Central Illustration
Key Words: chemotherapy-induced cardiotoxicity, QT interval, torsades de pointes
Abbreviations and acronyms: ECG, electrocardiogram; QTc, QT interval corrected for heart rate; TdP, torsades de pointes; TKI, tyrosine kinase inhibitor
Over the past 30 years, the development of efficacious treatment regimens has increased overall survival among cancer patients. However, many of these treatments can cause QT prolongation which can lead to life-threatening arrhythmias. Prolonged QT can lead to a polymorphic ventricular arrhythmia known as torsades de pointes (TdP) (1). The clinical presentation can be insidious varying between minimal symptoms to severe manifestations, including sudden cardiac death (1).
Patients with cancer are particularly vulnerable to QT prolongation. In this “How To,” we use a clinical case to show our approach to the diagnosis and management of QT prolongation in cancer patients.
Clinical Case
A 66-year-old female with a past medical history significant for acute myeloid leukemia, prior transient ischemic attack, hypertension, and hypothyroidism presented to the hospital with dizziness and falls. At the time of presentation, she was noted to have slurred speech and gait instability. She denied any loss of consciousness, chest pain, shortness of breath, or palpitations. Her initial electrocardiogram (ECG) showed sinus rhythm with frequent premature ventricular contractions and a QT interval, corrected for heart rate (QTc) of 487 ms. An ECG 2 months earlier showed a QTc of 455 ms.
How do we diagnose QT prolongation?
The QTc represents the time between ventricular depolarization and repolarization. On a standard 12-lead ECG, this measurement is usually taken from limb lead II and precordial lead V5 measuring from the beginning of the Q wave to the termination of the T-wave (2). The QT interval adjusts to heart rate and different formulas have been used to correct the QT interval. The Bazett (QTcB = QT/RR1/2) formula assumes an exponential relationship between the QT interval and the R to R interval (1). Bazett’s correction is most useful for heart rates between 60 and 100 beats/min with inaccuracies at slower (with overcorrection) and faster (with undercorrection) heart rates. The Fridericia formula (QTcF = QT/RR1/3) is similar, but has greater accuracy at faster heart rates. Linear formulas such as the Framingham (QTcFra = QT + 0.154 [1 − RR]) and Hodges (QTcH = QT + 0.00175 [(60 / RR) − 60]) have more uniform correction for heart rates above 90 beats/min, but are less commonly used (1).
Although definitions vary, prolongation of the QTc is generally defined as a QTc value >450 ms in males and >460 ms in females (2). The National Cancer Institute Common Terminology of Clinical Adverse Events v5.0 classifies QTc prolongation into 4 grades: grade 1 (QTc 450 to 480 ms), grade 2 (QTc 481 to 500 ms), grade 3 (QTc >501 ms; >60 ms change from baseline), and grade 4 (signs/symptoms of serious arrhythmia and TdP) (3).
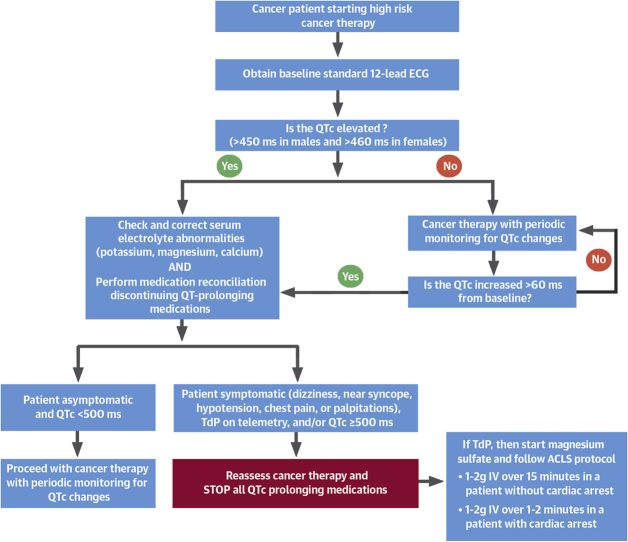
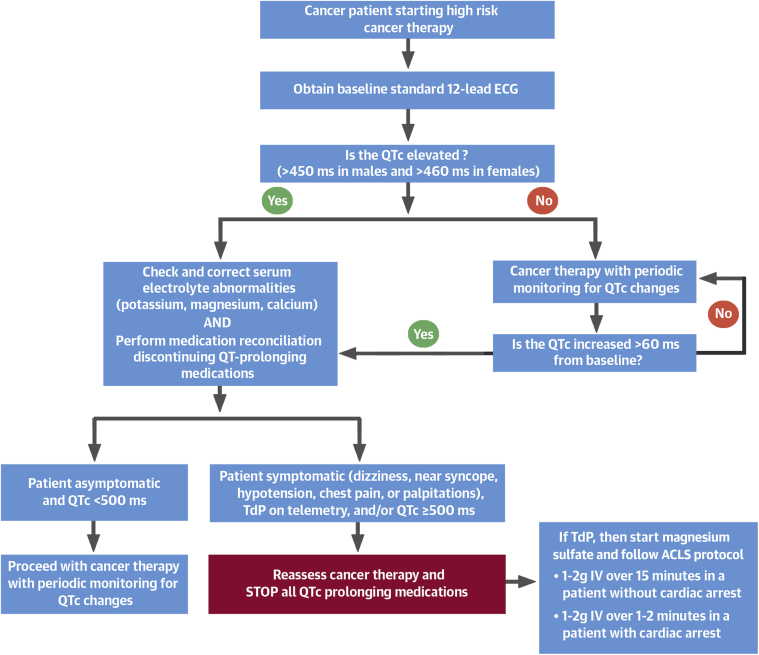
Most patients are recommended to obtain a pre-treatment ECG when they are scheduled to receive a potential cardiotoxic agent (Figure 1). Continuous cardiac monitoring in the inpatient setting is recommended for higher-risk patients. In the outpatient setting, patients should receive education and counseling regarding self-monitoring for symptoms of dizziness, presyncope, seizure, hypotension, chest pain, or palpitations with periodic monitoring (Figure 1).
Figure 1.
Algorithm to Monitor QTc Prolongation in Cancer Patients
This flowchart shows our approach to monitoring and treating QT prolongation in cancer patients. ACLS = advanced cardiovascular life support; ECG = electrocardiogram; IV = intravenous; TdP = torsades de pointes.
LEARNING POINTS
-
•
The QT interval should be measured on a 12-lead ECG from lead II and V5.
-
•
The preferred QT correction formula for heart rate is Fridericia due to fewer inaccuracies compared to Bazett.
-
•
Checking a baseline ECG before cancer therapy initiation and periodic cardiac monitoring are recommended in patients receiving potential cardiotoxic therapy.
Case Continued
Two months earlier, she was started on hydroxyurea, idarubicin, and cytarabine. Her treatment was complicated by neutropenic fever, for which she was started on antimicrobial therapy and additional medications including ondansetron, promethazine, voriconazole, moxifloxacin, and risperidone.
During her current hospitalization, the patient was found to have an elevated thyroid stimulating hormone (7.89 mIU/l; normal range 0.27 to 4.2 mIU/l) with a decreased total T3 (57 ng/dl; normal range 80 to 190 ng/dl). She also had hypokalemia (3.3 mEq/l; normal range 3.5 to 5 mEq/l) and hypomagnesemia (1.8 mg/dl; normal range 1.8 to 2.9 mg/dl).
An echocardiogram showed a new moderate-to-severe depressed left ventricular systolic function with a left ventricular ejection fraction of 35% and global hypokinesis. She underwent a left heart catheterization which showed no occlusive coronary artery disease.
What can cause QT prolongation in cancer patients?
Electrolyte abnormalities are common in cancer patients due to poor oral intake, emesis, diarrhea, and nephropathy and are an independent predictor for a worse prognosis (4). Electrolyte-wasting medications, such as corticosteroids and diuretics, should be minimized and medications which can prolong the QTc should be stopped whenever possible. Many oncologic therapies can cause QT prolongation. These include arsenic trioxide, anthracyclines, antimetabolites, tyrosine kinase inhibitors (TKIs), histone deacetylase inhibitors, cyclin-dependent kinase 4/6 inhibitors, and protein kinase C inhibitors (Table 1). An updated list of pharmacotherapies that can cause QT prolongation can be found online (5). There is significant heterogeneity in the degree of QT prolongation for each of these agents. For example, severe QTc prolongation (QTc >500 ms) was found in up to 40% of patients receiving arsenic trioxide, compared to 2.6% of patients receiving vandetanib (1).
Table 1.
Cancer and Non-Cancer Drugs Known to Cause Prolongation of the QTc
| Therapy | Drug Type | Drug |
|---|---|---|
| Cancer therapeutics | ||
| Antimetabolites | Capecitabine | |
| Anthracyclines | Epirubicin | |
| Antimicrotubule agents | Paclitaxel | |
| Tyrosine kinase inhibitors | Bosutinib | |
| Dasatinib | ||
| Lenvatinib∗ | ||
| Nilotinib∗ | ||
| Ponatinib | ||
| Vandetanib∗ | ||
| Pazopanib∗ | ||
| Sorafenib/sunitinib | ||
| Histone deacetylase inhibitors | Panobinostat∗ | |
| Romidepsin∗ | ||
| Vorinostat∗ | ||
| Proteasome inhibitors | Bortezomib | |
| CDK 4/6 inhibitor | Ribociclib∗ | |
| B-Raf inhibitor | Vemurafenib∗ | |
| Other | Arsenic trioxide∗ | |
| Non-cancer agents | ||
| Antiarrhythmic drugs | Amiodarone | |
| Disopyramide | ||
| Dofetilide∗ | ||
| Dronedarone∗ | ||
| Flecainide∗ | ||
| Ibutilide∗ | ||
| Procainamide | ||
| Quinidine | ||
| Sotalol∗ | ||
| Antibacterial and antifungal drugs | Moxifloxacin∗ | |
| Levofloxacin | ||
| Ciprofloxacin | ||
| Clarithromycin | ||
| Erythromycin | ||
| Azithromycin | ||
| Fluconazole | ||
| Pentamidine | ||
| Prokinetic and antiemetic drugs | Domperidone | |
| Chlorpromazine | ||
| Ondansetron∗ | ||
| Droperidol | ||
| Antipsychotics | Haloperidol∗ | |
| Thioridazine | ||
| Pimozide∗ | ||
| Antidepressants | Escitalopram | |
| Citalopram | ||
The medications included in this table have been identified to cause elevations in the QTc. For a more complete list of these medications, please visit www.crediblemeds.org.
The FDA package insert provides guidance regarding electrocardiographic monitoring.
The molecular mechanisms by which these cancer drugs can lead to prolongation of the QTc are multifactorial. Arsenic trioxide and TKIs appear to exert effects on the QTc through interaction with human Ether-à-go-go-related gene which encodes for a protein that mediates IKr, a potassium channel protein regulating repolarization of cardiomyocyte action potentials (1). Other reported mechanisms include cardiomyocyte-induced injury involving abnormal calcium homeostasis, mitochondrial injury, cardiac apoptosis, and inhibition of phosphatidylinositol 3-kinase (6).
Acquired QTc prolongation may be exacerbated by simultaneous use of drugs that can inhibit the cytochrome P450 enzyme such as azole antifungals, macrolides, and certain antivirals. Patients with renal and liver disease may also cause delayed excretion of the QT-prolonging medications (1,6). Our patient was on several medications that could cause QT prolongation both by direct and indirect mechanisms including ondansetron, promethazine, voriconazole, moxifloxacin, and risperidone.
Structural heart disease can also contribute to QT prolongation. A retrospective analysis of 239 patients with QT prolongation (>480 ms) found that one-quarter of the patients with acquired long QT syndrome had structural abnormalities. QT prolongation is thought to be caused by downregulation of potassium currents in hypertrophied and failing hearts (7).
Other non-cardiac systemic conditions, such as hypothyroidism, have also been associated with QT prolongation and ventricular arrhythmias in the setting of electrolyte imbalances or other concurrent QT prolonging medications. Timely diagnosis and treatment with thyroid replacement hormone have been shown to correct QT prolongation in these patients (8).
In our patient, QT prolongation was most likely due to a combination of risk factors, including electrolyte abnormalities, drug interactions, structural heart disease, and hypothyroidism.
LEARNING POINTS
-
•
Many commonly used cancer therapies can lead to QTc prolongation, particularly arsenic trioxide and TKIs.
-
•
Adjunctive medications used to treat side effects caused by cancer therapies can affect the QTc.
-
•
Structural heart disease and hypothyroidism can contribute to QT prolongation.
Case Continued
While on telemetry, the patient was noted to have ventricular bigeminy with short frequent runs of polymorphic ventricular tachycardia leading to a 20-s run of TdP. All nonessential QT-prolonging medications were discontinued, and electrolytes were corrected. She was transferred to the intensive care unit for closer monitoring.
How do we manage QT prolongation in TdP?
Once QTc prolongation is identified, serum electrolyte levels should be obtained and abnormalities should be corrected. If a patient develops TdP, intravenous magnesium sulfate should be administered immediately. In patients without congenital long-QT syndrome, mexiletine, a Class Ib antiarrhythmic, has been shown to shorten the QT interval, halt episodes of TdP, and prevent recurrence of refractory TdP (9). If signs of electrical instability persist, transfer to the intensive care unit is recommended with initiation of beta-adrenergic agents such as isoproterenol or temporary pacing to increase the heart rate (1).
The European Society of Cardiology has offered an expert consensus on cancer treatments and cardiotoxicity (10). However, there are no generally accepted consistent criteria regarding discontinuation of cancer therapeutics for patients who develop QT prolongation. Oncologists and cardiologists must make case-by-case decisions of continuing potentially life-prolonging cancer therapy balanced by the risk for lethal arrhythmias (10).
LEARNING POINTS
-
•
When QTc is elevated, check and correct electrolyte imbalances and perform a medication reconciliation to discontinue QT-prolonging agents.
-
•
Patients who develop TdP should be treated as per advanced cardiovascular life support (ACLS) protocol with intravenous magnesium sulfate and transfer to an intensive care unit for closer monitoring.
Case Continued
Before discharge, the patient had resolution of ventricular ectopy on telemetry. She was started on guideline-directed anti-remodeling medications. A repeat echocardiogram showed an improved left ventricular ejection fraction of 50%. A follow up 12-lead ECG showed sinus rhythm with a QTc of 442 ms.
Conclusions
Cancer patients have an increased risk of QTc prolongation due to multiple etiologies and a higher risk of mortality. Because the majority of QT monitoring for cancer patient is performed in the outpatient setting, clinicians must be aware of the common risk factors that can lead to QTc prolongation to prevent the development of TdP. Early recognition and definitive treatment of QTc prolongation may allow cancer patients to continue with their treatment in most cases. For higher-risk individuals, multispecialty discussions should be held to assess the risk and benefits tailored to the best interests of the patient.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Porta-Sanchez A., Gilbert C., Spears D. Incidence, diagnosis, and management of QT prolongation induced by cancer therapies: a systematic review. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giudicessi J.R., Noseworthy P.A., Ackerman M.J. The QT interval. Circulation. 2019;139:2711–2713. doi: 10.1161/CIRCULATIONAHA.119.039598. [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute Common Terminology of Clinical Adverse Events v5.0. National Cancer Institute Division of Cancer Treatment and Diagnosis website. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf Published November 27, 2017. Available at: Accessed January 11, 2021.
- 4.Rosner M.H., Dalkin A.C. Electrolyte disorders associated with cancer. Adv Chronic Kidney Dis. 2014;21:7–17. doi: 10.1053/j.ackd.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Woosley R.L., Heise C.W., Gallo T. QT drugs List. www.crediblemeds.org Available at: Accessed October 20, 2020.
- 6.Buza V., Rajagopalan B., Curtis A.B. Cancer treatment–induced arrhythmias: focus on chemotherapy and targeted therapies. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.117.005443. [DOI] [PubMed] [Google Scholar]
- 7.Weissler-Snir A., Gollob M.H., Chauhan V., Care M., Spears D.A. Evaluation of prolonged QT interval: structural heart disease mimicking long QT syndrome. Pacing Clin Electrophysiol. 2017;40:417–424. doi: 10.1111/pace.13040. [DOI] [PubMed] [Google Scholar]
- 8.Kweon K.H., Park B.H., Cho C.G. The effects of L-thyroxine treatment on QT dispersion in primary hypothyroidism. J Korean Med Sci. 2007;22:114–116. doi: 10.3346/jkms.2007.22.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Badri M., Patel A., Patel C. Mexiletine prevents recurrent torsades de pointes in acquired long QT syndrome refractory to conventional measures. J Am Coll Cardiol EP. 2015;1:315–322. doi: 10.1016/j.jacep.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Zamorano J.L., Lancellotti P., Rodriguez Munoz D. ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC) Eur Heart J. 2016;37:2768–2801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]