Abstract
Background
Adult survivors of Hodgkin lymphoma (HL) are at increased risk of cardiovascular (CV) events secondary to mediastinal radiation therapy (RT).
Objectives
In this group of patients, we assessed the association between cardiopulmonary exercise testing (CPET), as determined by percent-predicted peak Vo2 (ppVo2peak), and clinical outcomes, as well as the rate of ppVo2peak decline and sex differences.
Methods
All survivors of HL who were >10 years post chest RT and who underwent ≥1 CPET were enrolled from a single center. Traditional CV and treatment risk factors, along with CV events, were ascertained.
Results
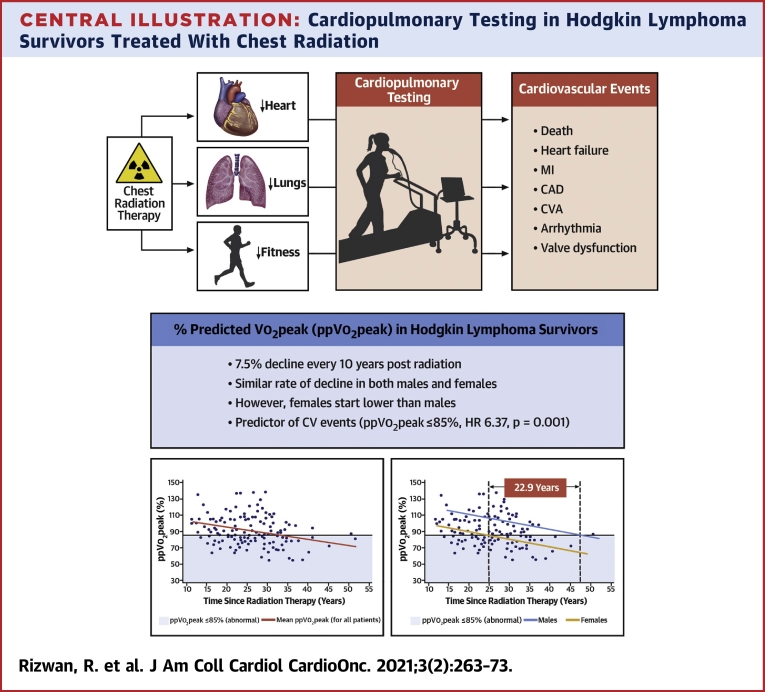
A total of 64 patients (67% female; median age 51 years [range 26 to 70 years]) with a median follow-up time after RT of 23 years (range 11 to 41 years), and 141 CPET studies, were included. Median initial ppVo2peak was 91% (range 58% to 138%). ppVo2peak in survivors declined by 7.5 percentage points every 10-year period after RT, as compared with age- and sex-based norms (P = 0.001), even after adjusting for hypertension and history of anthracycline. Both male and female patients had a similar rate of ppVo2peak decline. However, women had a lower ppVo2peak at all times, and they developed abnormal ppVo2peak (≤85%) on average earlier than men (24.1 years vs 47.0 years after RT). Patients with abnormal ppVo2peak vs normal ppVo2peak (>85%), had an increased risk of CV events (59% vs 16%). Abnormal ppVo2peak was independently associated with the risk of CV events (adjusted HR: 6.37; 95% CI: 2.06-19.80; P = 0.001).
Conclusions
Percent-predicted Vo2peak in long-term survivors of HL who were treated with chest RT progressively declined as compared with population- and sex-based norms. Importantly, women developed abnormal ppVo2peak more than 2 decades earlier than male survivors. Abnormal ppVo2peak was associated with an increased risk of CV events in this group of patients.
Key Words: cardiac events, cardiopulmonary test, Hodgkin lymphoma, radiation, sex, Vo2, women
Abbreviations and Acronyms: BCH, Boston Children’s Hospital; CPET, cardiopulmonary exercise testing; CV, cardiovascular; HL, Hodgkin lymphoma; LSM, least squares mean; ppVo2peak, percent-predicted peak volume oxygen; RER, respiratory exchange ratio; RT, radiation therapy
Central Illustration
Introduction
Children and adolescents with Hodgkin lymphoma (HL) have a 5-year survival rate of >95% (1). This high cure rate has enabled the emergence of >35,000 long-term survivors of HL currently living in the United States (2). However, cardiovascular (CV) sequelae resulting from exposure to curative mediastinal radiation therapy (RT) are among the leading causes of nonmalignant premature death in these long-term survivors. As compared with the general population, the CV morbidity and mortality in HL survivors of HL are 3- to 5-fold more common (2, 3, 4, 5, 6, 7, 8, 9, 10).
Many long-term survivors of HL experience exercise intolerance. Cardiopulmonary exercise testing (CPET) is considered the gold standard for assessment of CV fitness and functional capacity, and it provides valuable data on exercise duration and peak myocardial oxygen consumption (Vo2peak). Vo2peak has been correlated with all-cause mortality in patients with heart failure (11,12). Recent studies have shown the association of abnormal Vo2peak with all-cause mortality and CV mortality among all survivors of childhood cancers (13, 14, 15). Therefore, surveillance of long-term survivors of HL and other cancers with CPET may serve as an additional method of detecting early signs of CV disease and assessing its severity while providing additional information on cardiopulmonary function. Of note, changes in exercise capacity often develop slowly and even before onset of symptoms, but CPET measures can demonstrate subclinical cardiopulmonary limitations not apparent by history or physical examination.
Given that Vo2peak is affected by age, sex, and muscle mass, percent predicted Vo2peak (ppVo2peak) also obtained by CPET may be useful because it provides age-, sex-, and weight-adjusted values for assessment (16). Therefore, ppVo2peak measurements may potentially serve as an additional tool for surveillance of CV health and assessment of sex differences in long-term survivors of HL. Systematic analysis of changes in ppVo2peak over time and its effect on mortality may provide further insight into cardiopulmonary limitations in long-term survivors of HL. Quantifying the rate of ppVo2peak decline may also help guide the establishment of CV screening timelines. This study sought to assess changes in ppVo2peak over time and determine the association between abnormal ppVo2peak and adverse CV events (fatal and nonfatal). We hypothesized that ppVo2peak declines in HL survivors at a higher rate than in the general population. We also aimed to assess whether there is any difference in the rate of decline of ppVo2peak among male and female survivors.
Patients and Methods
At the Boston Children’s Hospital (BCH, Boston, Massachusetts) Cardiovascular Health for Cancer Survivors Clinic, adult survivors of HL who were treated with high-dose mediastinal radiation (>30 Gy) at any of the 3 Harvard Medical School teaching hospitals have been followed over the last decade (10). All participated in the prospective 3-year serial cardiac screening protocol of almost 200 asymptomatic survivors of HL, conducted by Chen et al (17). As part of the cardiac screening protocol, long-term survivors of HL additionally underwent prospective longitudinal screening by echocardiography and stress echocardiography at regular intervals along with routine cardiology visits (17). The subset of all HL patients (n = 64) who had undergone additional testing with CPET at BCH during the ensuing decade after the asymptomatic screening study formed the current study cohort of this retrospective review. The demographic features of this cohort were not different from the cohort reported by Chen et al, except that current study participants were slightly younger at the time of RT (Supplemental Table 1). HL survivors were generally seen annually at BCH, and CPETs were performed every 1 to 3 years to assess exercise tolerance in those patients who could exercise. CPETs were not performed to rule out ischemia. Patients’ characteristics, CV risk factors, and treatment data were extracted from clinical visits and/or the Dana-Farber Cancer Institute (Boston, Massachusetts) radiation oncology database and subsequently analyzed. The study was approved by the BCH and Dana-Farber Cancer Institute Institutional Review Boards.
CPET protocol
Stress testing and CPET were conducted according to standardized institutional (BCH) laboratory protocol (Supplemental Methods). The target respiratory exchange ratio (RER) was ≥1.09. Data from patients with an RER<1.09 were retained if maximal effort was demonstrated. Tracings were reviewed to assess for Vo2 plateau. Vo2peak data were obtained, along with ppVo2peak, the latter allowing for comparison with sex- and age-based norms with use of the Jones prediction equation (18). Pre-exercise spirometry values were collected immediately before testing through breath-by-breath expiratory gas analysis to evaluate pulmonary function, including percent-predicted FVC, percent-predicted FEV1, FEV1/FVC ratio, and presence of obstructive or restrictive lung disease patterns. Presence of obstructive or restrictive spirometry patterns was determined according to the BCH protocol, which was similar to the criteria used by Stenehjem et al (19). Resting echocardiograms performed within 6 months of CPET were used to quantify the LVEF and to assess its correlation with ppVo2peak values.
Analysis of ppVo2peak change over time after RT
The ppVo2peak values from all the CPET studies were extracted and used to estimate the rate of change in ppVo2peak with every ensuing decade of follow-up after RT.
Normal VERSUS abnormal ppVo2peak
The cohort was also divided into 2 groups on the basis of their ppVo2peak results with use of the following BCH clinical standard at the time these studies were performed: normal ppVo2peak (>85%) and abnormal ppVo2peak (≤85%). Patients’ characteristics and CV outcomes were compared between the groups. For patients who underwent serial testing (>1 test), patients were grouped in the abnormal ppVo2peak group (≤85%) if any of their ppVo2peak results were ≤85%. Two patients had a bicycle ppVo2peak only.
CV events (outcome or ENDPOINT)
The primary CV endpoint was a composite of coronary artery disease requiring stenting or coronary artery bypass grafting, myocardial infarction, CV death, cerebrovascular disease, moderate to severe valvular stenosis, cardiac valve replacement, New York Heart Association functional class II to IV heart failure or cardiomyopathy requiring medical management, atrial fibrillation or flutter, and complete heart block.
Statistical analysis
With inclusion of ppVo2peak values from all available CPET studies, mixed effects linear regression was used to estimate the mean rate of decline in ppVo2 over time from curative RT. Least squares mean (LSM) decline was reported for each 10-year increment. Prespecified interactions with sex, anthracycline use, and hypertension were examined. Cox regression was used to investigate the association between abnormal ppVo2peak and future CV events; abnormal ppVo2peak was treated as a time-varying covariate. Competing risk analysis was not necessary because no noncardiac deaths were observed during the study. Further details of statistical methods used are included in the Supplemental Methods section.
Results
Patient demographics
A total of 64 patients (with 141 total CPET studies with ppVo2peak), all of whom had chest RT and met entry criteria, were included in the study. Patients’ characteristics and test results of the cohort are displayed in Table 1. Median age was 51 years (range 26 to 71 years), and 43 (67%) were female. Median BMI was 25.6 kg/m2 (range 17.6 to 36.3 kg/m2). All participants were Caucasian except for 1, who was Asian. Median age at cancer diagnosis was 25 years (range 6 to 55 years), and 53 (83%) of patients had a diagnosis before the age of 35 years. Median time from RT exposure to CPET testing was 23 years (range 11 to 51 years). Anthracycline was administered to 26 (41%) patients in addition to RT, and 18 (28%) patients had prevalent hypertension at the time of CPET. Importantly, clinical characteristics of the study cohort did not differ significantly from the larger cohort of Chen et al, with the exception that the current group was slightly younger at time of cancer treatment (median age 24 years vs. 28 years) (Supplemental Table 1).
Table 1.
Patient Characteristics
| All Patients (N = 64) | Normal (>85%) ppVo2peak (n = 32) | Abnormal (≤85%) ppVo2peak (n = 32) | P Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age at first CPET post radiation therapy (yrs) | 51 (26-71) | 49 (26-71) | 52 (39-66) | 0.246 |
| Female | 43 (67) | 16 (50) | 27 (84) | 0.003 |
| Body mass index (kg/m2) | 25.6 (17.6-36.3) | 26.1 (20.1-35.6) | 23.7 (17.6-36.3) | 0.022 |
| Treatment characteristics | ||||
| Time since radiation therapy (yrs) | 23 (11-51) | 21 (11-34) | 27 (13-51) | 0.001 |
| Age at radiation therapy completion (yrs) | 25 (6-55) | 25 (6-55) | 20 (8-41) | 0.057 |
| Chest radiation therapy dose (cGy)∗ | 3,978 (2,540-7,700) | 3,928 (2,540-4,560) | 4,000 (2,600-7,700) | 0.101 |
| Radiation field | ||||
| Mantle | 16 (25) | 10 (31) | 6 (19) | 0.145 |
| Mantle and para-aortic | 35 (55) | 13 (41) | 22 (69) | |
| Mini-mantle | 1 (1.6) | 1 (3.1) | 0 (0) | |
| Mantle and cardiac | 4 (6.3) | 3 (9.4) | 1 (3.1) | |
| Chemotherapy received | 31 (48) | 19 (59) | 12 (39) | 0.080 |
| Anthracyclines | 26 (41) | 17 (53) | 9 (28) | 0.042 |
| Anthracycline dose (mg/m2)† | 240 (80-240) | 240 (160-240) | 160 (80-240) | 0.267 |
| Splenectomy | 41 (64) | 18 (56) | 23 (77) | 0.090 |
| Comorbidities | ||||
| Smoking 100+ cigarettes | 16 (25) | 5 (16) | 11 (34) | 0.083 |
| Smoking within 5 yrs of CPET | 5 (8) | 2 (6) | 3 (9) | 1.000 |
| Overweight or obese | 34 (53) | 21 (66) | 13 (41) | 0.045 |
| Elevated blood glucose level or diabetes mellitus medications | 0 (0) | 0 (0) | 0 (0) | N/A |
| Hypertension or hypertension medications | 18 (28) | 7 (22) | 11 (34) | 0.266 |
| Hyperlipidemia or hyperlipidemia medications | 36 (56) | 18 (56) | 18 (58) | 0.884 |
| Elevated high-sensitivity C-reactive protein | 16 (25) | 9 (28) | 7 (23) | 0.667 |
Values are median (range) or n (%). Note: For patients who underwent serial testing, only 1 CPET result was used for each patient. For subjects with no cardiovascular event, results from the first CPET was used; for patients with cardiovascular event(s), data from the first abnormal test were used.
CPET = cardiopulmonary exercise testing; N/A = not applicable; ppVo2peak = percent-predicted peak Vo2.
Available for only 30 patients in the Abnormal ppVO2peak group.
Available for 16 patients only.
CPET and Vo2peak results
All patients included underwent close exercise physiologist or provider supervision during CPET, and they demonstrated and self-confirmed maximal effort. The majority of patients had more than 1 CPET over the duration of follow-up of this study. Of the 64 patients, 48 (75%) had more than 1 CPET; 28 of these 48 patients had 2 tests, 12 had 3 tests, 7 had 4 tests, and 1 had 5 tests. RER was ≥1.09 in 94% of all CPET studies. No patients had premature CPET termination secondary to chest pain, presyncope, or ventricular tachycardia. At the time of CPET, subjects exercised for a median of 7.6 METS, with a Vo2peak of 26.4 cc/min/m2 (Table 2). The median ppVo2peak of the cohort was 85% (range 42%-138%), with 32 (50%) patients having an abnormal ppVo2peak of ≤85%. Fifteen CPET studies had an RER <1.09, and 3 of these studies had an RER <1.00. Only 4 of 10 patients with an RER <1.09 were able to achieve an RER ≥1.09 on subsequent retesting. Importantly, the ppVo2 of these 4 patients continued to decline despite achieving a slightly higher RER on a subsequent test.
Table 2.
CPET Data
| All Patients (N = 64) | Normal (>85%) ppVo2peak (n = 32) | Abnormal (≤85%) ppVo2peak (n = 32) | P Value | |
|---|---|---|---|---|
| Cardiovascular function | ||||
| Resting heart rate (beats/min) | 87 (55-127) | 82 (55-103) | 92 (70-127) | 0.001 |
| Peak heart rate (beats/min) | 163 (111-210) | 170 (150-210) | 154 (111-196) | <0.001 |
| Heart rate reserve (beats/min) (peak heart rate − resting heart rate) | 76.5 (30-141) | 91 (50-141) | 58 (30-126) | <0.001 |
| Heart rate 1 min post exercise (beats/min) | 136 (95-166) | 138 (95-166) | 132 (100-157) | 0.102 |
| Heart rate recovery difference at 1 min post exercise (beats/min) | 28 (7-71) | 32 (18-71) | 25 (7-46) | 0.001 |
| Abnormal heart rate recovery∗ | 9 (14) | 1 (3) | 8 (25) | 0.026 |
| Peak METS | 7.6 (3.8-12.8) | 9 (5-12.8) | 6.7 (3.8-9.5) | <0.001 |
| Peak O2 pulse (Vo2peak/heart rate) (mL/beat) | 11.4 (5.2-22.5) | 14.9 (8.9-22.5) | 9.3 (5.2-18.6) | <0.001 |
| % Predicted peak O2 pulse | 92 (43-139) | 103.5 (78-139) | 83 (43-107) | <0.001 |
| Vo2peak (L/min) | 26 (13-45) | 32 (17-45) | 23 (13-33) | <0.001 |
| ppVo2peak (%) | 85 (42-138) | 105 (86-138) | 76 (42-85) | <0.001 |
| RER | 1.16 (0.98-1.38) | 1.17 (1.00-1.38) | 1.16 (0.98-1.24) | 0.307 |
| VAT/ppVo2peak (%) | 52 (25-95) | 59 (46-95) | 45 (25-67) | <0.001 |
| Ve/Vco2 slope (nl <29) | 26 (20-37) | 26 (20-30) | 27 (20-37) | 0.226 |
| LVEF (%, echocardiogram ±6 months)† | 61 (37-71) | 62 (37-71) | 58 (47-65) | 0.098 |
| LVSD† | 3 (7.1) | 1 (4.2) | 2 (11) | 0.567 |
| Pulmonary function | ||||
| Median FEV1 (L) | 2.64 (0.91-4.63) | 3.14 (2.03-4.63) | 2.16 (0.91-3.82) | <0.001 |
| Predicted FEV1 (%) | 93 (44-129) | 95 (64-129) | 82 (44-111) | 0.001 |
| Breathing reserve (%) | 31 (-8 to 64) | 29 (-8 to 49) | 34 (3 to 64) | 0.016 |
| Breathing reserve ≤25% | 36 (56) | 17 (53) | 19 (59) | 0.614 |
| Spirometry pattern (type) | 0.001 | |||
| Normal | 34 (53) | 25 (78) | 9 (28) | |
| Obstructive | 20 (31) | 6 (19) | 14 (44) | |
| Restrictive | 1 (2) | 0 (0) | 1 (3) | |
| Both restrictive and obstructive | 9 (14) | 1 (3) | 8 (25) | |
| Moderate or severe pulmonary disease | 10 (16) | 0 (0) | 10 (31) | 0.001 |
Values are median (range) or n (%). For patients who underwent serial testing, only 1 CPET result was used for each patient in this table: for patients with no cardiovascular event, data from the first test were used. For patients with cardiovascular event(s), data from the first abnormal test were used.
CPET = cardiopulmonary exercise testing; LVSD = left ventricular systolic dysfunction, defined as LVEF <50%; nl = normal; ppVo2peak = percent-predicted peak Vo2; RER = respiratory exchange ratio; VAT = ventilatory anaerobic threshold; Ve/Vco2 = minute ventilation to carbon dioxide production.
Abnormal heart rate recovery: ≤18 beats/min decrease at 1 minute post exercise with passive recovery.
For all patients, n = 42; for the normal ppVo2peak group, n = 24; for the abnormal ppVo2peak group, n = 18; Fisher exact test was used to assess significance for LVSD.
In examining the rate of decline of ppVo2peak of the cohort 10 years after RT, LSM ppVo2peak was found to decrease by 7.5 percentage points every 10 years (P = 0.001, 95% CI: −12.0% to −3.1%) (Figure 1). The relationship between ppVo2peak and time since radiation exposure was significant even after adjusting for sex, history of anthracycline use, and history of hypertension (Table 3).
Figure 1.
Decline of ppVo2peak Over Time After Radiation Therapy
(Top) Least squares mean percent-predicted peak Vo2 (ppVo2peak) decreased by 7.5 percentage points every 10 year-interval after radiation therapy (P = 0.001) (0.75 percentage point decrease per year, assuming a linear decline after 10 years from radiation exposure). (Bottom) The percentage of abnormal cardiopulmonary exercise testing (CPET) results (ppVo2peak ≤85%, shaded) increased over time. At 40 years after radiation therapy, 100% of cardiopulmonary exercise testing results had abnormal ppVo2peak.
Table 3.
Mixed Effects Linear Regression Analysis to Estimate the Mean Decline in Percent-predicted Peak Vo2 for Each Additional Decade Since Radiation Exposure, Adjusting for Potential Confounders
| Least Squares Mean (95% Confidence Interval) | P Value | |
|---|---|---|
| Model 1: unadjusted | ||
| Mean change per 10 yrs | −7.5% (−12.0% to −3.1%) | 0.001 |
| Model 2: adjusted for sex | ||
| Mean change per 10 yrs | −9.2% (−13.0% to −5.4%) | <0.001 |
| Female | −21.0% (−28.1% to −13.9%) | <0.001 |
| Model 3: adjusted for anthracycline use | ||
| Mean change per 10 yrs | −8.7% (−14.3% to −3.1%) | 0.002 |
| Anthracycline use | −3.6% (−14.0% to 6.7%) | 0.493 |
| Model 4: adjusted for history of hypertension | ||
| Mean change per 10 yrs | −7.0% (−11.7% to −2.4%) | 0.003 |
| Hypertension | −3.5% (−13.0% to 5.9%) | 0.462 |
The analysis was performed with use of measurements from all cardiopulmonary exercise tests (n = 141).
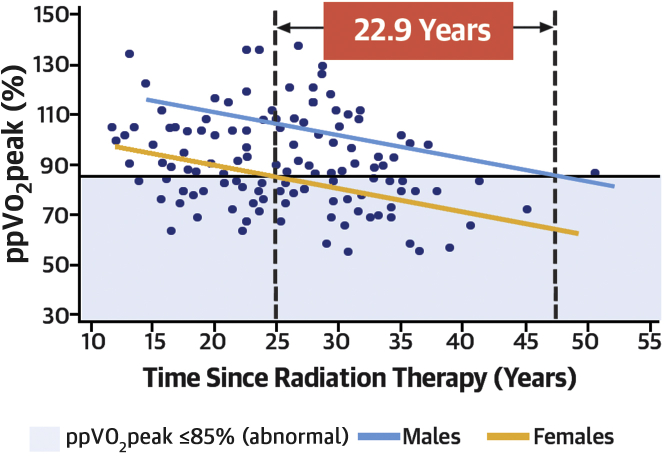
LSM ppVo2peak decreased for both male and female patients at a similar rate, but ppVo2peak was lower for female patients across all times. LSM ppVo2peak values in women were abnormal (≤85%) at 24.1 years after RT exposure, whereas in male patients, not until 47 years after RT did values become abnormal, ∼23 years later than in female patients (Figure 2). Treatment-related risk factors, including radiation fields, were not significantly different between male and female patients. Of note, female patients had fewer traditional cardiac risk factors than male patients, with women having lower lipid levels (P = 0.001) and a lower likelihood of being overweight or obese (P = 0.003). A detailed comparison of demographic factors is presented in Supplemental Table 2.
Figure 2.
Rate of ppVo2 Decline by Sex
Least squares mean percent-predicted peak Vo2 (ppVo2peak) decreased at a similar rate for both male (blue) and female (yellow) patients, but it was lower for female patients at all times. Least squares mean ppVo2peak in female patients was abnormal (≤85%) 22.9 years earlier than in male patients, as represented by the intersection of dotted vertical lines and the solid horizontal line.
The ppVo2peak decreased by 13.2 percentage points per 10 years (P < 0.001; 95% CI: −20.1% to −6.3%) for patients who were treated with RT alone, but it did not change significantly for those treated with both RT and anthracyclines (decline of 0.4 percentage point per 10 years; P = 0.940; 95% CI: −9.5% to 8.8%) (Supplemental Figure 1). Importantly, most of the latter group had a shorter follow-up time because combination therapy was introduced into standard HL treatment at least 15 years later than RT alone. The presence or absence of hypertension did not have any significant impact on the rate of change in ppVo2peak.
Comparison of patients with normal (>85%) VERSUS abnormal (≤85%) ppVo2peak
Patients with abnormal ppVo2peak vs normal ppVo2peak were more likely to have undergone mantle and para-aortic RT (73% vs. 41%; P = 0.009), less likely to have undergone combination RT and anthracycline therapy (28% vs. 53%; P = 0.042), and less likely to be overweight or obese at time of testing (41% vs. 66%; P = 0.045), with a lower BMI (23.7 vs. 26.1 kg/m2; P = 0.022) (Table 1). The relationship of BMI with ppVo2peak is presented in Supplemental Figure 2. Patients in the abnormal ppVo2peak group were more likely to be female (84% vs. 50%; P = 0.003) and have a longer duration of follow-up (median 27 years vs. 21 years; P = 0.001). On exercise stress testing, patients with abnormal ppVo2peak had a higher resting heart rate (92 beats/min vs. 82 beats/min; P = 0.001) and a lower peak heart rate (154 beats/min vs. 170 beats/min; P < 0.001), and they were more likely to have abnormal heart rate recovery (25% vs 3%; P = 0.026). From a pulmonary standpoint, patients with abnormal ppVo2peak had lower FEV1 (median 2.16 L vs. 3.14 L; P < 0.001) and predicted FEV1 (median 82% vs. 95%; P = 0.001). They were more likely to have moderate to severe pulmonary disease patterns on spirometry (31% vs. 0%; P = 0.001), most commonly obstructive findings (14 of 32; 44%). Tables 1 and 2 present detailed comparisons of patient-related factors and CPET results between groups.
CV events and prognostic value of ppVo2peak
There was a total of 24 CV events in the cohort (Table 4). Overall, patients with abnormal ppVo2peak experienced more CV events compared with patients with normal ppVo2peak (59% vs. 16%; P < 0.001).
Table 4.
Patient Characteristics at First Occurrence of Cardiovascular Events by ppVo2peak Status
| Total No. of Patients With Events | Patients With Normal (>85%) ppVo2peak | Patients With Abnormal (≤85%) ppVo2peak | P Value | |
|---|---|---|---|---|
| Cardiovascular events | 24 | 6 | 18 | — |
| Types of cardiovascular events | ||||
| Coronary artery disease | 7 | 1 | 6 | — |
| Valvular disease | 3 | 1 | 2 | |
| Sudden cardiac death | 1 | 0 | 1 | |
| Arrhythmia | 3 | 1 | 2 | |
| Cerebrovascular disease or stroke | 3 | 1 | 2 | |
| NYHA heart failure (II-IV) | 7 | 1 | 6 | |
| Patient age at first event (yrs) | 55 (21-69) | 52 (43-67) | 55 (21-69) | 0.764 |
| Time to event from radiation therapy (yrs) | 29 (0-45) | 24 (20-33) | 29 (0-45) | 0.443 |
Values are median (range) or n. Coronary artery disease and NYHA heart failure (functional class II to IV) were the most common cardiovascular events. There were no noncardiovascular deaths.
NYHA = New York Heart Association; ppVo2peak = percent-predicted peak Vo2.
In 6 patients, CV events occurred before their first CPET; these subjects were excluded from subsequent analysis. Of the remaining 58 patients, 26 patients with abnormal ppVo2peak were more likely to experience an adverse CV event, with 13 of 26 (50%) experiencing a CV event within 5 years of an abnormal ppVo2peak. After adjusting for hypertension and BMI, abnormal ppVo2peak was an independent predictor of future CV events in HL survivors (HR: 6.37; 95% CI: 2.06 to 19.80; P = 0.001) (Table 5). LVEF was found not to be correlated with ppVo2peak (Pearson correlation: 0.06; P = 0.55; median 60% [range 29% to 79%]).
Table 5.
Cox Regression Analysis Showing the Risk of Cardiovascular Events for Abnormal ppVo2peak
| Covariates (n = 58)∗ | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|
| Unadjusted | ||
| Abnormal (≤85%) ppVo2peak | 3.48 (1.24-9.77) | 0.018 |
| Adjusted for confounders | ||
| Abnormal (≤85%) ppVo2peak | 6.37 (2.06-19.8) | 0.001 |
| Hypertension† | 15.1 (4.39-51.9) | <0.001 |
| Overweight or obesity | 0.60 (0.20-1.83) | 0.371 |
CPET = cardiopulmonary exercise testing; ppVo2peak = percent-predicted peak Vo2.
Of the 64 patients, 6 were excluded because of the occurrence of a cardiovascular event before their first CPET.
Patients with a history of antihypertensive medication use were also included.
Discussion
The study represents one of the largest cohorts of long-term survivors of HL who were treated with chest RT and who were clinically phenotyped with serial CPET with ppVo2peak measurements, over a median follow-up after RT ranging from 11 to 51 years (Central Illustration). The long duration of follow-up after RT allowed correlation of ppVo2peak with adverse CV events in these patients. We observed a steady decline in ppVo2peak in survivors, as compared with age- and sex-based norms with each ensuing decade after RT. This finding demonstrated that HL survivors become progressively more limited than the general population as they age. Women survivors were especially affected, with significantly lower mean ppVo2peak than men across the follow-up time and a greater proportion of abnormal ppVo2peak. HL survivors with abnormal ppVo2peak were 6 times more likely to experience adverse CV events compared with survivors of HL with normal values. Overall, the association between abnormal ppVo2peak and increased risk of CV events highlights the importance of ppVo2peak in long-term survivors of HL.
Central Illustration.
Cardiopulmonary Testing in Hodgkin Lymphoma Survivors Treated With Chest Radiation
Long-term survivors of Hodgkin lymphoma survivors treated with high-dose mediastinal radiation therapy (>30 Gy) have progressive decline in cardiopulmonary function and fitness, quantitated by percent-predicted peak Vo2 (ppVo2peak) on cardiopulmonary exercise testing. (Left graph) A decline in ppVo2peak of 7.5 percentage points per 10 years was found. (Right graph) Despite a similar rate of decline between female patients (yellow line) and male patients (blue line), female survivors of Hodgkin lymphoma had lower percent-predicted peak Vo2 at all follow-up times and a higher frequency of abnormal percent-predicted peak Vo2. The percent-predicted peak Vo2 in female patients declined to abnormal levels almost 23 years earlier than in male patients. Light blue shading denotes an abnormal percent-predicted peak Vo2 region. HR = hazard ratio; MI = myocardial infarction; ↓ = decreased function.
Many previous cardiometabolic studies in survivors of childhood cancer have examined the correlation of CPET findings with echocardiographic measures between cancer survivors and healthy control subjects, without focusing on the association of Vo2peak with clinical outcomes (20,21). Recently, 2 large studies used CV fitness to demonstrate an association with mortality and CV measurements in cancer survivors. In a large single-center cohort analysis of 1,632 patients with all types of adult-onset cancer, Groarke et al (22) demonstrated an association with cardiorespiratory fitness (measured by METs) and all-cause mortality, including cancer and CV disease, although cardiometabolic data were not examined. In a large cohort of survivors of childhood cancer, Ness et al (15) demonstrated the association of exercise intolerance, as measured by CPET with Vo2peak, with overall mortality. The latter study, however, included survivors of all types of childhood cancer (<30% HL) who were exposed to RT and/or chemotherapy. In contrast, the current study examined a homogenous population of long-term survivors of HL all treated with chest RT, with or without chemotherapy. Furthermore, endpoints for adverse events in the current study focused on CV events (both fatal and nonfatal), compared with others where endpoints were all-cause mortality. In the study by Ness et al (15), 50% of the events were secondary cancers, and only 5 were cardiac events, without assessment of nonfatal CV events. The current study is also the first, to our knowledge, to describe the rate of decline of ppVo2peak in long-term survivors of HL, overall and by sex, with the determination that women develop abnormal ppVo2 almost 25 years earlier than men. In establishing a cut point (ppVo2peak ≤85%) where patients with decreased exercise tolerance were at increased risk for adverse CV outcomes, and delineating the impact of percentage of decrease in ppVo2peak on risk for CV events, these data may provide clinicians with further data for risk stratifying HL survivors for follow-up care and for tailoring risk assessment for these patients.
The overall decline in ppVo2peak over time is especially striking, as is the difference in ppVo2peak measurements between male and female patients, showing a consistently lower ppVo2peak in female patients throughout the years. Female HL survivors were more debilitated from the same RT and cancer therapy, despite having fewer traditional cardiac risk factors than their male counterparts. As seen in Figure 2, female patients had lower ppVo2peak at all time-points than male patients, but ppVo2peak in female patients declined to abnormal levels (≤85%) almost 23 years earlier.
Interestingly, patients who underwent mantle and para-aortic RT were more likely to have abnormal ppVo2peak although this was not statistically significant. Increased size of radiation fields may be associated with greater debility in patients and should be examined in larger RT data sets. However, contrary to our expectations, combination therapy with anthracycline and RT was less common in patients with abnormal ppVo2peak, thus warranting further exploration and validation. The findings of higher prevalence of elevated resting heart rate and abnormal heart rate recovery in patients with abnormal ppVo2peak are consistent with earlier studies of autonomic dysfunction in these patients (15,23). In addition, patients can have other comorbidities that further contribute to heart rate abnormalities such as impaired diastolic relaxation. Limited echocardiographic data performed at the time of CPET demonstrated abnormal mitral E/A Doppler ratio and higher A waves, suggesting greater frequency of diastolic dysfunction in patients with abnormal ppVo2peak. However, more systematic diastolic evaluation is warranted because this was outside the scope of this retrospective study. Importantly, there was no difference in LVEF and LV systolic dysfunction (LVEF ≤50%) between the normal and abnormal ppVo2peak groups. Because ppVo2peak is an integrative assessment of 3 different organ systems—cardiac, pulmonary, and musculoskeletal, one would not necessarily expect that echocardiographic indices alone could be a surrogate for other systems. Another noteworthy finding is the higher prevalence of abnormal spirometry patterns in nearly one-half of the cohort, particularly obstructive pulmonary disease, an observation that was also made by Ness (15) and should be explored in future studies. Of note, all our patients were exposed to high-dose chest RT (≥3,000 cGy), and some also had bleomycin, as compared with the study by Ness et al (15), where only ∼10% of participants had ≥3,000 cGy chest RT.
The correlation between abnormal ppVo2peak and adverse CV events underscores the potential importance of exercise testing and early detection of at-risk individuals in improving CV morbidity or mortality in long-term survivors of HL. Furthermore, higher maximal exercise capacity consistently has been shown to predict a lower risk of adverse cardiac events at all ages (22), and decline in exercise capacity with aging can be mitigated with regular exercise (13). Although this study was not targeted to quantitate exercise in HL survivors, there are data to show that exercise therapy improves CV fitness in patients with cancer (14). Several small exercise intervention trials in cancer survivors have demonstrated improvements in Vo2peak, as well as patient-reported physical function and fatigue (24). Our findings further support the importance of improving CV fitness for survivors and potentially the opportunity to alter long-term outcomes for high-risk patients. Our multivariable analysis demonstrated that ppVo2peak independently was associated with adverse CV events, even after adjusting for traditional cardiac risk factors. Therefore, CPET with ppVo2peak testing has the potential to improve management of long-term survivors of HL by identifying those at higher risk of CV events and opportunities for earlier exercise and medical intervention, even for those patients with normal LVEF. As with the larger cohort of HL survivors reported by Chen et al (17), the study reinforces the association of hypertension with occult CV disease and adverse CV events in long-term survivors of HL.
Study limitations
The results are from a single center, and the size of the cohort was relatively small. There may be selection bias, although our patients’ demographic features were similar to those of the larger HL cardiac cohort reported by Chen et al (17). By definition, a study of exercise CPET has a selection bias toward those who are more fit and able to exercise; therefore, our findings may not apply to more debilitated survivors. Systemic quantitative self-reported physical activity levels at the time of testing were not available for our participants; however, qualitatively, most survivors of HL were not sedentary in their jobs or lives. In addition, the Jones prediction equation for cycle ergometry results in a conservative estimate of the true number of patients with abnormal ppVo2peak. The use of the FRIEND (Fitness Registry and the Importance of Exercise National Database) treadmill equation will likely yield an even higher prevalence of patients with abnormal ppVo2peak than reported in this study (25). Therefore, the decline in ppVo2peak for survivors is at least 7.5 percentage points/10-year period. Because this is the first study that examined serial CPET with ppVo2peak in survivors of HL, future prospective studies using different treadmill equations will further inform the upper bound of the rate of decline in survivors of childhood cancers as they age. Intrinsic to studying survivors treated decades earlier, all patients were treated with high-dose chest RT, the standard of care of the time (26). Current advances in HL treatment have shifted away from mediastinal RT except in aggressive disease and instead rely on anthracycline as first-line therapy. Further studies are under way to evaluate the intermediate-term effects on more recently treated HL survivors. Another limitation is that even when testing was routinely ordered, not all patients underwent serial testing, so optimal intervals of testing could not be determined. There may also be a surveillance bias toward those patients who were more adherent to testing and follow-up in our cohort. Surveillance with CPET could potentially result in fewer CV events, but our numbers were insufficient to test this hypothesis. We also did not have sufficient clinical information on radiation dosing to the lungs or lung shielding to understand observed abnormal spirometry patterns more clearly. This should be an area for future investigation. Overall, additional studies in larger cohorts that shed light on cardiopulmonary function, with novel echocardiographic indices, and association with cardiac events are needed in these patients.
Conclusions
The ppVo2peak in long-term survivors of HL who were treated with chest RT progressively declined as compared with population- and sex-based norms. Women developed abnormal ppVo2peak more than 2 decades earlier than male survivors. Abnormal ppVo2peak was associated with an increased risk of CV events in these patients.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: Patients with a history of HL who were treated with chest RT experienced a 7.5 percentage point decline in ppVo2peak over a 10-year period compared with age- and sex-based population norms. After 23 years, 50% had an abnormal ppVo2peak, with female patients consistently having lower ppVo2peak than male patients. Although the rate of ppVo2peak decline in both male and female patients was similar, women had a lower median ppVo2peak than men after RT and developed abnormal ppVo2peak more than 2 decades earlier. Abnormal exercise ppVo2peak was associated with more than a 6-fold increased risk for future nonfatal and fatal CV events.
TRANSLATIONAL OUTLOOK: Assessment of ppVo2peak decline in long-term survivors of HL, especially in female survivors, may identify those at highest risk for subsequent adverse CV events. Future prospective studies with larger, multicenter cohorts are further needed to define the role of CPET with ppVo2peak in the routine evaluation of long-term cancer survivors who were treated with RT.
Funding Support and Author Disclosures
Drs. Ng and Alexander have received royalties from UpToDate. Dr Chen has received support from the Translational Research Fund for Cardiology and Oncology (Boston Children’s Hospital) and the National Cancer Institute (grant R01 CA196854). All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Acknowledgments
The authors thank all of our patients with Hodgkin lymphoma and their families. The authors are grateful to the staff of the Exercise Testing Lab at Boston Children’s Hospital for their work and would also like to thank and acknowledge Ellen S. Deng for her contribution to the final preparation of the manuscript for publication.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental Methods section as well as tables and figures, please see the online version of this paper.
Appendix
References
- 1.Lymphoma - Hodgkin - childhood – statistics. Cancer.Net. 2012 https://www.cancer.net/cancer-types/lymphoma-hodgkin-childhood/statistics Available: [Google Scholar]
- 2.Bhakta N., Liu Q., Yeo F. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol. 2016;17:1325–1334. doi: 10.1016/S1470-2045(16)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amini A., Murphy B., Cost C.R. Cardiac mortality in children and adolescents with Hodgkin’s lymphoma: a Surveillance, Epidemiology and End Results analysis. J Adolesc Young Adult Oncol. 2016;5:181–186. doi: 10.1089/jayao.2015.0067. [DOI] [PubMed] [Google Scholar]
- 4.Ng A.K. Review of the cardiac long-term effects of therapy for Hodgkin lymphoma. Br J Haematol. 2011;154:23–31. doi: 10.1111/j.1365-2141.2011.08713.x. [DOI] [PubMed] [Google Scholar]
- 5.Castellino S.M., Geiger A.M., Mertens A.C. Morbidity and mortality in long-term survivors of Hodgkin lymphoma: a report from the Childhood Cancer Survivor Study. Blood. 2011;117:1806–1816. doi: 10.1182/blood-2010-04-278796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Bruin M.L., Dorresteijn L.D.A., van’t Veer M.B. Increased risk of stroke and transient ischemic attack in 5-year survivors of Hodgkin lymphoma. J Natl Cancer Inst. 2009;101:928–937. doi: 10.1093/jnci/djp147. [DOI] [PubMed] [Google Scholar]
- 7.Chen M.H., Colan S.D., Diller L. Cardiovascular disease: cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res. 2011;108:619–628. doi: 10.1161/CIRCRESAHA.110.224519. [DOI] [PubMed] [Google Scholar]
- 8.Aleman B.M.P., van den Belt-Dusebout A.W., De Bruin M.L. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109:1878–1886. doi: 10.1182/blood-2006-07-034405. [DOI] [PubMed] [Google Scholar]
- 9.Bowers D.C., McNeil D.E., Liu Y. Stroke as a late treatment effect of Hodgkin’s disease: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2005;23:6508–6515. doi: 10.1200/JCO.2005.15.107. [DOI] [PubMed] [Google Scholar]
- 10.Galper S.L., Yu J.B., Mauch P.M. Clinically significant cardiac disease in patients with Hodgkin lymphoma treated with mediastinal irradiation. Blood. 2011;117:412–418. doi: 10.1182/blood-2010-06-291328. [DOI] [PubMed] [Google Scholar]
- 11.Malhotra R., Bakken K., D’Elia E. Cardiopulmonary exercise testing in heart failure. J Am Coll Cardiol HF. 2016;4:607–616. doi: 10.1016/j.jchf.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Keteyian S.J., Patel M., Kraus W.E. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol. 2016;67:780–789. doi: 10.1016/j.jacc.2015.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones L.W., Liu Q., Armstrong G.T. Exercise and risk of major cardiovascular events in adult survivors of childhood Hodgkin lymphoma: a report from the childhood cancer survivor study. J Clin Oncol. 2014;32:3643–3650. doi: 10.1200/JCO.2014.56.7511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott J.M., Nilsen T.S., Gupta D. Exercise therapy and cardiovascular toxicity in cancer. Circulation. 2018;137:1176–1191. doi: 10.1161/CIRCULATIONAHA.117.024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ness K.K., Plana J.C., Joshi V.M. Exercise intolerance, mortality, and organ system impairment in adult survivors of childhood cancer. J Clin Oncol. 2020;38:29–42. doi: 10.1200/JCO.19.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wasserman K., Hansen J.E., Sue D.Y., Stringer W., Whipp B.J. Normal values. In: Weinberg R., editor. Principles of Exercise Testing and Interpretation. 4th ed. Lippincott Williams & Wilkins; 2005. pp. 160–182. [Google Scholar]
- 17.Chen M.H., Blackington L.H., Zhou J. Blood pressure is associated with occult cardiovascular disease in prospectively studied Hodgkin lymphoma survivors after chest radiation. Leuk Lymphoma. 2014;55:2477–2483. doi: 10.3109/10428194.2013.879716. [DOI] [PubMed] [Google Scholar]
- 18.Jones N.L., Makrides L., Hitchcock C. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131:700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 19.Stenehjem J.S., Smeland K.B., Murbraech K. Cardiorespiratory fitness in long-term lymphoma survivors after high-dose chemotherapy with autologous stem cell transplantation. Br J Cancer. 2016;115:178–187. doi: 10.1038/bjc.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaudry R.I., Howden E.J., Foulkes S. Determinants of exercise intolerance in breast cancer patients prior to anthracycline chemotherapy. Physiol Rep. 2019;7 doi: 10.14814/phy2.13971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaneko S., Tham E.B., Haykowsky M.J. Impaired left ventricular reserve in childhood cancer survivors treated with anthracycline therapy. Pediatr Blood Cancer. 2016;63:1086–1090. doi: 10.1002/pbc.25933. [DOI] [PubMed] [Google Scholar]
- 22.Groarke J.D., Payne D.L., Claggett B. Association of post-diagnosis cardiorespiratory fitness with cause-specific mortality in cancer. Eur Heart J Qual Care Clin Outcomes. 2020;6:315–322. doi: 10.1093/ehjqcco/qcaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groarke J.D., Tanguturi V.K., Hainer J. Abnormal exercise response in long-term survivors of Hodgkin lymphoma treated with thoracic irradiation: evidence of cardiac autonomic dysfunction and impact on outcomes. J Am Coll Cardiol. 2015;65:573–583. doi: 10.1016/j.jacc.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Yu A.F., Jones L.W. Modulation of cardiovascular toxicity in Hodgkin lymphoma: potential role and mechanisms of aerobic training. Future Cardiol. 2015;11:441–452. doi: 10.2217/fca.15.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers J., Kaminsky L.A., Lima R., Christle J.W., Ashley E., Arena R. A reference equation for normal standards for VO2 max: analysis from the Fitness Registry and the Importance of Exercise National Database (FRIEND Registry) Prog Cardiovasc Dis. 2017;60:21–29. doi: 10.1016/j.pcad.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Thompson C.A., Mauck K., Havyer R. Care of the adult Hodgkin lymphoma survivor. Am J Med. 2011;124:1106–1112. doi: 10.1016/j.amjmed.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.