Abstract
Objectives
The purpose of this study was to assess osimertinib-associated cardiac adverse events (AEs) in a real-world setting, using a retrospective single-center cohort study in Japan.
Background
Cases of osimertinib-associated cardiac AEs have been reported but remain poorly understood.
Methods
A total of 123 cases of advanced non–small cell lung cancer (NSCLC) with confirmed EGFR mutations who received osimertinib monotherapy from 2014 to 2019 at the Osaka International Cancer Institute (Osaka, Japan) were evaluated. Cardiac AEs were defined according to Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Changes in left ventricular ejection fraction (LVEF) and rates of cancer therapeutics-related cardiac dysfunction (CTRCD), defined as a ≥10 % absolute decline in LVEF from baseline to a value of <53%, were further assessed in 36 patients in whom serial measurements of LVEF were obtained before and during osimertinib treatment.
Results
Severe cardiac AEs (CTCAE grade 3 or higher) occurred in 6 patients (4.9%) after osimertinib administration. These AEs included acute myocardial infarction (n = 1), heart failure with reduced LVEF (n = 3), and valvular heart disease (n = 2). Five of the 6 patients had a history of cardiovascular risk factors or disease. Myocardial biopsies in 2 of the patients showed cardiomyocyte hypertrophy and lipofuscin deposition. In 36 patients assessed with serial LVEF, LVEF declined from 69.4 ± 4.2% to 63.4 ± 10.5% with osimertinib therapy (p < 0.001). CTRCD occurred in 4 patients with a nadir LVEF of 40.3 ± 9.1% with osimertinib.
Conclusions
In this retrospective cohort analysis, the incidence of cardiac AEs in patients treated with osimertinib was 4.9%. Additional prospective data collected from patients with NSCLC treated with osimertinib will be important in understanding the incidence, pathophysiology, and management of cardiac AEs with osimertinib.
Key Words: cardiac adverse events, cardiac dysfunction, EGFR mutations, myocardial biopsy, non–small cell lung cancer, osimertinib
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; AE, adverse event; ARB, angiotensin II receptor blocker; CTCAE, common terminology criteria for adverse event; CTRCD, cancer therapeutics-related cardiac dysfunction; EGRF, epidermal growth factor receptor; HER, human epidermal growth factor receptor; LVIDd, left ventricular internal end-diastolic diameter; LVIDs, left ventricular internal end-systolic diameter; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NT-proBNP, N-terminal pro–B-type natriuretic peptide; NSCLC, non–small cell lung cancer; PASP, pulmonary artery systolic pressure; TKI, tyrosine kinase inhibitor; TR, tricuspid regurgitation; VEGF, vascular endothelial growth factor
Central Illustration
Osimertinib (Tagrisso, AstraZeneca) is an orally available, third-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) that selectively inhibits both EGFR TKI sensitizing and the EGFR T790M resistance mutation with less activity against wild-type EGFR (1). The AURA (A Phase I/II, Open-Label, Multicentre Study to Assess the Safety, Tolerability, Pharmacokinetics and Anti-tumour Activity of Ascending Doses of AZD9291 in Patients with Advanced Non Small Cell Lung Cancer w have Progressed Following Prior Therapy with an Epidermal GrowthFactor Receptor Tyrosine Kinase Inhibitor Agent) and FLAURA (AZD9291 Versus Gefitinib or Erlotinib in Patients With Locally Advanced or Metastatic Non-small Cell Lung Cancer) trials have consistently demonstrated superior clinical activity and relative safety of osimertinib in advanced non–small cell lung cancer (NSCLC) patients with EGFR mutations regardless of their EGFR T790M mutation status (2,3). The incidence of Grade 3 or higher Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 resulting in permanent discontinuation was lower for osimertinib than in the first-generation EGFR-TKIs (4). Most recently, patients with previously untreated, advanced EGFR mutant NSCLC had longer survival with osimertinib than those treated with EGFR TKIs, including gefitinib or erlotinib (3,5). With the success of these clinical trials, osimertinib has become the standard care for patients with advanced NSCLC with EGFR mutations and has become the first-line therapy for NSCLC patients with the EGFR T790M mutation who have progressed on previous EGFR-TKI treatment.
Safety information for osimertinib from international, large clinical trials have reported Grade 3 or higher corrected QT (QTc) prolongation in 1% of patients, any grade cardiac failure in 4%, and a decline in left ventricular ejection fraction (LVEF) >10% from baseline and to <50% in 3% of patients (3). However, in a post-marketing surveillance study of osimertinib in 3,578 Japanese NSCLC patients with EGFR mutations, the incidence of Grade 3 or higher QTc prolongation occurred in only 0.1% and other Grade 3 or higher cardiac adverse events (AEs) in 0.8% (6).
Recently, severe osimertinib-associated cardiac dysfunction was observed in NSCLC patients with EGFR mutations who were treated with osimertinib. These experiences prompted a detailed retrospective analysis in a single-center experience to further understand cardiac AEs associated with osimertinib in a real-world setting in EGFR mutation-carrying NSCLC patients.
Subjects and Methods
This was a retrospective, single-center, observational study conducted at the Osaka International Cancer Institute (OICI), Osaka, Japan. The study protocol and consent forms were approved by the local ethics committee at OICI (approval 19017). The electronic medical records of all NSCLC patients with an EGFR mutation treated with osimertinib between 2014 and 2019 at the OICI hospital were reviewed. Inclusion criteria were a diagnosis of histologically and cytologically confirmed NSCLC; clinical stage IIIB-IV cancer, according to the American Joint Committee for Cancer staging system, 8th edition; presence of a sensitizing, activating EGFR mutation; and treatment history of osimertinib monotherapy. Patients were excluded if they had discontinued osimertinib treatment within 2 weeks for any reason or if their medical records were unobtainable. All AEs associated with osimertinib were graded according to CTCAE version 5.0 criteria. AEs were adjudicated by a cardiologist based upon detailed medical record review.
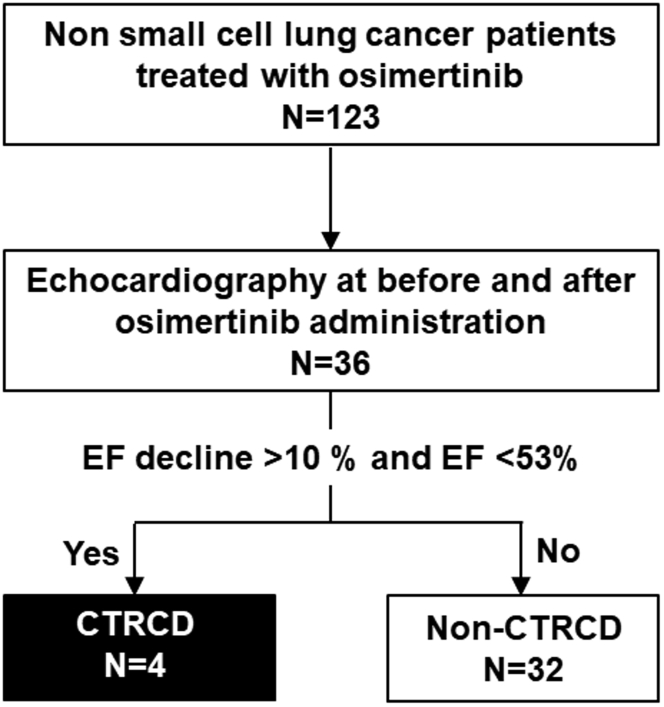
Within this cohort, there were 72 patients who underwent serial electrocardiography (ECG) and 36 who underwent echocardiography both before and after osimertinib administration. QTc and echocardiographic parameters were compared. Cancer therapeutics-related cardiac dysfunction (CTRCD) was defined, according to the American Society of Echocardiography/European Association of Cardiovascular Imaging, as a greater than 10 % decline in LVEF from baseline to a value of <53% (7). Patients’ characteristics and cardiac functions in the non-CTRCD group (n = 32) were compared with those in the CTRCD group (n = 4) (Figure 1).
Figure 1.
Study Cohort
CTRCD, as defined by a decrease in LVEF of more than 10 % to <53% LVEF, was observed in 4 patients (black box). The remaining 32 patients did not experience CTRCD (white box). CTRCD = cancer therapeutics-related cardiac dysfunction; LVEF = left ventricular ejection fraction; NSCLC = non–small cell lung cancer.
Electrocardiography and echocardiography
Twelve-lead ECGs were obtained using a standardized ECG machine (Fukuda Denshi, Tokyo, Japan) and QTc was performed using the Bazett formula. Echocardiography was performed using an IE33 ultrasonography machine (Philips, Stockton, California). LV end-diastolic and end-systolic diameters (LVIDd and LVIDs) were obtained by 2-dimensional (2D) imaging, and LVEF was obtained using the Teichholz method. Echocardiographic data were extracted from the records within 2 years before and 1 year after osimertinib initiation. The lowest LVEF data were extracted for analysis in 18 patients who underwent repeated echocardiography after osimertinib initiation.
Statistical analyses
Within- and between-group comparisons of changes in cardiac parameters were made and tested with the paired t-test, using Prism version 6.02 software (GraphPad, San Diego, California). Nonparametric comparisons were performed using the Mann-Whitney U test. Results were expressed as mean ± SD or median (interquartile range [IQR]), and a p value of <0.05 was considered statistically significant.
Results
Osimertinib-associated cardiac adverse events in NSCLC patients
From 2014 to 2019, 123 NSCLC patients with EGFR mutations were treated with osimertinib (Table 1). Seven of those patients were enrolled in clinical trials (AURA and FLAURA) (2,3). The median age of those patients was 69 years (interquartile range 33 to 86); 83 patients (67.5%) were female; and 122 patients (99.2%) had adenocarcinoma (Table 1). The EGFR mutation profiles were as follows: Exon (Ex.) 19 deletion (del), which occurred in 21.1% of patients; L858R, which occurred in 17.1% of patients; and resistance mutation T790M, which occurred in 59.4% of patients. Osimertinib was used as first-line therapy in 26.8% of patients. Grade 3 or higher CTCAE version 5.0 cardiac AEs were observed in 6 patients (4.9%) (Table 2). Five of those patients were female, and all 5 had a history of cardiovascular diseases and/or cardiovascular risk factors. Their cardiac AEs included acute myocardial infarction (n = 1), heart failure with reduced LVEF (n = 3), and valvular heart disease (n = 2) (Table 2). The 6 CTCAE grade 3 or higher cardiac AEs are described in detail below and presented in Table 2.
Table 1.
Patient Characteristics of Osimertinib-Treated NSCLC Patients (N = 123)
| Age, yrs | 69 (33-86) |
| Females | 83 (67.5) |
| Smoking status | |
| Former, current | 35 (28.5) |
| Histological type | |
| Adenocarcinoma | 122 (99.2) |
| EGFR mutation status at osimertinib administration | |
| Ex.19 del | 26 (21.1) |
| L858R | 21 (17.1) |
| T790M | 1 (0.8) |
| Ex.19 del + T790M | 36 (29.3) |
| L858R + T790M | 36 (29.3) |
| G719X | 3 (2.4) |
| Osimertinib treatment line | |
| 1st | 33 (26.8) |
| 2nd | 32 (26.0) |
| 3rd | 19 (15.4) |
| >4th | 39 (31.7) |
| Medical history | |
| Hypertension | 35 (28.5) |
| Diabetes mellitus | 3 (2.4) |
| Dyslipidemia | 11 (8.9) |
| Hyperuricemia | 1 (0.8) |
| Arrhythmia | 6 (4.9) |
| Heart failure | 1 (0.8) |
| Vasospastic angina | 1 (0.8) |
| Thoracic aortic aneurysm | 2 (1.6) |
| Atrial septal defect | 1 (0.8) |
| Valvular disease (moderate and over) | 4 (3.3) |
| VTE | 2 (1.6) |
| Medication | |
| Beta-blocker | 5 (4.1) |
| CCB | 17 (13.8) |
| ACE inhibitor | 1 (0.8) |
| ARB | 15 (12.2) |
| MRA | 3 (2.4) |
| Loop diuretic agent | 3 (2.4) |
Values are median (interquartile range) or n (%).
ACE = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; CCB = calcium channel blocker; MRA = mineralocorticoid receptor antagonist; VTE = venous thromboembolism.
Table 2.
Cases of Osimertinib-Induced Cardiac Adverse Events
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age, yrs | 78 | 71 | 68 | 64 | 52 | 71 |
| Sex | Female | Female | Male | Female | Female | Female |
| EGFR mutation | L858R | L858R+T790M | Ex.19 del+T790M | L858R+T790M | L858R | L858R |
| Osimertinib line | 2nd | 3rd | 3rd | 3rd | 1st | 1st |
| Osimertinib effect | PR | PR | PR | PR | PR | NE |
| Tobacco | No | No | No | Yes (former) | Yes (former) | No |
| CVD risk/history | HTN Aortic aneurysm |
HTN | Moderate AR HU |
Moderate MR | Obesity | HTN DM |
| Daily medications | Carvedilol 20 mg; Nifedipine CR 40 mg; Azilsartan 40 mg; Rosuvastatin 5 mg |
Amlodipine 5 mg | Allopurinol 100 mg; Prednisolone 5 mg | Prednisolone 7.5 mg | - | Candesartan 8 mg |
| Symptoms | Exertional dyspnea; leg edema |
Fatigue | Leg edema; fatigue |
Facial/leg edema | - | Chest pain; dyspnea |
| Cardiac event | Heart failure; QT prolongation |
MR progression; mitral valve prolapse |
TR progression | EF decline; HTN; MR progression |
EF decline | Acute myocardial infarction |
| CTCAE Grade | 3 | 3 | 3 | 3 | 3 | 4 |
| Time to event | 3 months | 3 months | 1 month | 9 months | 2 weeks | 2 months |
| NT-proBNP or BNP, pg/ml | NT-proBNP 18,890 | BNP 21.9 | NT-proBNP 450 | BNP 227.9 | NT-proBNP 36 | BNP 423.4 |
| LVEF before osimertinib, % | 61 | 82 | 74 | 72 | 63 | 69 |
| LVEF after osimertinib initiation, % | 28 | 74 | 60 | 50 | 41 | 42 |
| CTRCD | Yes | No | No | Yes | Yes | Yes |
| RV biopsy | Yes | No | No | No | Yes | No |
| Valvular disease | MR; (−) → severe (3–4) |
MR; trace (0–1) → severe (3–4), prolapse |
TR; trace (0–1) → moderate–severe (3-4) |
MR; mild–moderate (2–3) → moderate (3) |
- | - |
| Osimertinib treatment | Discontinued | Discontinued | Reduced dosage from 80 mg to 40 mg every other day | Temporarily held and resumed at 80 mg daily | Discontinued | Discontinued |
| Subsequent cancer therapy | Gefitinib | Gefitinib | Osimertinib rechallenge | Osimertinib rechallenge | Afatinib | Erlotinib |
| Treatment for cardiac event (daily medications) | Furosemide 40 mg; Spironolactone 50 mg; Candesartan 2 mg; Carvedilol 5 mg; Tolvaptan 7.5 mg |
Furosemide 20 mg | Furosemide 40 mg; Tolvaptan 3.75 mg |
Furosemide 20 mg; Spironolactone 25 mg | Candesartan 4 mg | PCI for LAD 6 |
| Return of LVEF to baseline | No 48% after 9 months |
Yes 74% after 6 months |
Yes 72% after 2 months |
Yes 62% after 14 months |
Yes 63% after 2 months |
No 54% after 7 months |
AR = aortic valve regurgitation; BNP = brain natriuretic peptide; CTCAE = common terminology criteria of adverse event; CTRCD = cancer therapeutics-related cardiac dysfunction; CVD = cardiovascular disease; DM = diabetes mellitus; LVEF = left ventricular ejection fraction; HTN = hypertension; HU = hyperuricemia; LAD = left anterior descending coronary artery; MR = mitral regurgitation; NA = not available; NE = not evaluable; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PCI = percutaneous coronary intervention; PR = partial response; RV = right ventricle; TR = tricuspid regurgitation.
Case series
Case 1
A 78-year-old female patient with advanced NSCLC harboring an EGFR L858R mutation was treated with osimertinib as the second-line therapy. She had a history of hypertension for more than 30 years and had undergone aortic graft replacement due to thoracic aortic aneurysm. Her daily medications were azilsartan 40 mg; nifedipine 40 mg; carvedilol 20 mg; and rosuvastatin 5 mg. After 3 months of osimertinib treatment, she presented with dyspnea. An ECG showed that the QTc was prolonged to 562 ms. N-terminal pro–B-type natriuretic peptide (NT-proBNP) and troponin I levels were elevated at 18,890 pg/ml and 0.416 ng/ml, respectively. Echocardiography revealed severely reduced LVEF at 28% and an increased LVIDd/LVIDs at 44/38 mm, with moderate to severe mitral regurgitation (MR). Before osimertinib administration, her baseline LVEF was 61% and LVIDd/LVIDs were 44/29 mm. Coronary angiography revealed no significant coronary artery stenosis, and myocardial biopsy was performed to further understand the pathophysiology of her heart failure. Histopathology analysis of right ventricle biopsy specimens revealed moderately hypertrophied cardiomyocytes with disarray and deposition of lipofuscin (Figure 2A). Mild interstitial edema and fibrotic changes were observed, but no inflammatory cell infiltration was detected. Due to her long-term history of hypertension, it was believed that her cardiac dysfunction was accelerated with osimertinib in the setting of hypertensive heart disease. Osimertinib was discontinued, and furosemide 40 mg, spironolactone 50 mg, tolvaptan 7.5 mg, carvedilol 5 mg, and candesartan 2 mg, daily were initiated and resulted in an improvement of her heart failure symptoms. Her LVEF remained at 26% after 3 months of osimertinib discontinuation but gradually improved to 48% after 9 months.
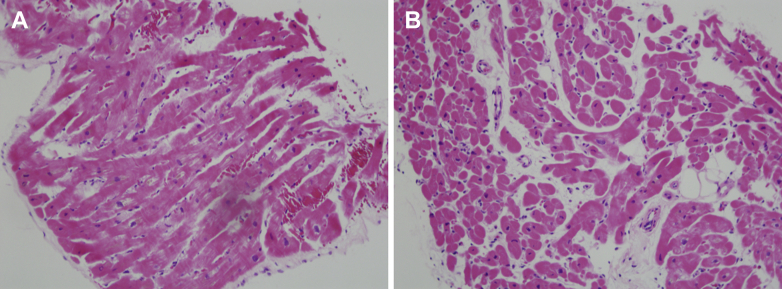
Figure 2.
Histology Assessment of Myocardial Biopsy Specimens
Hematoxylin-eosin-stained specimens of myocardial biopsy are shown at an original magnification of ×200. (A) In Case 1, cardiomyocytes were moderately hypertrophied with disarray and deposition of lipofuscin. There was slight interstitial edema and fibrosis but no inflammatory cell infiltration. (B) In Case 5, cardiomyocytes were mildly hypertrophied with focal vacuolization and deposition of lipofuscin, indicative of myocyte damage. Interstitial edema and fatty infiltration with partial fibrosis were observed around the vasculature, whereas lymphocyte infiltration was modest.
Case 2
A 72-year-old female patient was treated with osimertinib as a third-line therapy for advanced NSCLC harboring the EGFR L858R and T790M mutations. She had had a history of hypertension for 15 years, which was treated with amlodipine 5 mg daily. After 3 months of osimertinib, she presented with fatigue. Echocardiography revealed severe MR with mitral valve prolapse and LVEF of 74%, which was not detected by the echocardiography obtained before osimertinib treatment. Osimertinib was discontinued, and furosemide 20 mg, was started. After 1 month, her symptoms improved, but the MR persisted. Given the severity of MR, 250 mg of gefitinib was started as the fourth-line therapy. Four months after gefitinib therapy, her MR did not worsen, and her advanced NSCLC did not progress.
Case 3
A 68-year-old male was treated with osimertinib as the third-line therapy for advanced NSCLC harboring the EGFR Ex.19 del. and T790M mutations. He was previously treated with gefitinib and erlotinib for more than 7 years before osimertinib. He had a history of hepatitis B but no history of cardiovascular disease. After 3 months of osimertinib, he presented with fatigue and pitting edema of the lower extremities. ECG showed QTc prolongation, and serum laboratory tests showed severe hyponatremia (120 mEq/l). Echocardiography revealed severe tricuspid valve regurgitation (TR) and mild pulmonary hypertension (estimated pulmonary artery systolic pressure: 45 mm Hg), which had not been observed before osimertinib administration. Osimertinib was discontinued, and tolvaptan 3.75 mg, and furosemide 40 mg daily, were initiated. After 1 month, fatigue and edema improved, but severe TR persisted. Because of the presence of the EGFR T790M mutation and lack of alternative EGFR-TKIs to consider, low-dose osimertinib (40 mg daily) was initiated with careful follow-up. After 6 months of re-administration of osimertinib, TR and NSCLC did not progress.
Case 4
A 64-year-old female was treated with osimertinib as the third-line therapy for advanced NSCLC harboring the EGFR L858R and T790M mutations. She had a history of untreated moderate MR. Before osimertinib initiation, she had received erlotinib and experienced erlotinib-associated interstitial pneumonitis, which was treated with prednisolone. After 9 months of osimertinib, she presented with fatigue and pitting edema of her lower extremities. NT-proBNP was elevated at 227.9 pg/ml and LVEF decreased from 72% to 50%. Osimertinib was discontinued, and she was treated with furosemide 20 mg; and spironolactone 25 mg daily. After 1 month of close observation, her symptoms resolved, LVEF improved to 58%, and NT-proBNP decreased to 75.2 pg/ml. Osimertinib was restarted at 80 mg daily after LVEF improvement. Follow-up showed there were no signs of additional cardiac dysfunction; LVEF was most recently reported at 62% after 6 months.
Case 5
A 52-year-old woman was treated with osimertinib as the first-line therapy for her advanced NSCLC harboring the EGFR L858R mutation. She had no history of cardiovascular risk factors or disease except for obesity (body mass index: 29.7 kg/m2). After 2 weeks of osimertinib, screening echocardiography revealed a reduction of LVEF from 63% to 41% without cardiac symptoms. An ECG showed normal QTc, and NT-proBNP and troponin I concentrations were also within normal ranges at 36 pg/ml and <0.01 ng/ml, respectively. Coronary angiography revealed no significant stenosis in her coronary arteries, and right ventricular myocardial biopsy was performed. Biopsy analysis revealed mildly hypertrophied cardiomyocytes with deposition of lipofuscin, focal vacuolization, and focal degradation (Figure 2B). Edematous changes and fatty infiltration with partial fibrosis around the vasculature were detected in the interstitial space, and a small amount of inflammatory cell infiltration was observed, but there were no signs of cardiomyocyte death. After osimertinib was discontinued and candesartan 4 mg daily, was initiated, the LVEF recovered to 63%. The patient was then treated with afatinib, a second-generation of EGFR-TKI, and her cardiac function remained stable.
Case 6
A 71-year-old woman was treated with osimertinib as the first-line therapy for advanced case of NSCLC harboring the EGFR L858R mutation. She had a long-standing history of hypertension, which had been treated with 8 mg of candesartan daily, and diabetes mellitus. After 2 months of osimertinib, she presented with sudden dyspnea at rest following chest pain. ECG showed QS pattern in V1–4. Her creatine phosphokinase concentration was 163 U/l, her creatine kinase-myocardial band was 7.2 ng/ml, and her troponin I level was elevated at 2.945 ng/ml. Echocardiography showed anteroseptal hypokinesis and an LVEF of 42%. Coronary angiography revealed 99% stenosis with delayed flow in the left anterior descending artery. Percutaneous coronary intervention of the left anterior descending artery was performed. The patient was then treated with erlotinib instead of osimertinib.
Osimertinib rechallenge or discontinuation
In each of the above cases, the decision regarding the risks and benefits of continuing osimertinib was made on an individual basis, after detailed consideration of the patients’ histories and preferences. For the patient described in case 1, LVEF recovery was delayed, and gefitinib was started 8 months after the AE. The patient in case 2 refused osimertinib re-administration and also received gefitinib. Osimertinib was re-administered to the patients described in cases 3 and 4, given the presence of the T790M mutation, whereas patients were treated with other EGFR-TKIs in cases 5 and 6. Altogether, osimertinib was discontinued in 4 of 6 patients and restarted in 2 patients; in one of those patients, osimertinib was re-administered at the reduced dose and at the original dose in the other patient.
Assessment of serial changes QTc and LVEF in NSCLC patients treated with osimertinib
Among the 123 NSCLC patients treated with osimertinib, 72 patients underwent serial ECG before and after osimertinib initiation. The average QTc was significantly prolonged from 421.9 ± 23.0 ms to 442.4 ± 33.2 ms (p < 0.001) over a median of 116 days (interquartile range 16 to 851 days) after osimertinib initiation. Grade 3 QTc prolongation >501 ms was observed in 2 patients (562 and 517 ms, respectively); Grade 2 (QTc between 481 and 500 ms), and Grade 1 (between 450 and 480 ms) in 18 patients. No episodes of fatal arrhythmia were documented in this cohort.
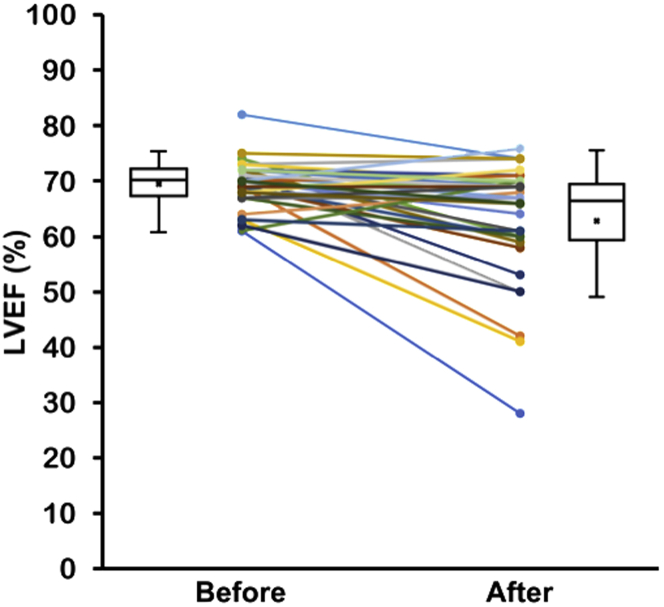
Among the 123 patients, 36 NSCLC patients were identified who underwent echocardiography both before and after osimertinib administration within a 1 years’ time period (Figure 1). Echocardiography was performed in 10 patients as part of the protocol for the FLAURA study or other clinical trials and in 12 patients for suspected cardiovascular complications. The patient characteristics of these 36 patients with serial echocardiography are displayed in Table 3. The median age was 68 years old; 72.2% were female; and 47.2% had hypertension. Overall, LVEF significantly decreased after osimertinib treatment from 69.4 ± 4.2% at baseline to 63.4 ± 10.5% (p < 0.001) (Figure 3, Table 4), and LVIDd/LVIDs also significantly increased from 42.6 ± 4.5/26.3 ± 3.3 mm to 44.5 ± 5.2/29.1 ± 5.6 mm (p = 0.005) (Table 4). Although the changes in LVEF were relatively small and the average LVEF was still in the normal range, the declines in LVEF in this subgroup of patients were statistically significant.
Table 3.
Characteristics of 36 NSCLC Patients Treated With Osimertinib with Serial Echocardiography Monitoring
| All (N = 36) | Non-CTRCD (n = 32) | CTRCD (n = 4) | |
|---|---|---|---|
| Age, yrs | 68 (63–74) | 68 (63–74) | 68 (55–76) |
| Females | 26 (72.2) | 22 (68.8) | 4 (100) |
| Smoking | 12 (33.3) | 10 (34.3) | 2 (50) |
| Medical history | |||
| Hypertension | 17 (47.2) | 15 (46.9) | 2 (50) |
| Diabetes mellitus | 1 (2.8) | 0 | 1 (25) |
| Dyslipidemia | 5 (13.9) | 5 (15.6) | 0 |
| Hyperuricemia | 1 (2.8) | 1 (3.1) | 0 |
| Atrial fibrillation | 2 (5.6) | 2 (6.2) | 0 |
| Vasospastic angina | 1 (2.8) | 1 (3.1) | 0 |
| Thoracic aortic aneurysm | 1 (2.8) | 0 | 1 (25) |
| Atrial septal defect | 1 (2.8) | 1 (3.1) | 0 |
| Valvular disease (moderate or greater) | 4 (11.1) | 3 (3.9) | 1 (25) |
| Medication | |||
| Beta-blocker | 2 (5.6) | 1 (3.1) | 1 (25) |
| CCB | 9 (25) | 8 (25.0) | 1 (25) |
| ACE inhibitor | 0 | 0 | 0 |
| ARB | 8 (22.2) | 6 (18.8) | 2 (50) |
| MRA | 1 (2.8) | 1 (3.1) | 0 |
| Loop diuretic | 1 (2.8) | 1 (3.1) | 0 |
Values are median (interquartile range) or n (%).
ACE = angiotensin-converting enzyme; ARB = angiotensin II receptor blocker; CCB = calcium channel blocker; CTRCD = cancer therapeutics-related cardiac dysfunction; LVEF = left ventricular ejection fraction; MRA = mineralocorticoid receptor antagonist.
Figure 3.
Changes in LVEF Before and After Osimertinib Administration
Echocardiography was performed within at 2 years before and 1 year after osimertinib initiation. The LVEF of each individual patient is indicated as a round dot. Mean LVEFs are indicated cross marks in the boxes. Overall, LVEF significantly decreased after osimertinib (p < 0.001). Abbreviations as in Figure 1.
Table 4.
Comparison of Echocardiographic Parameters Before and After Osimertinib Administration
| NSCLC (N = 36) |
Non-CTRCD (n = 32) |
CTRCD (n = 4) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | p Value∗ | Before | After | p Value† | Before | After | p Value‡ | |
| LVEF, % | 69.4 ± 4.2 | 63.4 ± 10.5 | <0.001 | 69.8 ± 4.0 | 67.9 ± 5.5 | 0.004 | 66.3 ± 5.1 | 40.3 ± 9.1 | <0.001 |
| LVIDd, mm | 42.6 ± 4.5 | 44.5 ± 5.2 | 0.005 | 42.4 ± 4.4 | 43.9 ± 4.8 | 0.003 | 44.0 ± 4.9 | 48.8 ± 7.2 | 0.344 |
| LVIDs, mm | 26.3 ± 3.3 | 29.1 ± 5.6 | <0.001 | 26.0 ± 3.1 | 27.8 ± 3.9 | <0.001 | 28.2 ± 4.3 | 39.3 ± 7.5 | 0.001 |
| E peak, cm/s | 66.7 ± 18.0 | 73.4 ± 19.2 | 0.017 | 67.3 ± 18.2 | 73.1 ± 17.9 | 0.035 | 62.4 ± 18.1 | 75.7 ± 31.4 | 0.858 |
| Dct, ms | 222.6 ± 54.0 | 217.9 ± 39.2 | 0.625 | 225.2 ± 55.9 | 216.6 ± 31.3 | 0.333 | 201.5 ± 32.4 | 228.5 ± 87.3 | 0.817 |
| E/A ratio | 0.90 ± 0.26 | 1.04 ± 0.36 | 0.013 | 0.90 ± 0.28 | 1.04 ± 0.38 | 0.047 | 0.70 ± 0.14 | 0.98 ± 0.26 | 0.870 |
| HR, beats/min | 74.2 ± 10.8 | 75.1 ± 14.6 | 0.600 | 74.3 ± 11.3 | 74.3 ± 14.5 | 0.875 | 73.5 ± 7.0 | 81.6 ± 16.5 | 0.442 |
Values are mean ± SD.
CTRCD = cancer therapeutics-related cardiac dysfunction; Dct = deceleration time; E peak = peak velocity of early transmitral flow; E/A ratio = peak velocities of early-to-late ratio of transmitral flow; HR = heart rate; LVEF = left ventricular ejection fraction; LVIDd = left ventricular end-diastolic dimension; LVIDs = left ventricular end-systolic dimension.
p value comparing echocardiographic parameters before and after osimertinib in 36 NSCLC patients with serial monitoring.
p value comparing parameters before and after in 32 patients without CTRCD.
p value comparing parameters between after in 32 non-CTRCD and after in 4 with CTRCD.
Among the 36 patients, 4 patients experienced declines in LVEF of > 10% to <53% after osimertinib (mean LVEF: 40.3 ± 9.1%) (Figure 1, Table 4), corresponding to a widely accepted definition of CTRCD (7). These patients were also categorized as CTCAE grade 3 of LVEF decreased. As expected, patients with CTRCD had a significantly lower LVEF and larger LVIDd/lVIDs than those who did not have CTRCD (Table 4). However, the early mitral inflow velocity and mitral annular early diastolic velocity (E/e′) ratio was similar in both groups with analyzable diastolic function parameters; this did not change with osimertinib (12.8 ± 4.6% and 12.6 ± 3.3%, respectively, in the non-CTRCD group [n = 28] and 14.6 ± 6.6% and 15.7 ± 7.9%, respectively, in the CTRCD group [n = 3]).
Discussion
A retrospective analysis of cardiac AEs was performed in NSCLC patients harboring EGFR mutations treated with osimertinib. This is the first study to describe osimertinib-associated cardiac AEs in a real-world Japanese NSCLC population (Central Illustration). Overall, we observed a 4.9% incidence of grade 3 or higher CTCAE version 5.0 cardiac AEs. Five of the 6 patients were female, and all 5 had a history of cardiovascular disease and/or cardiovascular risk factors. Our observations suggest that cardiac AEs may occur more commonly in patients with pre-existing cardiovascular disease. However, the present cohort was small, and these were single-center retrospective analyses where data were not collected in a standardized, prospective manner. Further investigations are necessary to further understand the associations between osimertinib and cardiac AEs in NSCLC patients harboring EGFR mutations, including defining the predictors, natural history, and potential mechanisms of toxicity.
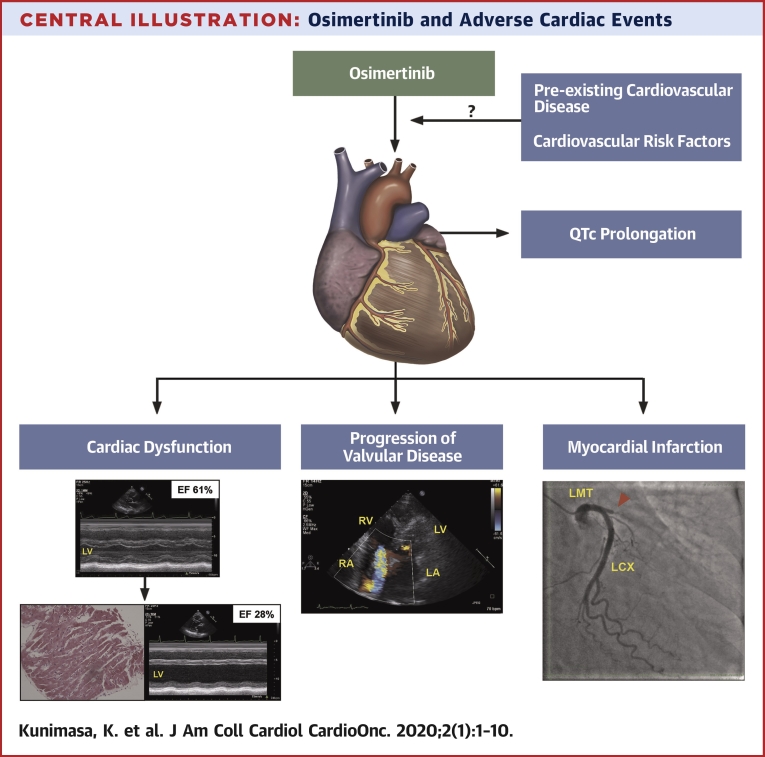
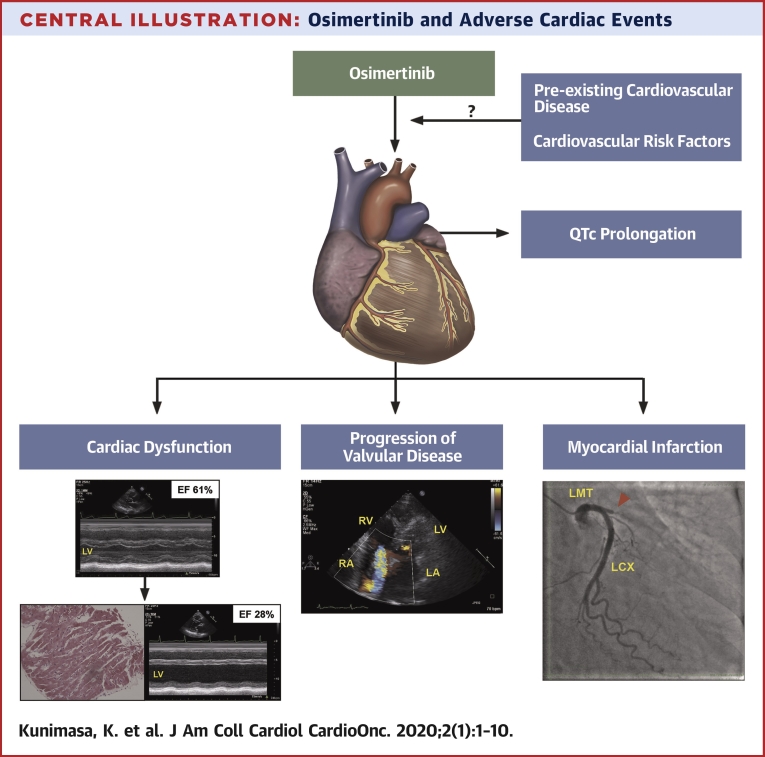
Central Illustration.
Osimertinib and Adverse Cardiac Events
In this retrospective analysis, the incidence of cardiac adverse events was 4.9% including cardiac systolic dysfunction, progression of valvular disease, myocardial infarction, and QTc prolongation. Further investigations are necessary to clarify the associations between osimertinib and cardiac AEs in NSCLC patients harboring EGFR mutations. AE = adverse events; other abbreviations as in Figures 1 and 2.
Commonly reported osimertinib-associated AEs in Phase 1 to 3 clinical trials include diarrhea, rash, nausea, and decreased appetite. Most concerning AEs reported to date include interstitial lung disease and QTc prolongation (2, 3, 4). Generally, osimertinib has a more favorable toxicity profile than earlier generation EGFR-TKIs because it has a lower affinity for wild-type EGFR (1,4). A post-marketing surveillance study of osimertinib in 3,578 Japanese NSCLC patients with EGFR mutations suggested that the incidence of grade 3 or higher QTc prolongation occurred in only 0.1% and other grade 3 or higher cardiac AEs in only 0.8% of patients (6). However, U.S. Food and Drug Administration (FDA) reports suggest that cardiomyopathy associated with osimertinib occurred in 1.9% of patients (16 of 833), and LVEF declined ≥ 10% to <50% in 4.0% of patients (26 of 655) (FDA Tagrisso prescribing information [reference ID: 4077625; revised: March 2017]).
Our study indicates that osimertinib-associated cardiac AEs occur at a rate (4.9%) similar to that reported by the FDA, but not by other large retrospective analyses (6). Because 5 of 6 cases had pre-existing cardiovascular risk factors or disease, osimertinib may further worsen underlying cardiac pathology. Unfortunately, only a small subset of patients underwent serial echocardiography before and after osimertinib administration, limiting a more detailed understanding of the changes in cardiac function that occur with osimertinib. Functional assessment before and at standardized intervals during treatment with osimertinib can help elucidate the true rate of cardiac dysfunction.
The common histopathological findings of cases 1 and 5 were myocyte hypertrophy and lipofuscin deposition, which is seen in heart failure (8). Both of the cases had pre-existing cardiovascular risk factors, including either hypertension or obesity, which might have also worsened or resulted in cardiomyocyte hypertrophy. Focal vacuolization and degeneration of myocytes were also observed in case 5, which is suggestive of myocyte damage (9), potentially from osimertinib. There are 2 published case reports of osimertinib-associated cardiac dysfunction (10,11), and myocarditis with lymphocyte infiltration without myocyte hypertrophy has been described (10). Although a few lymphocytes infiltrated the specimen of case 5, typical myocyte degeneration/necrosis with adjacent mononuclear cell infiltration was not observed in either case 1 or 5. Nevertheless, the histopathologic findings from these 2 cases suggest that osimertinib does not result in a pattern of myocyte death seen with anthracyclines nor myocarditis seen with immune checkpoint inhibitors and that cardiac dysfunction associated with osimertinib may result from functional inhibition of myocyte contractility without marked cell death or inflammation.
Recent guidelines for heart failure suggest that early detection of cardiac dysfunction and cardioprotection in the setting of cardiac risk factors before symptomatic heart failure may be beneficial (12). This study raises the question of whether there is a need for routine monitoring of cardiac function with osimertinib to detect asymptomatic declines in LVEF and whether such declines should be treated with pharmacologic intervention.
Our findings also suggest statistically significant increases in the QTc interval. Although a strong emphasis has been placed on osimertinib-associated QTc prolongation because of its reported frequency, no fatal episodes of arrhythmia associated with osimertinib in this cohort were detected. Other recent reports have suggested an increased odds ratio for heart failure, atrial fibrillation, and QTc prolongation in osimertinib compared with other TKIs based on the FAERS (FDA Events Reporting System) (13). No new onset of atrial fibrillation associated with osimertinib was detected in the present cohort; however, as suggested by the FAERS study, we did observe an increased risk of heart failure.
Study limitations
First, this is a retrospective single-center study. As such, the sample size was small. However, a detailed, careful review of each individual patient’s medical record was performed. Second, there was no established, standardized protocol for cardiovascular monitoring and follow-up in this population. There may be selection bias in those patients who underwent serial echocardiography or electrocardiogram evaluation, and as such, the true incidence of cardiac dysfunction may also be underestimated. The timing of follow-up echocardiography was also highly variable, from 5 to 327 days, potentially resulting in additional detection bias. Third, LVEF was quantified using a 2D Teichholz method and not by biplane Simpson’s method. Fourth, the case series of Grade 3 cardiac AEs was small, and biopsy data were not available in all patients, limiting the interpretation of our findings.
Conclusions
In this retrospective cohort study, the incidence of cardiac AEs in patients treated with osimertinib was similar to that observed in the clinical trials but unexpectedly higher than that observed in the post-marketing surveillance in Japan. Additional prospective data collection of advanced NSCLC patients treated with osimertinib will be important in understanding the incidence, pathophysiology, and management of cardiac AEs with osimertinib.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: QTc prolongation is well described with osimertinib. However, the incidence of other cardiac AEs is less well described. Among 123 NSCLC patients treated with osimertinib, cardiac AEs occurred in 4.9%. These included acute myocardial infarction, heart failure, and valvular diseases. These AEs may occur more commonly in patients with pre-existing cardiovascular disease or risk factors.
TRANSLATIONAL OUTLOOK: Further studies should focus on elucidating the mechanisms underlying cardiac AEs associated with osimertinib. Moreover, prospective studies with standardized data collection are needed to define the epidemiology of osimertinib associated cardiac disease, and clinical studies are needed to understand strategies to prevent and treat cardiac AEs.
Acknowledgment
The authors thank Dr. Hatsue Ueda, Department of Pathology, National Cerebral and Cardiovascular Center, Suita, Japan, for valuable comments on myocardial biopsies.
Footnotes
Supported by Japan Society for the Promotion of Science KAKEN grant JP16K09470 to Dr. Oka. Dr. Nishino has received speaker honoraria from AstraZeneca, Chugai Pharmaceutical Co., Boehringer Ingelheim, and Novartis Pharmaceuticals; and has received research support from Nippon Boehringer Ingelheim. Dr. Imamura has received honoraria and research support from AstraZeneca. Dr. Kumagai has received speaker honoraria and research support from AstraZeneca, Chugai Pharmaceutical, Pfizer, Nippon Boehringer Ingelheim, and Delta-Fly Pharma. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: CardioOncologyauthor instructions page.
References
- 1.Cross D.A., Ashton S.E., Ghiorghiu S. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok T.S., Wu Y.L., Ahn M.J. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soria J.C., Ohe Y., Vansteenkiste J. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 4.Mezquita L., Varga A., Planchard D. Safety of osimertinib in EGFR-mutated non–small cell lung cancer. Expert Opin Drug Saf. 2018;17:1239–1248. doi: 10.1080/14740338.2018.1549222. [DOI] [PubMed] [Google Scholar]
- 5.Ramalingam S.S., Vansteenkiste J., Planchard D. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 6.AstraZeneca K.K. Tagrisso tablets 40 mg, Tagrisso tablets 80 mg, the result of the use-results surveys final report. 2019. https://www.mhlw.go.jp/content/11120000/000484135.pdf [in Japanese]. Available at:
- 7.Plana J.C., Galderisi M., Barac A. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Kakimoto Y., Okada C., Kawabe N. Myocardial lipofuscin accumulation in ageing and sudden cardiac death. Sci Rep. 2019;9:3304. doi: 10.1038/s41598-019-40250-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zuppinger C., Timolati F., Suter T.M. Pathophysiology and diagnosis of cancer drug induced cardiomyopathy. Cardiovasc Toxicol. 2007;7:61–66. doi: 10.1007/s12012-007-0016-2. [DOI] [PubMed] [Google Scholar]
- 10.Oyakawa T., Nakashima K., Naito T. Cardiac Dysfunction caused by osimertinib. J Thorac Oncol. 2017;12:e159–e160. doi: 10.1016/j.jtho.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe H., Ichihara E., Kano H., Ninomiya K., Tanimoto M., Kiura K. Congestive heart failure during osimertinib treatment for epidermal growth factor receptor (EGFR)-mutant non–small cell lung cancer (NSCLC) Intern Med. 2017;56:2195–2197. doi: 10.2169/internalmedicine.8344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldberg L.R., Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation. 2006;113:2851–2860. doi: 10.1161/CIRCULATIONAHA.105.600437. [DOI] [PubMed] [Google Scholar]
- 13.Anand K., Ensor J., Trachtenberg B., Bernicker E.H. Osimertinib-induced cardiotoxicity: a retrospective review of the FDA adverse events reporting system (FAERS) J Am Coll Cardiol CardioOnc. 2019;1:172–178. doi: 10.1016/j.jaccao.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]