 10.1155/2015/869242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verstovsek S., Mesa R.A., Gotlib J. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med. 2012;366 doi: 10.1056/NEJMoa1110557. 799–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaFarge C.G., Miettinen O.S. The estimation of oxygen consumption. Cardiovasc Res. 1970;4:23–30. doi: 10.1093/cvr/4.1.23. [DOI] [PubMed] [Google Scholar]
- 7.Greenfield G., McPherson S., Mills K., McMullin M.F. The ruxolitinib effect: understanding how molecular pathogenesis and epigenetic dysregulation impact therapeutic efficacy in myeloproliferative neoplasms. J Transl Med. 2018;16:360. doi: 10.1186/s12967-018-1729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]