A 78-year-old woman with hypertension, type 2 diabetes mellitus, and hyperlipidemia was diagnosed with stage 3C, BRAF wild-type left knee melanoma. Following local excision and sentinel lymph node surgery, she received adjuvant pembrolizumab (2 mg/kg every 3 weeks), a monoclonal antibody against the programmed cell death receptor-1 (PD-1). Two weeks after the second pembrolizumab dose, she presented to the emergency department with a 5-day history of exertional dyspnea and dysphagia. Cardiovascular examination revealed an irregular heart rate (105 to 140 beats/min) and signs of heart failure (HF). Neurological examination demonstrated bilateral asymmetric ptosis with fatigability and proximal muscle weakness. High-sensitivity troponin T (3,075 ng/l, 99th percentile <15 ng/l, Roche Diagnostics, Indianapolis, Indiana), N-terminal pro–B-type natriuretic peptide (4,246 pg/ml, reference range [RR] <300 pg/ml for age >75 years, Roche Diagnostics, Basel, Switzerland), and creatine kinase (2,487 U/l, RR <149 U/l, Abbott Diagnostics, Abbott Park, Illinois) were markedly elevated. Electrocardiogram showed atrial tachycardia with new right bundle branch and left anterior fascicular block. Transthoracic echocardiogram revealed a left ventricular ejection fraction (LVEF) of 65% with hypokinesis of the basal-to-mid-inferior wall and basal inferoseptum. Initial cardiovascular magnetic resonance (CMR) imaging was nondiagnostic due to patient noncompliance. Coronary angiography showed no flow-limiting disease. Single-fiber electromyography of the frontalis muscle revealed abnormalities compatible with myasthenia gravis (MG); acetylcholine receptor antibody (AchR-Ab) was negative.
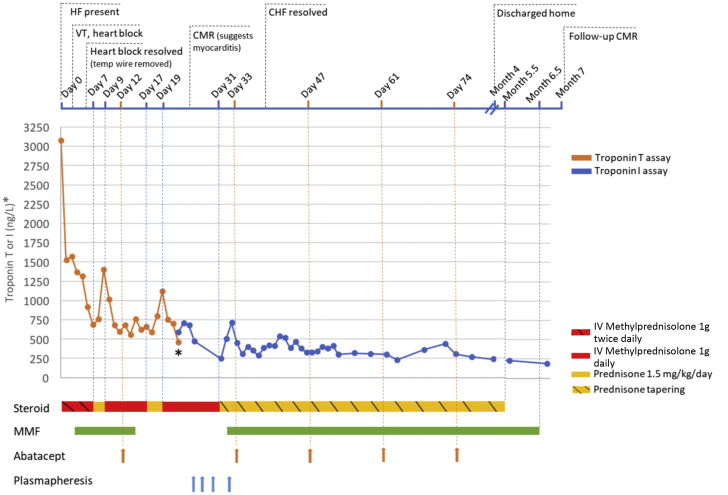
She was diagnosed with immune-related adverse events related to immune checkpoint inhibitor (ICI) including myocarditis based on the European Society of Cardiology (ESC) criteria (1) and neuromuscular complications. She was started on intravenous methylprednisolone (1,000 mg twice daily). On day 2 of admission, she developed monomorphic ventricular tachycardia and received intravenous amiodarone. Later in the day, she developed high-grade atrioventricular block and R-on-T polymorphic ventricular tachycardia requiring resuscitation with 4 shocks and a temporary pacemaker. She stabilized with intravenous methylprednisolone (1,000 mg once daily); however, switching to oral prednisone resulted in a recurrent rise in troponin (Figure 1). As a result, mycophenolate mofetil (MMF) (750 mg twice daily) was added. Due to persistent troponin elevation in the context of a malignant presentation, she was started on intravenous abatacept and received 5 doses (10 mg/kg/dose) ∼2 weeks apart. She was transferred to our facility after the first dose of abatacept. A repeat CMR at that time demonstrated features of myocarditis (Figure 2). She was started on spironolactone and intravenous furosemide for HF with preserved LVEF (56% by CMR) and bisoprolol for paroxysmal atrial tachycardia. After 2 doses of abatacept, her HF symptoms significantly improved, supplemental oxygen was discontinued, and she transitioned to oral diuretics. Her B-type natriuretic peptide peaked at 623 pg/ml (RR <100 pg/ml) during abatacept and fell to 269 pg/ml immediately post-treatment. She received plasmapheresis for persistent neurological symptoms with good response. After abatacept therapy and plasmapheresis, her high-sensitivity troponin I (hsTnI) level stabilized and gradually decreased on a slow prednisone taper. No recurrence of ventricular arrhythmias or heart block was noted. She was discharged home after a 4-month stay in the hospital and rehabilitation center. Her discharge hsTnI remained elevated (272 ng/l, 99th percentile <27 ng/l, Abbott Diagnostics). Prednisone was slowly tapered (10 mg per 1 to 2 weeks) and discontinued at 5.5 months after index admission, and MMF was stopped a month later. Two weeks later, her functional status improved (New York Heart Association functional class II), but hsTnI remained elevated (106 ng/l). To investigate this persistent troponin elevation, repeat analysis of multiple banked specimens from this patient was performed. We excluded false-positive troponin I due to pre-analytical factors (e.g., specimen mix-up), random errors, hemolysis, or fibrin microclots. The presence of interfering heterophilic antibodies was also excluded. To investigate the presence of macrotroponin, the sample with measured hsTnI of 106 ng/l was treated with polyethylene glycol (PEG) 6000 and protein G, and the recovery of hsTnI post-treatment was 15% and 50%, respectively, suggesting possible partial contribution of macrotroponin (immunoglobulin G [IgG]-troponin complex) to the elevated hsTnI measurements. Repeat CMR showed stable late gadolinium enhancement (LGE), with no myocardial edema (Figure 2); there was, however, mild worsening biventricular function (LVEF from 56% to 50% and right ventricular ejection fraction from 61% to 46%), along with increased myocardial native T1 levels without elevated T2 levels (Figure 2), suggestive of myocardial fibrosis. Her cardiac medications were continued with slow tapering of her furosemide doses. Surveillance CT scans of her melanoma showed no disease recurrence.
Figure 1.
Timeline of Troponin Response and Immunosuppressive Treatment After Hospital Admission (Day 0)
The time points of corticosteroid, mycophenolate mofetil (MMF), abatacept, and plasmapheresis administration are illustrated. The asterisk denotes change from troponin T to troponin I assay due to patient transfer to a different health care institution. HF = heart failure; CMR = cardiac magnetic resonance; IV = intravenous; VT = ventricular tachycardia.
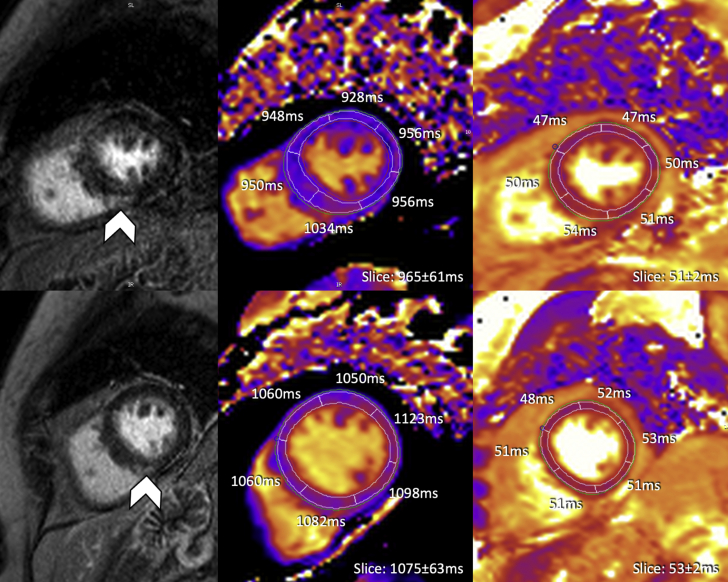
Figure 2.
Initial and Follow-Up CMR Imaging at Our Institution
Initial cardiovascular magnetic resonance (CMR) was performed on day 25 post-admission, after the patient was treated with high-dose immunosuppression; the top leftimage showed focal mid-myocardial to subepicardial late gadolinium enhancement (LGE) within the mid-ventricular inferoseptal myocardium (arrowhead). Segmental T1 (modified Look-Locker inversion recovery [MOLLI] 5(3)3) values were predominately within the normal local range (volunteer cohort 1,006 ± 24 ms) with somewhat higher T1 values in the segment matching described LGE (top middle image). All segmental T2 (T2-prep SSFP) values were within local normal range (volunteer cohort 52 ± 3 ms) (top rightimage). Follow-up CMR at month 7 after initial presentation (bottom 3 images) identified unchanged LGE (arrowhead) compared with initial CMR; all segmental T1 values were significantly higher than at baseline and above normal ranges, whereas T2 values remained within normal limits.
ICIs amplify the host T cell response against tumor antigens (2). By targeting specific inhibitory signals in the T cell regulatory pathways, such as cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4), PD-1, and programmed cell death ligand 1 (PD-L1), ICIs disrupt the immune tolerance and restore the antitumor T-cell response (2). ICIs are however associated with immune-related adverse events, of which myocarditis is the most concerning cardiovascular toxicity (2). Although the incidence of myocarditis is relatively uncommon (0.4% to 1.1%), it is associated with up to 46% mortality (3). Whereas prior studies have focused on acute presentations, management, and prognosis in patients with ICI myocarditis, there are limited published reports on prolonged persistent myocarditis despite aggressive immunosuppressive therapy. We present a challenging case of severe and persistent, “smoldering” myocarditis associated with a PD-1 inhibitor.
Our patient met the criteria for clinically suspect myocarditis based on the ESC criteria using a combination of clinical, biomarker, and imaging features (1). Endomyocardial biopsy was not performed due to CMR findings, and procedural risks in a patient on prolonged high-dose corticosteroids. It has been increasingly recognized that ICI myocarditis can present with overlapping neuromuscular complications, with a prior study demonstrating 11% and 25% incidence of concurrent MG and myositis, respectively (3). Shared antigens between cardiac and skeletal muscle cells may contribute to this overlapping presentation (4). Although generalized MG is usually associated with positive AchR-Ab in 80% to 85% of the cases, only 50% of the patients with de novo ICI-induced MG are AchR-Ab positive (5). Because myocarditis with MG/myositis is associated with a poor prognosis (4), multidisciplinary collaboration in the management is essential.
Although steroids remain the first-line treatment for ICI myocarditis, current evidence remains lacking on adjunctive immunosuppressive therapies in patients with inadequate response to corticosteroids. Mycophenolate, antithymocyte globulin, and infliximab are recommended, but the evidence relies mostly on anecdotal experience (6). Infliximab was not used in this patient due to HF at presentation (6). Plasmapheresis or intravenous immunoglobulins, which deplete pathogenic autoantibodies, can be considered when patients present with concurrent neuromuscular toxicities as in our patient (6). Evidence is also emerging on the use of novel T cell (e.g., abatacept, alemtuzumab) targeted therapies (7,8). Abatacept is a CTLA-4 agonist that induces profound T cell anergy (7). A recent case demonstrated its efficacy in treating steroid-refractory myocarditis due to nivolumab, and this was attributed to the inhibitory effects of abatacept on T cell costimulation upstream of the PD-1/PD-L1 pathways (7). We are not aware of prior reports on the use of abatacept in pembrolizumab-associated myocarditis. The unique aspects in this patient’s management include the use of both abatacept and MMF as second-line immunomodulatory agents, and the persistently elevated hsTnI despite 6.5 months of immunosuppression. This illustrates that ICI myocarditis, even after single-agent checkpoint blockade, can have a prolonged course. In this patient, hsTnI level remained elevated at 7 months following her index admission, and she developed worsening biventricular systolic function. Ultimately, given the long-term risks of steroids, the relatively low level of persistent hsTnI elevation, the partial contribution of macrotroponin formation, and most importantly, the patient’s overall clinical stability, steroids were gradually tapered and eventually discontinued. However, it is crucial to follow these patients closely, particularly after stopping immunosuppression.
Cardiac biomarkers, especially troponin, play a key role in myocarditis diagnosis and management. It is important to consider the clinical context and the testing method when interpreting troponin in ICI myocarditis. Although troponins are widely used as markers of myocardial injury, they are not specific for ICI myocarditis, and other common causes should be considered (9). In terms of the testing method itself, there is potential cross-reaction of the troponin T immunoassay with isoforms expressed in regenerating skeletal muscles (9). This is especially relevant in patients who present with an overlap of myocarditis and myositis. As a result, troponin I is a more specific marker of myocardial injury (9). Our patient had persistently elevated hsTnI despite being on immunosuppression for a total of 6.5 months. Because immunosuppressive therapy was continued for a prolonged period based on persistent troponin elevation, it was important to rule out causes of false-positive troponin such as specimen mix-up, fibrin microclots, and heterophilic antibodies. Macrotroponin, which is troponin in complex with antibody (typically auto-troponin IgG), has been reported to cause falsely increased or decreased troponin I or T, and can be found in both normal population and patients with cardiac diseases (10). Presence of macrotroponin is often investigated by treatment with PEG, protein G, or size exclusion chromatography. Our analysis using PEG and protein G assays suggests only partial contribution of macrotroponin formation to the troponin measurement, which means there was still ongoing myocardial necrosis. This is also confirmed by the worsening of myocardial function and increase in myocardial native-T1 values with CMR (without increased T2 values) at follow-up despite immunosuppressive therapy (Figure 2). The mechanism of macrotroponin formation, and the relationship between macrotroponin formation and troponin release, half-life in circulation, and the corresponding effect on overall troponin concentration are not well understood. Additional research is needed to elucidate the potential role of macrotroponin complexes in ICI myocarditis and its influence on chronically elevated troponin measurements.
In conclusion, we present a case of severe myocarditis associated with pembrolizumab, with overlapping syndrome of MG. We highlight the potential refractory nature of the disease course with persistent elevation in troponin, despite plasmapheresis, abatacept, and prolonged use of MMF and corticosteroid. This case also demonstrates the importance of assessing other causes of persistently elevated troponin especially when using this biomarker to guide management.
Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Caforio A.L., Pankuweit S., Arbustini E. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 2.Ball S., Ghosh R.K., Wongsaengsak S. Cardiovascular toxicities of immune checkpoint inhibitors: JACC review topic of the week. J Am Coll Cardiol. 2019;74:1714–1727. doi: 10.1016/j.jacc.2019.07.079. [DOI] [PubMed] [Google Scholar]
- 3.Moslehi J.J., Salem J.E., Sosman J.A., Lebrun-Vignes B., Johnson D.B. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puwanant A., Isfort M., Lacomis D., Zivkovic S.A. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul Disord. 2019;29:127–133. doi: 10.1016/j.nmd.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Makarious D., Horwood K., Coward J.I.G. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer. 2017;82:128–136. doi: 10.1016/j.ejca.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Brahmer J.R., Lacchetti C., Schneider B.J. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714–1768. doi: 10.1200/JCO.2017.77.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salem J.E., Allenbach Y., Vozy A. Abatacept for severe immune checkpoint inhibitor-associated myocarditis. N Engl J Med. 2019;380:2377–2379. doi: 10.1056/NEJMc1901677. [DOI] [PubMed] [Google Scholar]
- 8.Esfahani K., Buhlaiga N., Thebault P., Lapointe R., Johnson N.A., Miller W.H., Jr. Alemtuzumab for immune-related myocarditis due to PD-1 therapy. N Engl J Med. 2019;380:2375–2376. doi: 10.1056/NEJMc1903064. [DOI] [PubMed] [Google Scholar]
- 9.Bonaca M.P., Olenchock B.A., Salem J.E. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation. 2019;140:80–91. doi: 10.1161/CIRCULATIONAHA.118.034497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warner J.V., Marshall G.A. High incidence of macrotroponin I with a high-sensitivity troponin I assay. Clin Chem Lab Med. 2016;54:1821–1829. doi: 10.1515/cclm-2015-1276. [DOI] [PubMed] [Google Scholar]